Submitted:
29 February 2024
Posted:
01 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
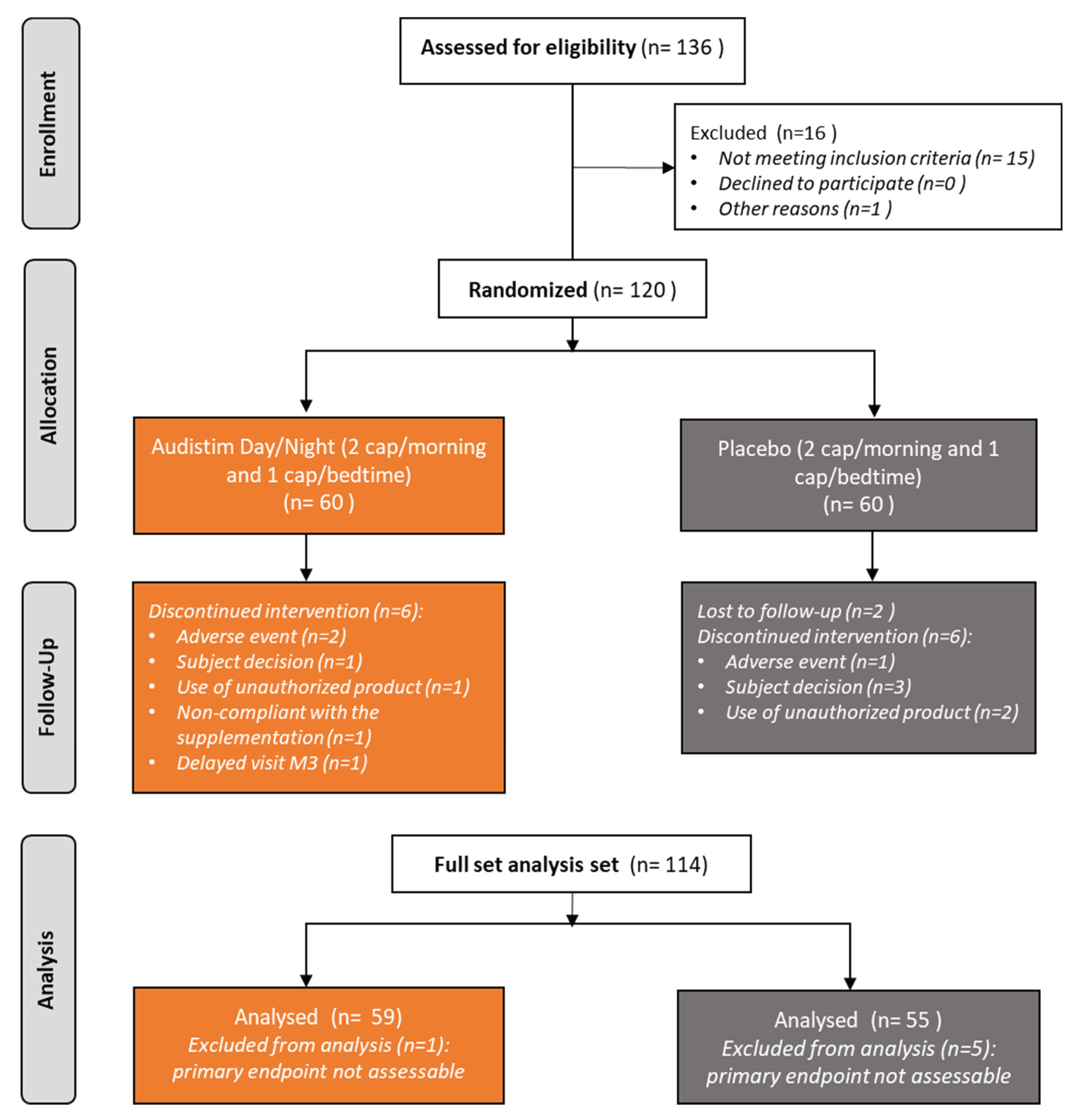
3.1. Patient Disposition
3.2. Description of Patients and Tinnitus at Baseline
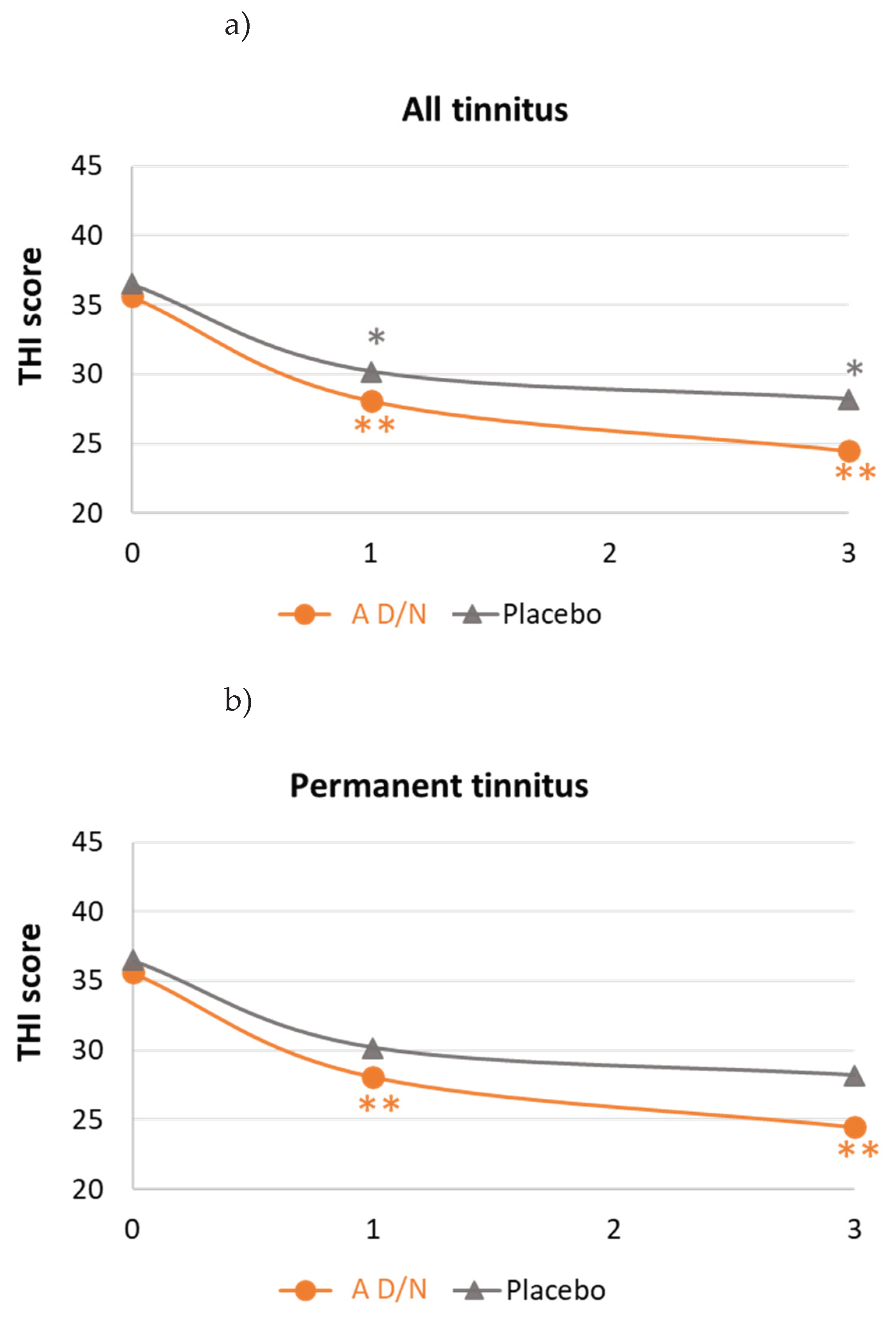
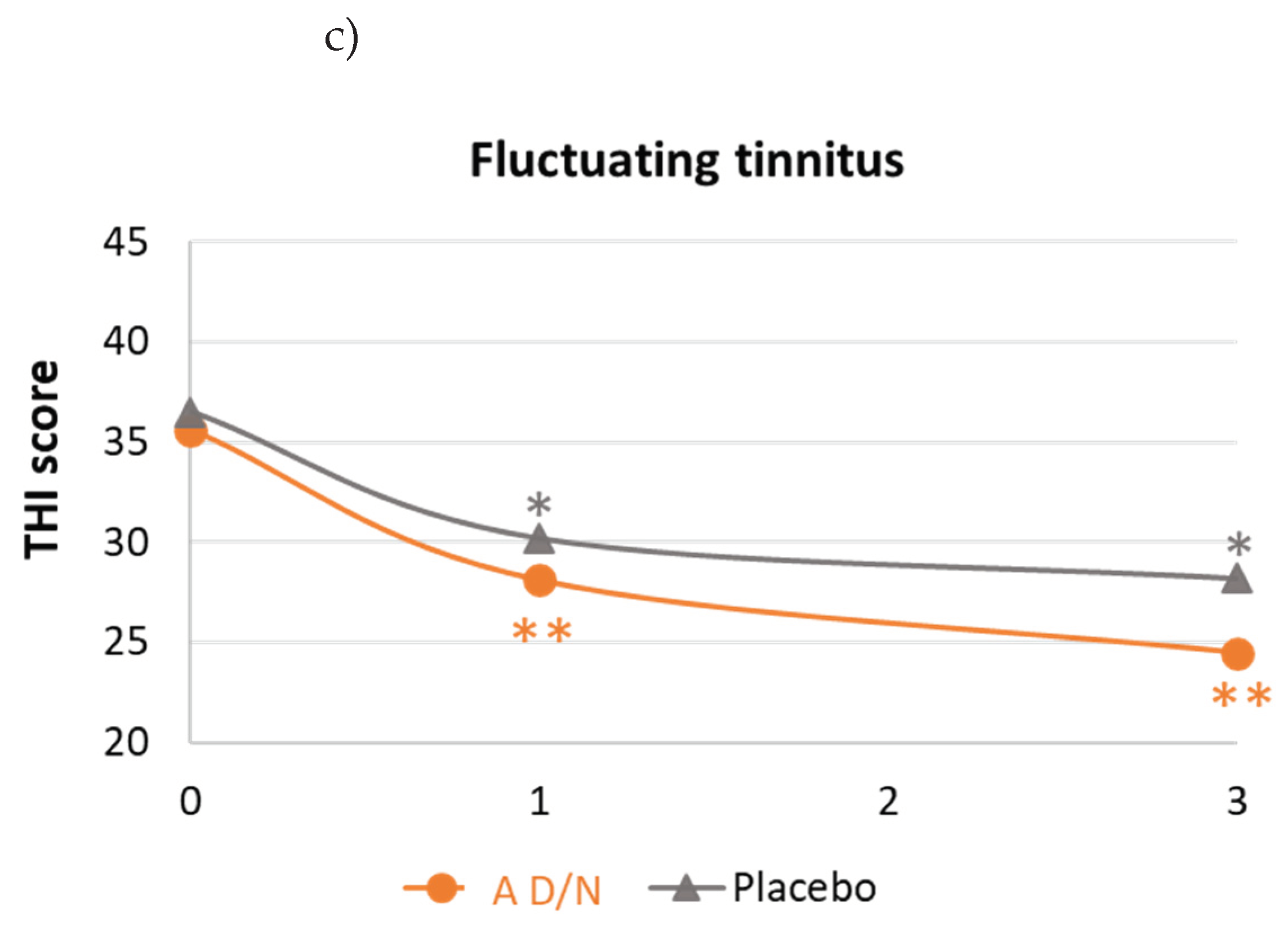
3.3. Changes in Tinnitus-Related Handicap under Dietary Supplementation
3.4. Psychological Stress, Sleep Quality and Subjective Assessment of Tinnitus improvement
3.4.1. Psychological Stress and Sleep Quality
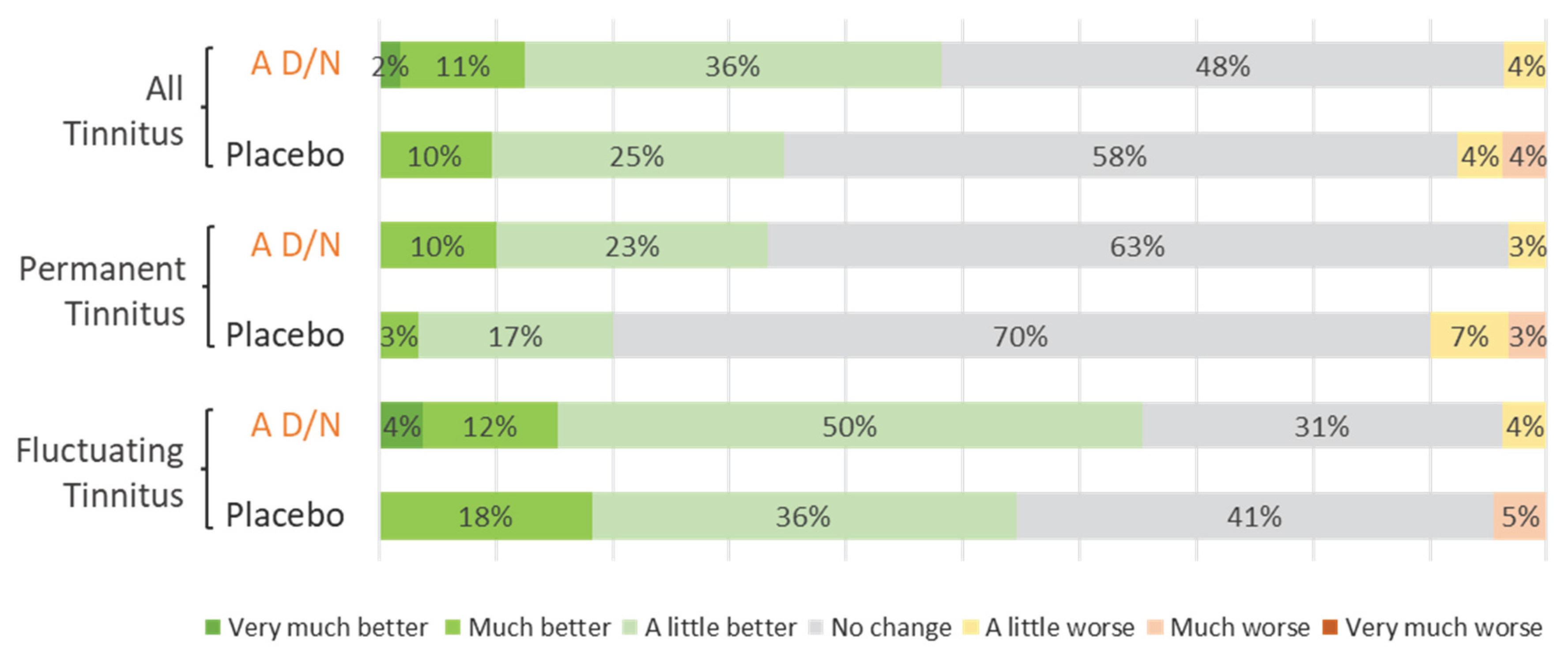
3.4.2. Subjective Assessment
3.5. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Author 1, A.B.; ICD11, 2019, Tinnitus. Available online: https://icd.who.int/ct11/icd11_mms/en/remease.
- McFadden, D., 1982. Tinnitus: Facts, Theories, and Treatments. National Academy of Sciences Press, Washington DC.
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Langguth, B.; Kreuzer, P.M.; Kleinjung, T.; De Ridder, D. Tinnitus: causes and clinical management. Lancet Neurol. 2013, 12, 920–930. [Google Scholar] [CrossRef] [PubMed]
- De Ridder D, Schlee W, Vanneste S; et al. Tinnitus and tinnitus disorder: Theoretical and operational definitions (an international multidisciplinary proposal). Prog Brain Res. 2021, 260, 1–25. [Google Scholar] [CrossRef]
- Jarach CM, Lugo A, Scala M; et al. Global Prevalence and Incidence of Tinnitus: A Systematic Review and Meta-analysis. JAMA Neurol. 2022, 79, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Biswas R, Lugo A, Akeroyd MA, Schlee W, Gallus S, Hall DA. Tinnitus prevalence in Europe: a multi-country cross-sectional population study. Lancet Reg Health Eur. 2022, 12, 100250. [CrossRef]
- Tunkel DE, Bauer CA, Sun GH; et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg 2014, 151 (Suppl. S2), S1–S40. [Google Scholar] [CrossRef]
- Bauer, CA. Tinnitus. N Engl J Med. 2018, 378, 1224–1231. [Google Scholar] [CrossRef]
- Henry JA, Reavis KM, Griest SE; et al. Tinnitus: An Epidemiologic Perspective. Otolaryngol Clin North Am. 2020, 53, 481–499. [Google Scholar] [CrossRef]
- Méric C, Gartner M, Collet L, Chéry-Croze S. Psychopathological Profile of Tinnitus Sufferers: Evidence Concerning the Relationship between Tinnitus Features and Impact on Life. Audiology and Neurotology 1998, 3, 240–252. [CrossRef]
- Tinnitus Retraining Therapy Trial Research Group, Scherer RW, Formby C. Effect of Tinnitus Retraining Therapy vs Standard of Care on Tinnitus-Related Quality of Life: A Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg. 2019, 145, 597–608. [Google Scholar] [CrossRef]
- Meikle M, Taylor-Walsh E. Characteristics of tinnitus and related observations in over 1800 tinnitus clinic patients. J. Laryngol Otol Suppl 1984, 9, 17–21. [Google Scholar] [CrossRef]
- Reavis K.M., Henry J.A., Marshall L.M., Carlson K.F. Prevalence of self-reported depression symptoms and perceived anxiety among community-dwelling U.S. adults reporting tinnitus. Perspect. ASHA Spec. Interest Groups 2020, 5, 959–970. [CrossRef]
- Langguth B, Elgoyhen AB, Cederroth CR. Therapeutic Approaches to the Treatment of Tinnitus. Annu Rev Pharmacol Toxicol. 2019, 59, 291–313. [Google Scholar] [CrossRef]
- Chen JJ, Chen YW, Zeng BY; et al. Efficacy of pharmacologic treatment in tinnitus patients without specific or treatable origin: A network meta-analysis of randomised controlled trials. EClinicalMedicine. 2021, 39, 101080. [Google Scholar] [CrossRef] [PubMed]
- Langguth B, Kleinjung T, Schlee W, Vanneste S, De Ridder D. Tinnitus Guidelines and Their Evidence Base. J. Clin. Med 2023, 12, 3087. [CrossRef] [PubMed]
- Person OC, Junior FVA, Altoé J, Portes LM, Lopes PR, Puga ME dos S. O que revisões sistemáticas Cochrane dizem sobre terapêutica para zumbido? ABCS Health Sciences 2022, 47, e022301. [CrossRef]
- Petridou AI, Zagora ET, Petridis P; et al. The Effect of Antioxidant Supplementation in Patients with Tinnitus and Normal Hearing or Hearing Loss: A Randomized, Double-Blind, Placebo Controlled Trial. Nutrients. 2019, 11, 3037. [Google Scholar] [CrossRef]
- Frachet B, Portmann D, Allaert F. Observational Study to assess the effect of Audistim® on the quality of life of patients presenting with chronic tinnitus. Revue de laryngologie - otologie - rhinologie 2017, 138. [Google Scholar]
- Van Becelaere T, Zahti H, Polet T, Glorieux P, Portmann D, Rigaudier F, Herpin F, Decat M. Improving quality of life in subjective tinnitus patients with Audistim®. Rev Laryngol Otol Rhinol. 2019, 140, 1, 3–7.
- Newman CW, Sandridge SA, Jacobson GP. Psychometric adequacy of the Tinnitus Handicap Inventory (THI) for evaluating treatment outcome. J Am Acad Audiol. 1998, 9, 153–160. [Google Scholar] [PubMed]
- Kikidis D, Vassou E, Schlee W; et al. Methodological Aspects of Randomized Controlled Trials for Tinnitus: A Systematic Review and How a Decision Support System Could Overcome Barriers. J Clin Med. 2021, 10, 1737. [Google Scholar] [CrossRef] [PubMed]
- Ghulyan-Bédikian V, Paolino M, Giorgetti-D’Esclercs F, Paolino F. [Psychometric properties of a French adaptation of the Tinnitus Handicap Inventory]. Encephale. 2010, 36, 390–396. [CrossRef] [PubMed]
- Available online: https://www.anses.fr/fr/content/les-r%C3%A9f%C3%A9rences-nutritionnelles-en-vitamines-et-min%C3%A9raux.
- Zeman F, Koller M, Figueiredo R; et al. Tinnitus handicap inventory for evaluating treatment effects: which changes are clinically relevant? Otolaryngol Head Neck Surg. 2011, 145, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Lemyre, L.; Tessier, R. La mesure de stress psychologique en recherche de première lgne : concept, modèle et mesure. Canadian Family Psysician – Le Médecin de famille canadien 2003, 49, 1166–1168. [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [CrossRef]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: Statistical Principles for Clinical Trials E9. London, England: European Medicines Agency; 1998. 1998.
- Cohen, J. Things I have learned (so far). Am. Psychol. 1990, 45, 1304–1312. [Google Scholar] [CrossRef]
- Wilson BS, Tucci DL, Merson MH, O’Donoghue GM. Global hearing health care: new findings and perspectives. Lancet. 2017, 390, 2503–2515. [CrossRef]
- Oppitz SJ, Garcia MV, Bruno RS; et al. Supplementation with açaí (Euterpe Oleracea Martius) for the treatment of chronic tinnitus: effects on perception, anxiety levels and oxidative metabolism biomarkers. Codas. 2022, 34, e20210076. [Google Scholar] [CrossRef]
- Sereda M, Xia J, Scutt P, Hilton MP, El Refaie A, Hoare DJ. Ginkgo biloba for tinnitus. Cochrane Database Syst Rev. 2022, 11, CD013514. [CrossRef]
- Hosseinzadeh A, Kamrava SK, Moore BCJ; et al. Molecular Aspects of Melatonin Treatment in Tinnitus: A Review. Curr Drug Targets. 2019, 20, 1112–1128. [Google Scholar] [CrossRef] [PubMed]
- Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016, 61, 253–278. [CrossRef] [PubMed]
- Community herbal monograph on Melissa officinalis L. folium. EMA/HMPC/196745/2012.
- Landgrebe M, Azevedo A, Baguley D; et al. Methodological aspects of clinical trials in tinnitus: a proposal for an international standard. J Psychosom Res. 2012, 73, 112–121. [Google Scholar] [CrossRef] [PubMed]
| Patient description | A D/N (n=59) | Placebo (n=55) | p-value |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 54.6 (12.5) | 52.9 (10.1) | 0.4281* |
| Median (IQR) | 59.0 (43.0 ; 65.0) | 53.0 (44.0 ; 59.0) | |
| Gender (% male/% female) | 37.3/ 62.7 | 47.3 / 52.7 | 0.2806** |
| Concomitant disorder (Yes) (n (%)) | 23 (39.0) | 13 (23.6) | 0.1693** |
| Clinical abnormalities (Yes) (n (%)) | 1 (1.7) | 5 (9.1) | 0.1047** |
| Tinnitus description | A D/N(n=59) | Placebo(n=55) | p-value |
|---|---|---|---|
| Duration (years) Mean (SD) Median (IQR) |
9.5 (9.7) 6.0 (3.0 ; 13.0) |
9.0 (9.3) 5.0 (2.0 ; 15.0) |
0.7862* |
| Main aetiology | |||
| Idiopathic (n (%)) | 25 / 42.4 | 18 / 32.7 | 0.7529** |
| Acoustic trauma (n (%)) | 18 / 30.5 | 20 / 36.4 | 0.5075** |
| ENT/Viral infection (n (%)) | 6 / 10.2 | 6 / 10.9 | 0.7529** |
| Presbycusis (n (%)) | 5 / 8.5 | 2 / 3.6 | 0.4404** |
| Vascular disorders (n (%)) | 2 /3.4 | 5 / 9.1 | 0.2597** |
| Location | |||
| Both ears (n (%)) | 37 / 62.7 | 38 / 69.1 | 0.2105** |
| Right or Left ear (n (%)) | 19 / 32.2 | 17 / 30.9 | |
| Head (n (%)) | 3 / 5.1 | 0 /0.0 | |
| Tinnitus onset | |||
| Permanent (n (%)) | 32 / 54.2 | 31 / 56.4 | 0.8195** |
| Fluctuating (n (%)) | 27 / 45.8 | 24 / 43.6 | |
| Associated symptoms (Yes) (n (%)) | 41 / 69.5 | 36 / 65.5 | |
| Hypoacusis/hyperacusis | 25 / 42.4 | 25 / 45.5 | 0.8885** |
| Headache | 13 / 22.0 | 8 / 14.5 | 0.3027** |
| Annoyance (0-10 VAS) | |||
| Mean (SD) Median (IQR) |
5.3 (1.5) 5.0 (4.0 ; 6.7) |
4.9 (1.8) 5.0 (3.0 ; 7.0) |
0.1913* |
| THI total score | |||
| Mean (SD) | 38.9 (16.2) | 35.7 (17.5) | 0.3180* |
| Median (IQR) | 36.0 (26.0 ; 54.0) | 30.0 (22.0 ; 48.0) | |
| THI classification | |||
| No handicap (n (%)) | 7 (11.9) | 7 (12.7) | 0.6329** |
| Mild handicap (n (%)) | 23 (39.0) | 23 (41.8) | |
| Moderate handicap (n (%)) | 23 (39.0) | 16 (29.1) | |
| Severe handicap (n (%)) | 6 (10.2) | 9 (16.4) | |
| Catastrophic handicap (n (%)) | 0 (0.0) | 0 (0.0) |
| A D/N | Placebo | p-value | |
|---|---|---|---|
| All tinnitus (n=104) | (n=59) | (n=55) | |
| Changes in THI scores | |||
| Mean (SD) | -13.2 (16.0) | -6.2 (14.4) | 0.0158* |
| 95%CI | -17.4/-9.1 | -10.1/-2.3 | |
| THI score reduction ≥20% | |||
| n (%) | 40 (67.8) | 26 (47.3) | 0.0266** |
| Permanent tinnitus (n=63) | (n=32) | (n=31) | |
| Changes in THI scores | |||
| Mean (SD) | -15.0 (16.3) | -4. (12.8) | 0.0065* |
| 95%CI | -20.9/-9.1 | -9.3/0.1 | |
| THI score reduction ≥20% | |||
| n (%) | 23 (71.9) | 15 (48.4) | 0.0568** |
| Fluctuating tinnitus (n=51) | (n=27) | (n=24) | |
| Changes in THI scores | |||
| Mean (SD) | -11.1 (15.7) | -8.3 (16.3) | 0.5384* |
| 95%CI | -17.3/-4.9 | -15.2/-1.5 | |
| THI score reduction ≥20% | |||
| n (%) | 17 (63.0) | 11 (45.8) | 0.2198** |
| A D/N | Placebo | A D/N vs Placebo | |||||
|---|---|---|---|---|---|---|---|
|
Baseline Mean (SD) |
Final Mean (SD) |
p-value* | Baseline Mean (SD) |
Final Mean (SD) |
p-value* | p-value** | |
| All tinnitus | (n=56) | (n=52) | |||||
| MSP-9 scores | 35.4 (12.6) | 31.4 (11.2) | 0.008 | 34.1 (11.2) | 32.2 (11.1) | 0.1947 | 0.3190 |
| PSQI scores | 7.4 (3.5) | 6.0 (3.2) | 0.0014 | 7.3 (3.5) | 6.3 (3.0) | 0.0374 | 0.3979 |
| Permanent tinnitus | (n=30) | (n=30) | |||||
| MSP-9 scores | 33.5 (10.4) | 29.4 (9.8) | 0.0655 | 32.5 (10.8) | 31.8 (11.1) | 0.6561 | 0.2062 |
| PSQI scores | 6.7 (3.6) | 5.3 (2.5) | 0.0154 | 6.4 (3.4) | 6.0 (3.4) | 0.4077 | 0.1855 |
| Fluctuating tinnitus | (n=26) | (n=22) | |||||
| MSP-9 scores | 37.5 (14.5) | 33.7 (12.4) | 0.0586 | 36.1 (11.5) | 32.6 (11.4) | 0.2026 | 0.9359 |
| PSQI scores | 8.2 (3.3) | 6.7 (3.8) | 0.0385 | 8.4 (3.3) | 6.8 (2.3) | 0.0458 | 0.9128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





