Submitted:
29 February 2024
Posted:
01 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patient Collective and Ethics Statement
2.2. Data Evaluation and Statistics
3. Results
3.1. Patient Collective
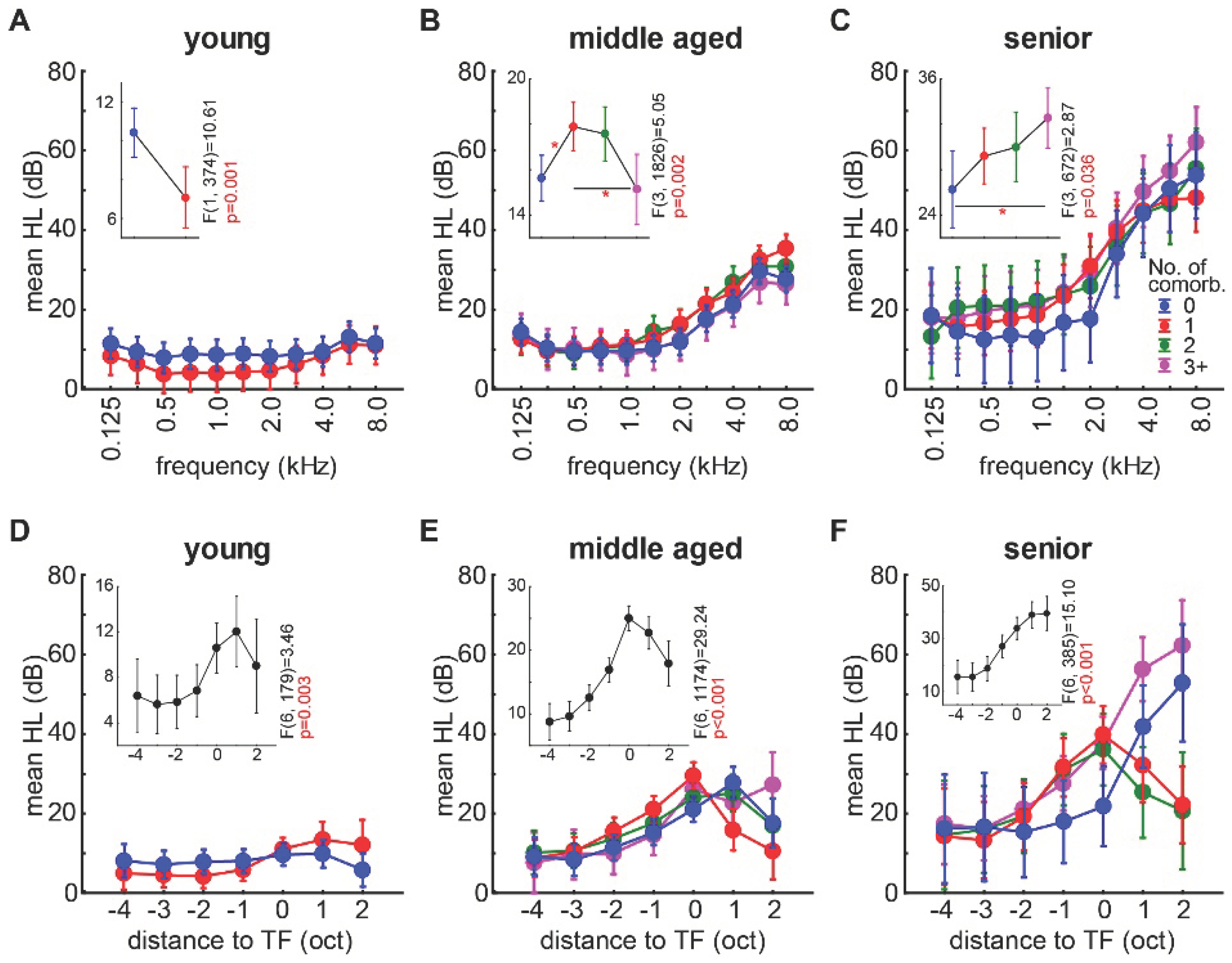
3.2. Correlation of the Audiometric Data of Tinnitus Patients with and without the Presence of Non-Auditory Comorbidities
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biswas, R.; Lugo, A.; Akeroyd, M.A.; Schlee, W.; Gallus, S.; Hall, D.A. Tinnitus prevalence in Europe: a multi-country cross-sectional population study. The Lancet Regional Health - Europe 2022, 12, 100250. [CrossRef]
- Eggermont, J.J. Central tinnitus. Auris Nasus Larynx 2003, 30 Suppl, S7-12. [CrossRef]
- Schaette, R.; McAlpine, D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci 2011, 31, 13452-13457. [CrossRef]
- Savastano, M. Tinnitus with or without hearing loss: are its characteristics different? Eur Arch Otorhinolaryngol 2008, 265, 1295-1300. [CrossRef]
- Hebert, S.; Canlon, B.; Hasson, D. Emotional exhaustion as a predictor of tinnitus. Psychother Psychosom 2012, 81, 324-326. [CrossRef]
- Hebert, S.; Canlon, B.; Hasson, D.; Magnusson Hanson, L.L.; Westerlund, H.; Theorell, T. Tinnitus severity is reduced with reduction of depressive mood–a prospective population study in Sweden. PloS one 2012, 7, e37733. [CrossRef]
- Tziridis, K.; Friedrich, J.; Brueggemann, P.; Mazurek, B.; Schulze, H. Estimation of Tinnitus-Related Socioeconomic Costs in Germany. Int J Environ Res Public Health 2022, 19. [CrossRef]
- Eggermont, J.J.; Roberts, L.E. The neuroscience of tinnitus. Trends Neurosci 2004, 27, 676-682. [CrossRef]
- Gerken, G.M. Central tinnitus and lateral inhibition: an auditory brainstem model. Hear Res 1996, 97, 75-83. [CrossRef]
- Rüttiger, L.; Singer, W.; Panford-Walsh, R.; Matsumoto, M.; Lee, S.C.; Zuccotti, A.; Zimmermann, U.; Jaumann, M.; Rohbock, K.; Xiong, H. The reduced cochlear output and the failure to adapt the central auditory response causes tinnitus in noise exposed rats. PloS one 2013, 8, e57247. [CrossRef]
- Krauss, P.; Tziridis, K.; Metzner, C.; Schilling, A.; Hoppe, U.; Schulze, H. Stochastic resonance controlled upregulation of internal noise after hearing loss as a putative cause of tinnitus-related neuronal hyperactivity. Frontiers in neuroscience 2016, 10, 597. [CrossRef]
- Hickox, A.E.; Liberman, M.C. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol 2014, 111, 552-564. [CrossRef]
- Tziridis, K.; Forster, J.; Buchheidt-Dorfler, I.; Krauss, P.; Schilling, A.; Wendler, O.; Sterna, E.; Schulze, H. Tinnitus development is associated with synaptopathy of inner hair cells in Mongolian gerbils. Eur J Neurosci 2021, 54, 4768-4780. [CrossRef]
- Mazurek, B.; Haupt, H.; Olze, H.; Szczepek, A.J. Stress and tinnitus—from bedside to bench and back. Frontiers in systems neuroscience 2012, 6, 47. [CrossRef]
- Schilling, A.; Sedley, W.; Gerum, R.; Metzner, C.; Tziridis, K.; Maier, A.; Schulze, H.; Zeng, F.-G.; Friston, K.J.; Krauss, P. Predictive coding and stochastic resonance as fundamental principles of auditory phantom perception. Brain 2023, 146, 4809-4825. [CrossRef]
- Knipper, M.; Ruettiger, L.; Schick, B.; Dlugaiczyk, J. Glycine receptor agonists for the treatment of phantom phenomena. 2011, US20110077239A1.
- Engineer, N.D.; Riley, J.R.; Seale, J.D.; Vrana, W.A.; Shetake, J.A.; Sudanagunta, S.P.; Borland, M.S.; Kilgard, M.P. Reversing pathological neural activity using targeted plasticity. Nature 2011, 470, 101-104. [CrossRef]
- Tziridis, K.; Brunner, S.; Schilling, A.; Krauss, P.; Schulze, H. Spectrally Matched Near-Threshold Noise for Subjective Tinnitus Loudness Attenuation Based on Stochastic Resonance. Front Neurosci 2022, 16, 831581. [CrossRef]
- Knipper, M.; van Dijk, P.; Schulze, H.; Mazurek, B.; Krauss, P.; Scheper, V.; Warnecke, A.; Schlee, W.; Schwabe, K.; Singer, W.; et al. The Neural Bases of Tinnitus: Lessons from Deafness and Cochlear Implants. J Neurosci 2020, 40, 7190-7202. [CrossRef]
- Távora-Vieira, D.; Marino, R.; Krishnaswamy, J.; Kuthbutheen, J.; Rajan, G.P. Cochlear implantation for unilateral deafness with and without tinnitus: a case series. The Laryngoscope 2013, 123, 1251-1255. [CrossRef]
- Tass, P.A.; Adamchic, I.; Freund, H.-J.; von Stackelberg, T.; Hauptmann, C. Counteracting tinnitus by acoustic coordinated reset neuromodulation. Restorative neurology and neuroscience 2012, 30, 137-159. [CrossRef]
- Mazurek, B.; Hesse, G.; Sattel, H.; Kratzsch, V.; Lahmann, C.; Dobel, C. S3 Guideline: Chronic Tinnitus: German Society for Otorhinolaryngology, Head and Neck Surgery e. V.(DGHNO-KHC). Hno 2022, 70, 795-827. [CrossRef]
- Shekhawat, G.S.; Searchfield, G.D.; Stinear, C.M. Role of hearing aids in tinnitus intervention: a scoping review. Journal of the American Academy of Audiology 2013, 24, 747-762. [CrossRef]
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. The Lancet 2013, 382, 1600-1607. [CrossRef]
- Bauer, C.A. Tinnitus. New England Journal of Medicine 2018, 378, 1224-1231. [CrossRef]
- Beukes, E.W.; Manchaiah, V.; Allen, P.M.; Andersson, G.; Baguley, D.M. Chapter 4 - Exploring tinnitus heterogeneity. In Progress in Brain Research, Schlee, W., Langguth, B., Kleinjung, T., Vanneste, S., De Ridder, D., Eds.; Elsevier: 2021; Volume 260, pp. 79-99. [CrossRef]
- Mazurek, B.; Rose, M.; Schulze, H.; Dobel, C. Systems Medicine Approach for Tinnitus with Comorbid Disorders. Nutrients 2022, 14, 4320. [CrossRef]
- Cederroth, C.R.; Gallus, S.; Hall, D.A.; Kleinjung, T.; Langguth, B.; Maruotti, A.; Meyer, M.; Norena, A.; Probst, T.; Pryss, R. Towards an understanding of tinnitus heterogeneity. Frontiers in aging neuroscience 2019, 11, 53. [CrossRef]
- Besser, J.; Stropahl, M.; Urry, E.; Launer, S. Comorbidities of hearing loss and the implications of multimorbidity for audiological care. Hearing research 2018, 369, 3-14. [CrossRef]
- Wattamwar, K.; Qian, Z.J.; Otter, J.; Leskowitz, M.J.; Caruana, F.F.; Siedlecki, B.; Spitzer, J.B.; Lalwani, A.K. Association of cardiovascular comorbidities with hearing loss in the older old. JAMA Otolaryngology–Head & Neck Surgery 2018, 144, 623-629. [CrossRef]
- Stam, M.; Kostense, P.J.; Lemke, U.; Merkus, P.; Smit, J.H.; Festen, J.M.; Kramer, S.E. Comorbidity in adults with hearing difficulties: which chronic medical conditions are related to hearing impairment? International journal of audiology 2014, 53, 392-401. [CrossRef]
- Gibrin, P.C.D.; Melo, J.J.; Marchiori, L. Prevalence of tinnitus complaints and probable association with hearing loss, diabetes mellitus and hypertension in elderly. In Proceedings of the CoDAS, 2013; pp. 176-180. [CrossRef]
- Fackrell, K.; Fearnley, C.; Hoare, D.J.; Sereda, M. Hyperacusis Questionnaire as a Tool for Measuring Hypersensitivity to Sound in a Tinnitus Research Population. Biomed Res Int 2015, 2015, 290425. [CrossRef]
- Rhee, J.; Lee, D.; Suh, M.W.; Lee, J.H.; Hong, Y.-C.; Oh, S.H.; Park, M.K. Prevalence, associated factors, and comorbidities of tinnitus in adolescents. PLOS ONE 2020, 15, e0236723. [CrossRef]
- Basso, L.; Boecking, B.; Brueggemann, P.; Pedersen, N.L.; Canlon, B.; Cederroth, C.R.; Mazurek, B. Subjective hearing ability, physical and mental comorbidities in individuals with bothersome tinnitus in a Swedish population sample. Progress in Brain Research 2021, 260, 51-78. [CrossRef]
- Bhatt, I.S.; Washnik, N.J.; Kingsbury, S.; Deshpande, A.K.; Kingsbury, H.; Bhagavan, S.G.; Michel, K.; Dias, R.; Torkamani, A. Identifying Health-Related Conditions Associated with Tinnitus in Young Adults. Audiology Research 2023, 13, 546-562. [CrossRef]
- Belli, S.; Belli, H.; Bahcebasi, T.; Ozcetin, A.; Alpay, E.; Ertem, U. Assessment of psychopathological aspects and psychiatric comorbidities in patients affected by tinnitus. European Archives of Oto-Rhino-Laryngology 2008, 265, 279-285. [CrossRef]
- Hébert, S. Psychological comorbidities of tinnitus. The Behavioral Neuroscience of Tinnitus 2021, 349-359. [CrossRef]
- Malakouti, S.K.; Mahmoudian, M.N.S.; Alifattahi, N.; Salehi, M. Comorbidity of chronic tinnitus and mental disorders. The international tinnitus journal 2011, 16, 118-122.
- Pavaci, S.; Tortorella, F.; Fioretti, A.B.; Angelone, A.M.; Businco, L.D.R.; Lauriello, M.; Eibenstein, A. Analysis of the audiological characteristics and comorbidity in patients with chronic tinnitus. Audiology Research 2019, 9, 231. [CrossRef]
- Basso, L.; Boecking, B.; Brueggemann, P.; Pedersen, N.L.; Canlon, B.; Cederroth, C.R.; Mazurek, B. Gender-specific risk factors and comorbidities of bothersome tinnitus. Frontiers in neuroscience 2020, 14, 706. [CrossRef]
- Zirke, N.; Seydel, C.; Szczepek, A.J.; Olze, H.; Haupt, H.; Mazurek, B. Psychological comorbidity in patients with chronic tinnitus: analysis and comparison with chronic pain, asthma or atopic dermatitis patients. Quality of Life Research 2013, 22, 263-272. [CrossRef]
- Boecking, B.; von Sass, J.; Sieveking, A.; Schaefer, C.; Brueggemann, P.; Rose, M.; Mazurek, B. Tinnitus-related distress and pain perceptions in patients with chronic tinnitus–Do psychological factors constitute a link? PLoS One 2020, 15, e0234807. [CrossRef]
- Biehl, R.; Boecking, B.; Brueggemann, P.; Grosse, R.; Mazurek, B. Personality Traits, Perceived Stress, and Tinnitus-Related Distress in Patients With Chronic Tinnitus: Support for a Vulnerability-Stress Model. Front Psychol 2019, 10, 3093. [CrossRef]
- Schilling, A.; Krauss, P.; Hannemann, R.; Schulze, H.; Tziridis, K. Reduktion der Tinnituslautstärke. HNO 2020, 69, 891-898. [CrossRef]
- Shetty, H.N.; Pottackal, J.M. Gain adjustment at tinnitus pitch to manage both tinnitus and speech perception in noise. Journal of Otology 2019, 14, 141-148. [CrossRef]
- McNeill, C.; Távora-Vieira, D.; Alnafjan, F.; Searchfield, G.D.; Welch, D. Tinnitus pitch, masking, and the effectiveness of hearing aids for tinnitus therapy. International journal of audiology 2012, 51, 914-919. [CrossRef]
- Vielsmeier, V.; Lehner, A.; Strutz, J.; Steffens, T.; Kreuzer, P.M.; Schecklmann, M.; Landgrebe, M.; Langguth, B.; Kleinjung, T. The Relevance of the High Frequency Audiometry in Tinnitus Patients with Normal Hearing in Conventional Pure-Tone Audiometry. [CrossRef]
- Martines, F.; Bentivegna, D.; Martines, E.; Sciacca, V.; Martinciglio, G. Assessing audiological, pathophysiological and psychological variables in tinnitus patients with or without hearing loss. European Archives of Oto-Rhino-Laryngology 2010, 267, 1685-1693. [CrossRef]
- Langguth, B.; Landgrebe, M.; Schlee, W.; Schecklmann, M.; Vielsmeier, V.; Steffens, T.; Staudinger, S.; Frick, H.; Frick, U. Different patterns of hearing loss among tinnitus patients: a latent class analysis of a large sample. Frontiers in neurology 2017, 8, 46. [CrossRef]
- Selye, H. Stress and disease. Science 1955, 122, 625-631. [CrossRef]
| Comorbidity Category | Young Patients | Middle Aged Patients | Senior Patients | Σ and % of all patients |
|---|---|---|---|---|
| endocrine system / metabolic diseases | 1/18 | 24/85 | 15/33 | 40/136 (29.4%) |
| psychiatric / behavioral disorders | 4/18 | 20/85 | 4/33 | 28/136 (20.6%) |
| diseases of the central nervous system | 0/18 | 6/85 | 3/33 | 9/136 (6.6%) |
| Diseases of the circulatory system | 0/18 | 22/85 | 19/33 | 41/136 (30.1%) |
| Diseases of the respiratory system | 0/18 | 5/85 | 5/33 | 10/136 (7.4%) |
| Diseases of the digestive system | 0/18 | 9/85 | 1/33 | 10/136 (7.4%) |
| muscle-skeletal diseases | 1/18 | 19/85 | 11/33 | 31/136 (22.8%) |
| Σ all comorbidity classes | 6 | 105 | 58 | 169 comorbidities in 136 patients |
| Age Group | 0 Comorbidities | 1 Comorbidity | 2 Comorbidities | 3+ Comorbidities |
|---|---|---|---|---|
| young | 11 | 7 | 0 | 0 |
| middle | 28 | 25 | 20 | 12 |
| senior | 6 | 11 | 7 | 9 |
| category | age group | frequency | comorbidity presence | interaction |
|---|---|---|---|---|
| endocrine system / metabolism | middle | F(10, 1144)=7.64, p<0.001 | F(1, 1144)=7.69, p=0.006with (24): 12.97 dBwithout (61): 17.00 dB | F(10, 1144)=0.73, p=0.70 |
| senior | F(10, 694)=9.64, p<0.001 | F(1, 694)=4.55, p=0.034with (15): 20.73 dBwithout (18): 27.51 dB | F(10, 694)=0.14, p=0.99 | |
| psychiatric / behavioral | middle | F(10, 1144)=16.06, p<0.001 | F(1, 1144)=1.11, p=0.29with (20): 15.44 dBwithout (65): 16.74 dB | F(10, 1144)=0.40, p=0.95 |
| senior | F(10, 694)=3.75, p<0.001 | F(1, 694)=0.02, p=0.88with (4): 27.05 dBwithout (29): 26.26 dB | F(10, 694)=0.07, p=0.99 | |
| central nervous system | middle | F(10, 1144)=9.19, p<0.001 | F(1, 1144)=0.87, p=0.35with (6): 18.17 dBwithout (79): 16.61 dB | F(10, 1144)=0.06, p=0.99 |
| senior | F(10, 694)=12.53, p<0.001 | F(1, 694)=1.96, p=0.16with (3): 26.65 dBwithout (30): 30.11 dB | F(10, 694)=0.19, p=0.99 | |
| circulatory system | middle | F(10, 1144)=20.23, p<0.001 | F(1, 1144)=0.35, p=0.55with (22): 16.05 dBwithout (63): 16.78 dB | F(10, 1144)=0,41, p=0.94 |
| senior | F(10, 694)=12.65, p<0.001 | F(1, 694)=20.09, p<0.001with (19): 33.42 dBwithout (14): 22.39 dB | F(10, 694)=1.32, p=0.22 | |
| respiratory system | middle | F(10, 1144)=1.76, p=0.06 | F(1, 1144)=0.55, p=0.46with (5): 14.64 dBwithout (80): 16.74 dB | F(10, 1144)=0.39, p=0.95 |
| senior | F(10, 694)=17.19, p<0.001 | F(1, 694)=3.59, p=0.06with (5): 26.60 dBwithout (28): 30.37 dB | F(10, 694)=0.33, p=0.97 | |
| digestive system | middle | F(10, 1144)=3.81, p<0.001 | F(1, 1144)=3.95, p=0.047with (9): 22.23 dBwithout (76): 16.59 dB | F(10, 1144)=0.14, p=0.99 |
| muscle-skeletal system | middle | F(10, 1144)=25.37, p<0.001 | F(1,1144)=66.60,p<0.001with (19): 26.12 dBwithout (66): 15.72 dB | F(10, 1144)=2.29, p=0.01 |
| senior | F(10, 694)=3.26, p<0.001 | F(1, 694)=1.06, p=0.30with (11): 31.32 dBwithout (22): 25.99 dB | F(10, 694)=0.17, p=0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





