Submitted:
29 February 2024
Posted:
01 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction of Taste Peptides from Straw Mushrooms
2.3. Purification of the Peptides by Ultrafiltration
2.4. Identification of the Taste Peptides
2.5. Molecular Docking (MD) of the Identified Peptides and T1R1/T1R3
2.6. Sensory Assessors
2.7. The Saltiness Was Compared by QDA Method
2.8. Comparing the Relative Saltiness Using the Two- Alternative Forced-Choice (2-AFC) Method
2.9. Rating the Saltiness Intensity Using the General Labeled Magnitude Scale (gLMS)
2.10. Evaluation of the Saltiness Enhancement Effect of Synthetic Peptides and Their Threshold Determination
2.11. Dose-Response Evaluation of the Saltiness Taste for Synthetic Peptides Solutions with NaCl Solutions
2.12. E-Tongue Analysis
2.13. Statistical Analysis
3. Results and Discussion
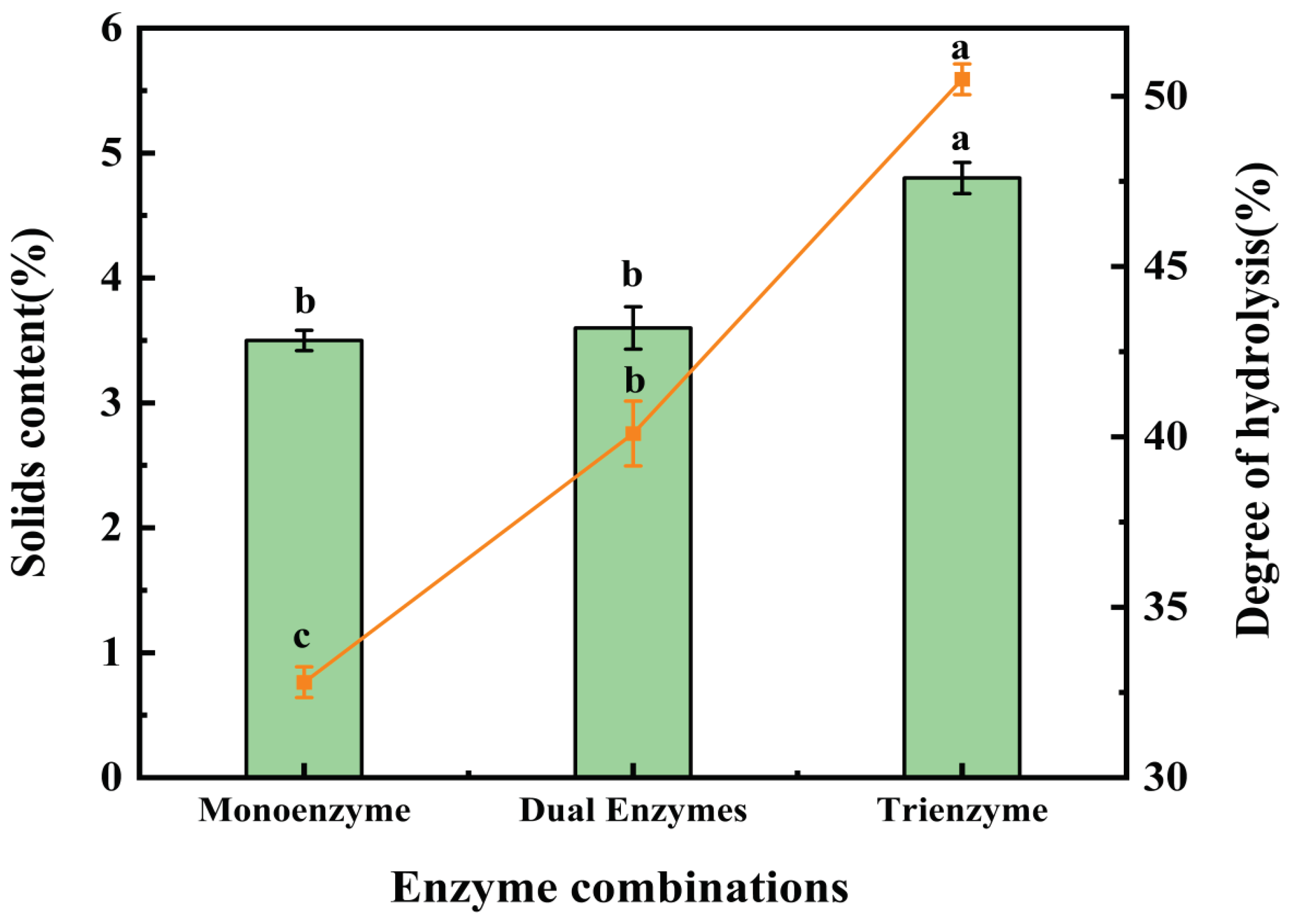
3.1. Solid Content and Degree of Hydrolysis of Straw Mushroom Peptides
3.2. Free Amino Acid Content and Molecular Weight Distribution of Straw Mushroom Peptides
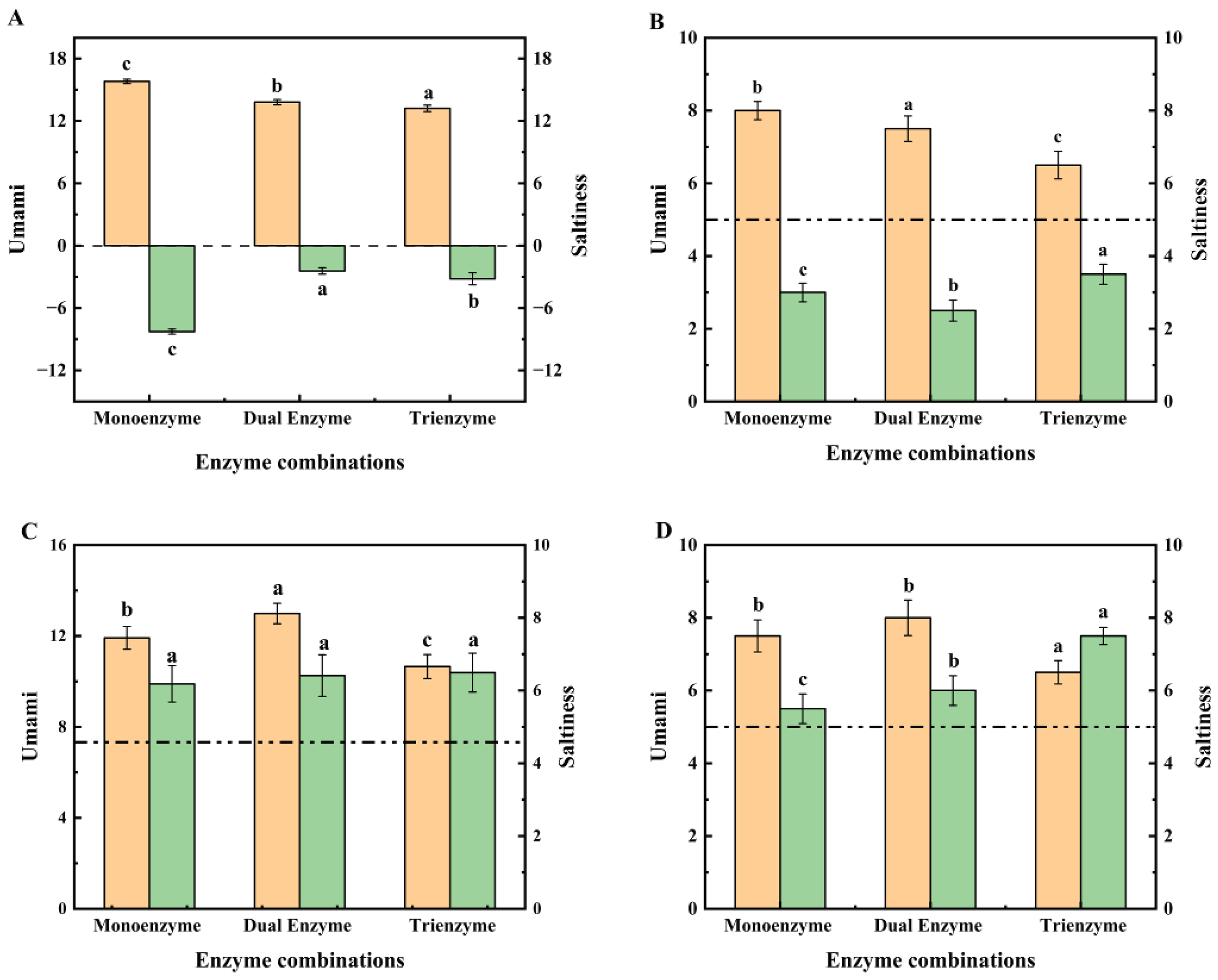
3.3. Evaluation of the Saltiness Enhancement Effect of Enzyme Solution of Straw Mushroom.
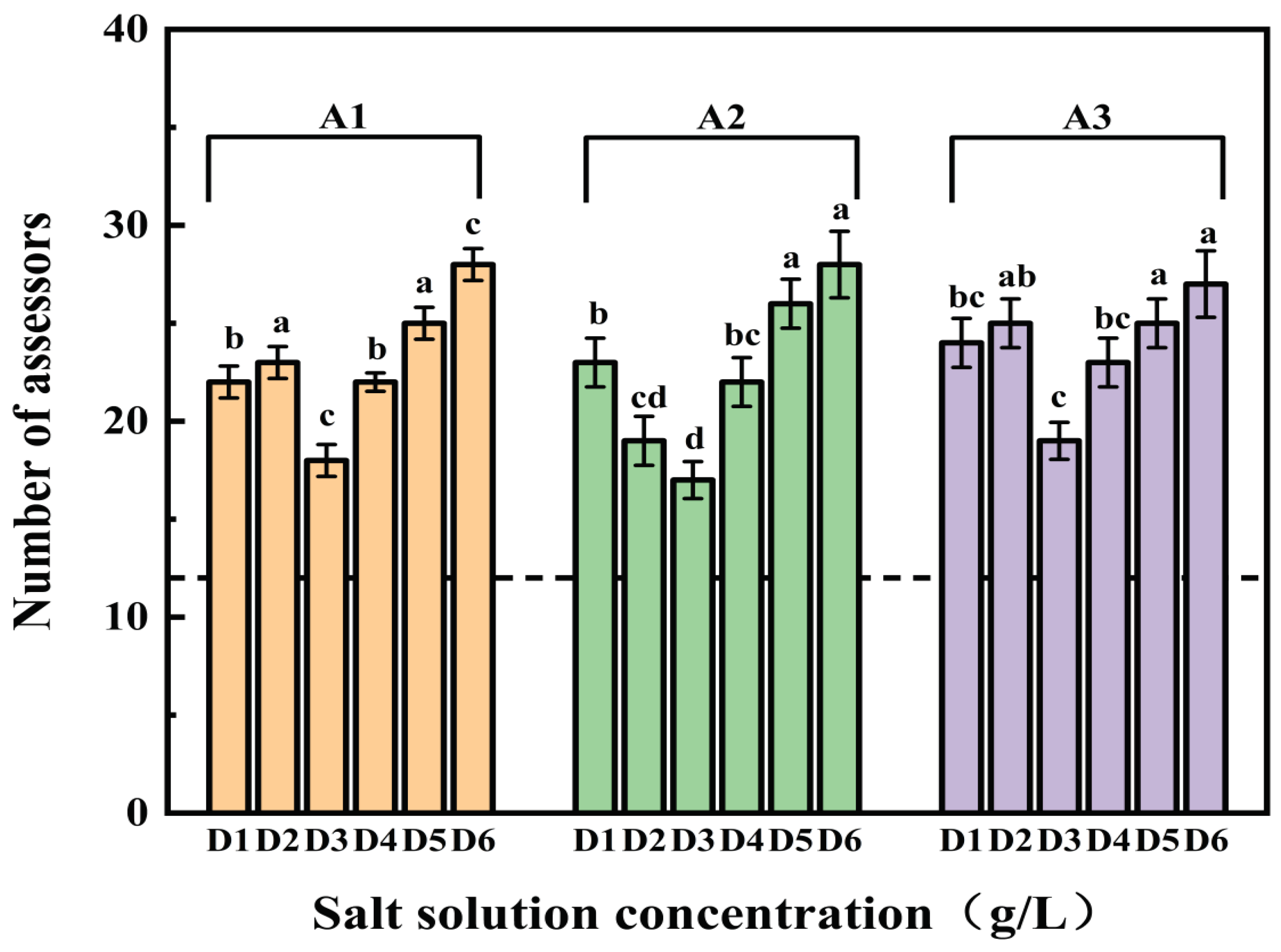
3.4. Comparison of Saltiness Intensity Using the 2-AFC
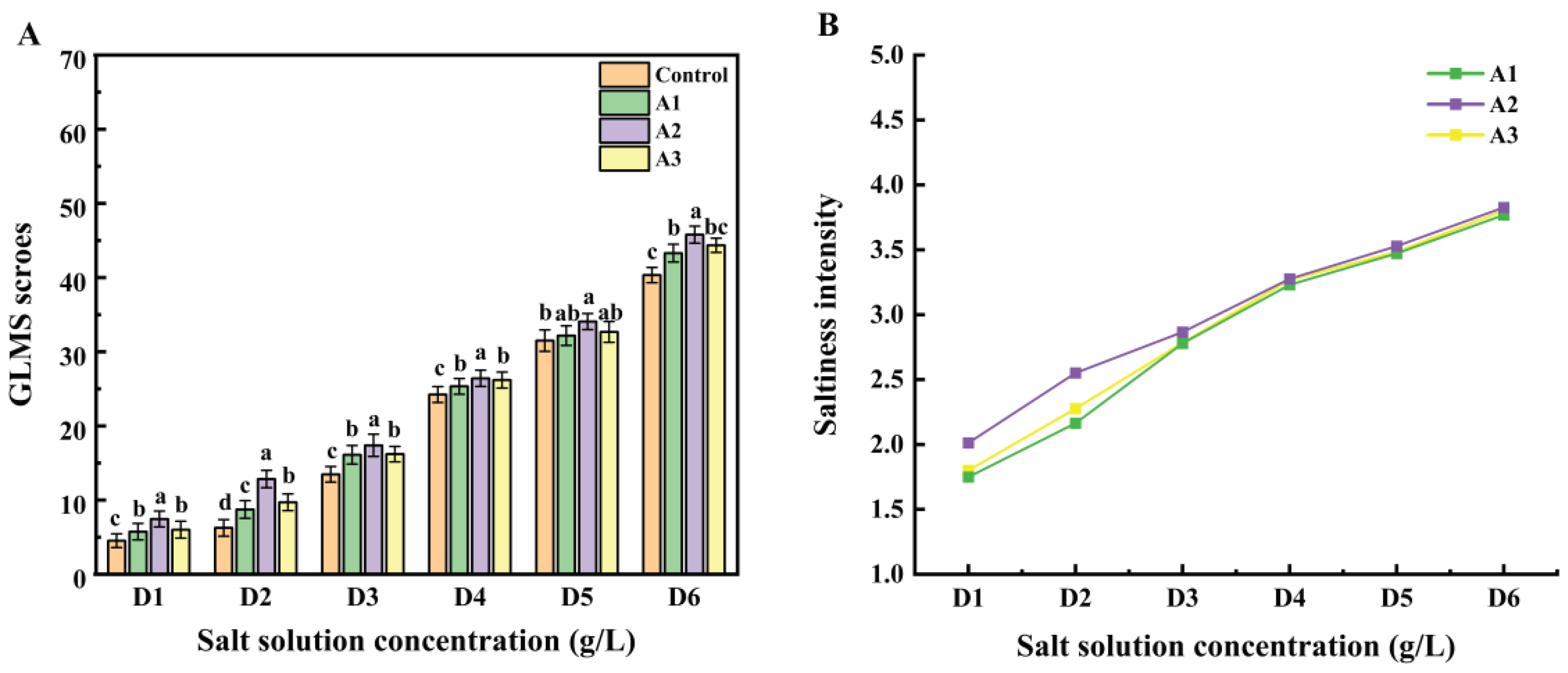
3.5. Saltiness Intensity Evaluation Using the gLMS
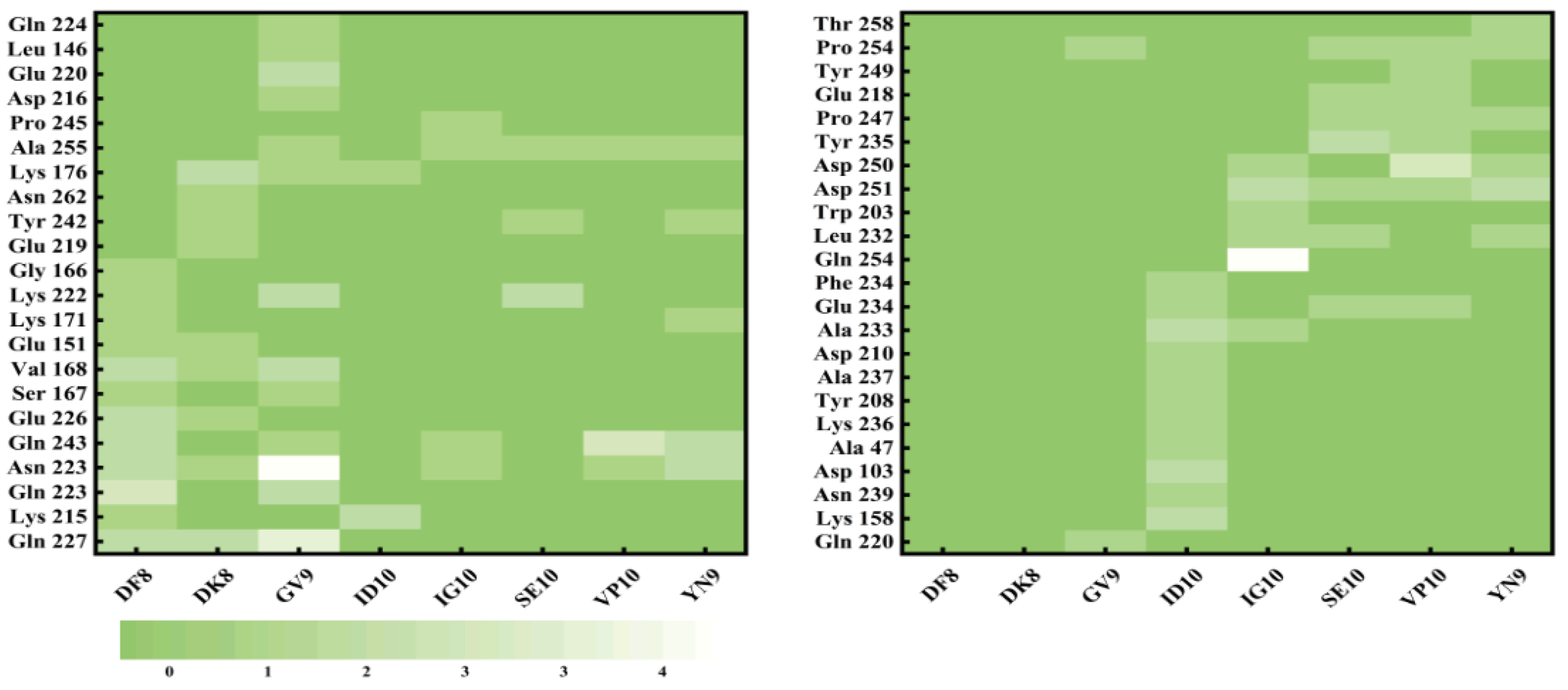
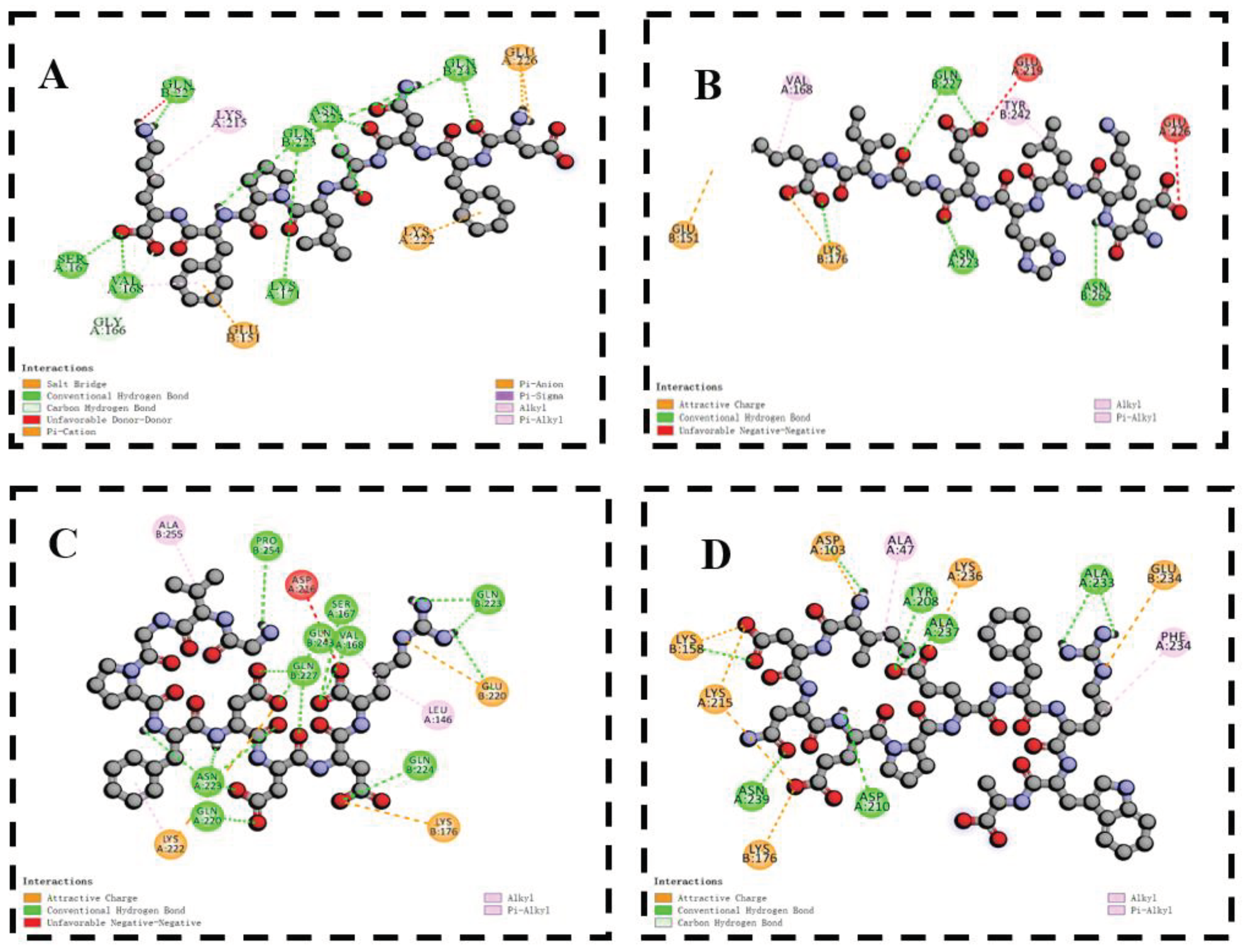
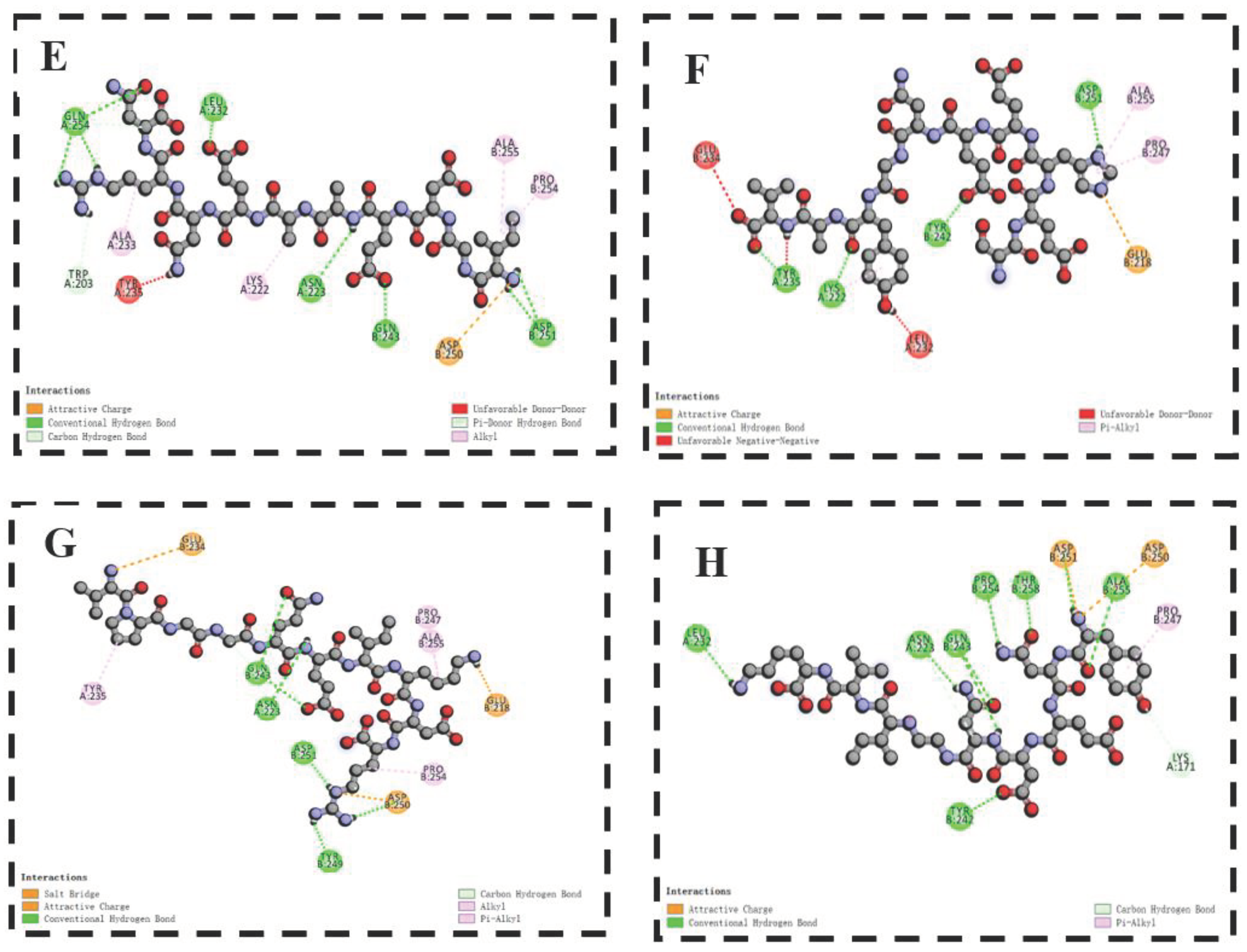
3.6. Identification and Molecular Docking of Peptides
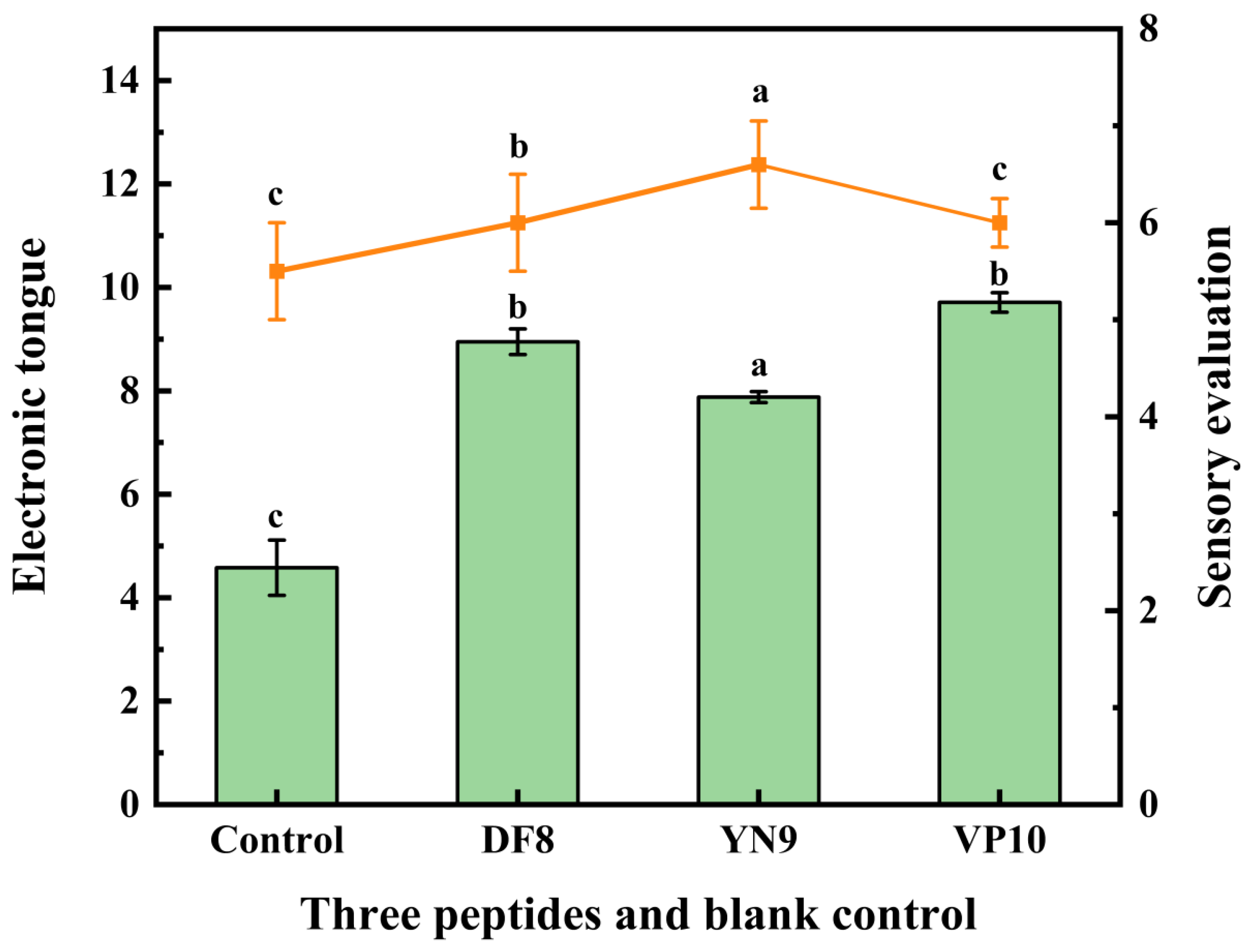
3.7. Effect of Synthetic Peptides on Saltiness in Salt Solutions
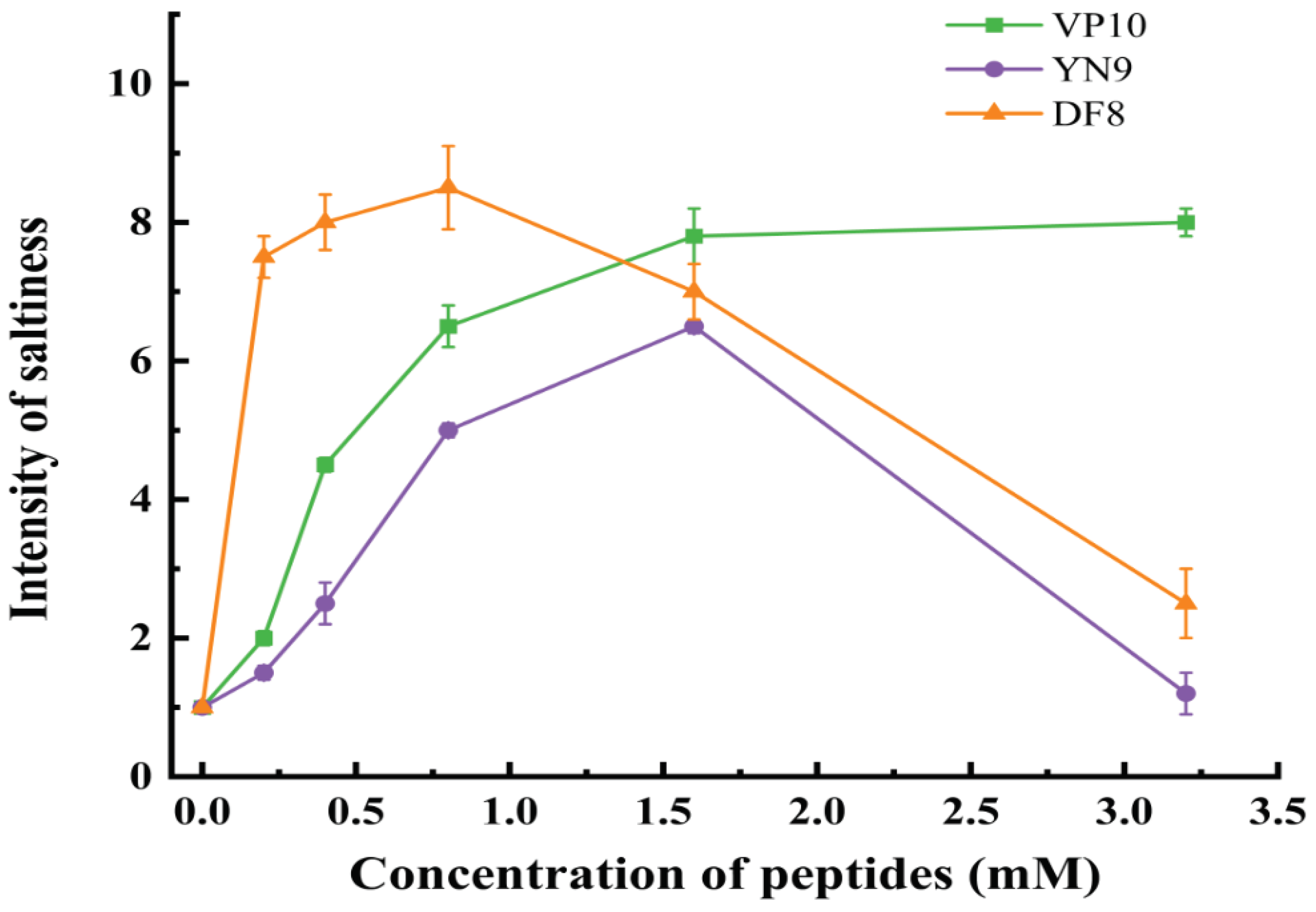
3.8. Dose-Feedback Saltiness Enhancement Effects of Synthetic Peptides
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jimenez-Maroto, L.A., T. Sato, and S.A. Rankin, Saltiness potentiation in white bread by substituting sodium chloride with a fermented soy ingredient. Journal of Cereal Science, 2013. 58(2): p. 313-317. [CrossRef]
- Pujols, K.D., et al., Low-sodium roasted peanuts: effects of salt mixtures (NaCl,KCl and glycine) on consumer perception and purchase intent. International Journal of Food Science & Technology, 2019. 54(9): p. 2754-2762. [CrossRef]
- D’Elia, L., et al., Habitual salt intake and risk of gastric cancer: A meta-analysis of prospective studies. Clinical Nutrition, 2012. 31(4): p. 489-498. [CrossRef]
- Messerli, et al., Salt and heart disease: a second round of "bad science"? The Lancet, 2018.
- Le, B., et al., Salt taste receptors and associated salty/salt taste-enhancing peptides: A comprehensive review of structure and function. Trends in Food Science & Technology, 2022. 129: p. 657-666. [CrossRef]
- Chen, Y.P., et al., Saltiness-Enhancing Peptides Isolated from the Chinese Commercial Fermented Soybean Curds with Potential Applications in Salt Reduction. J Agric Food Chem, 2021. 69(35): p. 10272-10280. [CrossRef]
- Schindler, A., et al., Discovery of salt taste enhancing arginyl dipeptides in protein digests and fermented fish sauces by means of a sensomics approach. J Agric Food Chem, 2011. 59(23): p. 12578-88. [CrossRef]
- Ueda, Y., et al., Characteristic flavor constituents in water extract of garlic. Agricultural and Biological Chemistry, 1990. 54(1): p. 163-169.
- Xiang, F., Research progress on food salt reduction strategies. Food and fermentation industry, 2023: p. 1-10.
- Moore, A., C.R. Luckett, and J.P. Munafo, Jr., Taste-Active Dipeptides from Hydrolyzed Mushroom Protein Enhance Saltiness. J Agric Food Chem, 2021. 69(40): p. 11947-11959. [CrossRef]
- Yu, B., et al., Maillard-reacted peptides from glucosamine-induced glycation exhibit a pronounced salt taste-enhancing effect. Food Chem, 2022. 374: p. 131776. [CrossRef]
- Son, M. and T.H. Park, The bioelectronic nose and tongue using olfactory and taste receptors: Analytical tools for food quality and safety assessment. Biotechnology Advances, 2017: p. S0734975017301763. [CrossRef]
- Shigemura and Noriatsu, Angiotensin II Modulates Salty and Sweet Taste Sensitivities. Journal of Neuroscience, 2013. 33: p. 6267-6277.
- A, T.S., et al., Quantitative proteomics and SWATH-MS to elucidate peri-receptor mechanisms in human salt taste sensitivity. Food Chemistry, 2018. 254: p. 95-102.
- Niki, M., et al., Gustatory signaling in the periphery: detection, transmission, and modulation of taste information. Biological & Pharmaceutical Bulletin, 2010. 33(11): p. 1772-7. [CrossRef]
- Guo, M., et al., Collagen Glycopeptides from Transglutaminase-Induced Glycosylation Exhibit a Significant Salt Taste-Enhancing Effect. J Agric Food Chem, 2023. 71(22): p. 8558-8568. [CrossRef]
- Xie, X., et al., The enhancement and mechanism of the perception of saltiness by umami peptide from Ruditapes philippinarum and ham. Food Chem, 2023. 405(Pt A): p. 134886. [CrossRef]
- Shan, Y., et al., Decoding of the Saltiness Enhancement Taste Peptides from the Yeast Extract and Molecular Docking to the Taste Receptor T1R1/T1R3. J Agric Food Chem, 2022. 70(47): p. 14898-14906.
- Zhang, J., et al., Identification and virtual screening of novel umami peptides from chicken soup by molecular docking. Food Chem, 2023. 404(Pt A): p. 134414. [CrossRef]
- Song, S., et al., Identification of novel umami peptides from Boletus edulis and its mechanism via sensory analysis and molecular simulation approaches. Food Chem, 2023. 398: p. 133835. [CrossRef]
- Sun, X., et al., The enhancement of the perception of saltiness by umami sensation elicited by flavor enhancers in salt solutions. Food Res Int, 2022. 157: p. 111287. [CrossRef]
- ISO, Sensory analysis -General guidance for the selection, training and monitoring of assessors. 2012.
- Zhang, N., et al., Sensory-Guided Analysis of Key Taste-Active Compounds in Pufferfish (Takifugu obscurus). J Agric Food Chem, 2019. 67(50): p. 13809-13816. [CrossRef]
- GB/T12315, Sensory analysis-Methodology-Ranking. 2008.
- ISO, Sensory analysis — Methodology — Paired comparison test. ISO, 2005.
- Blackman, J., A. Saliba, and L. Schmidtke, Sweetness acceptance of novices, experienced consumers and winemakers in Hunter Valley Semillon wines. Food Quality and Preference, 2010. 21(7): p. 679-683. [CrossRef]
- Zhang Huimin, D.Y., Zhang Ruifen, et al., Modification effects of different inhibitors on bitter melon powder flavour perception. Food Science, 2018. 39(10): p. 298-303.
- Green, B.G., et al., Evaluating the 'Labeled Magnitude Scale' for Measuring Sensations of Taste and Smell. Chemical Senses, 1996. 21(3): p. 323-334. [CrossRef]
- L.M. Bartoshuka, V.B. Duffya,b, K. Fasta, B.G. Greena,c, J. Prutkina, D.J. Snydera, Labeled scales (e.g., category, Likert, VAS) and invalid across-group comparisons: what we have learned from genetic variation in taste. Food Quality and Preference, 2003. [CrossRef]
- Zhao, J., et al., Isolation, identification and characterization of taste peptides from fermented broad bean paste. Food Funct, 2022. 13(16): p. 8730-8740.
- Yan, F., et al., Small Peptides Hydrolyzed from Pea Protein and Their Maillard Reaction Products as Taste Modifiers: Saltiness, Umami, and Kokumi Enhancement. Food and Bioprocess Technology, 2021. 14(6): p. 1132-1141. [CrossRef]
- Sun, H., Z. Chen, and Z. Zhou, Optimization of Soybean Protein Isolate Extraction by Response Surface Method and Complex Enzymatic Hydrolysis for Preparing Active Peptides. China Condiment, 2022. 46(3): p. 82-87.
- Luo, B., Protein Extraction from Chlorella and Peptide Preparation by Enzyme Hydrolysis Food Industry, 2023. 44(8): p. 95-98.
- Caffall, K.H. and D. Mohnen, The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res, 2009. 344(14): p. 1879-900. [CrossRef]
- Xu, X., et al., Lignocellulose degradation patterns, structural changes, and enzyme secretion by Inonotus obliquus on straw biomass under submerged fermentation. Bioresour Technol, 2017. 241: p. 415-423. [CrossRef]
- Zhao, G., et al., Effects of applying cellulase and starch on the fermentation characteristics and microbial communities of Napier grass (Pennisetum purpureum Schum.) silage. J Anim Sci Technol, 2021. 63(6): p. 1301-1313. [CrossRef]
- Feyisa, M.M. and P. Yadav, Role of Live Microbes for Fermentation and Enhancement of Feeding Value of Wheat Straw as Animal Fodder. The Asia Journal of Applied Microbiology, 2021. 8. [CrossRef]
- Silvestre, T., et al., Performance of dairy cows fed normal- or reduced-starch diets supplemented with an exogenous enzyme preparation. J Dairy Sci, 2022. 105(3): p. 2288-2300. [CrossRef]
- Gu,Z and Y. Yang, Research progress in flavor components of edible fungus. Food Industry Science and Technology, 2013. 34(5): p. 363-367.
- Yamaguchi, S., et al., Measurement of the relative taste intensity of some L-α-amino acids and 5'-nucleotides. Journal of Food Science, 2010. 36(6): p. 846-849.
- Xu, X.S.Z.S., S, Effects of Different Treatments on the Release of Flavor Substances from Straw Mushroom (Volvariella volvacea). Food Science, 2017. 39(12): p. 107-111.
- Adcock, S.A. and J.A. Mccammon, Molecular dynamics: survey of methods for simulating the activity of proteins. Chemical Reviews, 2006. 106(5): p. 1589. [CrossRef]
- Sun, X., et al., The enhancement of the perception of saltiness by umami sensation elicited by flavor enhancers in salt solutions. Food Research International, 2022. 157. [CrossRef]
- Zhang, H., et al., Taste Modification of Bitter Melon (Momordica charantia) Powder by Different Bitterness Inhibitors. Food Science, 2018. 39(10): p. 298-303.
- Liang, L., et al., Characteristics of umami peptides identified from porcine bone soup and molecular docking to the taste receptor T1R1/T1R3. Food Chem, 2022. 387: p. 132870.
- Liu, H., L.T. Da, and Y. Liu, Understanding the molecular mechanism of umami recognition by T1R1-T1R3 using molecular dynamics simulations. Biochem Biophys Res Commun, 2019. 514(3): p. 967-973. [CrossRef]
- Dang, Y., et al., Comparison of umami taste peptides in water-soluble extractions of Jinhua and Parma hams. LWT - Food Science and Technology, 2015. 60(2): p. 1179-1186. [CrossRef]
- Su, G., et al., Effect of transglutaminase on taste characteristics of pea protein hydrolysates through altering the composition of amino acids and peptides. Food Bioscience, 2023. 56. [CrossRef]
- Chang, R., et al., Ion-exchange purification, nano-HPLC–MS/MS identification and molecular dynamics simulation of novel umami peptides from fermented grain wine (Huangjiu). Journal of Food Composition and Analysis, 2024. 125.
- A, Y.Z., et al., Isolation, characterization and molecular docking of novel umami and umami-enhancing peptides from Ruditapes philippinarum. Food Chemistry, 2020.
- Li, C., et al., A rapid selection strategy for umami peptide screening based on machine learning and molecular docking. Food Chem, 2023. 404(Pt A): p. 134562. [CrossRef]
- Wang, W., et al., Identification and comparison of umami-peptides in commercially available dry-cured Spanish mackerels (Scomberomorus niphonius). Food Chem, 2022. 380: p. 132175. [CrossRef]
- Zhou, H., et al., Design, virtual screening, molecular docking and molecular dynamics studies of novel urushiol derivatives as potential HDAC2 selective inhibitors. Gene, 2017. 637: p. 63-71. [CrossRef]
- Chen, S., J. Chang, and M. Sun, Study on the Salt-reducing Effect of Agaricus bisporus Extract on Three Kinds of Broth. Science and Technology of Food Industry, 2023. 44(15): p. 69-77.
- Wang, K., et al., Evaluation of eight kinds of flavor enhancer of umami taste by an electronic tongue. Food Sci Nutr, 2021. 9(4): p. 2095-2104. [CrossRef]
- Moon, S.Y. and E.C.Y. Li-Chan, Changes in aroma characteristics of simulated beef flavour by soy protein isolate assessed by descriptive sensory analysis and gas chromatography. Food Research International, 2007. 40(10): p. 1239-1248. [CrossRef]
- Zhuang, M., et al., Sequence, taste and umami-enhancing effect of the peptides separated from soy sauce. Food Chem, 2016. 206: p. 174-81. [CrossRef]
| Amino acids |
Content(mg/ml) | ||
|---|---|---|---|
| Monoenzyme | Dual Enzyme | Trienzyme | |
| Bitterness | 1.13±0.01a | 0.88±0.01b | 1.06±0.02c |
| Sweetness | 0.52±0.01b | 0.51±0.01b | 0.61±0.02a |
| umami | 0.52±0.02b | 0.53±0.02b | 0.65±0.01a |
| tasteless | 0.38±0.01b | 0.34±0.01b | 0.41±0.02a |
| Total | 2.55±0.01c | 2.26±0.01b | 2.74±0.02a |
| Peptide | Length | affinity (kcal/mol) | |
|---|---|---|---|
| 1 | DFNALPFK | 8 | -9.2 |
| 2 | VPGGQEIKDR | 10 | -8.9 |
| 3 | YNEDNGIVK | 9 | -8.8 |
| 4 | IDNEPEFRWA | 10 | -8.6 |
| 5 | GVGPFDDDR | 9 | -8.7 |
| 6 | SEHEENGYAV | 10 | -8.7 |
| 7 | DKLHEGIK | 8 | -8.6 |
| 8 | IGDEAAENRN | 10 | -8.3 |
| Peptides | Taste attribute | Threshold value (mM) | |
|---|---|---|---|
| water | 0.5%NaCl | ||
| DFNALPFK | sore, astringency, umami | 0.38±0.03b | 0.22±0.02c |
| YNEDNGIVK | sore, astringency | 0.45±0.02b | 0.33±0.05b |
| VPGGQEIKDR | sore, astringency, weak umami | 0.56±0.04a | 0.41±0.03a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





