1. Introduction
Si-Wu-Tang (SWT) is one of the widely applied Traditional Chinese Medicine (TCM) formula [
1]. The formula contains a combination of 4 herbs: Radix Paeoniae Alba, Angelica siniensis, Rhizoma Chuanxiong, and Rehmannia glutinosa, and has been considered having the actions of blood-tonifying (buxie) decoction [
2]. SWT has been applied for the treatment of women’s diseases such as cutaneous pruritus and chronic inflammation [
3]. Aqueous extract of SWT was reported to have antipruritic and anti-inflammatory effects in mice [
4]. Additionally, this formula is also used for treating other gynecological diseases, such as relief of menstrual irregularity, dysmenorrhea, uterine bleeding, climacteric syndrome, and other estrogen-related diseases, in China and East Asia [
5,
6,
7,
8,
9,
10]. SWT has also been beneficial to other physiological or pathological conditions. It has been shown to improve the antioxidant level and positively regulate the lipid profile, liver function, and skin integrity and texture in healthy adults [
11]. The main function of SWT applied in gynecology is nourishing blood and activating the blood circulation for menoxenia, dysmenorrhea, and amenorrhea.
Indirect evidences have suggested the beneficial effects of SWT on bone health. Pharmacological studies have shown that SWT extract protects against radiation-induced bone marrow damage in an animal model [
12,
13]. Additionally, since SWT revealed anti-inflammatory and anti-oxidant effects in several different clinical and biological studies [
14,
15], these results suggest the potential application of this medicine on treating osteoporosis possibly through increasing bone formation and/or suppressing bone resorption. A more recent study provided some direct evidences about SWT effects on bone cells. Wu et al. reported that SWT extract increased alkaline phosphatase (ALP), bone morphogenic protein-2 (BMP-2), and osteopontin (OPN) expression and bone mineralization in the differentiated osteoblast culture16. Phosphatidylinositol 3-kinase (PI3K), Akt, and NF-κB signaling pathways were involved in the SWT-mediated increase in gene expression and bone mineralization [
16]. Moreover, oral administration of SWT extract every two days for four weeks prevented bone loss induced by ovariectomy in mice [
16]. Those data, provide preliminary evidences that SWT may be used to stimulate bone formation for the treatment of osteoporosis. However, the effects of SWT on cellular and systemic levels in bone systems still remained mostly elusive.
In this study, we investigated the effects of SWT on the in vitro models of bone resorption and bone formation. We employed the -glycerol phosphate induced pre-osteoblast MC3T3-E1 osteogenic differentiation as a cell model of bone formation, while Receptor activator of nuclear factor kappa-B ligand (RANKL)-induced RAW267.4 cell osteoclast differentiation as a cell model of bone resorption. Our results implied that SWT impaired osteoclast differentiation and bone resorption, yet upregulated cell proliferation and osteoblast functions or bone formation.
2. Materials and Methods
2.1. The preparation of SWT extract
The ingredients of SWT included 11.25 g of Rehmannia glutinosa, 11.25 g of Angelica sinensis, 11.25 g of Radix Paeoniae Alba and 11.25 g of Rhizoma Chuanxiong. An extract of SWT was prepared by decocting the dried prescription of herbs with boiling water (1300 ml) for 50 minutes. The obtained suspension was separated by filtration and condensed to the concentration of 360 ml (1 g/ ml) solution and then stored at 4C before use.
2.2. Cell culture and treatment
The murine preosteoblastic calvarial cell line MC3T3-E1 subclone 4 was obtained from ATCC (ATCC®, USA, CRL-2593™). Cells were cultured in α-Minimum Essential Medium (α-MEM) (10490-01, Gibco, Thermo Fisher Scientific, Inc.) supplemented with 10% (v/v) fetal bovine serum (FBS) (10437-028, Gibco) and 1% (v/v) penicillin-streptomycin (P/S) (15140-122, Gibco) at 37°C in a humidified atmosphere containing 5% CO2. To obtain osteoblasts, MC3T3-E1 cells were grown for 7 days in α-MEM containing 1% FBS, 1% P/S, 10 mM β-glycerol phosphate (G9422, Sigma Aldrich, USA), and 50 μg/ml L-ascorbic acid (A5960, Sigma Aldrich) [
17] (differentiated medium).
The murine macrophage cell line RAW264.7 subclone 2 was obtained from ATCC (ATCC® TIB-71™). Cells were grown in DMEM (Gibco, Thermo Fisher Scientific Inc.) supplemented with 10% FBS and 1% P/S, and incubated at 37ºC in 5% CO2 humidified air. The medium was changed every 3 days. To obtain osteoclasts, RAW264.7 cells were grown for 6 days in DMEM containing 10% FBS, 1% P/S, and 50 ng/ml mRANKL (PeproTech, Rocky Hill, NJ, USA) [
18].
2.3. Cell viability MTT assay
MC3T3-E1 and RAW264.7 were plated into 96-well plates and grown in differentiation medium for the indicated days. Cells were treated with different concentrations of SWT for 24 or 48 h according to the experimental design. Subsequently, the medium was removed, and cells were cultured in 100 μL fresh medium containing 10% 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) for 4 h at 37°C. The supernatant was removed, and the formazan crystals were dissolved in 100 μL of dimethyl sulfoxide (DMSO). Absorbance was recorded at 595 nm using a microplate reader.
2.4. Reverse transcription-quantitative polymerase chain reaction assay
Total RNA was extracted using RNAzol®RT reagent (RN190, MRC, Molecular Research Center, Inc., USA), according to the manufacturer’s protocol. cDNA was synthesized using a IQ2 MMLV RT-Script kit (Bio-Genesis Technologies Inc., TW). Quantitative polymerase chain reaction (qPCR) was performed using SYBR-Green (Applied Biosystems™, Thermo Fisher Scientific Inc., USA), and data collection was conducted using an ABI 7300 (Applied Biosystems; Thermo Fisher Scientific Inc.). The PCR cycling conditions were as follows: 95ºC for 2 min, followed by 40 cycles at 95ºC for 20 s, 58ºC or 53ºC for 20 s, and 72ºC for 40 s, and a final extension step of 95ºC for 15 s, 60ºC for 1 min, 95ºC for 15 s, and 60ºC for 15 s. Primer sequences were as follow (see
Table 1): Relative fold changes of gene expression were normalized using gapdh and results were plotted and analyzed using Prism 9 software (GraphPad Software Inc.). GAPDH was used as an internal control for normalization. Gene expression was calculated using the delta-delta Ct method.
2.5. Statistical analysis
Statistical analysis was performed using Prism 9 software (GraphPad Software Inc., San Diego, CA, USA). The values given are means ± standard errors of the mean (SEM) of at least three independent experiments. Statistical analysis was performed using one-way ANOVA test followed by Tukey’s multiple comparison test, or Student’s t-test. p < 0.05 was considered statistically significant.
3. Results
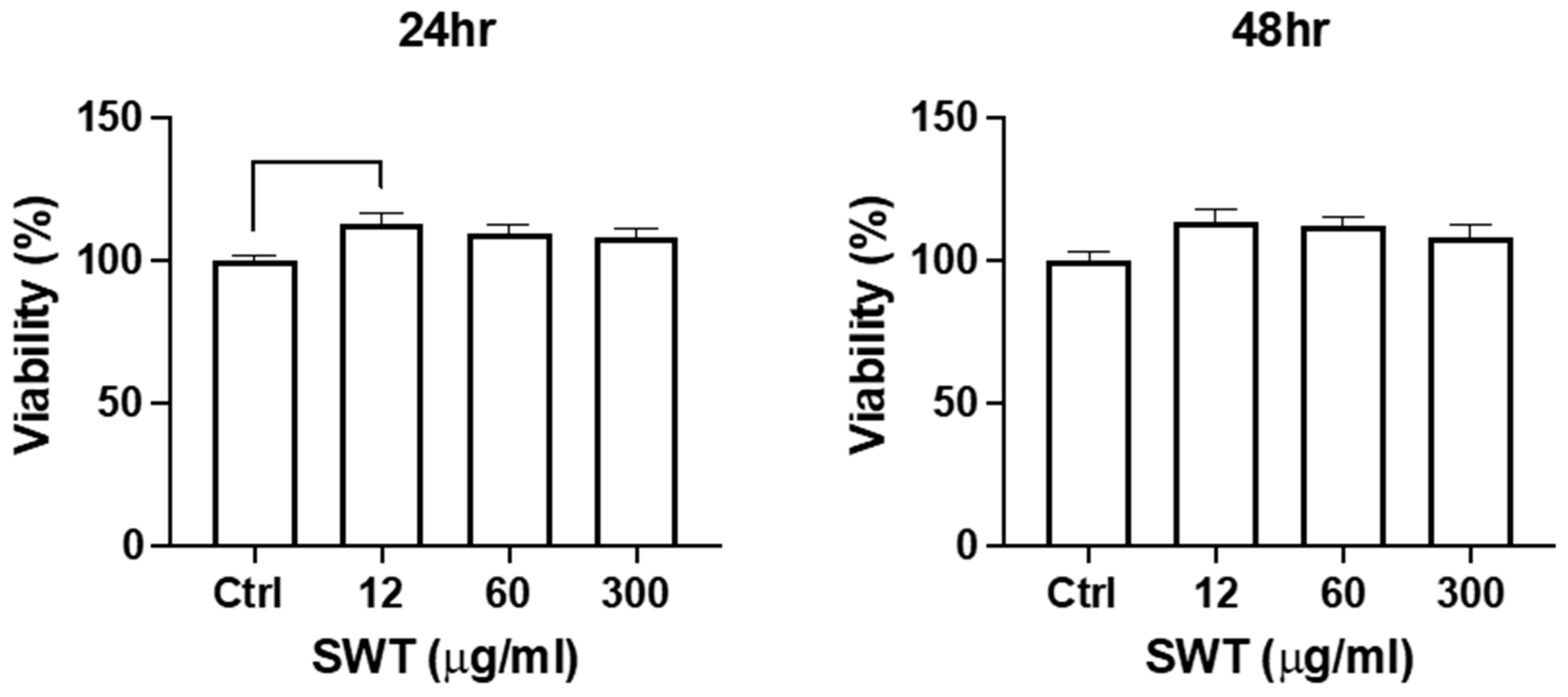
3.1. SWT promoted cell viability during osteoblast differentiation
MTT cell viability assay was performed to examine whether SWT stimulates cell proliferation or affect cell survival of the bone cells. BGP induced osteogenic differentiation of pre-osteoblast MC3T3-E1 cells as a cell model of osteoblast differentiation. After 7 days incubation in differentiation medium, a significant percentage of the pre-osteoblasts switch into late stage of osteoblastogenesis, expressing osteogenic markers, including ALP and OPN, and starting to execute bone formation. SWT was added at this stage, and its effects on cell proliferation or cell survival of differentiating osteoblast were first examined. Three different concentrations of SWT were used to treat for 24 or 48 hours. The MTT results revealed that SWT stimulated the increase of cell number at all treatment conditions, including different dosages and incubation times (
Figure 1). Differences dosages or incubation times did not generate different degrees of impacts on cell viability in differentiating osteoblasts.
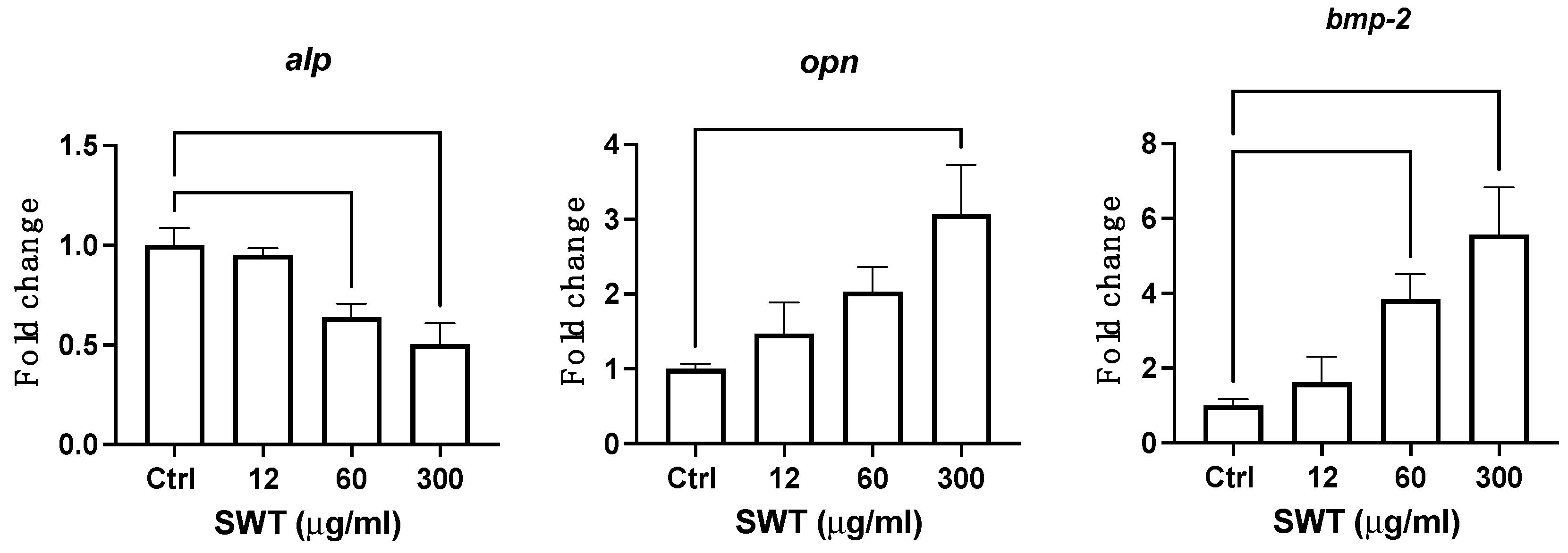
3.2. SWT altered the expression of essential bone formation genes in the differentiating osteoblast
SWT also affected the differentiation of osteoblast and bone formation. The expression of essential genes for osteoblast differentiation were measured in different dosages, including 12, 60, 300 ug/ml, of SWT treatment starting from the eighth day of the differentiation induction for 24 hours. BMP-2 is a critical transcription factor driving osteoblstogenesis, and promote cell proliferation of pre-osteoblasts. ALP is a key enzyme for matrix maturation and mineralization, while OPN is expressed in mineralizing tissue and functions as a mineralization regulator. Thus, those two genes were considered as markers for matrix maturation and mineralization stages of osteoblastogenesis, respectively. Interestingly, the expression of both bmp2 and opn genes showed a trend of increase with higher dosage, as shown in
Figure 2. The treatment with 300 ug/ml SWT significantly upregulated the expression of those two genes. Curiously, SWT induced inhibitory effects on alp gene expression. Our results suggested that SWT alters osteoblast differentiation and bone formation.
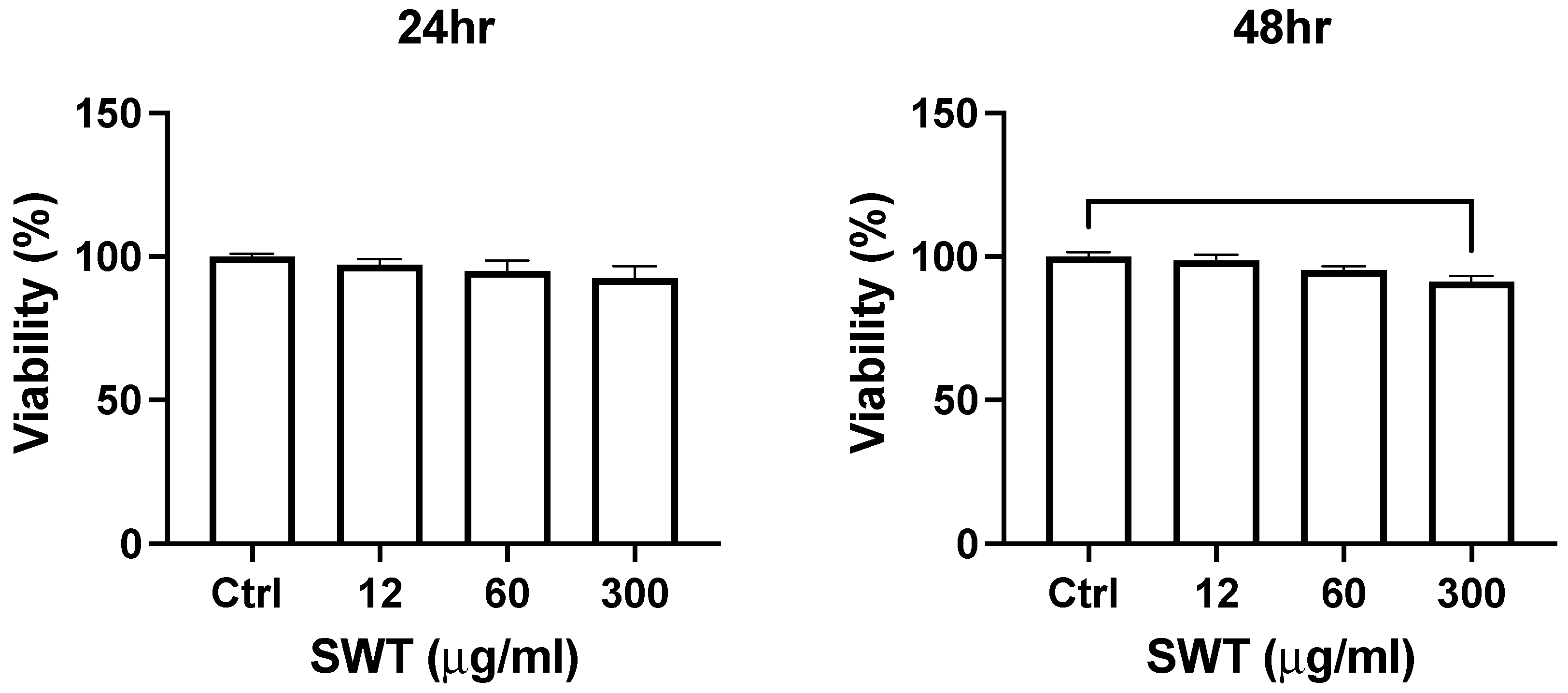
3.3. SWT decreased cell number during osteoclast differentiation
The influence of SWT on cell viability in osteoclast was also determined. RANKL-induced osteoclastogenesis from macrophage RAW264.7 cells were employed as a cell model for osteoclast differentiation and bone resorption. After 6 days of differentiation induction, the differentiating osteoclasts were treated with different concentrations of SWT for 24 or 48 hours.
Figure 3 reported the cell numbers determined by MTT assay. Lower concentration of SWT (12 or 60 ug/ml) did not change the cell viability. Only high concentration of SWT (300 ug/ml) significantly decreased the cell number. Different duration of treatment did not affect the results. As this differentiation process does not involve cell proliferation, our data suggested that SWT suppress cell survival in differentiating osteoclasts.
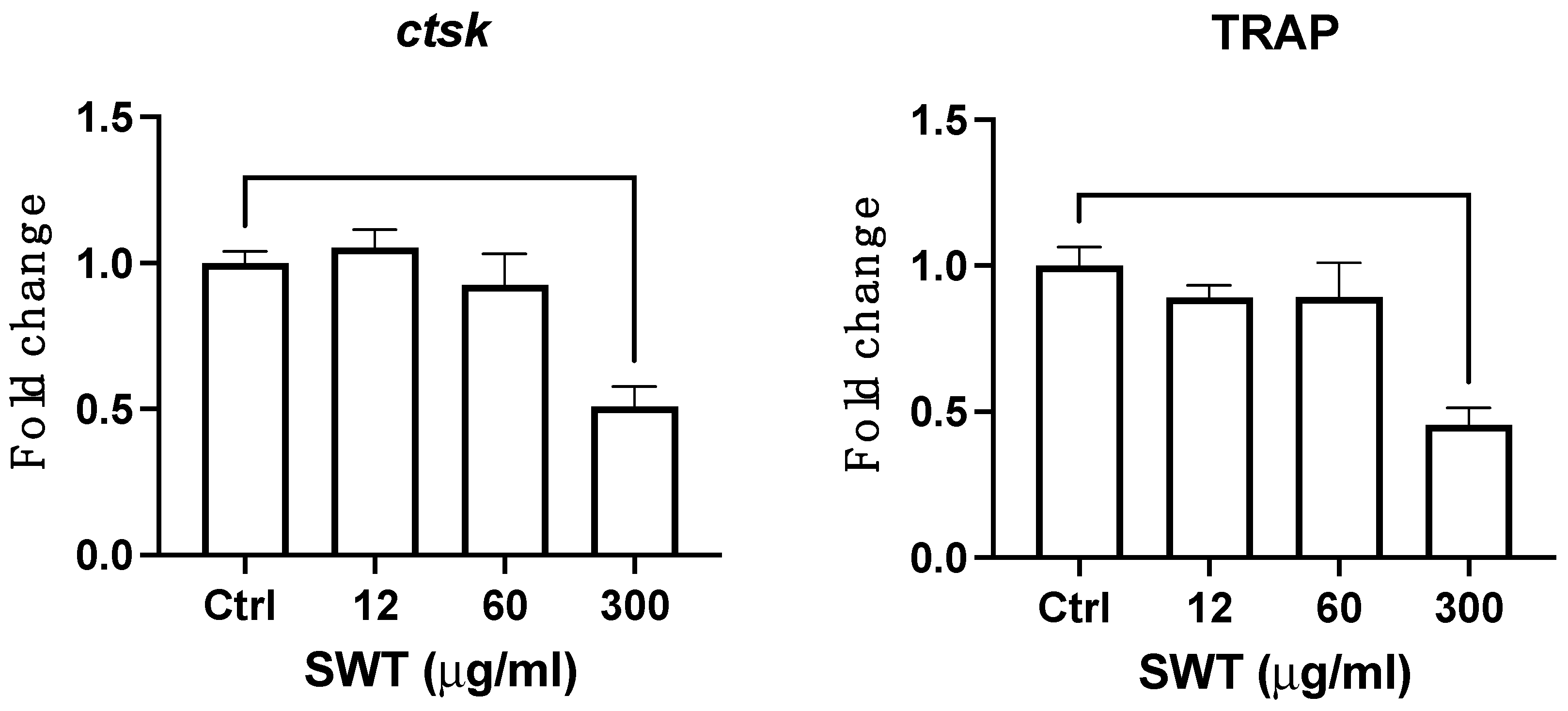
3.4. The expression of critical bone resorptive genes were disrupted by SWT in differentiating osteoclasts
Cathepsin K (ctsk) and Tartrate-resistant acid phosphatase (TRAP) are critical effectors of bone resorption. The expression of those two genes were measured to determine the influence of SWT on osteoclast differentiation and bone resorption. The results were demonstrated as
Figure 4. Consistent with the MTT results, only high concentration (300 ug/ml) of SWT impaired the expression of both genes. Combining the MTT and qPCR results, the decrease of cell survival and downregulating the bone resorptive genes strongly indicates that SWT negatively regulates osteoclast differentiation and bone resorption.
4. Discussion
SWT has multiple applications in various clinical conditions. Although the application of this medicine in bone diseases, such as osteoporosis, has been previously suggested, cellular and biochemical basis of this potential application were not fully understood. Previous study has reported a supportive role on bone formation in differentiated osteoblasts. In this study we examined the effects of SWT on differentiation and cellular functions in both osteoblast and osteoclast in vitro differentiation models. Our data showed that SWT promoted the cell viability in differentiating pre-osteoblasts, possibly attributed to promotion of cell proliferation or enhancing cell survival. The culture was composed of pre-osteoblasts in different stages, including proliferation, matrix maturation and mineralization. The upregulation of bmp2 gene by SWT suggested that cell proliferation was promoted.
The increase of bmp2 gene expression generally followed by the upregulation of alp gene. Previous study also showed the upregulation of alp gene by SWT treatment. However, our data revealed the decrease of alp gene expression, suggesting that SWT also influence alternative signaling pathways that regulate alp gene expression, such as Wnt/PI3K/Akt/β-catenin pathway. The differences between our results and previous study suggested different cell properties of osteoblast in different differentiation status. Additionally, our results suggested that SWT regulated osteoblast differentiation in a stage-dependent manner. Although alp gene was downregulated by SWT, the other 2 essential bone formation genes that were tested, including bmp2 and opn genes, all transcriptionally activated by SWT treatment. Combining the results, SWT appeared to be a positive regulator of osteoblast differentiation and bone formation.
Our data also demonstrated that cell viability and the expression of critical bone resorptive genes were all downregulated by SWT. The results suggested that SWT decrease osteoclast number and inhibit osteoclast differentiation and bone resorption. Collectively, our results implied that SWT positive regulates osteoblastogenesis and bone formation, yet negatively regulates osteoclastogenesis and bone resorption. The results provide supportive evidences for SWT applications for treating osteoporosis and other destructive bone diseases.
Author Contributions
Conceptualization, Shuenn-Yun Wu; methodology, Yu-Hsu Chen; writing—original draft preparation, Ying-Chou Hsieh; supervision, Yu-Pao Hsu; project administration, Ching-Hsiao Yu; funding acquisition, Ming-Te Cheng. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Taoyuan General Hospital, Ministry of Health and Welfare, Taiwan, grant number PTH111060, PTH112019.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We gratefully acknowledge support by CHIMERA BIOSCIENCE INC.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guo, Y.; Jiang, Q.; Gui, D.; et al. Chinese Herbal Formulas Si-Wu-Tang and Er-Miao-San Synergistically Ameliorated Hyperuricemia and Renal Impairment in Rats Induced by Adenine and Potassium Oxonate. Cell Physiol Biochem 2015, 37, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.D.; Lu, X.Q.; Ma, Z.C.; et al. Preliminary study on hematopoietic constituents of Si-Wu-Tang. Zhongguo Zhong Yao Za Zhi 2004, 29, 546–549. [Google Scholar] [PubMed]
- Xie, M. Modern Study of the Medical Formulae in Traditional Chinese Medicine; Xue Yue Press: Beijing, 1997; pp. 591–602. [Google Scholar]
- Dai, Y.; But, P.P.; Chan, Y.P.; et al. Antipruritic and anti-inflammatory effects of aqueous extract from Si-Wu-Tang. Biol Pharm Bull 2002, 25, 1175–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ravula, R.; Wang, Z.; et al. Traditional Chinese medicinal formula Si-Wu-Tang prevents oxidative damage by activating Nrf2-mediated detoxifying/antioxidant genes. Cell & Bioscience 2014, 4, 8. [Google Scholar] [CrossRef]
- Ohta, H.; Ni, J.W.; Matsumoto, K.; et al. Peony and its major constituent, paeoniflorin, improved radial maze performance impaired by scopolamine in rats. Pharmacology Biochemistry and Behavior 1993, 45, 719–23. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.C.; Tsai, Y.Z.; Lee, C.J.; et al. Elucidation of the effects of Si-Wu Tang on menstrual disorder patterns through activation of aromatase and antioxidation. Evidence-Based Complementary and Alternative Medicine 2019, Article ID 4761651. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, R.; Wang, Y. Study on the anti-breast cancer mechanism and bioactive components of Si-Wu-Tang by cell type-specific molecular network. Evidence-Based Complementary and Alternative Medicine 2020, Article ID 2345970. [Google Scholar] [CrossRef]
- Chang, P.J.; Lin, C.C.; Chen, Y.C.; et al. Use of herbal dietary supplement Si-Wu-Tang and health-related quality of life in postpartum women: a population-based correlational study. Evidence-Based Complementary and Alternative Medicine 2013, Article ID 790474. [Google Scholar] [CrossRef]
- Liang, Q.D.; Gao, Y.; Tan, H.L.; et al. Effects of four Si-Wu-Tang’s constituents and their combination on irradiated mice. Biological & Pharmaceutical Bulletin 2006, 29, 1378–82. [Google Scholar] [CrossRef]
- Chiu, H.F.; Wu, Y.H.; Shen, Y.C.; et al. Antioxidant and physiological effects of Si-Wu-Tang on skin and liver: a randomized, double-blind, placebo-controlled clinical trial. Chinese Medicine 2016, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Oh, H.; Yang, J.A.; et al. Radioprotective effects of two traditional Chinese medicine prescriptions: si-wu-tang and si-jun-zi-tang. Am J Chin Med 1999, 27, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Ho, Y.H.; Lin, C.C. Protection of mouse bone marrow by Si-Wu-Tang against whole body irradiation. J Ethnopharmacol 1996, 52, 113–117. [Google Scholar] [CrossRef]
- Kim, J.L.; Kang, S.W.; Kang, M.K.; et al. Osteoblastogenesis and osteoprotection enhanced by flavonolignan silibinin in osteoblasts and osteoclasts. J Cell Biochem 2012, 113, 247–259. [Google Scholar] [CrossRef]
- Nazrun, A.S.; Norazlina, M.; Norliza, M.; et al. The anti-inflammatory role of vitamin E in prevention of osteoporosis. Adv Pharmacol Sci 2012, 2012, 142702. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Chen, P.C.; Li, T.M.; et al. Si-Wu-Tang extract stimulates bone formation through PI3K/Akt/NF-κB signaling pathways in osteoblasts. BMC Complement Altern Med 2013, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.Z.; Teng, X.; Zhang, Z.B.; et al. Mangiferin inhibits apoptosis and oxidative stress via BMP2/Smad-1 signaling in dexamethasone-induced MC3T3-E1 cells. Int J Mol Med 2018, 41, 2517–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Peng, S.Y.; Cheng, M.T.; et al. Different susceptibilities of osteoclasts and osteoblasts to glucocorticoid-induced oxidative stress and mitochondrial alterations. Chin J Physiol 2019, 62, 70–79. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).