1. Introduction
The UN has Responsible Consumption and Production as its 12
th Sustainable Development Goal (SDG). One way of pursuing this is in the responsible exploitation of otherwise waste materials. Organic wastes are highly biodegradable and have a high energy recovery rate [
1]. As this waste material can provide fuel without removing further carbon from the Earth’s crust, this also addresses SDG number 7 (Affordable and Clean Energy) and SDG number 13 (Climate Action).
A further UN SDG is number 6, which calls for clean water and sanitation. As this goal is taken up, more wastewater treatment will take place everywhere on Earth. The primary sludge from sewage treatment plant principally consists of raw solids derived from bottom primary clarifier; this contains high amounts of fats and protein and lower amounts of carbohydrate [
2]. This composition results in a greater gas yield from primary sludge compared to waste activated sludge. Waste activated sludge, on the other hand, is produced in a secondary digestion process and is more difficult to digest compared to primary sludge. The pursuit of AD to process waste material from non-sewage sources such as waste paper has also grown but this still often uses activated sludge as a source of microorganisms to start the process [
3].
Foaming occurs during the anaerobic digestion process as bubbles of the gas produced are trapped in the liquid contents of the AD vessel. If the liquid surface tension is low enough, the bubbles do not burst in time and hence foam rises to fill the vessel and even escape into the outlets used to remove the gas. This decreases process efficiency and is often associated with the waste activated sludge digester [
4]. Moeller and Görsch [
5] found 80% (i.e. 12 of the 16 of biogas plants studied) showed excessive foam formation during the anaerobic digestion process. Subramanian, et al. [
6] found 32 out of 39 sludge anaerobic digestion tanks studied in the United States experienced foaming problems while Kougias, Boe, O-Thong, Kristensen and Angelidaki [
4] reported foaming problems in 15 of the 16 large scale sludge anaerobic digestion plants surveyed in Denmark. Excessive foaming causes reduction in working volume thereby reducing gas yield and organic matter removal efficiency, which in turn has a negative impact in terms of process efficiency and operational cost. Research by Kougias, Boe, O-Thong, Kristensen and Angelidaki [
4] found up to 50% of biogas production is lost. Excessive foaming caused system failure with the anaerobic digestion system shutdown which led to a massive cleaning operation and restarting of the reactors, thus increasing labour and operational cost. Economic loss and safety hazards were seen in an anaerobic digestion plant in Sweden due to foaming that caused the plant to shut down for 10 weeks. This led to up to 40% loss in gas production and economic loss of 150000 USD [
7] . During an episode of excessive foaming in an anaerobic digestion plant, the roof of the digester was damaged and the estimated repair costs were € 500,000 [
5]. During foaming, the gas produced in digester is not efficiently discharged from liquid phase. This causes a dispersed system and gathers at the surface forming a stable mucus layer known as conventional foam and when it fills the whole reactor, it is known as rapid volume expansion [
8]. It can be observed during conventional foaming that the moisture content of the substrate becomes uneven thereby reducing the moisture content at the bottom of the digester.
Foaming is influenced by feedstock mix, excessive degassing, temperature fluctuation and change in viscosity [
9]. Ganidi, et al. [
10] notes that foaming leads to additional cost of electricity in the digestion process. It was concluded that problems associated with foaming are a higher solid concentration at the digester top due to inverse solid profile in active volume of digester, which results in instability. Westlund, Hagland and Rothman [
7] found a biogas loss of 40% after anaerobic digestion for 10 weeks. Jiang, et al. [
11] found foam microorganisms are caused by VFA accumulation of sludge in the anaerobic digestion system thereby reducing methane yield.
Temperature is an important factor for volume expansion and foam formation, and also temperature impact foam formation [
12]. AD is operated at optimum temperatures that either encourage mesophilic bacteria (35°C) or thermophilic bacteria (55°C) [
3]. Methanogenic activity is higher in mesophilic digestion, which improves process stability with reduced foaming, than it is in thermophilic digestion [
13]. Mesophilic anaerobic digestion has less chance of foam fromation compared to thermophilic anaerobic digestion due to lower presence of ammonia, alkalinity and volatile fatty acids present in the digester [
14]. Weiland [
15] notes that thermophilic digestion does not adapt to temperature fluctuation unlike mesophilic digestion. Furthermore, the higher temperature associated with thermophilic digestion makes it more susceptible to ammonia inhibition thereby causing more foaming and decreased gas compared to mesophilic digestion. Foaming triggered by temperature is caused by temperature fluctuation [
8]. The researchers concluded that temperature fluctuation has greater effect in thermophilic conditions at as low a temperature as 1°C which affects microbial actions. It is therefore necessary to control temperature during anaerobic digestion to circumvent fluctuation. Siebels [
14], studying the foaming phenomenon in a bench-scale anaerobic digester, notes that temperature fluctuation produces instability in a thermophilic digester by distorting activities of volatile acid and methane forming bacteria. It was later concluded that temperatures as low as 2°C led to instability in the thermophilic digester to create excessive foaming.
Anaerobic digestion relies on microorganism activities during digestion and foaming indicates instability in anaerobic digestion which may lead to a system failure. Jiang, McIlroy, Qi, Petriglieri, Yashiro, Kondrotaite and Nielsen [
11] found substantial correlation between foam formation and microorganism activities. They found filamentous bacteria to be the main foam forming microorganism of sludge anaerobic digestion and ammonium and total nitrogen contents are positively correlated with foam forming microorganisms. Zhang, et al. [
16] found high concentration of ammonia nitrogen causes proton imbalance and potassium deficiency and destroys microbial activities, leading the accumulation of volatile fatty acid and affecting the surface tension of the digestate. Pagilla, et al. [
17], in the causes and effects of foam in anaerobic sludge digesters, found foaming increases with extreme level of filamentous microorganisms present in sludge, causing instability and digester failure and gas mixed digesters are more prone to foaming than mechanically mixed digesters. Kong, et al. [
18] found the instability in digesters caused foaming in OFMSW due to high organic loading, shock load and restart-up. It was concluded that the reduction in methanogenic actions in anaerobic digestion cause the surface activities of sludge to increase by 50% during foaming. Nguyen, et al. [
19] found that the profusion of methanogen at the top of the reactor during anaerobic digestion caused severe foaming compared to the bottom. They later concluded that the lack of mixing contributed greatly to foaming. Inadequate mixing and mechanical failure such as accidental power failure, damage to the mixing device and lack of circulation in the water bath creates insufficient and discontinuous mixing which then distorts equilibrium between bubble formation causing change in gas retention and then foaming [20-22]. Lienen, et al. [
23] concluded that substrate overloading and inappropriate mixing both contribute to foaming; in stable anaerobic digestion system, the composition of the substrate does not have effect on foaming. Ross and Ellis [
24] in a laboratory scale investigation found that foaming in anaerobic digestion is linked to organic loading. Kovalovszki, et al. [
25] found that the reduction in microorganisms increases foaming. Westlund, Hagland and Rothman [
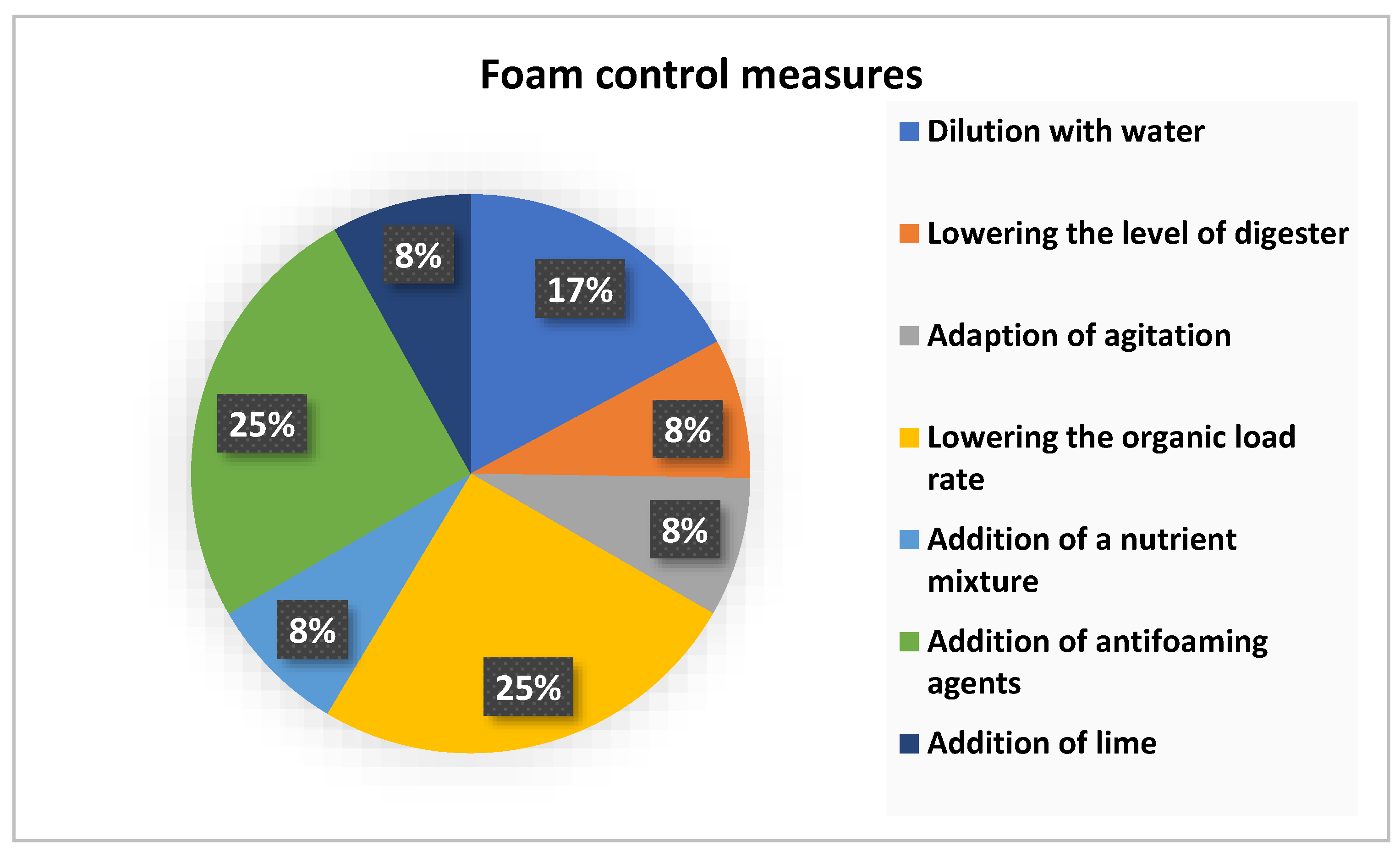
7] suggested that eliminating foaming by decreasing sludge load, separated stabilisation of sludge, lowering sludge level in digester, adding an anti-foaming agent, installed mixer and thermal pre-treatment of sludge.
Temperature changes the behaviour of sludge thereby causing blockages in anaerobic digesters [
26]. Digester sludge can be categorised with 2.5% to 12% TS at temperatures between 20°C and 60°C. Schneider and Gerber [
27] note that the rheological properties of digestate is said to be non-Newtonian; a shear-thinning flow with particle-loaded fluid and the reduction in mixing time and shear rate lowered gas yield. Mönch-Tegeder, et al. [
28] concluded that reduction in particle size through mechanical pre-treatment resulted in lower viscosity thereby increasing gas yield compared to substrates with a longer particle size. Furthermore, particle size is inversely proportional to total solid content and an increase in total solid content increased viscosity thereby reducing gas yield. Garuti, et al. [
29] found pre-treatment, reduction of particle quantity, or removing solids reduced viscosity of digestate leading to higher gas yield. In non-Newtonian fluids, viscosity changes under force to either more liquid or solid, with viscosity higher in high solid content than low solid content [
30]. The flow of sludge with high solid content affects foam stability due to yield stress and viscosity and sludge need more yield stress to deform due to their viscous nature enabling increased accumulation of bubbles in the sludge there by increasing chances of rapid volume expansion. This explains why sludge with low solid content has less foaming than sludge with high solid content, thus increasing chances of conventional foaming.
2. Foam Control in Anaerobic Digestion
Foaming in anaerobic digestion has led to the need for investigation of anaerobic digestion foam control as seen in
Figure 1. Foam can be controlled in the anaerobic digestion process using antifoaming and defoaming methods, and the efficiency of foam control agents depends on their chemical composition, the type of foam produced and the chemical composition of substrate in the reactor [
31]. Antifoaming agents or antifoams and defoamers are sometimes used interchangeably, describing compounds used to suppress foam either before or after foam formation. The economic viability of foam control in the anaerobic digestion process depends on the antifoaming agent dose, the cost of antifoaming and its influence during digestion. Although antifoaming can be used as a foam management process it does not tackle the root cause of foaming which is normally process instabilities in anaerobic digestion.
Defoaming can be categorised as mechanical, chemical and biological methods [
8]. Mechanical defoamers are typically used as emergency treatment after the foam has already formed. High intensity mixing and high-pressure water jetting are used during this process which is energy intensive [
32]. However, diluting the concentration of microorganism with the jet water has a negative effect on anaerobic digestion and therefore the methane yield. Generally, shear force is applied to the foam, and the lamellae are destroyed, which peels some of the surfactant off the lamellae to promote foaming collapse. However, this method is not always effective, and even when it is effective, it can often only remove foam over short timeframes. This method also has no long-term defoaming effect, and the addition of excessive amounts of water causes the TS content to decline, diluting the concentrations of microorganisms and ultimately negatively affecting the reactor [
33].
The effects of chemical defoaming agents decline following repeated use and cause inhibition to anaerobic digestion system due to excess volatile fatty acids.
Biological methods prevent foaming by reducing the biological activity of foam enhancing microorganisms by reducing organic loading rate, eliminating substrate components that advance the growth of foam foaming microorganism or by adding bacteriophages to infect and swallow foam-forming microorganisms such as filamentous bacteria in activated sludge [
34], [
35] and [
4]. The biological methods require restricting microbial activities with reduction of feeding over 2-3 days.
Out of the types of defoaming methods outlined, biological methods are the most efficient, economical, and environmentally friendly method. However, they do not target the root cause of foaming which is normally process instability in anaerobic digestion.
3. Research Outlook
Foaming is a common phenomenon during AD that seriously affects the operational safety, economic benefits, and environmental sanitation of AD plants [
8]. Exploring solutions to foaming not only has scientific value but also has practical significance, as such work can provide technical reserves for promoting the innocuous treatment of organic waste and capacity building for resource utilization. The causes of foaming, foaming risk prediction methods, and foaming control methods have been extensively explored in previous studies. However, certain process parameters, state parameters, or microorganisms related to foaming have yet to be identified. Although the continuous monitoring of some parameters is helpful for assessing the risk of foaming, quantitative information remains scarce. Furthermore, defoaming methods are mostly remedial and cannot prevent the generation of foam from their sources. After the cause of foaming has been identified, it will be more targeted to explore foaming risk evaluation methods and foaming control methods; thus, future studies should focus on identifying the causes of foaming. Foaming is a manifestation of the instability of the AD process, and microorganisms are the main functional body ensuring AD performance. Thus, foaming mechanisms need to be explored at the microbial community level. Future studies should go beyond identifying and comparing the microbes in sludge and in the foam layer before and after foaming and instead collect a large number of foam samples from similar AD reactors to identify common microorganisms, characterize changes in the concentrations of these microorganisms during the foaming process, and assess whether these microorganisms contribute to foaming. However, elucidating foaming mechanisms at the microbial community level might be particularly challenging given that microorganisms are characterized by their high diversity and redundancy and because the same species may have different functions under different environmental conditions. By contrast, metabolites are associated with the performance of organisms following specific environmental stimuli or perturbation and can more accurately reflect the state of the biological system, which more closely reflects phenotype (Putri et al., 2013). The metabolites of AD, extracellular polymeric substances EPS, and soluble microbial products SMP are likely important contributors to the foaming process. Future research should combine the separation and extraction methods of EPS and SMP. With the novel in situ Fourier transform infrared spectroscopy-two-dimensional correlation spectroscopy, fluorescence excitation-emission matrix spectroscopy, and metabolomics methods, we can analyse the functional groups and cross-linked aggregate structure to determine the EPS and SMP that may contribute to foaming. Next, the succession law of microorganisms involved in the production and consumption of these common metabolites during changes in foam volume can be determined, and this work may enhance our understanding of foaming mechanisms. With this knowledge, we can develop targeted foaming risk prediction methods and metabolic barrier methods for defoaming. The feasibility of the above research plan requires verification. Currently, there are many organic waste AD plants in operation around the world, and the number of proposed projects continues to increase. In light of the discussion in
Section 2, there is a need to begin optimizing reactor design operating parameters and strengthening operation management to avoid the risk of foaming. If a biogas plant seeks to accept a new substrate, it should first characterize its physical and chemical properties, organic components, and surface activity as well as evaluate its effect on feed load and digestion performance. If necessary, lab-scale experiments should be carried out. The temperature in the reactor should be strictly controlled to avoid temperature fluctuations in the digestion tank caused by feed or heating system failure. Finally, daily monitoring should be conducted to help prevent foaming. The daily monitoring of surface tension, viscosity, foaming potential FP/ foaming stability FS, intermediate metabolites (such as volatile fatty acid VFA), and other indicators in AD plants, as well as characterizing their changes, would help managers trace the source of foaming when these indicators remain abnormal over an extended period and would help eliminate foaming problems.