Submitted:
29 February 2024
Posted:
29 February 2024
You are already at the latest version
Abstract
Keywords:
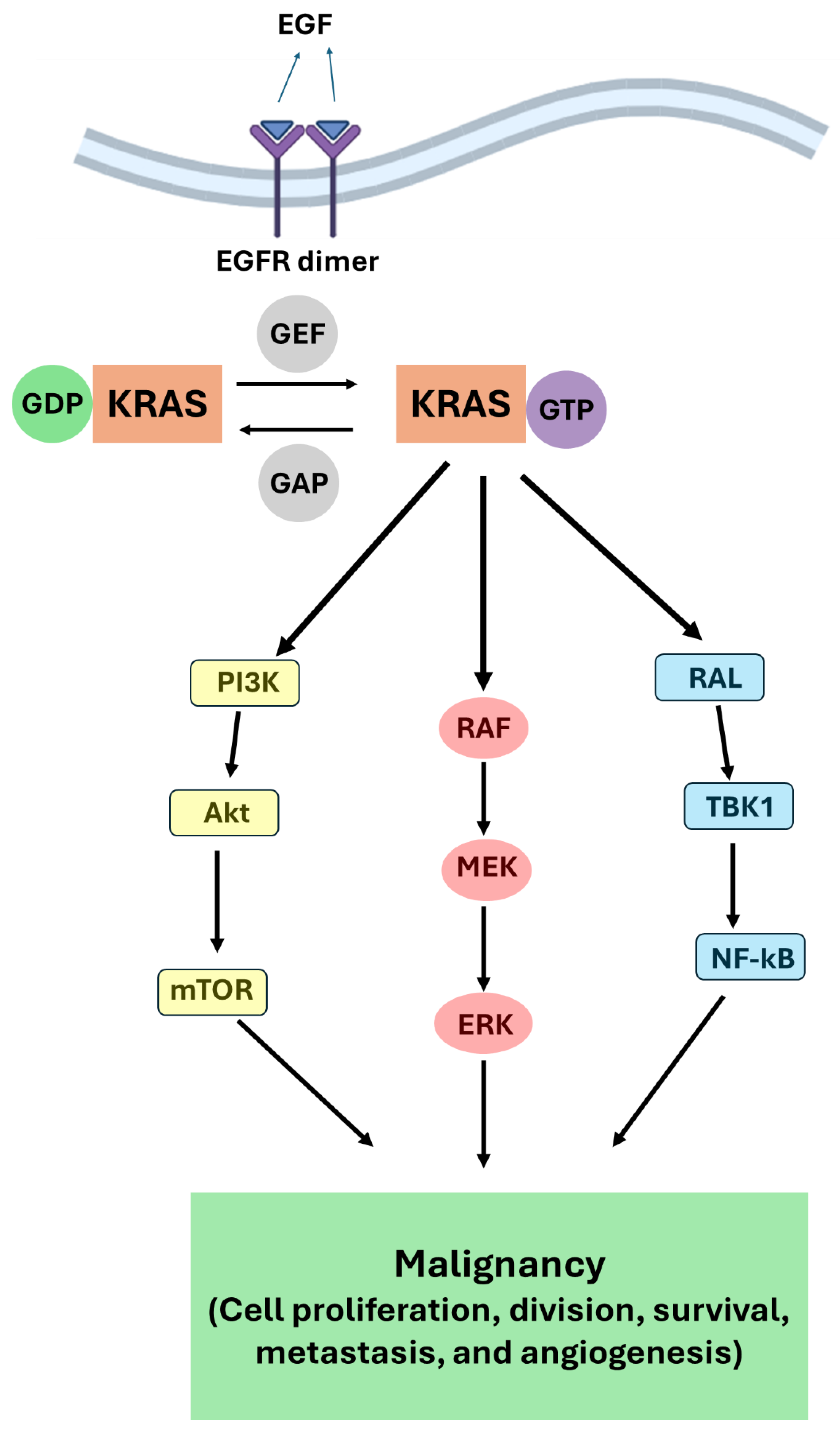
1. Introduction
2. Methods
3. Results
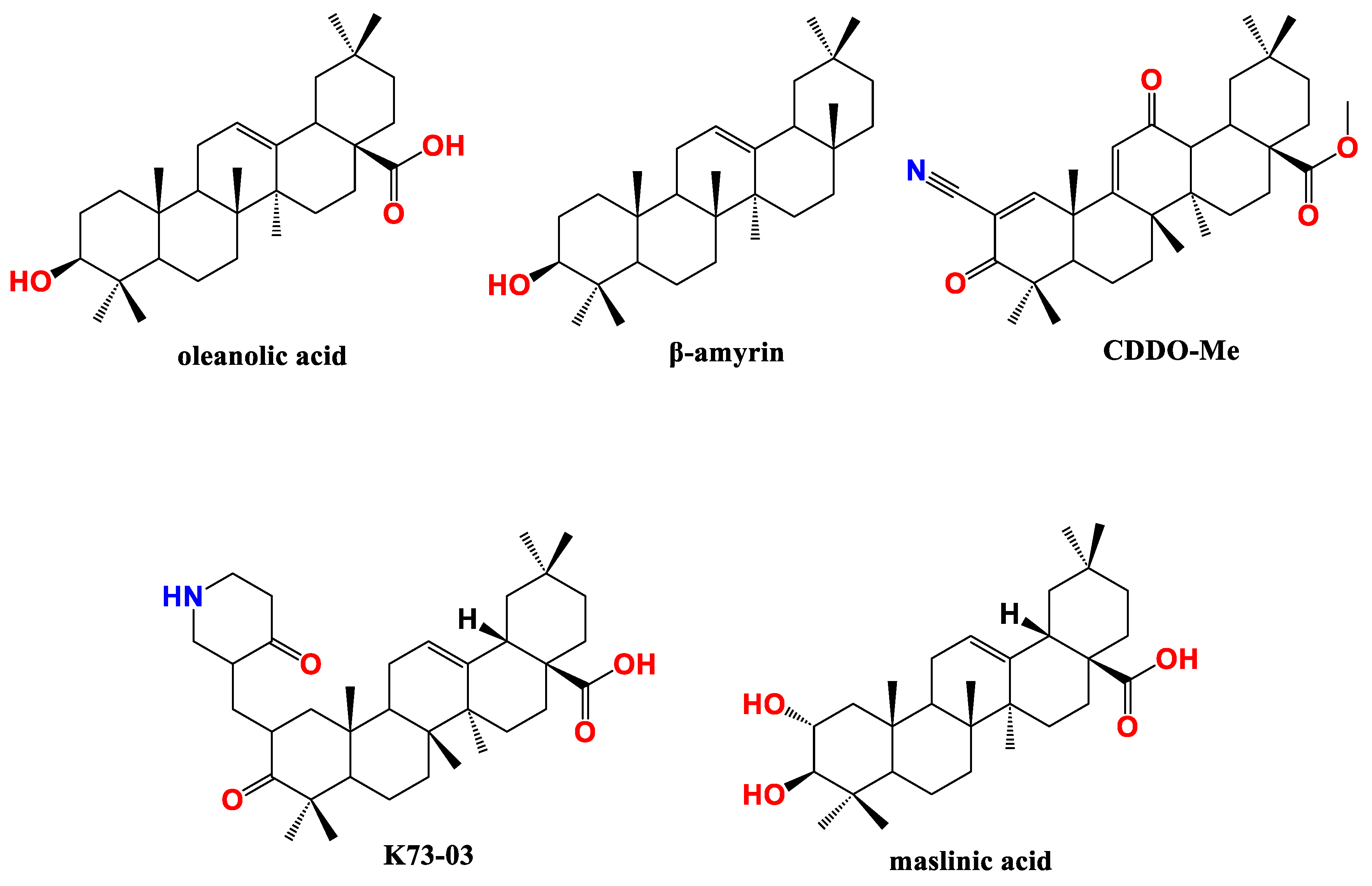
3.1. Oleanane type PTs in pancreatic cancer
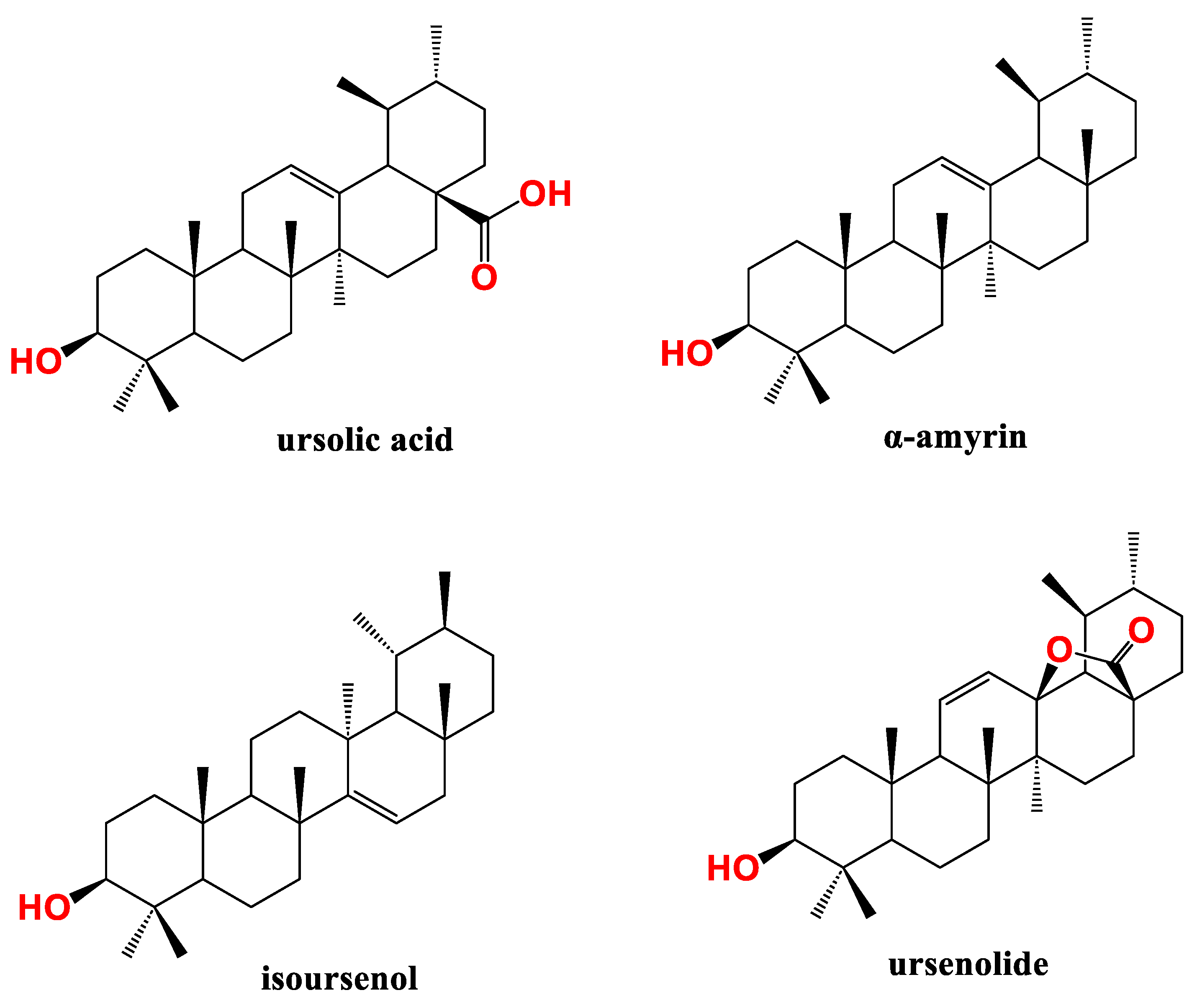
3.2. Ursane PTs in pancreatic cancer
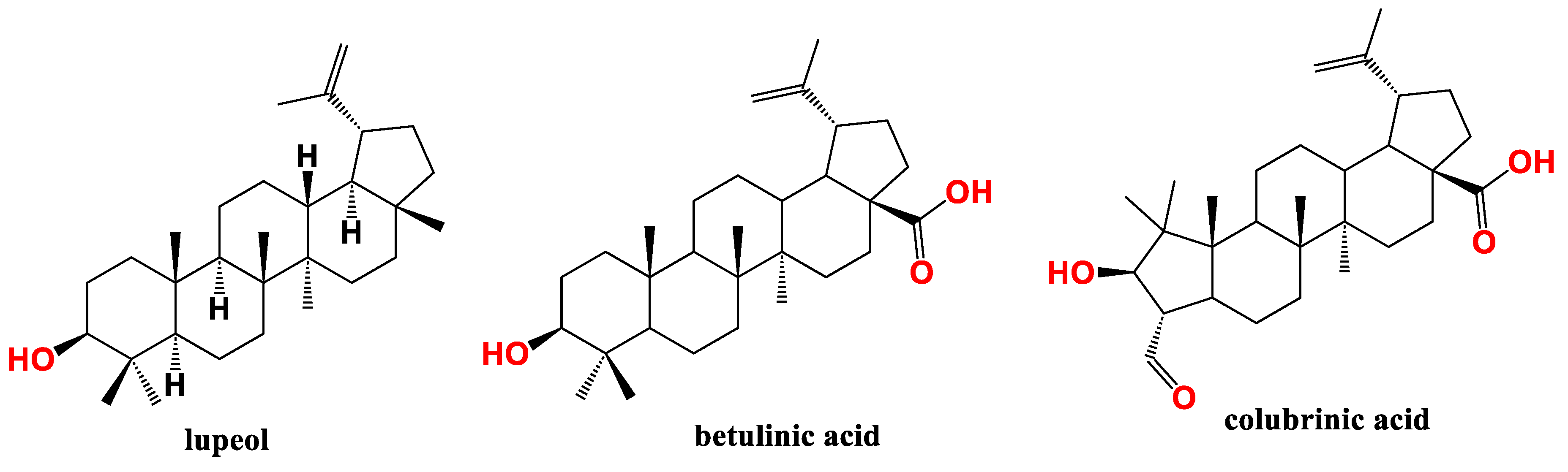
3.3. Lupane type PTs in pancreatic cancer
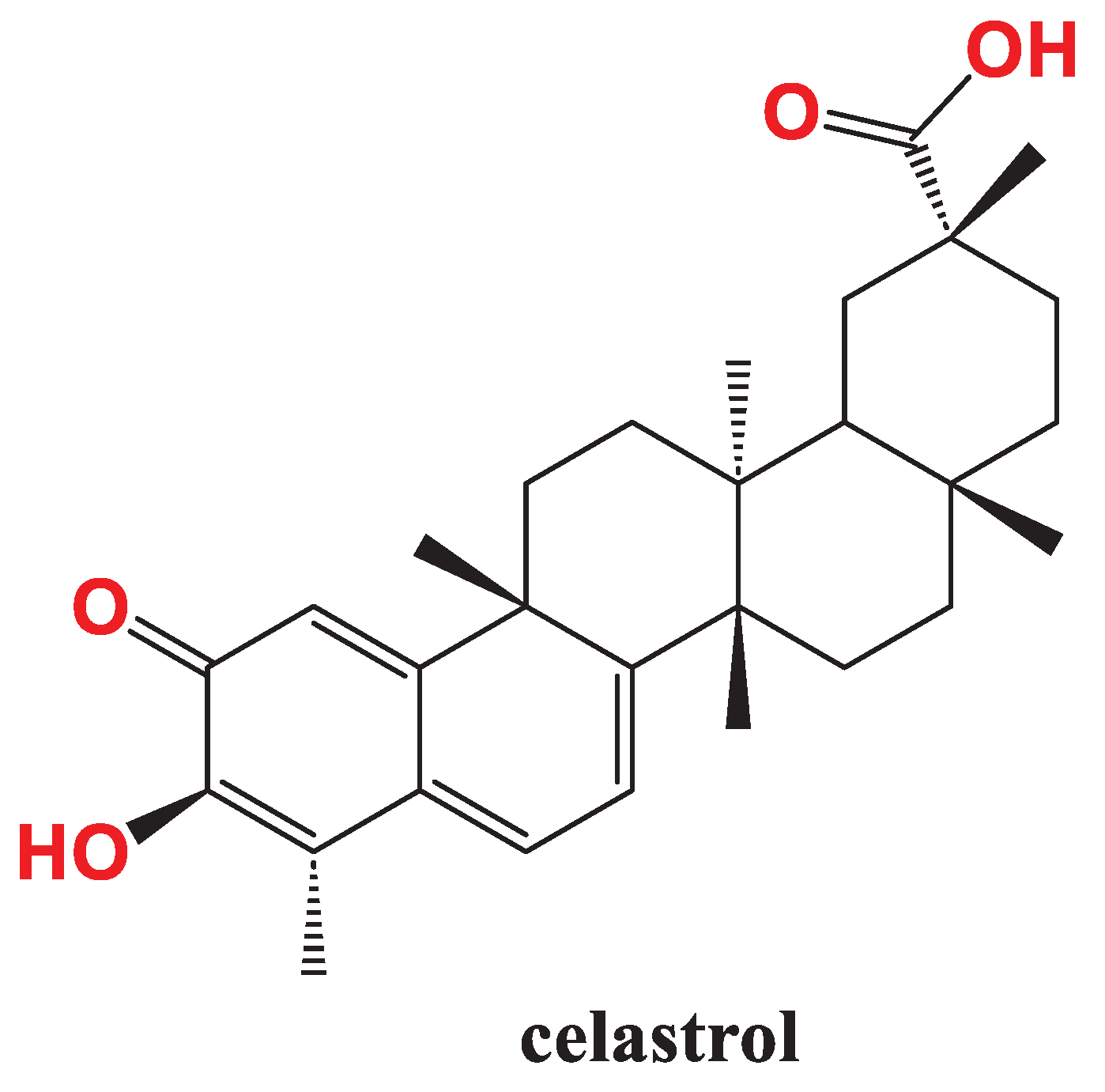
3.4. Friedelane type PTs in pancreatic cancer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA. Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Lucenteforte, E.; Silverman, D.T.; Petersen, G.; Bracci, P.M.; Ji, B.T.; Negri, E.; Li, D.; Risch, H.A.; Olson, S.H.; et al. Cigarette Smoking and Pancreatic Cancer: An Analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Genkinger, J.M.; Spiegelman, D.; Anderson, K.E.; Bergkvist, L.; Bernstein, L.; van den Brandt, P.A.; English, D.R.; Freudenheim, J.L.; Fuchs, C.S.; Giles, G.G.; et al. Alcohol Intake and Pancreatic Cancer Risk: A Pooled Analysis of Fourteen Cohort Studies. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2009, 18, 765–776. [Google Scholar] [CrossRef]
- Johansen, D.; Stocks, T.; Jonsson, H.; Lindkvist, B.; Björge, T.; Concin, H.; Almquist, M.; Häggström, C.; Engeland, A.; Ulmer, H.; et al. Metabolic Factors and the Risk of Pancreatic Cancer: A Prospective Analysis of Almost 580,000 Men and Women in the Metabolic Syndrome and Cancer Project. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2010, 19, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Tanaka, A.; Radwan, M.O.; Hamasaki, A.; Ejima, A.; Obata, E.; Koga, R.; Tateishi, H.; Okamoto, Y.; Fujita, M.; Nakao, M.; et al. A Novel Inhibitor of Farnesyltransferase with a Zinc Site Recognition Moiety and a Farnesyl Group. Bioorg. Med. Chem. Lett. 2017, 27, 3862–3866. [Google Scholar] [CrossRef]
- Klochkov, S.G.; Neganova, M.E.; Yarla, N.S.; Parvathaneni, M.; Sharma, B.; Tarasov, V.V.; Barreto, G.; Bachurin, S.O.; Ashraf, G.M.; Aliev, G. Implications of Farnesyltransferase and Its Inhibitors as a Promising Strategy for Cancer Therapy. Semin. Cancer Biol. 2019, 56, 128–134. [Google Scholar] [CrossRef]
- Aguirre, A.J.; Bardeesy, N.; Sinha, M.; Lopez, L.; Tuveson, D.A.; Horner, J.; Redston, M.S.; DePinho, R.A. Activated Kras and Ink4a/Arf Deficiency Cooperate to Produce Metastatic Pancreatic Ductal Adenocarcinoma. Genes Dev. 2003, 17, 3112–3126. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Wang, L.; Multani, A.S.; Combs, C.; Deramaudt, T.B.; Hruban, R.H.; Rustgi, A.K.; Chang, S.; Tuveson, D.A. Trp53R172H and KrasG12D Cooperate to Promote Chromosomal Instability and Widely Metastatic Pancreatic Ductal Adenocarcinoma in Mice. Cancer Cell 2005, 7, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Bardeesy, N.; Cheng, K.-H.; Berger, J.H.; Chu, G.C.; Pahler, J.; Olson, P.; Hezel, A.F.; Horner, J.; Lauwers, G.Y.; Hanahan, D.; et al. Smad4 Is Dispensable for Normal Pancreas Development yet Critical in Progression and Tumor Biology of Pancreas Cancer. Genes Dev. 2006, 20, 3130–3146. [Google Scholar] [CrossRef] [PubMed]
- Izeradjene, K.; Combs, C.; Best, M.; Gopinathan, A.; Wagner, A.; Grady, W.M.; Deng, C.-X.; Hruban, R.H.; Adsay, N.V.; Tuveson, D.A.; et al. Kras(G12D) and Smad4/Dpc4 Haploinsufficiency Cooperate to Induce Mucinous Cystic Neoplasms and Invasive Adenocarcinoma of the Pancreas. Cancer Cell 2007, 11, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, H.N.; Jeong, M.S.; Jang, S.B. Oncogenic KRAS: Signaling and Drug Resistance. Cancers 2021, 13, 5599. [Google Scholar] [CrossRef] [PubMed]
- Merz, V.; Gaule, M.; Zecchetto, C.; Cavaliere, A.; Casalino, S.; Pesoni, C.; Contarelli, S.; Sabbadini, F.; Bertolini, M.; Mangiameli, D.; et al. Targeting KRAS: The Elephant in the Room of Epithelial Cancers. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Jancík, S.; Drábek, J.; Radzioch, D.; Hajdúch, M. Clinical Relevance of KRAS in Human Cancers. J. Biomed. Biotechnol. 2010, 2010, 150960. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR Signaling Pathway and mTOR Inhibitors in Cancer: Progress and Challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, D.R.; Novick, S.; Kahn, J.M.; Leonardi, P.; Pellicer, A. Rsc: A Novel Oncogene with Structural and Functional Homology with the Gene Family of Exchange Factors for Ral. Oncogene 1997, 14, 1295–1305. [Google Scholar] [CrossRef]
- Lim, K.-H.; O’Hayer, K.; Adam, S.J.; Kendall, S.D.; Campbell, P.M.; Der, C.J.; Counter, C.M. Divergent Roles for RalA and RalB in Malignant Growth of Human Pancreatic Carcinoma Cells. Curr. Biol. CB 2006, 16, 2385–2394. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Li, X. Targeting the Untargetable KRAS in Cancer Therapy. Acta Pharm. Sin. B 2019, 9, 871–879. [Google Scholar] [CrossRef]
- Carbone, C.; Melisi, D. NF-κB as a Target for Pancreatic Cancer Therapy. Expert Opin. Ther. Targets 2012, 16 Suppl 2, S1-10. [Google Scholar] [CrossRef]
- Henry, D.O.; Moskalenko, S.A.; Kaur, K.J.; Fu, M.; Pestell, R.G.; Camonis, J.H.; White, M.A. Ral GTPases Contribute to Regulation of Cyclin D1 through Activation of NF-κB. Mol. Cell. Biol. 2000, 20, 8084–8092. [Google Scholar] [CrossRef]
- Kolbeinsson, H.M.; Chandana, S.; Wright, G.P.; Chung, M. Pancreatic Cancer: A Review of Current Treatment and Novel Therapies. J. Investig. Surg. Off. J. Acad. Surg. Res. 2023, 36, 2129884. [Google Scholar] [CrossRef]
- Groot, V.P.; Rezaee, N.; Wu, W.; Cameron, J.L.; Fishman, E.K.; Hruban, R.H.; Weiss, M.J.; Zheng, L.; Wolfgang, C.L.; He, J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2018, 267, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Halbrook, C.J.; Lyssiotis, C.A.; Pasca di Magliano, M.; Maitra, A. Pancreatic Cancer: Advances and Challenges. Cell 2023, 186, 1729–1754. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Koehn, F.E.; Carter, G.T. The Evolving Role of Natural Products in Drug Discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Plants as a Source of Anti-Cancer Agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospecting 2021, 11, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca Alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar] [PubMed]
- Shah, Z.; Gohar, U.F.; Jamshed, I.; Mushtaq, A.; Mukhtar, H.; Zia-UI-Haq, M.; Toma, S.I.; Manea, R.; Moga, M.; Popovici, B. Podophyllotoxin: History, Recent Advances and Future Prospects. Biomolecules 2021, 11, 603. [Google Scholar] [CrossRef]
- Ali, T.F.S.; Ciftci, H.I.; Radwan, M.O.; Koga, R.; Ohsugi, T.; Okiyama, Y.; Honma, T.; Nakata, A.; Ito, A.; Yoshida, M.; et al. New SIRT2 Inhibitors: Histidine-Based Bleomycin Spin-Off. Bioorg. Med. Chem. 2019, 27, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Koba, M.; Konopa, J. [Actinomycin D and its mechanisms of action]. Postepy Hig. Med. Doswiadczalnej Online 2005, 59, 290–298. [Google Scholar]
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From Marine Origin to Therapeutics: The Antitumor Potential of Marine Algae-Derived Compounds. Front. Pharmacol. 2018, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T. A Compressive Review about Taxol®: History and Future Challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Gao, G.; Zou, G.; Yu, H.; Zheng, X. Natural Products as Adjunctive Treatment for Pancreatic Cancer: Recent Trends and Advancements. BioMed Res. Int. 2017, 2017, e8412508. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, N.; Zhang, Y.; Huang, X.; Wang, Y. The Therapeutic Potential of Natural Products for Treating Pancreatic Cancer. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Ha, J.; Kim, J.; Cho, Y.; Ahn, J.; Cheon, C.; Kim, S.-H.; Ko, S.-G.; Kim, B. Natural Products for Pancreatic Cancer Treatment: From Traditional Medicine to Modern Drug Discovery. Nutrients 2021, 13, 3801. [Google Scholar] [CrossRef]
- Hosseini, M.; Hassanian, S.M.; Mohammadzadeh, E.; ShahidSales, S.; Maftouh, M.; Fayazbakhsh, H.; Khazaei, M.; Avan, A. Therapeutic Potential of Curcumin in Treatment of Pancreatic Cancer: Current Status and Future Perspectives. J. Cell. Biochem. 2017, 118, 1634–1638. [Google Scholar] [CrossRef]
- Rambam Health Care Campus Phase II Trial of Gemcitabine and Curcumin in Patients With Advanced Pancreatic Cancer; clinicaltrials.gov, 2010.
- Yang, L.; Yang, L.; Tian, W.; Li, J.; Liu, J.; Zhu, M.; Zhang, Y.; Yang, Y.; Liu, F.; Zhang, Q.; et al. Resveratrol Plays Dual Roles in Pancreatic Cancer Cells. J. Cancer Res. Clin. Oncol. 2014, 140, 749–755. [Google Scholar] [CrossRef]
- Qin, T.; Cheng, L.; Xiao, Y.; Qian, W.; Li, J.; Wu, Z.; Wang, Z.; Xu, Q.; Duan, W.; Wong, L.; et al. NAF-1 Inhibition by Resveratrol Suppresses Cancer Stem Cell-Like Properties and the Invasion of Pancreatic Cancer. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Ratajczak, K.; Glatzel-Plucińska, N.; Ratajczak-Wielgomas, K.; Nowińska, K.; Borska, S. Effect of Resveratrol Treatment on Human Pancreatic Cancer Cells through Alterations of Bcl-2 Family Members. Molecules 2021, 26, 6560. [Google Scholar] [CrossRef]
- Radwan, M.O.; Ismail, M.A.H.; El-Mekkawy, S.; Ismail, N.S.M.; Hanna, A.G. Synthesis and Biological Activity of New 18β-Glycyrrhetinic Acid Derivatives. Arab. J. Chem. 2016, 9, 390–399. [Google Scholar] [CrossRef]
- Tsai, S.-J.; Yin, M.-C. Antioxidative and Anti-Inflammatory Protection of Oleanolic Acid and Ursolic Acid in PC12 Cells. J. Food Sci. 2008, 73, H174-178. [Google Scholar] [CrossRef] [PubMed]
- Somova, L.I.; Shode, F.O.; Ramnanan, P.; Nadar, A. Antihypertensive, Antiatherosclerotic and Antioxidant Activity of Triterpenoids Isolated from Olea Europaea, Subspecies Africana Leaves. J. Ethnopharmacol. 2003, 84, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Kadasah, S.F.; Radwan, M.O. Overview of Ursolic Acid Potential for the Treatment of Metabolic Disorders, Autoimmune Diseases, and Cancers via Nuclear Receptor Pathways. Biomedicines 2023, 11, 2845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Wang, X.; Tian, Z.; Qi, D.; Li, Y.; Jiang, H. Antihypertensive Activity of Oleanolic Acid Is Mediated via Downregulation of Secretory Phospholipase A2 and Fatty Acid Synthase in Spontaneously Hypertensive Rats. Int. J. Mol. Med. 2020, 46, 2019–2034. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rebolledo, G.A.; Siordia-Reyes, A.G.; Meckes-Fischer, M.; Jiménez-Arellanes, A. Hepatoprotective Properties of Oleanolic and Ursolic Acids in Antitubercular Drug-Induced Liver Damage. Asian Pac. J. Trop. Med. 2016, 9, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-B.; Xiao, Y.-H.; Zhang, Q.-Y.; Zhou, M.; Liao, S.-G. Hepatoprotective Natural Triterpenoids. Eur. J. Med. Chem. 2018, 145, 691–716. [Google Scholar] [CrossRef]
- Radwan, M.O.; Kadasah, S.F.; Aljubiri, S.M.; Alrefaei, A.F.; El-Maghrabey, M.H.; El Hamd, M.A.; Tateishi, H.; Otsuka, M.; Fujita, M. Harnessing Oleanolic Acid and Its Derivatives as Modulators of Metabolic Nuclear Receptors. Biomolecules 2023, 13, 1465. [Google Scholar] [CrossRef]
- Pompei, R.; Laconi, S.; Ingianni, A. Antiviral Properties of Glycyrrhizic Acid and Its Semisynthetic Derivatives. Mini Rev. Med. Chem. 2009, 9, 996–1001. [Google Scholar] [CrossRef]
- Sun, Z.-G.; Zhao, T.-T.; Lu, N.; Yang, Y.-A.; Zhu, H.-L. Research Progress of Glycyrrhizic Acid on Antiviral Activity. Mini Rev. Med. Chem. 2019, 19, 826–832. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Y.; Huang, C.; Lv, X.; Liu, L.; Wang, Y.; Li, J. Antifibrosis Effects of Triterpene Acids of Eriobotrya Japonica (Thunb.) Lindl. Leaf in a Rat Model of Bleomycin-Induced Pulmonary Fibrosis. J. Pharm. Pharmacol. 2012, 64, 1751–1760. [Google Scholar] [CrossRef]
- Lee, M.K.; Lee, K.Y.; Jeon, H.Y.; Sung, S.H.; Kim, Y.C. Antifibrotic Activity of Triterpenoids from the Aerial Parts of Euscaphis Japonica on Hepatic Stellate Cells. J. Enzyme Inhib. Med. Chem. 2009, 24, 1276–1279. [Google Scholar] [CrossRef]
- Somensi, L.B.; Costa, P.; Boeing, T.; Mariano, L.N.B.; Longo, B.; Magalhães, C.G.; Duarte, L.P.; Maciel E Silva, A.T.; de Souza, P.; de Andrade, S.F.; et al. Gastroprotective Properties of Lupeol-Derived Ester: Pre-Clinical Evidences of Lupeol-Stearate as a Potent Antiulcer Agent. Chem. Biol. Interact. 2020, 321, 108964. [Google Scholar] [CrossRef]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as Potentially Cytotoxic Compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef]
- I. Ciftci, H.; O. Radwan, M.; E. Ozturk, S.; Ulusoy, N.G.; Sozer, E.; E. Ellakwa, D.; Ocak, Z.; Can, M.; F.S. Ali, T.; I. Abd-Alla, H.; et al. Design, Synthesis and Biological Evaluation of Pentacyclic Triterpene Derivatives: Optimization of Anti-ABL Kinase Activity. Molecules 2019, 24, 3535–3535. [Google Scholar] [CrossRef]
- Tang, Z.-Y.; Li, Y.; Tang, Y.-T.; Ma, X.-D.; Tang, Z.-Y. Anticancer Activity of Oleanolic Acid and Its Derivatives: Recent Advances in Evidence, Target Profiling and Mechanisms of Action. Biomed. Pharmacother. Biomedecine Pharmacother. 2022, 145, 112397. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.O.; Abd-Alla, H.I.; Alsaggaf, A.T.; El-Mezayen, H.; Abourehab, M.A.S.; El-Beeh, M.E.; Tateishi, H.; Otsuka, M.; Fujita, M. Gypsogenin Battling for a Front Position in the Pentacyclic Triterpenes Game of Thrones on Anti-Cancer Therapy: A Critical Review—Dedicated to the Memory of Professor Hanaa M. Rady. Molecules 2023, 28, 5677. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Honma, Y.; Urano, T.; Suzumiya, J. Japanese Apricot Extract (MK615) Potentiates Bendamustine-Induced Apoptosis via Impairment of the DNA Damage Response in Lymphoma Cells. Oncol. Lett. 2017, 14, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Kawakami, K.; Akimoto, M.; Takenaga, K.; Suzumiya, J.; Honma, Y. Antitumor Effect of Japanese Apricot Extract (MK615) on Human Cancer Cells in Vitro and in Vivo through a Reactive Oxygen Species-Dependent Mechanism. Tumori 2013, 99, 239–248. [Google Scholar] [CrossRef]
- Cheng, S.; Eliaz, I.; Lin, J.; Sliva, D. Triterpenes from Poria Cocos Suppress Growth and Invasiveness of Pancreatic Cancer Cells through the Downregulation of MMP-7 Erratum in /Ijo/44/5/1781. Int. J. Oncol. 2013, 42, 1869–1874. [Google Scholar] [CrossRef]
- Cheng, S.; Castillo, V.; Sliva, D. CDC20 Associated with Cancer Metastasis and Novel Mushroom-derived CDC20 Inhibitors with Antimetastatic Activity. Int. J. Oncol. 2019, 54, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Peron, G.; Ferrari, S.; Gandin, V.; Bramucci, M.; Quassinti, L.; Mártonfi, P.; Maggi, F. Phytochemical Investigations and Antiproliferative Secondary Metabolites from Thymus Alternans Growing in Slovakia. Pharm. Biol. 2017, 55, 1162–1170. [Google Scholar] [CrossRef]
- Wei, J.; Liu, H.; Liu, M.; Wu, N.; Zhao, J.; Xiao, L.; Han, L.; Chu, E.; Lin, X. Oleanolic Acid Potentiates the Antitumor Activity of 5-Fluorouracil in Pancreatic Cancer Cells. Oncol. Rep. 2012, 28, 1339–1345. [Google Scholar] [CrossRef]
- Esmaeili, H.; Nasrollahzadeh Sabet, M.; Mosaed, R.; Chamanara, M.; Hadi, S.; Hazrati, E.; Farhadi, A.; Heidari, M.F.; Behroozi, J. Oleanolic Acid Increases the Anticancer Potency of Doxorubicin in Pancreatic Cancer Cells. J. Biochem. Mol. Toxicol. 2023, 37, e23426. [Google Scholar] [CrossRef] [PubMed]
- Deeb, D.; Gao, X.; Liu, Y.; Varma, N.; Arbab, A.; Gautam, S. Inhibition of Telomerase Activity by Oleanane Triterpenoid CDDO-Me in Pancreatic Cancer Cells Is ROS-Dependent. Molecules 2013, 18, 3250–3265. [Google Scholar] [CrossRef]
- Chen, F.; Long, Q.; Fu, D.; Zhu, D.; Ji, Y.; Han, L.; Zhang, B.; Xu, Q.; Liu, B.; Li, Y.; et al. Targeting SPINK1 in the Damaged Tumour Microenvironment Alleviates Therapeutic Resistance. Nat. Commun. 2018, 9, 4315. [Google Scholar] [CrossRef] [PubMed]
- Shopit, A.; Li, X.; Tang, Z.; Awsh, M.; Shobet, L.; Niu, M.; Wang, H.; Mousa, H.; Alshwmi, M.; Tesfaldet, T.; et al. miR-421 up-Regulation by the Oleanolic Acid Derivative K73-03 Regulates Epigenetically SPINK1 Transcription in Pancreatic Cancer Cells Leading to Metabolic Changes and Enhanced Apoptosis. Pharmacol. Res. 2020, 161, 105130. [Google Scholar] [CrossRef]
- Shopit, A.; Li, X.; Wang, S.; Awsh, M.; Safi, M.; Chu, P.; Jia, J.; Al-Radhi, M.; Baldi, S.; Wang, F.; et al. Enhancement of Gemcitabine Efficacy by K73-03 via Epigenetically Regulation of miR-421/SPINK1 in Gemcitabine Resistant Pancreatic Cancer Cells. Phytomedicine Int. J. Phytother. Phytopharm. 2021, 91, 153711. [Google Scholar] [CrossRef]
- Zhou, Z.; Dong, Y.; Li, N.; Niu, M.; Wang, S.; Zhou, Y.; Sun, Z.; Chu, P.; Tang, Z. An Oleanolic Acid Derivative, K73-03, Inhibits Pancreatic Cancer Cells Proliferation in Vitro and in Vivo via Blocking EGFR/Akt Pathway. Cell Biol. Int. 2022, 46, 1801–1813. [Google Scholar] [CrossRef]
- Li, C.; Yang, Z.; Zhai, C.; Qiu, W.; Li, D.; Yi, Z.; Wang, L.; Tang, J.; Qian, M.; Luo, J.; et al. Maslinic Acid Potentiates the Anti-Tumor Activity of Tumor Necrosis Factor a by Inhibiting NF- B Signaling Pathway. 2010. [Google Scholar] [CrossRef]
- Lin, C.; Yan, S.; Yin, M. Inhibitory Effects of Maslinic Acid upon Human Esophagus, Stomach and Pancreatic Cancer Cells. J. Funct. Foods 2014, 11, 581–588. [Google Scholar] [CrossRef]
- Aguilera-Garrido, A.; Arranz, E.; Gálvez-Ruiz, M.J.; Marchal, J.A.; Galisteo-González, F.; Giblin, L. Solid Lipid Nanoparticles to Improve Bioaccessibility and Permeability of Orally Administered Maslinic Acid. Drug Deliv. 2022, 29, 1971–1982. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Garrido, A.; Graván, P.; Navarro-Marchal, S.A.; Medina-O’Donnell, M.; Parra, A.; Gálvez-Ruiz, M.J.; Marchal, J.A.; Galisteo-González, F. Maslinic Acid Solid Lipid Nanoparticles as Hydrophobic Anticancer Drug Carriers: Formulation, in Vitro Activity and in Vivo Biodistribution. Biomed. Pharmacother. 2023, 163, 114828. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, X.; Yang, X. Ursolic Acid Inhibits Growth and Induces Apoptosis in Gemcitabine-Resistant Human Pancreatic Cancer via the JNK and PI3K/Akt/NF-κB Pathways. Oncol. Rep. 2012, 28, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Yadav, V.R.; Sung, B.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Ursolic Acid Inhibits the Growth of Human Pancreatic Cancer and Enhances the Antitumor Potential of Gemcitabine in an Orthotopic Mouse Model through Suppression of the Inflammatory Microenvironment. Oncotarget 2016, 7, 13182–13196. [Google Scholar] [CrossRef] [PubMed]
- Shahab, U.; Ahmad, M.K.; Mahdi, A.A.; Waseem, M.; Arif, B.; Moinuddin, null; Ahmad, S. The Receptor for Advanced Glycation End Products: A Fuel to Pancreatic Cancer. Semin. Cancer Biol. 2018, 49, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Swami, P.; Thiyagarajan, S.; Vidger, A.; Indurthi, V.S.K.; Vetter, S.W.; Leclerc, E. RAGE Up-Regulation Differently Affects Cell Proliferation and Migration in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2020, 21, 7723. [Google Scholar] [CrossRef] [PubMed]
- Olaoba, O.T.; Kadasah, S.; Vetter, S.W.; Leclerc, E. RAGE Signaling in Melanoma Tumors. Int. J. Mol. Sci. 2020, 21, 8989. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Chen, S.-Y.; Weng, M.-H.; Yen, G.-C. Ursolic Acid Restores Sensitivity to Gemcitabine through the RAGE/NF-κB/MDR1 Axis in Pancreatic Cancer Cells and in a Mouse Xenograft Model. J. Food Drug Anal. 2021, 29, 262–274. [Google Scholar] [CrossRef]
- Kang, R.; Hou, W.; Zhang, Q.; Chen, R.; Lee, Y.J.; Bartlett, D.L.; Lotze, M.T.; Tang, D.; Zeh, H.J. RAGE Is Essential for Oncogenic KRAS-Mediated Hypoxic Signaling in Pancreatic Cancer. Cell Death Dis. 2014, 5, e1480. [Google Scholar] [CrossRef]
- Tawila, A.M.; Sun, S.; Kim, M.J.; Omar, A.M.; Dibwe, D.F.; Awale, S. A Triterpene Lactone from Callistemon Citrinus Inhibits the PANC-1 Human Pancreatic Cancer Cells Viability through Suppression of Unfolded Protein Response. Chem. Biodivers. 2020, 17, e2000495. [Google Scholar] [CrossRef]
- Saleem, M.; Kaur, S.; Kweon, M.-H.; Adhami, V.M.; Afaq, F.; Mukhtar, H. Lupeol, a Fruit and Vegetable Based Triterpene, Induces Apoptotic Death of Human Pancreatic Adenocarcinoma Cells via Inhibition of Ras Signaling Pathway. Carcinogenesis 2005, 26, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, I.; Saleem, M.; Adhami, V.M.; Hafeez, B.B.; Mukhtar, H. Suppression of cFLIP by Lupeol, a Dietary Triterpene, Is Sufficient to Overcome Resistance to TRAIL-Mediated Apoptosis in Chemoresistant Human Pancreatic Cancer Cells. Cancer Res. 2009, 69, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Ganaie, A.A.; Siddique, H.R.; Sheikh, I.A.; Parray, A.; Wang, L.; Panyam, J.; Villalta, P.W.; Deng, Y.; Konety, B.R.; Saleem, M. A Novel Terpenoid Class for Prevention and Treatment of KRAS -driven Cancers: Comprehensive Analysis Using in Situ, in Vitro, and in Vivo Model Systems. Mol. Carcinog. 2020, 59, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Thayer, S.P.; di Magliano, M.P.; Heiser, P.W.; Nielsen, C.M.; Roberts, D.J.; Lauwers, G.Y.; Qi, Y.P.; Gysin, S.; Fernández-del Castillo, C.; Yajnik, V.; et al. Hedgehog Is an Early and Late Mediator of Pancreatic Cancer Tumorigenesis. Nature 2003, 425, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.A.; Tateno, C.; Hosoya, T.; Koyano, T.; Kowithayakorn, T.; Ishibashi, M. Hedgehog/GLI-Mediated Transcriptional Inhibitors from Zizyphus Cambodiana. Bioorg. Med. Chem. 2008, 16, 9420–9424. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhu, H.; Weng, M.; Wang, C.; Sun, L. Chemopreventive Effect of Betulinic Acid via mTOR -Caspases/Bcl2/Bax Apoptotic Signaling in Pancreatic Cancer. BMC Complement. Med. Ther. 2020, 20, 178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hu, T.; Gao, L.; Su, P.; Zhang, Y.; Zhao, Y.; Chen, S.; Tu, L.; Song, Y.; Wang, X.; et al. Friedelane-Type Triterpene Cyclase in Celastrol Biosynthesis from Tripterygium Wilfordii and Its Application for Triterpenes Biosynthesis in Yeast. New Phytol. 2019, 223, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha, S.H.; Moudgil, K.D. Celastrol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Sung, B.; Prasad, S.; Kannappan, R.; Cho, S.-G.; Liu, M.; Chaturvedi, M.M.; Aggarwal, B.B. Celastrol Suppresses Invasion of Colon and Pancreatic Cancer Cells through the Downregulation of Expression of CXCR4 Chemokine Receptor. J. Mol. Med. 2010, 88, 1243–1253. [Google Scholar] [CrossRef]
- Marchesi, F.; Monti, P.; Leone, B.E.; Zerbi, A.; Vecchi, A.; Piemonti, L.; Mantovani, A.; Allavena, P. Increased Survival, Proliferation, and Migration in Metastatic Human Pancreatic Tumor Cells Expressing Functional CXCR4. Cancer Res. 2004, 64, 8420–8427. [Google Scholar] [CrossRef]
- Chakravarthy, R.; Clemens, M.J.; Pirianov, G.; Perdios, N.; Mudan, S.; Cartwright, J.E.; Elia, A. Role of the eIF4E Binding Protein 4E-BP1 in Regulation of the Sensitivity of Human Pancreatic Cancer Cells to TRAIL and Celastrol-induced Apoptosis. Biol. Cell 2013, 105, 414–429. [Google Scholar] [CrossRef] [PubMed]
- Youns, M.; Askoura, M.; Abbas, H.A.; Attia, G.H.; Khayyat, A.N.; Goda, R.M.; Almalki, A.J.; Khafagy, E.-S.; Hegazy, W.A.H. Celastrol Modulates Multiple Signaling Pathways to Inhibit Proliferation of Pancreatic Cancer via DDIT3 and ATF3 Up-Regulation and RRM2 and MCM4 Down-Regulation. OncoTargets Ther. 2021, 14, 3849–3860. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




