1. Introduction
Oxidative stress is a term used to designate an imbalance between the production of reactive oxygen species (ROS) and antioxidant levels; the production of ROS exceeds the quantity of antioxidants to neutralise it [
1,
2,
3]. The ROS family includes superoxide, hydroxy radical, peroxide radical, radical alkoxyl, hydroperoxyl radical, and non-intermediary radicals (hydrogen peroxide, hypochlorite acid, nitrogen peroxide, and peroxynitrite). These compounds are unstable due to their free electrons [
1,
2,
3]. There are multiple sources of ROS production, such as cellular respiration, inflammatory responses, metabolism of drugs and xenobiotics, exposure to ionising radiation (RX and UV), monoamine oxidase activity, purine catabolism, uric acid production, catalytic reactions supported by different enzymes (xanthine oxidase, NADPH oxidase, nitric oxide synthase, heme oxidase), nitrogen peroxide formation and protein synthesis in the rough endoplasmic reticulum (RER) [
4,
5,
6].
The body has enzymatic and non-enzymatic antioxidant mechanisms. Most non-enzymatic mechanisms are obtained from exogenous sources (vitamin C and E), and the rest are synthesised in the body, such as transferrin, ceruloplasmin and the glutathione reduction system (tripeptide, L-glutamate, L-cysteine, and glycine). Among all the antioxidant mechanisms, superoxide dismutase, peroxidases, catalase, and thioredoxin are extremely valuable [
4,
5,
6].
ROS production during pregnancy and its medical complications, including RPL, has been studied [
7,
8,
9]. ROS production in a healthy placenta increases due to new tissue formation and increased metabolic rate, but higher production and availability of antioxidant enzymes and metabolites compensate for it [
8,
9]. Increased levels of oxidative stress are associated with several gestational pathologies such as gestational diabetes, miscarriage, recurrent pregnancy loss, defects during embryogenesis, formation of teratomas, slowed intrauterine growth and preeclampsia [
10,
11,
12,
13,
14,
15,
16]. Excessive ROS production and insufficient antioxidants can kill the embryo [
8,
9,
10,
11,
12,
13,
14]. Therefore, antioxidant uptake and production are upregulated during pregnancy. The body produces higher catalase levels, glutathione peroxidase, and Cu/Zn or Mn superoxide dismutase [
16,
17,
18]. Genetic associations with detoxifying enzymes and other proteins involved in inflammation and radical elimination were reported in normal and medical complications during pregnancy [
19,
20]. However, most analyses were performed in Caucasian and Asian populations, and brief reports in admixed populations were contradictory [
20].
Epoxy hydrolases are critical enzymes transforming epoxide-containing lipids (modified by ROS) by adding water [
21]. At least four enzymes catalyse the reaction; however, the most important ones are the microsomal one, mEPH or EPHX1, and the soluble one, EPHX2 [
22]. Both enzymes have been involved in cardiovascular diseases and other medical conditions [
21,
22]. In addition, gene variants of the EPHX1, particularly rs1051740, have been shown to affect enzyme activity [
21,
22]. The decrease in enzyme activity may involve several processes, including female sexual hormonal regulation and endometrial tissue [
22]. It is currently unknown whether gene polymorphisms in the mEPH gene play a role in RPL or if there are possible associations with other polymorphisms.
Nitric oxide metabolites in pregnancy and pregnancy complications have been extensively studied [
23,
24], including RPL [
25,
26,
27,
28,
29]. A possible association between endothelial nitric oxide synthase and RPL has been suggested, especially in exon 8, rs1799983 (+ 894G > T). Nonetheless, questions remain on the impact of the polymorphism in the tissue, its association with specific races or populations and its possible relation to other genes [
26,
27,
28,
29]. It is important to note that admixed populations are particular and involve different gene segregations. The admixed Venezuelan population differs from other Latin American populations [
30].
Several analyses of nitric oxide metabolites and synthase enzymes in plasma, serum and placenta have been controversial. Alemán and colleagues [
31] in Vargas Hospital, Caracas, reported an increased amount of nitrite serum levels in preeclampsia patients compared to healthy pregnant women, as well as increased expression of NOS enzymes (eNOS and iNOS) in the placenta of these patients compared to control. These results differ from other reports independently of the ethnic difference. [
32,
33]. Our group demonstrated, as other authors [
32,
33], a significant reduction in serum levels of nitric oxide products (nitrites and nitrates) of preeclamptic patients compared to a control group [
34].
Genetic studies in different populations are essential to validate the impact of polymorphisms in diseases like RPL. The study aimed to evaluate certain aspects of oxidative metabolism in patients with frequent miscarriages by determining genetic polymorphisms in microsomal epoxy hydroxylase (mEPH o EPHX1, T612C, rs1051740) and nitric oxide synthase 3 (eNOS. G894T, rs1799983) in the admixed Venezuelan women with primary RPL.
2. Materials and Methods
The study involved 63 patients with criteria of primary RPL. Patients with RPL were screened for immunological and hormonal factors and had no alterations in paraclinical analysis (no infectious or genetic diseases). All patients suffered from primary RPL. The study included 26 healthy women with normal pregnancies and no medical conditions (no viral disease, hypertension, diabetes, metabolic syndrome or hormonal disbalances). Written consent was obtained from all individuals interested in the study. The Ethical Committee of the Institute of Immunology, Faculty of Medicine, Caracas, Venezuela, approved the study (number 20052308).
Ten mL of venous blood was collected in EDTA tubes from all individuals, and the cDNA was extracted from the buffy coat the same day. Genomic DNA isolation was done following the instructions of a commercial kit: AxyPrep Blood Genomic DNA Miniprep Kit (Axygen Biosciences). This kit can purify up to 12 µg of genomic DNA in 250 µl of non-coagulated blood. Briefly, 500 µL of AP1 lysis buffet was mixed with 200 µL of buffy coat in a 1.5 mL Eppendorf tube by vertexing for 10 seconds. Then. 100 µL of AP2 buffet was added and vortexed for 20 seconds to encourage protein precipitation before centrifuging for 10 minutes 12.000 at room temperature. The supernatant was added to the AxyPrep column. Then, the column was centrifuged for 2 minutes at 8.000 rpm. The eluate was discarded, and the column was loaded again with 700 µL of W1A washing buffer and centrifuged at 8.000 rpm for 1 minute. The eluate was discarded, and the column was loaded with W2 washing buffer and centrifuged at 12.000 rpm for 1 minute. This step was repeated with the W2 washing buffer. The column was then placed in a 1.5 mL Eppendorf tube, 200 µL of TE elution buffer was added, and after incubating it for 5 minutes, the tube was centrifuged at 12.000 rpm for 1 minute to obtain genomic DNA.
DNA concentration was calculated using the spectrophotometer Gene Quant II for DNA/RNA (Amersham Pharmacy Biotech® calculator). The purity of the DNA was checked using the standard 260 nm and 280 nm for nucleotides and proteins, respectively. The ratio 260/280 of the samples was ≥1.8. The required DNA concentration was adjusted using nuclease-free water (Promega®). DNA concentration was expressed in µg/ml and was automatically calculated by the spectrophotometer. Purified DNA was stored at -20ºC until needed.
The DNA was amplified via PCR using the following primers.
mEPH forward 5’GATCGATAAGTTCCGTTTCACC 3’
reverse 5’ATCCTTAGTCTTGAAGTGAGGAT 3’
eNOS, forward 5’AAGGCAGGAGACAGTGGATG 3’
reverse 5’CAGTCAATCCCTTTGGTGCT 3’.
For the analysis of the SNP rs1051740 (mEPH), the protocol of Chen et al. was used [
35]. Briefly, DNA (20 ng) was mixed with a 40 µL reaction mixture containing 1.5 mM MgCl2, 100 ng of each primer, 500 µM deoxyribonucleoside triphosphate and 0.6 IU Taq DNA polymerase (Promega®). The DNA was amplified via polymerase chain reaction (PCR) using a thermal cycler Minicycler ™ (MJ Research) consisting of an initial single cycle of 10 min at 95°C followed by 35 cycles of 30 s at 94°C, 20 s at 52°C and 5 s at 72°C. Then, PCR products were treated with
Eco RV (Promega®). The digested samples were then loaded on an ethidium bromide–stained gel, and the products were visualised using UV transillumination. RFLP products are for wild homozygous TT genotypes: two bands. 140pb/22pb, for heterozygous CT: 162pb/140pb/22pb and for the mutated homozygous CC 162 pb. The molecular weight marker was the
Hae III digested ΦX174 (Promega®).
The protocol described by Leeson et al. [
36] was used for the analysis of rs1799983 SNP with minor modifications. The sample of genomic DNA, 50 ng, was added to a mixture of 50 mmol/L KCl, 10 mmol/L Tris (pH 8.3), 0.2 mmol/L of each dNTP, 10 nmol of each primer, and 2U of Taq DNA polymerase, (Promega®) total volume 30µL. The amplification was carried out using the Minicycler™. The protocol was denaturation at 95°C for 10 minutes, followed by 35 cycles of 30 seconds at 63°C and 45 seconds at 72 °C. Ten microliters of the PCR products were digested with 2U
Dpn II (Promega®), which cuts when the T allele is present at position 894 (corresponding to Asp298). The RFLP products were GG: wild homozygous 248pb. GT: Heterozygous 248pb/160pb/88pb. TT: mutated homozygous 160pb/88pb. The RFLP products were visualised and analysed as described before.
Statistical Analysis of Results
The program GraphPad Prism was used for calculation. The results were analysed using Chi-square with Yate’s correction.
3. Results
The general data of the women who participated in the study is represented in
Table 1. The group of controls were from the same area as the patients. As compared to controls, patients have a significantly lower pregnancy duration (p<0.001) and a higher amount of pregnancy complications (p<0.05).
The SNP analysis of mEPH is illustrated in
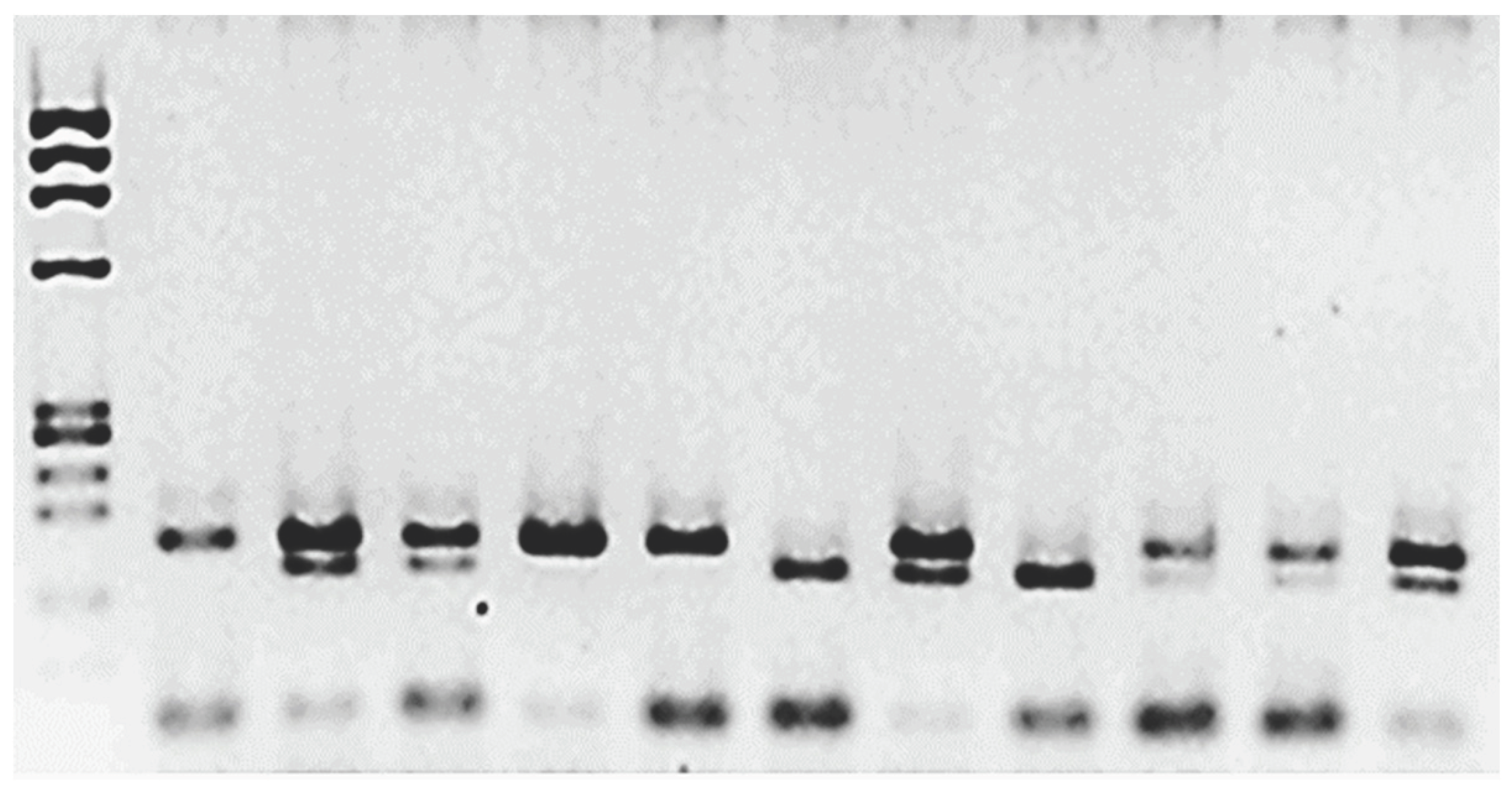
Figure 1. The frequency of the T allele in mEPH was 0.808 for controls and 0.6 for patients. On the other hand, the allelic frequency of the C allele was 0.192 for control and 0.4 for patients. The difference was significant (p=0.046).
Electrophoresis of the amplified and digested PCR products. The first column corresponds to the MW marker ΦX174/Hae III. The second is the amplified product, which was not treated with Eco RV enzyme; two bands, 162 bp and 22 bp, are observed. The rest of the samples were treated with the restriction enzyme. Columns 3, 4, 8, and 12 correspond to heterozygotes 162/142/22 bp. Columns 5, 10, and 11 correspond to the two bands (wt TT): 140 bp/22 bp. The mutated homozygous are columns 7 and 9, CC 162 bp.
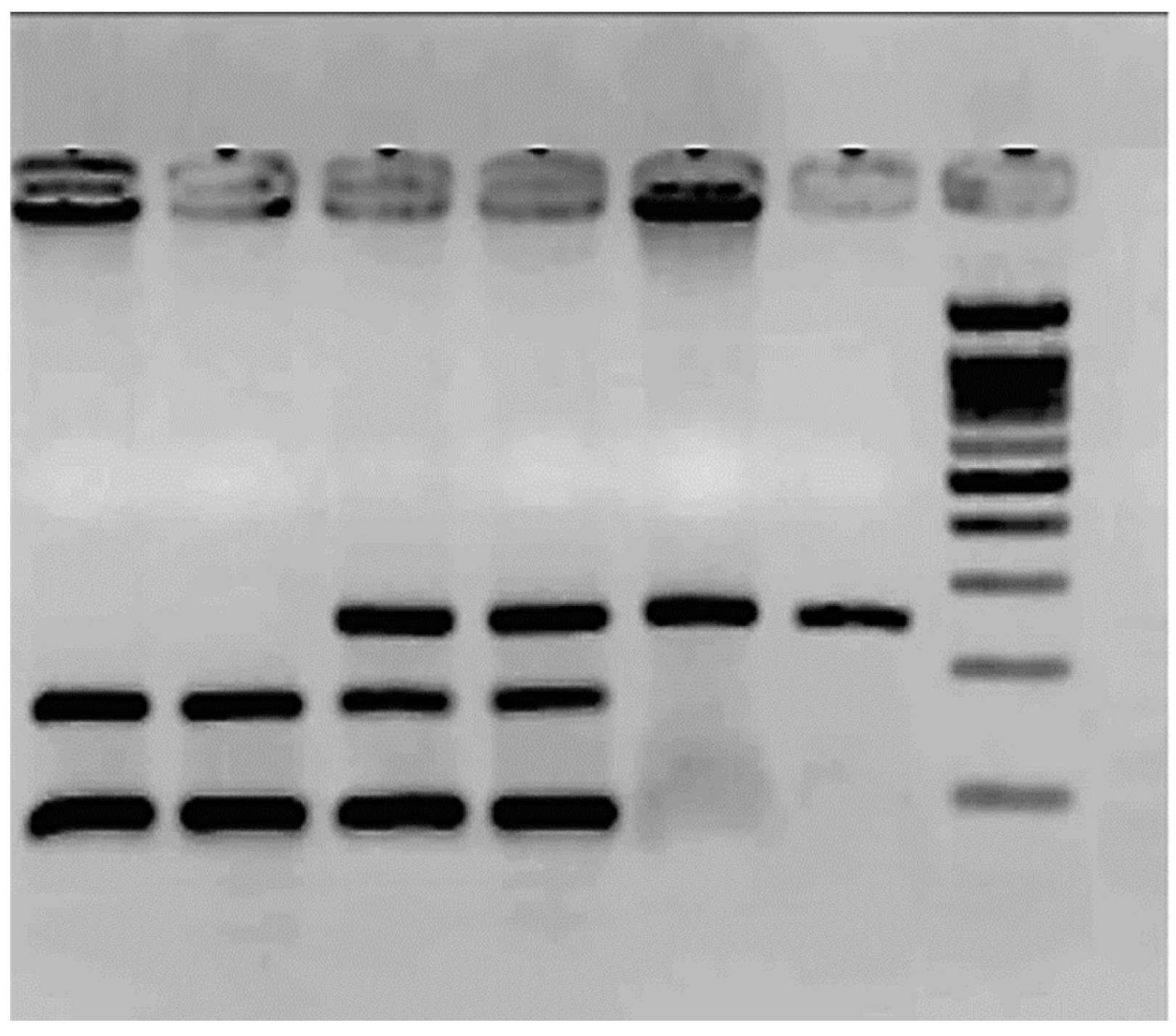
Figure 2 shows the results of the SNP rs1799983. The frequency of the G allele was 0.8 in controls and 0.7 in patients; for the T allele, (p=0.3). However, the TT homozygote is only observed in 6 patients; see Table 3. There is a high number of heterozygotes in the control group.
The molecular weight ladder (ΦX174/Hae III) is the last column. The rest corresponds to PCR products treated with the enzyme Dpn II. Columns 1 and 2 correspond to two TT: mutated homozygous 160 bp /88 bp. Columns 3 and 4 to GT heterozygous 248 bp/160 bp/88 bp and columns 5 and 6 to GG wild type homozygous, 248 bp.
Table 2 displays the results of the genetic polymorphism of mEPH. There were significant differences (p<0.05) in normal and mutated homozygotes when the normal and mutated gene frequencies were analysed between controls and patients.
The homozygous mutation was only observed in patients and was found to be statistically significant (p<0.05). However, the mutated T allele frequency was not substantial (p=0.4), likely due to a high number of heterozygotes in the control group.
Table 4 represents both polymorphisms. It was observed that there is a significant difference between patients and the control group when both polymorphisms are present. In the control group, only one individual has the mutated allele for mEPH and is a heterozygote for eNOS. However, four patients have the mutated gene mEPH and are heterozygotes for eNOS. Patients’ probability of double mutated alleles is significantly higher (p<0.05). The number of heterozygotes for both genes was similar in the two groups; on the contrary, the number of homozygotes with wild-type genes was lower in patients than in controls, although it was insignificant.
4. Discussion
Microsomal Epoxy hydroxylase (EPHX1. EC 3.3.2.9.0) is a highly conserved enzyme that conjugates the toxic products of trans dihydro diol and epoxides to be inert products excreted from the organism. Humans gave two EPHX enzymes, microsomal EPHX (EPHX1) and soluble EPHX (EPHX2). EPHX1 mutations have been associated with many types of cancer: preeclampsia and hypercholesterolemia or increased serum concentration of bile acid [
37]. Certain genetic variations of soluble epoxy hydrolase (EPHX2) are responsible for increased oxidative metabolism and the inactivation of epoxyeicosatrienoic acids (EETs), which play a role in vascularisation. The decrease in enzyme activity of EPHX2 has been involved in hypertension [
36] and in preeclampsia [
39,
40,
41]. The similarities between both enzymes, EPHX1 and EPHX2, suggest similar regulation and possible biological compensation [
21,
22,
42]. The role of both enzymes and their polymorphisms in RPL should be carefully analysed.
In the menstrual cycle, EPHX1 is regulated in the endometrium by progesterone and is involved in estrogen production [
43], but its role in implantation is less known [
44]. In this study, we observed that the frequency of the polymorphic variant His113His (rs1051740) of the mEPH was higher in patients than in controls. As reported, the genotype has been associated with a lower enzymatic activity [
21,
22], possibly related to a higher incidence of implantation failure and vascular complications in pregnancy. This is the first report of mEPH in RPL and in admix population.
Evidence suggests that a reduction in nitric oxide production related to polymorphisms of the eNOS enzyme may be related to implantation failure and frequent miscarriages [
20,
24,
25,
26,
27,
28,
33]. The polymorphism can affect the response of the vascular endothelium to oxidative stress and, therefore, promote vascular remodelling and cause pregnancy complications. In the present report, the polymorphic variant Asp298Asp (rs1799983) was only observed in a group of patients. There is a significant association (p<0.05) between the presence of the allele and the likelihood of miscarriage. In Asian populations, there are reports associating rs1799983 with frequent miscarriage. However, in other European countries, no clear association has been found [
20,
26]. Even though a review by Shi and coworkers [
20] mentions studies performed in admix populations in other geographic areas, the genetic characteristics of the admixed Venezuelan population differ. Even though most genes in the Venezuelan admix population are Caucasian, the African and mixed Amerindian genes differ from other Latin American populations [
28]. The present report is the first in this admixed population.
A combined analysis of both polymorphisms was intended to visualise the impact of the mutated alleles. Interestingly, a group of patients presented both polymorphisms, suggesting a possible association of this clinical entity. It is essential to consider the analysis of the polymorphisms of both enzymes to screen patients with RPL since it can be an interesting genetic marker to differentiate at least a subgroup of these patients. Genetic screening will most probably provide new treatment options for these patients.
5. Conclusions
The mutated homozygous genotypes, SNPs rs1051740 and rs1799983, are significantly increased in the admixed Venezuelan women with RPL.
6. Limitations of the Study
The number of samples was the main limitation. RPL samples were from patients whose medical history and follow-up were for over two years. Most of the RPL patients were attended by the fertility clinic without success. The controls were from the same gynaecological centres where the RPL patients were attended.
Author Contributions
Conceptualization, J.V.G and M.J.P..; methodology, M.J.P..; validation, J.B.D.S.., M.J.P.; J.V.G.; and C.V.D.S..; formal analysis, J.V.G; J.B.D.S..; investigation, M.J.P.; J.V.G..; resources, J.V.G.; J.B.D.S.; data curation, M.J.P.; C.V.D.S.; writing—original draft preparation, J.V.G.; C.V.D.S..; writing review and editing, J.V.G.; C.V.D.S.; J.B.D.S.; supervision, J.B.D.S.; project administration, J.V.G..; funding acquisition, J.V.G.. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: “This research was funded by the Universidad Central de Venezuela’s Counsel for Scientific and Human Development (CDCH-UCV), grant number PG 09-6599-2006/1.
Institutional Review Board Statement
The study was conducted following the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the Institute of Immunology, Faculty of Medicine, Universidad Central de Venezuela (protocol code 20052308, date of approval 15/09/2005).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The crude data is available from the authors.
Acknowledgments
The authors would like to thank the patients and controls involved in the study. Also, the authors are grateful to Drs Nancy Larocca and Félix Toro for their valuable discussions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007; 39:44-84. [CrossRef]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines. 2023 Oct 29;11(11):2925. [CrossRef]
- Averill-Bates D. Reactive oxygen species and cell signalling. Review. Biochim Biophys Acta Mol Cell Res. 2024 Feb;1871(2):119573. [CrossRef]
- Pokharel, M.D.; Garcia-Flores, A.; Marciano, D.; Franco, M.C.; Fineman, J.R.; Aggarwal, S.; Wang, T.; Black, S.M. Mitochondrial network dynamics in pulmonary disease: Bridging the gap between inflammation, oxidative stress, and bioenergetics. Redox Biol. 2024 Jan 20;70:103049. [CrossRef]
- An, G.; Park, J.; Song, J.; Hong, T.; Song, G.; Lim, W. Relevance of the endoplasmic reticulum-mitochondria axis in cancer diagnosis and therapy. Exp Mol Med. 2024 Feb;56(1):40-50. [CrossRef]
- Dyachenko, E.I.; Bel’skaya, L.V. The Role of Amino Acids in Non-Enzymatic Antioxidant Mechanisms in Cancer: A Review. Metabolites. 2023 Dec 31;14(1):28. [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. [CrossRef]
- Pereira, A.C.; Martel, F. Oxidative stress in pregnancy and fertility pathologies. Cell Biol Toxicol. 2014; 30:301-12. [CrossRef]
- Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative Stress in Pregnancy. Biomolecules. 2023 Dec 9;13(12):1768. [CrossRef]
- Haram, K.; Mortensen, J.H.; Myking, O.; Magann, E.F.; Morrison, J.C. The Role of Oxidative Stress. Adhesion Molecules and Antioxidants in Preeclampsia. Curr Hypertens Rev. 2019;15(2):105-112. [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; Tan, B. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediators Inflamm. 2021 Sep 27;2021:9962860. [CrossRef]
- Joó, J.G.; Sulyok, E.; Bódis, J.; Kornya L. Disrupted Balance of the Oxidant-Antioxidant System in the Pathophysiology of Female Reproduction: Oxidative Stress and Adverse Pregnancy Outcomes. Curr Issues Mol Biol. 2023 Oct 4;45(10):8091-8111. [CrossRef]
- Tuuli, M.G.; Longtine, M.S.; Nelson, D.M. Review: oxygen and trophoblast biology - a source of controversy. Placenta. 2011;32(Suppl 2):S109-18. [CrossRef]
- Myatt, L. Review: reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31;(Suppl l):S66-9. [CrossRef]
- Choi, S.; Kim, J.A.; Li, H.Y.; Lee, S.J.; Seok, Y.S.; Kim, T.H.; et al. Altered redox state modulates endothelial KCa2.3 and KCa3.1 levels in normal pregnancy and preeclampsia. Antioxid Redox Signal. 2019 Feb 1;30(4):505-519. [CrossRef]
- Poston, L.; Igosheva, N.; Mistry, H.D.; Seed, P.T.; Shennan, A.H.; Rana, S. et al. Role of oxidative stress and antioxidant supplementation in pregnancy disorders. Am J Clin Nutr. 2011;94:1980S-5S. [CrossRef]
- Diniz, M.S.; Magalhães, C.C.; Tocantins, C.; Grilo, L.F.; Teixeira, J.; Pereira, S.P. Nurturing through Nutrition: Exploring the Role of Antioxidants in Maternal Diet during Pregnancy to Mitigate Developmental Programming of Chronic Diseases. Nutrients. 2023 Oct 31;15(21):4623. [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; Tan, B. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediators Inflamm. 2021 Sep 27;2021:9962860. [CrossRef]
- Parveen, F.; Faridi, R.M.; Das, V.; Tripathi, G.; Agrawal S. Genetic association of phase I and phase II detoxification genes with recurrent miscarriages among North Indian women. Mol Hum Reprod. 2010; 16: 207-214. [CrossRef]
- Shi, X.; Xie, X.; Jia, Y.; Li, S. Maternal genetic polymorphisms and unexplained recurrent miscarriage: a systematic review and meta-analysis. Clin Genet. 2017 Feb;91(2):265-284. [CrossRef]
- Hosagrahara, V.P.; Rettie, A.E.; Hassett, C.; Omiecinski, C.J. Functional analysis of human microsomal epoxide hydrolase genetic variants. Chem Biol Interact. 2004 Nov 20;150(2):149-59. [CrossRef]
- Gautheron, J.; Jéru, I. The Multifaceted Role of Epoxide Hydrolases in Human Health and Disease. Int J Mol Sci. 2020 Dec 22;22(1):13. [CrossRef]
- Luo, Y.; Zhu, Y.; Basang, W.; Wang, X.; Li, C.; Zhou, X. Roles of Nitric Oxide in the Regulation of Reproduction: A Review. Front Endocrinol (Lausanne). 2021 Nov 19;12:752410. [CrossRef]
- Li, Q.; Chen, S.; Dong, X.; Fu, S.; Zhang, T.; Zheng, W.; Tian, Y.; Huang, D. The Progress of Research on Genetic Factors of Recurrent Pregnancy Loss. Genet Res (Camb). 2023 Mar 24;2023:9164374. [CrossRef]
- Cao, Y.; Zhang, Z.; Xu, J.; Wang, J.; Yuan, W.; Shen Y. et al. Genetic association studies of endothelial nitric oxide synthase gene polymorphisms in women with unexplained recurrent pregnancy loss: a systematic and meta-analysis. Mol Biol Rep. 2014;41(6):3981-9. [CrossRef]
- Pereza, N.; Peterlin, B.; Volk, M.; Kapović, M.; Ostojić, S. A critical update on endothelial nitric oxide synthase gene variations in women with idiopathic recurrent spontaneous abortion: genetic association study, systematic review and meta-analyses. Mol Hum Reprod. 2015 May;21(5):466-78. [CrossRef]
- Azani, A.; Hosseinzadeh, A.; Azadkhah, R.; Zonouzi, A.A.P.; Zonouzi, A.P.; Aftabi, Y. et al. Association of endothelial nitric oxide synthase gene variants (-786 T>C. intron 4 b/a VNTR and 894 G>T) with idiopathic recurrent pregnancy loss: A case-control study with haplotype and in silico analysis. Eur J Obstet Gynecol Reprod Biol. 2017; 215:93-100. [CrossRef]
- Zhao, X.; Li, Q.; Yu, F.; Lin, L.; Yin, W.; Li, J.; Feng, X. Gene polymorphism associated with endothelial nitric oxide synthase (4VNTR, G894T, C786T) and unexplained recurrent spontaneous abortion risk: A meta-analysis. Medicine (Baltimore). 2019 Jan;98(4):e14175. [CrossRef]
- Golestanpour, H.: Bahrami, R.; Dastgheib, S.A.; Tabatabaei, R.S.; Javaheri, A.; Karimi-Zarchi, M.; Mirjalili, S.R.; Neamatzadeh, H. A meta-analysis for association of eNOS VNTR 4b/a, - 786 T > C and + 894G > T polymorphisms with risk of recurrent pregnancy loss. Arch Gynecol Obstet. 2021 Nov;304(5):1135-1151. [CrossRef]
- Conesa, A.; Fernández-Mestre, M.; Padrón, D.; Toro F.; Silva, N.; Tassinari, P.; Blanca, I.; Martin, M.P.; Carrington, M.; Layrisse, Z. Distribution of killer cell immunoglobulin-like receptor genes in the mestizo population from Venezuela. Tissue Antigens. 2010 Jun;75(6):724-9. [CrossRef]
- Alemán, I.; Ramírez A. M.; Hung, A.; Ramírez, C. Endothelial and inducible nitric oxide synthase expression in Venezuelan patients with pre-eclampsia. Invest Clin. 2008 Sep;49(3):321-30.
- Hao, F.; Tang, L.C.; Sun, J.X.; Li, W.X.; Zhao, Y.; Xu, X.H.; Jin L.P. Decreased nitric oxide content mediated by asymmetrical dimethylarginine and protein l-arginine methyltransferase 3 in macrophages induces trophoblast apoptosis: a potential cause of recurrent miscarriage. Hum Reprod. 2021 Nov 18;36(12):3049-3061. [CrossRef]
- Zhu, X.Z.; Deng, Z.M.; Dai, F.F.; Liu, H.; Cheng, Y.X. The impact of early pregnancy metabolic disorders on pregnancy outcome and the specific mechanism. Eur J Med Res. 2023 Jun 24;28(1):197. [CrossRef]
- Garmendia, J.V.; Gutiérrez, Y.; Blanca, I.; Bianco, N.E.; De Sanctis, J.B. Nitric oxide in different types of hypertension during pregnancy. Clin Sci (Lond). 1997; 93:413-21. [CrossRef]
- Cheng, S.L.; Yu, C.J.; Chen, C.J.; Yang, P.C. Genetic polymorphism of epoxide hydrolase and glutathione S-transferase in COPD. Eur Respir J. 2004 Jun;23(6):818-24. [CrossRef]
- Leeson, C.P.; Hingorani, A.D.; Mullen, M.J.; Jeerooburkhan, N.; Kattenhorn, M.; Cole, T.J.; Muller, D.P.; Lucas, A.; Humphries, S.E.; Deanfield, J.E. Glu298Asp endothelial nitric oxide synthase gene polymorphism interacts with environmental and dietary factors to influence endothelial function. Circ Res. 2002 Jun 14;90(11):1153-8. [CrossRef]
- Václaviková, R.; Hughes, D.J.; Souček, P. Microsomal epoxide Hydrolase 1 (EPHX1): gene. structure. Function. and role in human disease. Gene. 2015; 571:1-8. [CrossRef]
- Jiang, H.; Quilley, J.; Doumad, A.B.; Zhu, A.G.; Falck, J.R.; Hammock, B.D.; Stier, C.T. Jr; Carroll, M.A. Increases in plasma trans-EETs and blood pressure reduction in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2011 Jun;300(6):H1990-6. [CrossRef]
- Dalle Vedove, F.; Fava, C.; Jiang, H.; Zanconato, G.; Quilley, J.; Brunelli, M.; Guglielmi, V.; Vattemi, G.; Minuz, P. Increased epoxyeicosatrienoic acids and reduced soluble epoxide hydrolase expression in the preeclamptic placenta. J Hypertens. 2016 Jul;34(7):1364-70. [CrossRef]
- Sari, I.; Pinarbasi, H.; Pinarbasi, E.; Yildiz, C. Association between soluble epoxy hydrolase gene and preeclampsia. Hypertens Pregnancy 2017;36:315-325. [CrossRef]
- Sarı, İ.; Ökten, H.; Aktan, Ç.; Cihan, E. Association of the sEH gene promoter polymorphisms and haplotypes with preeclampsia. J Med Biochem. 2020 Oct 2;39(4):428-435. [CrossRef]
- Morisseau, C. The Role of Hydrolases in Biology and Xenobiotics Metabolism. Int J Mol Sci. 2022 Apr 28;23(9):4870. [CrossRef]
- Hattori, N.; Fujiwara, H.; Maeda, M.; Fujii, S.; Ueda, M. Epoxide hydrolase affects estrogen production in the human ovary. Endocrinology. 2000 Sep;141(9):3353-65. [CrossRef]
- Popp; S.L.; Abele, I.S.; Buck, M.B.; Stope2, M.B.; Block LJ. Hanifi-Moghaddam et al. Microsomal epoxide hydrolase expression in the endometrial uterine corpus is regulated by progesterone during the menstrual cycle. J Mol Histol 2010; 41:111-119. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).