Submitted:
29 February 2024
Posted:
01 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
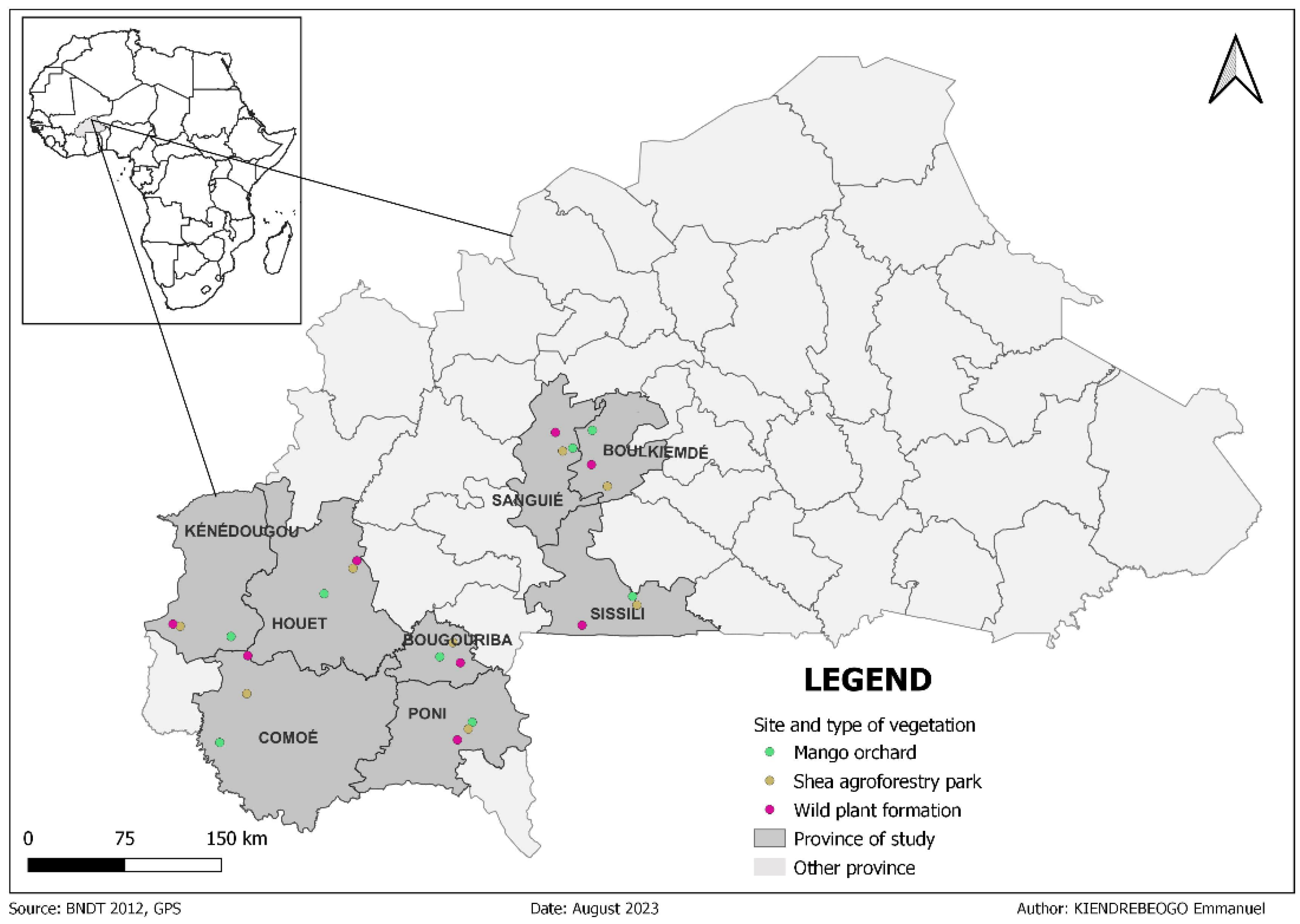
2.1. Sample Collection
2.2. Molecular Analyses
2.3. Data Analysis
2.3.1. Flies Abundance and Genetic Diversity Analysis
2.3.2. Analysis of Genetic Relations between Sampling Populations
2.3.3. Analysis of Genetic Structure
3. Results
3.1. Abundance and Diversity
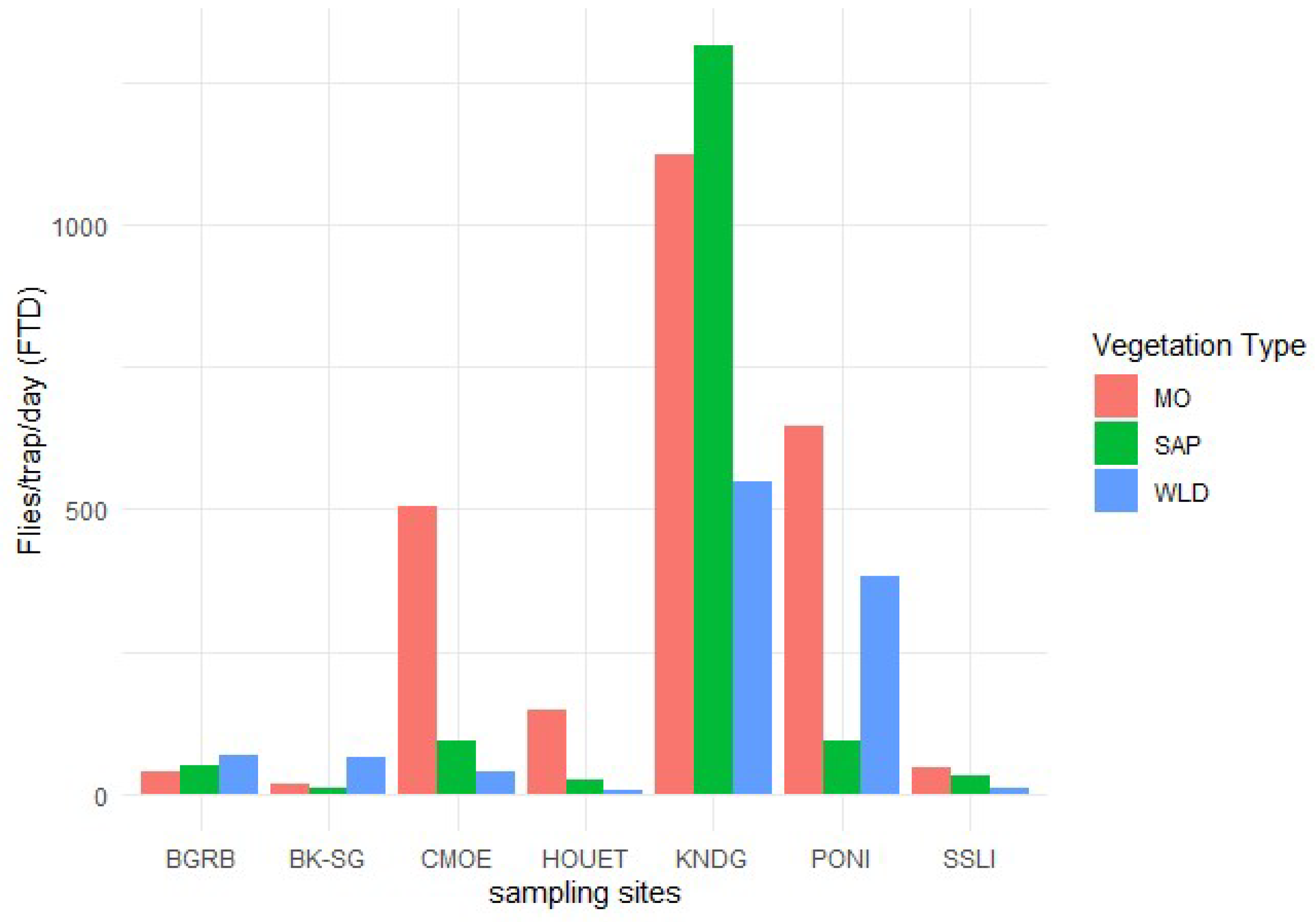
3.1.1. Abundance of Bactrocera Dorsalis Pests in Burkina Faso
3.1.2. Polymorphism of Microsatellite Markers and Genetic Diversity
3.2. Population Structure
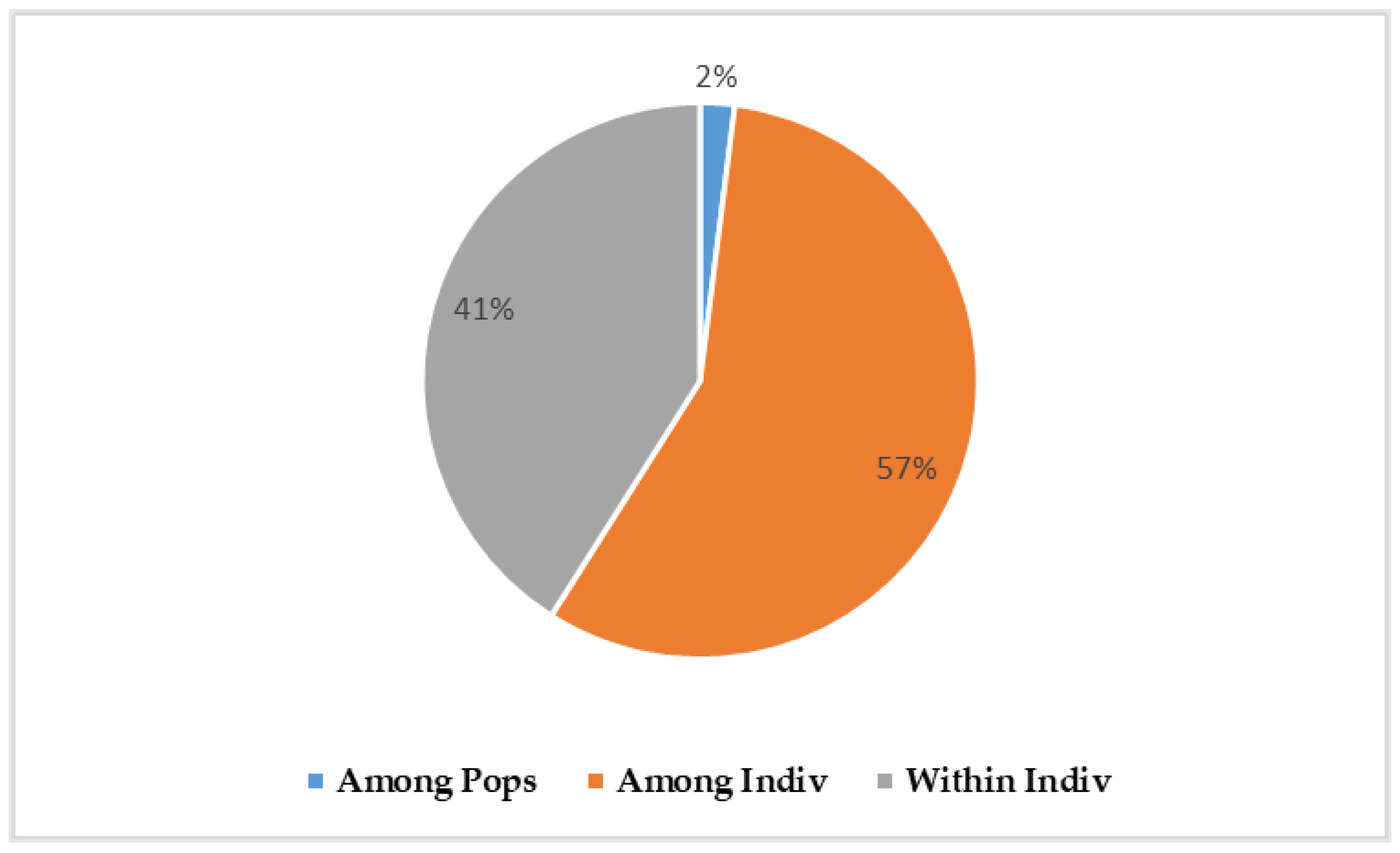
3.2.1. Genetic Differentiation
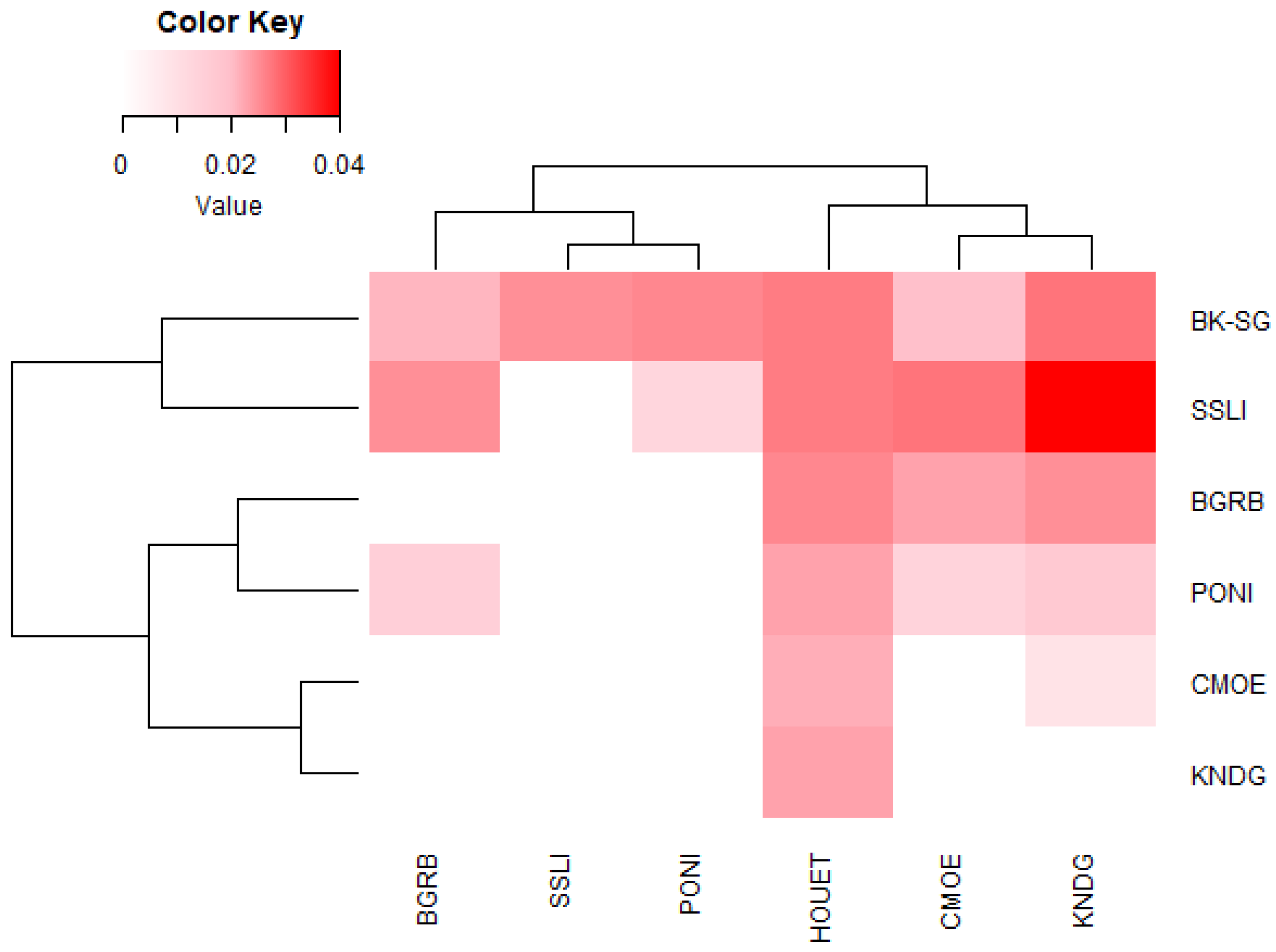
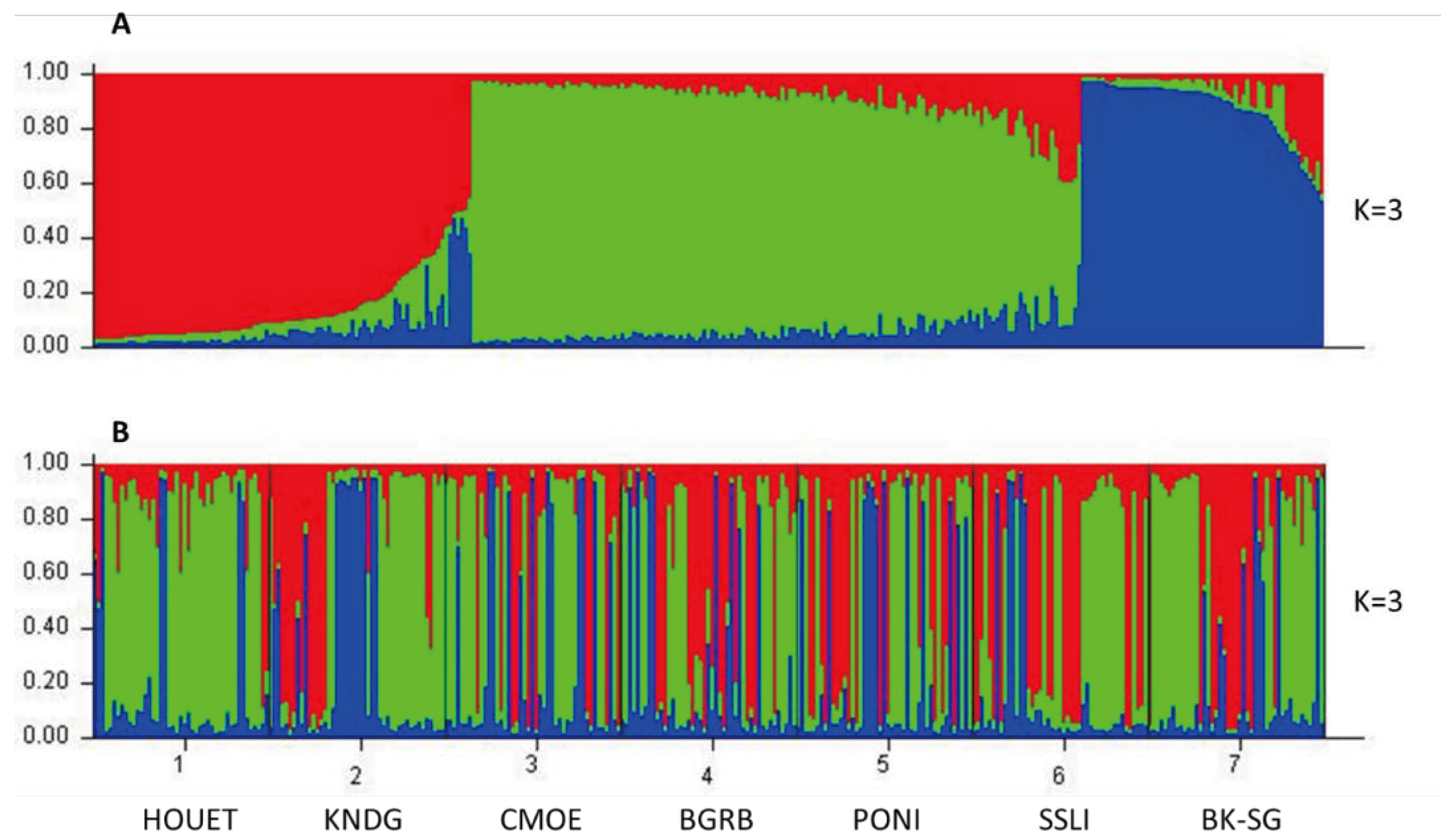
3.2.2. Multivariate Analysis and Genetic Clustering
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zida:, S. Zida: S. Nacro, R. Dabiré, L. Moquet, H. Delatte, and I. Somda, “Host range and species diversity of Tephritidae of three plant formations in Western Burkina Faso,” Bull. Entomol. Res., vol. 110, no. 6, pp. 732–742, 2020. [CrossRef]
- IPPC, “ISPM 27 Diagnostic protocols for regulated pests DP29 : Bactrocera dorsalis,” Int. Plant Prot. Conv., vol. ISPM 27, no. February, p. 34, 2019.
- H. Liu et al., “Invasion, expansion, and control of Bactrocera dorsalis (Hendel) in China,” J. Integr. Agric., vol. 18, no. 4, pp. 771–787, 2019. [CrossRef]
- T. Dohino et al., “Phytosanitary Treatments against Bactrocera dorsalis (Diptera: Tephritidae): Current Situation and Future Prospects,” J. Econ. Entomol., vol. 110, no. 1, pp. 67–79, 2017. [CrossRef]
- Y. jia Qin et al., “Population structure of a global agricultural invasive pest , Bactrocera dorsalis ( Diptera : Tephritidae ),” Evol. Appl., vol. 11, no. April, pp. 1990–2003, 2018. [CrossRef]
- S. Ekesi, M. De Meyer, S. A. Mohamed, M. Virgilio, and C. Borgemeister, “Taxonomy, Ecology, and Management of Native and Exotic Fruit Fly Species in Africa,” Annu. Rev. Entomol., vol. 61, pp. 219–238, 2016. [CrossRef]
- A. Loomans, M. Diakaki, M. Kinkar, M. Schenk, and S. Vos, “Pest survey card on Bactrocera dorsalis,” EFSA Support. Publ., vol. 16, no. 9, pp. 2–25, 2019. [CrossRef]
- R. I. Vargas, J. C. Piñero, and L. Leblanc, “An overview of pest species of Bactrocera fruit flies (Diptera: Tephritidae) and the integration of biopesticides with other biological approaches for their management with a focus on the pacific region,” Insects, vol. 6, no. 2, pp. 297–318, 2015. [CrossRef]
- M. K. Schutze et al., “Synonymization of key pest species within the Bactrocera dorsalis species complex (Diptera: Tephritidae): Taxonomic changes based on a review of 20 years of integrative morphological, molecular, cytogenetic, behavioural and chemoecological data,” Syst. Entomol., vol. 40, no. 2, pp. 456–471, 2015. [CrossRef]
- S. Jaffar, S. A. H. Rizvi, and Y. Lu, “Understanding the Invasion, Ecological Adaptations, and Management Strategies of Bactrocera dorsalis in China: A Review,” Horticulturae, vol. 9, no. 9, p. 1004, 2023. [CrossRef]
- S. A. Lux, R. S. Copeland, I. M. White, A. Manrakhan, and M. K. Billah, “A New Invasive Fruit Fly Species from the Bactrocera dorsalis (Hendel) Group Detected in East Africa,” Int. J. Trop. Insect Sci., vol. 23, no. 04, pp. 355–361, 2003. [CrossRef]
- R. A. I. Drew, K. Tsuruta, and I. M. White, “A new species of pest fruit fly (Diptera: Tephritidae: Dacinae) from Sri Lanka and Africa,” African Entomol., vol. 13, no. 1, pp. 149–154, 2005.
- K. B. Badii, M. K. Billah, K. Afreh Nuamah, D. Obeng Ofori, and G. Nyarko, “Review of the pest status, economic impact and management of fruit-infesting flies (Diptera: Tephritidae) in Africa,” African J. Agric. Res., vol. 10, no. 12, pp. 1488–1498, 2015. [CrossRef]
- CABI, “Bactrocera dorsalis (Oriental fruit fly),” Invasive Species Compendium, 2021. https://www.cabi.org/isc/datasheet/17685 (accessed May 25, 2022).
- Zida, S. Nacro, R. Dabiré, and I. Somda, “Co-Existence of Bactrocera dorsalis Hendel (Diptera: Tephritidae) and Ceratitis cosyra Walker (Diptera: Tephritidae) in the Mango Orchards in Western Burkina Faso,” Adv. Entomol., vol. 08, no. 01, pp. 46–55, 2020. [CrossRef]
- W. K. Heve, T. A. Adjadeh, and M. K. Billah, “Overview and future research needs for development of effective biocontrol strategies for management of Bactrocera dorsalis Hendel ( Diptera : Tephritidae ) in sub-Saharan Africa,” Pest Manag Sci, vol. 77, no. February, pp. 1–14, 2021. [CrossRef]
- S. Ekesi, M. K. Billah, P. W. Nderitu, S. A. Lux, and I. Rwomushana, “Evidence for competitive displacement of ceratitis cosyra by the invasive fruit fly bactrocera invadens (Diptera: Tephritidae) on mango and mechanisms contributing to the displacement,” J. Econ. Entomol., vol. 102, no. 3, pp. 981–991, 2009. [CrossRef]
- R. Mutamiswa, C. Nyamukondiwa, G. Chikowore, and F. Chidawanyika, “Overview of oriental fruit fly , Bactrocera dorsalis ( Hendel ) ( Diptera : Tephritidae ) in Africa : From invasion , bio-ecology to sustainable management,” Crop Prot., vol. 141, no. September 2020, pp. 1–17, 2021. [CrossRef]
- S. N. Ouédraogo, J. F. Vayssières, A. Rémy Dabiré, and C. Rouland-Lefèvre, “Biodiversité des mouches des fruits (Diptera: Tephritidae) en vergers de manguiers de l’ouest du Burkina Faso: structure et comparaison des communautés de différents sites,” Fruits, vol. 66, no. 6, pp. 393–404, 2011. [CrossRef]
- S. Isasawin, N. Aketarawong, and S. Thanaphum, “Characterization and evaluation of microsatellite markers in a strain of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae), with a genetic sexing character used in sterile insect population control,” Eur. J. Entomol., vol. 109, no. 3, pp. 331–338, 2012. [CrossRef]
- M. Sutantawong, W. Orankanok, W. R. Enkerlin, V. Wornoayporn, and C. Caceres, “The sterile insect technique for control of the oriental fruit fly, Bactrocera dorsalis (Hendel), in mango orchards in Ratchaburi Province, Thailand,” Proc. 6th Int. Fruit Fly Symp., vol. 6-10 May, no. May, pp. 223–232, 2002, [Online]. Available: https://nucleus.iaea.org/sites/naipc/twd/Documents/6thISFFEI_Proceedings/SUTANTAWONG.pdf.
- P. T. Leftwich et al., “Genetic pest management and the background genetics of release strains,” Philos. Trans. R. Soc. Lond. B. Biol. Sci., vol. 376, no. 1818, p. 20190805, 2021. [CrossRef]
- M. Legros, J. M. Marshall, S. Macfadyen, K. R. Hayes, A. Sheppard, and L. G. Barrett, “Gene drive strategies of pest control in agricultural systems: Challenges and opportunities,” Evol. Appl., vol. 14, no. 9, pp. 2162–2178, 2021. [CrossRef]
- R. F. Medina, “Gene drives and the management of agricultural pests,” J. Responsible Innov., vol. 5, pp. S255–S262, 2018. [CrossRef]
- S. Choudhary, N. Naaz, C. S. Prabhakar, and M. Lemtur, “Genetic analysis of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae) populations based on mitochondrial cox1 and nad1 gene sequences from India and other Asian countries,” Genetica, vol. 144, no. 5, pp. 611–623, Oct. 2016. [CrossRef]
- National Academy of Sciences, Gene Drives on the Horizon. Washington, D.C.: National Academies Press, 2016.
- W. Shi, C. Kerdelhué, and H. Ye, “Genetic structure and inferences on potential source areas for Bactrocera dorsalis (Hendel) based on mitochondrial and microsatellite markers,” PLoS One, vol. 7, no. 5, pp. 1–15, 2012. [CrossRef]
- G. T. McQuate and R. I. Vargas, “Assessment of attractiveness of plants as roosting sites for the melon fly, Bactrocera cucurbitae, and oriental fruit fly, Bactrocera dorsalis,” J. Insect Sci., vol. 7, no. 57, p. 57, 2007. [CrossRef]
- N. Aketarawong, M. Bonizzoni, A. R. Malacrida, G. Gasperi, and S. Thanaphum, “Seventeen novel microsatellite markers from an enriched library of the pest species Bactrocera dorsalis sensu stricto,” Mol. Ecol. Notes, vol. 6, no. 4, pp. 1138–1140, 2006. [CrossRef]
- F. M. Khamis et al., “Uncovering the tracks of a recent and rapid invasion: The case of the fruit fly pest Bactrocera invadens (Diptera: Tephritidae) in Africa,” Mol. Ecol., vol. 18, no. 23, pp. 4798–4810, 2009. [CrossRef]
- C. Yi, C. Zheng, L. Zeng, and Y. Xu, “High genetic diversity in the offshore island populations of the tephritid fruit fly Bactrocera dorsalis,” BMC Ecol., vol. 16, no. 1, pp. 1–12, 2016. [CrossRef]
- P. E. Peakall, R. and Smouse, “GenAlEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research—An Update,” Bioinforma. Appl. Note, vol. 28, pp. 2537–2539, 2012. [CrossRef]
- M. Nei, “Chapter 9: Genetic Distance Between Populations,” in The American Naturalist, vol. 106, no. 949, Columbia University Press, 1972, pp. 208–253.
- T. Jombart, S. Devillard, and F. Balloux, “Discriminant analysis of principal components: a new method for the analysis of genetically structured populations,” BMC Genet., vol. 11, no. 1, p. 94, Aug. 2010. [CrossRef]
- D. A. Earl and B. M. vonHoldt, “STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method,” Conserv. Genet. Resour., vol. 4, no. 2, pp. 359–361, 2012. [CrossRef]
- G. Evanno, S. Regnaut, and J. Goudet, “Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study.,” Mol. Ecol., vol. 14, no. 8, pp. 2611–20, Jul. 2005. [CrossRef]
- R. K. Ramasamy, S. Ramasamy, B. B. Bindroo, and V. G. Naik, “STRUCTURE PLOT: A program for drawing elegant STRUCTURE bar plots in user friendly interface,” Springerplus, vol. 3, no. 1, pp. 1–3, 2014. [CrossRef]
- M. K. Konopiński, “Shannon diversity index: a call to replace the original Shannon’s formula with unbiased estimator in the population genetics studies,” PeerJ, vol. 8, no. 6, p. e9391, Jun. 2020. [CrossRef]
- Zida, S. Nacro, R. Dabiré, and I. Somda, “Seasonal Abundance and Diversity of Fruit Flies (Diptera: Tephritidae) in Three Types of Plant Formations in Western Burkina Faso, West Africa,” Ann. Entomol. Soc. Am., vol. 113, no. 5, pp. 343–354, Sep. 2020. [CrossRef]
- M. Faye, A. Ndiaye, I. Diallo, and P. M. Sembene, “Genetic identification of populations of Bactrocera dorsalis ( Diptera Tephritidae ) in the Niayes and Lower Casamance areas in Senegal,” J. Appl. Biosci., vol. 158, pp. 16351–16362, 2021.
- Diallo, A. Ndiaye, E. Faye, M. Faye, and M. Sembene, “Does landscape factors drive the genetic diversity and structure of populations of fruit flies, Senegal? Exploratory study of the case of Bactrocera dorsalis (Hendel, 1912) in the Niayes, Senegal,” J. Entomol. Zool. Stud., vol. 9, no. 6, pp. 47–52, 2021. [CrossRef]
- Zida, S. Nacro, R. Dabiré, S. N. Ouédraogo, and I. Somda, “Shea fruit-infesting fruit flies (Diptera: Tephritidae) and evaluation of infestation level according to the ethno-varieties in Western Burkina Faso,” Int. J. Trop. Insect Sci., vol. 40, no. 3, pp. 493–501, Sep. 2020. [CrossRef]
- D. Gnanvossou et al., “Diversity and seasonal abundance of tephritid fruit flies in three agro-ecosystems in Benin, West Africa,” J. Appl. Entomol., vol. 141, no. 10, pp. 798–809, 2017. [CrossRef]
- J.-F. Vayssières, S. Korie, and D. Ayegnon, “Correlation of fruit fly ( Diptera Tephritidae ) infestation of major mango cultivars in Borgou ( Benin ) with abiotic and biotic factors and assessment of damage,” Crop Prot., vol. 28, no. 6, pp. 477–488, 2009.
- T. De Meeûs, Initiation à la génétique des populations naturelles : Applications aux parasites et à leurs vecteurs., IRD Éditio. Marseille : IRD, 2012.
- S. Wright, “Variability Within and Among Natural Populations,” in Evolution and the Genetics of Populations, vol. 4, Chicago, IL, USA: University of Chicago Press., 1978.
- R. E. Stinner, C. S. Barfield, J. L. Stimac, and L. Dohse, “Dispersal and movement of insect pests.,” Annu. Rev. Entomol. Vol. 28, no. 68, pp. 319–335, 1983. [CrossRef]
- G. C. Lanzaro et al., “Selection of sites for field trials of genetically engineered mosquitoes with gene drive,” Evol. Appl., vol. 14, no. 9, pp. 2147–2161, 2021. [CrossRef]
- Y. Cao, B. Li, N. Chen, D. Yang, L. Li, and T. Liu, “Evaluation of Reference Genes for Quantitative Reverse Transcription Polymerase Chain Reaction in Bactrocera dorsalis (Diptera: Tephritidae) Subjected to Various Phytosanitary Treatments,” Insects, vol. 12, no. 10, p. 945, Oct. 2021. [CrossRef]

| Locus | N | Na | Ne | I | Ho | He | Ht | Fis | Fit | Fst | Nm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bd15 | 44,286 | 1,286 | 1,007 | 0,018 | 0,003 | 0,006 | 0,006 | 0,491 | 0,499 | 0,015 | 16,819 |
| Bd19 | 44,286 | 4 | 3,131 | 1,239 | 0,622 | 0,673 | 0,7 | 0,075 | 0,111 | 0,039 | 6,139 |
| Bi1 | 44,143 | 3 | 1,527 | 0,611 | 0,356 | 0,34 | 0,345 | -0,044 | -0,03 | 0,014 | 17,553 |
| Bi5 | 44,429 | 3,571 | 1,454 | 0,594 | 0,276 | 0,304 | 0,31 | 0,092 | 0,109 | 0,019 | 12,915 |
| Bi8 | 44,714 | 2 | 1,147 | 0,223 | 0,035 | 0,118 | 0,125 | 0,704 | 0,719 | 0,052 | 4,561 |
| Bi10 | 44,143 | 3 | 1,834 | 0,773 | 0,292 | 0,447 | 0,462 | 0,347 | 0,368 | 0,032 | 7,577 |
| MS12A | 43,857 | 3 | 1,894 | 0,787 | 0,589 | 0,47 | 0,477 | -0,253 | -0,233 | 0,016 | 15,846 |
| Bd85b | 45 | 3,714 | 2,613 | 1,055 | 0,438 | 0,616 | 0,644 | 0,288 | 0,32 | 0,044 | 5,368 |
| MS4 | 44,286 | 4,429 | 3,013 | 1,235 | 0,791 | 0,664 | 0,68 | -0,191 | -0,164 | 0,023 | 10,732 |
| MS3 | 44 | 3,143 | 1,623 | 0,688 | 0,162 | 0,378 | 0,384 | 0,571 | 0,577 | 0,015 | 16,091 |
| Mean | 44,314 | 3,114 | 1,924 | 0,722 | 0,356 | 0,402 | 0,413 | 0,208 | 0,228 | 0,027 | 11,36 |
| SE | 0,111 | 0,130 | 0,089 | 0,047 | 0,031 | 0,026 | 0,048 | 0,103 | 0,101 | 0,004 | 1,623 |
| Pop | N | Na | Ne | I | Ho | He | uHe | F | PPL |
|---|---|---|---|---|---|---|---|---|---|
| HOUET | 44,6 | 3,3 | 1,895 | 0,718 | 0,347 | 0,390 | 0,394 | 0,128 | 80% |
| KNDG | 44,8 | 3,5 | 1,963 | 0,741 | 0,328 | 0,397 | 0,402 | 0,202 | 80% |
| CMOE | 43,2 | 3,2 | 2,032 | 0,771 | 0,348 | 0,431 | 0,436 | 0,269 | 100% |
| BGRB | 44,6 | 3,1 | 1,916 | 0,740 | 0,384 | 0,423 | 0,428 | 0,167 | 90% |
| PONI | 44,5 | 2,9 | 1,926 | 0,716 | 0,365 | 0,398 | 0,402 | 0,159 | 90% |
| SSLI | 44,6 | 2,8 | 1,819 | 0,657 | 0,354 | 0,370 | 0,374 | 0,164 | 90% |
| BK-SG | 43,9 | 3,0 | 1,919 | 0,712 | 0,369 | 0,403 | 0,407 | 0,062 | 90% |
| Mean | 44,3 | 3,1 | 1,924 | 0,722 | 0,356 | 0,402 | 0,406 | 0,166 | 88.6 % |
| Pop | BGRB | BK-SG | CMOE | HOUET | KNDG | PONI | SSLI |
| BGRB | - | 16.416 | 20.583 | 11.114 | 12.250 | 22.477 | 12.908 |
| BK-SG | 0.015 | - | 17.607 | 11.655 | 11.114 | 13.639 | 12.250 |
| CMOE | 0.012 | 0.014 | - | 14.456 | 22.478 | 27.528 | 13.639 |
| HOUET | 0.022 | 0.021 | 0.017 | - | 14.456 | 13.639 | 14.456 |
| KNDG | 0.020 | 0.022 | 0.011 | 0.017 | - | 17.607 | 10.620 |
| PONI | 0.011 | 0.018 | 0.009 | 0.018 | 0.014 | - | 24.75 |
| SSLI | 0.019 | 0.020 | 0.018 | 0.017 | 0.023 | 0.010 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





