Submitted:
29 February 2024
Posted:
01 March 2024
You are already at the latest version
Abstract
Keywords:
Introduction
2. Materials and Methods
2.1. Tick Collection and Identification, and DNA Extraction
2.2. Screening for Borrelia burgdorferi s.l. DNA
2.3. Screening for Babesia spp. DNA
2.4. Statistical Analysis
2.5. Sequence Analysis
2.6. Phylogenetic Analysis
3. Results
3.1. Collection and Identification of Ticks
3.2. Detection of Borrelia burgdorferi s.l. DNA
3.3. Genetic Diversity of B. burgdorferi s.l. V4 16S Amplicon Sequence Variants
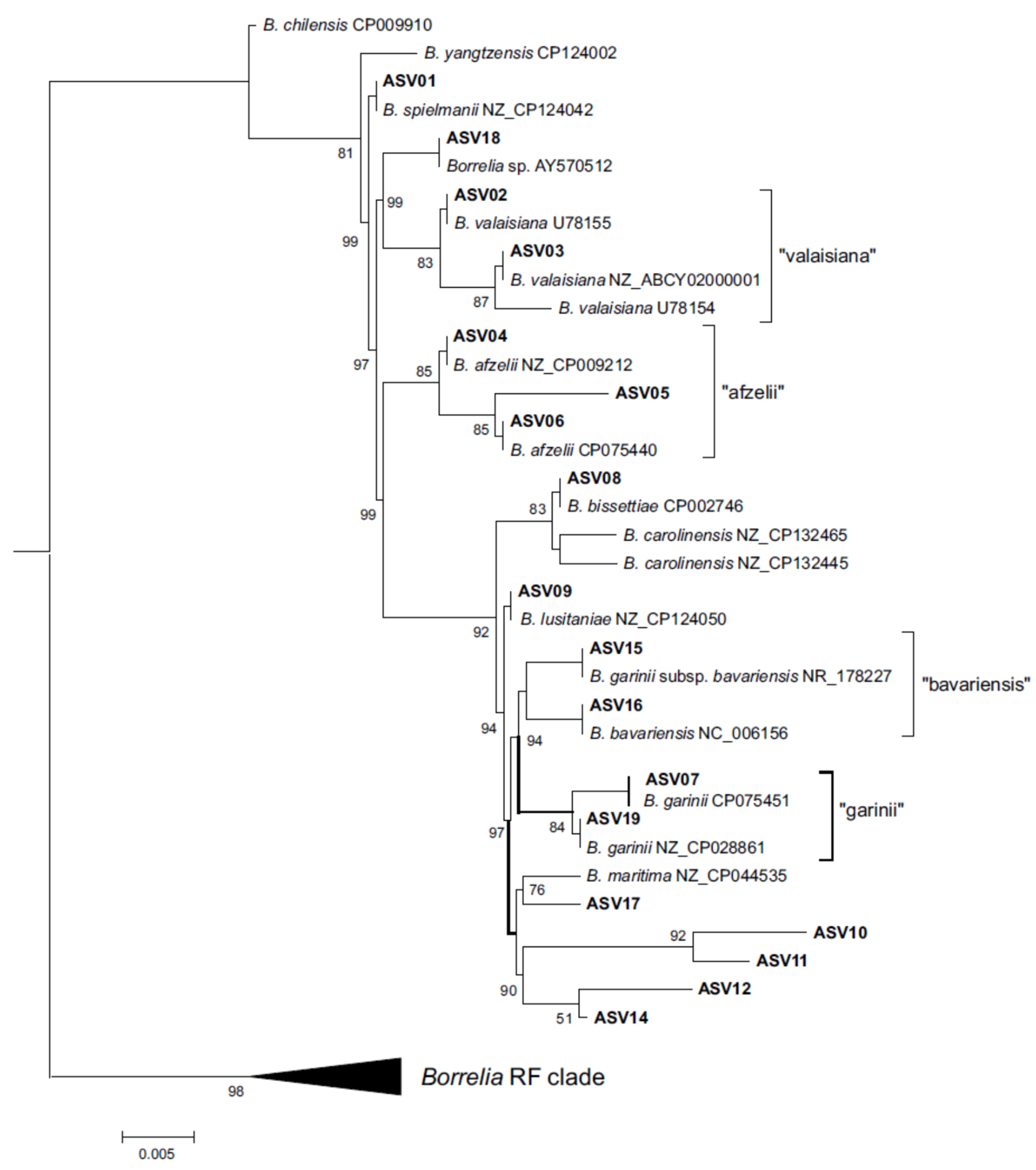
3.3. Presence of Babesia spp. DNA in Ixodes ricinus
3.4. Co-occurrence of Borrelia burgdorferi s.l. and Babesia spp. DNA in Ixodes ricinus
3.5. Co-occurrence of Borrelia burgdorferi s.l. and Borrelia miyamotoi in Ixodes ricinus
4. Discussion
Supplementary Materials
Funding
Acknowledgements
References
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubálek, Z.; Földvári, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Śpitalská, E.; et al. 2014. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: new hazards and relevance for public health. Front. Public Health. 2014, 2, 1–26. [Google Scholar] [CrossRef]
- Noden, B.H.; Roselli, M.A.; Los, S.R. Effect of urbanization on presence, abundance, and coinfection of bacteria and protozoa in ticks in the US Great Plains. J. Med. Entomol. 2022, 59, 957–968. [Google Scholar] [CrossRef]
- Ginsberg, H.S. Potential effects of mixed infections in ticks on transmission dynamics of pathogens: Comparative analysis of published records. Exp. Appl. Acarol. 2008, 46, 29–41. [Google Scholar] [CrossRef]
- Moutailler, S.; Valiente Moro, C.; Vaumourin, E.; Michelet, L.; Tran, FH.; Devillers, E.; et al. Co-infection of ticks: The rule rather than the exception. PLoS Negl Trop Dis. 2016, 10, e0004539. [Google Scholar] [CrossRef] [PubMed]
- Welc-Falęciak, R.; Kowalec, M.; Karbowiak, G.; Bajer, A.; Behnke, J.M.; Siński, E. Rickettsiaceae and Anaplasmataceae infections in Ixodes ricinus ticks from urban and natural forested areas of Poland. Parasites Vectors 2014, 7, 121. [Google Scholar] [CrossRef]
- Kowalec, M.; Szewczyk, T.; Welc-Falȩciak, R.; Siński, E.; Karbowiak, G.; Bajer, A. Ticks and the city—are there any differences between city parks and natural forests in terms of tick abundance and prevalence of spirochaetes? Parasites Vectors 2017, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Richtrová, E.; Míchalová, P.; Lukavská, A.; Navrátil, J.; Kybicová, K. 2022. Borrelia bugdorferi sensu lato infection in Ixodes ricinus ticks in urban green areas in Prague. Ticks Tick Borne Dis. 2022, 13, 102053. [Google Scholar] [CrossRef]
- Bajer, A.; Dwużnik-Szarek, D. The Specificity of Babesia-tick vector interactions: Recent advances and pitfalls in molecular and field studies. Parasites Vectors 2021, 14, 507. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Spielman, A.; Telford, S.R., 3rd; Sikand, V.K.; McKay, K.; Christianson, D.; Pollack, R.J.; Brassard, P.; Magera, J.; Ryan, R.; et al. Persistent parasitemia after acute babesiosis. N. Engl. J. Med. 1998, 339, 160–165. [Google Scholar] [CrossRef]
- Dunn, J.M.; Krause, P.J.; Davis, S.; Vannier, E.G.; Fitzpatrick, M.C.; Rollend, L.; Belperron, A.A.; States, S.L.; Stacey, A.; Bockenstedt, L.K.; et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS One 2014, 9, e115494. [Google Scholar] [CrossRef]
- Little, E.A.H.; Molaei, G. Passive tick surveillance: exploring spatiotemporal associations of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Babesia microti (Piroplasmida: Babesiidae), and Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae) infection in Ixodes scapularis (Acari: Ixodidae). Vector Borne Zoonotic Dis. 2020, 20, 177–186. [Google Scholar] [CrossRef]
- Zembsch, T.E.; Lee, X.; Bron, G.M.; Bartholomay, L.C.; Paskewitz, S.M. Coinfection of Ixodes scapularis (Acari: Ixodidae) nymphs with Babesia spp. (Piroplasmida: Babesiidae) and Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) in Wisconsin. J Med Entomol. 2021, 58, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Hersh, M.H.; Ostfeld, R.S.; McHenry, D.J.; Tibbetts, M.; Brunner, J.L.; Killilea, M.E.; LoGiudice, K.; Schmidt, K.A.; Keesing, F. Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS ONE 2014, 9, e99348. [Google Scholar] [CrossRef]
- Diuk-Wasser, M.A.; Vannier, E.; Krause, P.J. Coinfection by Ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends Parasitol. 2015, 32, 30–42. [Google Scholar] [CrossRef]
- Jahfari, S.; Hofhuis, A.; Fonville, M.; van der Giessen, J.; van Pelt, W.; Sprong, H. Molecular detection of tick-borne pathogens in humans with tick bites and erythema migrans, in the Netherlands. PLoS Neglected Trop. Dis. 2016, 10, e0005042. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.J.; Vayssier-Taussat, M.; Estrada-Peña, A.; Potkonjak, A.; Mihalca, A.D.; Zeller, H. Tick-borne diseases and co-infection: Current considerations. Ticks Tick Borne Dis. 2021, 12, 101607. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Telford, S.R., III; Spielman, A.; Sikand, V.; Ryan, R.; Christianson, D.; Burke, G.; Brassard, P.; Pollack. , et al. Concurrent Lyme disease and babesiosis: Evidence for increased severity and duration of illness. JAMA 1996, 275, 1657–1660. [Google Scholar] [CrossRef]
- Martínez-Balzano, C.; Hess, M.; Malhotra, A.; Lenox, R. Severe babesiosis and Borrelia burgdorferi co-infection. QJM Int. J. Med. 2015, 108, 141–143. [Google Scholar] [CrossRef]
- Djokic, V.; Akoolo, L.; Primus, S.; Schlachter, S.; Kelly, K.; Bhanot, P.; Parveen, N. Protozoan parasite Babesia microti subverts adaptive immunity and enhances Lyme disease severity. Front. Microbiol. 2019, 10, 1596. [Google Scholar] [CrossRef]
- Parveen, N.; Bhanot, P. Babesia microti—Borrelia burgdorferi coinfection. Pathogens 2019, 8, 117. [Google Scholar] [CrossRef]
- Hildebrandt, A. , Zintl, A., Montero, E., Hunfeld, K.P., Gray, J. 2021. Human babesiosis in Europe. Pathogens 2021, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Liberska, J.; Michalik, J.; Pers-Kamczyc, E.; Wierzbicka, A.; Lane, R. S.; Rączka, G.; Opalińska, P.; Skorupski, M.; Dabert, M. Prevalence of Babesia canis DNA in Ixodes ricinus ticks collected in forest and urban ecosystems in west-central Poland. Ticks Tick Borne Dis. 2021, 12, 101786. [Google Scholar] [CrossRef] [PubMed]
- Siuda, K. Kleszcze (Acari: Ixodida) Polski. II. Systematyka i rozmieszczenie. Monografie Parazytologiczne vol, 12. Polish Parasitological Society, Warsaw, Poland 1993.
- Trzebny, A.; Slodkowicz-Kowalska, A.; Becnel, J.J.; Sanscrainte, N.; Dabert, M. A new method of metabarcoding Microsporidia and their hosts reveals high levels of microsporidian infections in mosquitoes (Culicidae). Mol. Ecol. Resour. 2020, 20, 1486–1504. [Google Scholar] [CrossRef]
- Liberska, J.A.; Michalik, J.F.; Dabert, M. Exposure of dogs and cats to Borrelia miyamotoi infected Ixodes ricinus ticks in urban areas of the city of Poznań, west-central Poland. Ticks Tick Borne Dis. 2023, 14, 102188. [Google Scholar] [CrossRef]
- Wodecka, B.; Leońska, A.; Skotarczak, B. A comparative analysis of molecular markers for the detection and identification of Borrelia spirochetes in Ixodes ricinus. J. Med. Microbiol. 2010, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Zahler, M.; Rinder, H.; Schein, E.; Gothe, R. Detection of a new pathogenic Babesia microti-like species in dogs. Vet Parasitol. 2000, 89, 241–248. [Google Scholar] [CrossRef]
- Li, Y.; Teng, L.; Shang, L.; Ma, R.; Cai, J.; Zhao, Q.; Li, J.; Liu, Q. Detection of Theileria and Babesia sp. in Ixodid ticks from Qinghai Province, Northwestern China. J Anim Vet Adv. 2013, 12, 775–778. [Google Scholar] [CrossRef]
- Nicholas, K.B.; Nicholas, H.B. Jr. GeneDoc: A tool for editing and annotating multiple sequence alignments. Pittsburgh Supercomputing Center’s National Resource for Biomedical Supercomputing. 1997. Available online: http://www.nrbsc. org/downloads/ (accessed on 2 March 2007).
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Hansford, K.M.; Wheeler, B.W.; Tschirren, B.; Medlock, J.M. Questing Ixodes ricinus ticks and Borrelia spp. in urban green space across Europe: A review. Zoonoses Public Health, 2022, 69, 153–166. [Google Scholar] [CrossRef]
- Chvostáč, M.; Špitalská, E.; Václav, R.; Vaculová, T.; Minichová, L.; Derdáková, M. Seasonal patterns in the prevalence and diversity of tick-borne Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Rickettsia spp. in an urban temperate forest in south western Slovakia. Int. J. Environ. Res. Public Health 2018, 15, 994. [Google Scholar] [CrossRef]
- Vaculová, T.; Derdáková, M.; Špitalská, E.; Václav, R.; Chvostáč, M.; Rusňáková Tarageľová, V. Simultaneous occurrence of Borrelia miyamotoi, Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Rickettsia helvetica in Ixodes ricinus ticks in urban foci in Bratislava, Slovakia. Acta Parasitol. 2019, 64, 19–30. [Google Scholar] [CrossRef]
- Pangrácová, L.; Derdáková, M.; Pekárik, L.; Hviščová, I.; Víchová, B.; Stanko, M.; Hlavatá, H.; Peťko, B. Ixodes ricinus abundance and its infection with the tick-borne pathogens in urban and suburban areas of Eastern Slovakia. Parasites Vectors 2013, 16, 238. [Google Scholar] [CrossRef]
- Pejchalová, K.; Žakovská, A.; Mejzlíková, M.; Halouzka, J.; Dendis, M. Isolation, cultivation and identification of Borrelia burgdorferi genospecies from Ixodes ricinus ticks from the city of Brno, Czech Republic. Ann. Agric. Environ. Med. 2007, 14, 75–79. [Google Scholar]
- Venclíková, K.; Mendel, J.; Betášová, L.; Blažejová, H.; Jedličková, P.; Straková, P.; Hubálek, Z.; Rudolf, I. Neglected tick-borne pathogens in the Czech Republic, 2011-2014. Ticks Tick Borne Dis. 2016, 7, 107–112 https://101016/jttbdis201509004. [Google Scholar] [CrossRef]
- Žygutienė, M.; Alekseev, A.; Dubinina, H.; Kazlauskienė, R. Evidence for a risk of tick borne infection in the city parks of Vilnius, Lithuania. Ekologija 2008, 54, 40–43. [Google Scholar] [CrossRef]
- Glass, A.; Springer, A.; Raulf, M.K.; Fingerle, V.; Strube, C. 15-year Borrelia prevalence and species distribution monitoring in Ixodes ricinus/inopinatus populations in the city of Hanover, Germany. Ticks Tick Borne Dis. 2023, 14, 102074. [Google Scholar] [CrossRef] [PubMed]
- Stańczak, J.; Gabre, R.M.; Kruminis-Łozowska, W.; Racewicz, M.; Kubica-Biernat, B. Ixodes ricinus as a vector of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti in urban and suburban forests. Ann. Agric. Environ. Med. 2004, 11, 109–114. [Google Scholar] [PubMed]
- Kubiak, K.; Dziekońska-Rynko, J.; Szymańska, H.; Kubiak, D.; Dmitryjuk, M.; Dzika, E. Questing Ixodes ricinus ticks (Acari, Ixodidae) as a vector of Borrelia burgdorferi sensu lato and Borrelia miyamotoi in an urban area of north-eastern Poland. Exp. Appl. Acarol. 2019, 78, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Grochowska, A.; Dunaj-Małyszko, J.; Pancewicz, S.; Czupryna, P.; Milewski, R.; Majewski, P.; Moniuszko-Malinowska, A. Prevalence of tick-borne pathogens in questing Ixodes ricinus and Dermacentor reticulatus ticks collected from recreational areas in northeastern Poland with analysis of environmental factors. Pathogens. 2022, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, A.; Bertolotti, L.; Gern, L.; Gray, J. Ecology of Borrelia burgdorferi sensu lato in Europe: transmission dynamics in multi-host systems, influence of molecular processes and effects of climate change. FEMS Microbiol Rev. 2015, 36, 837–861. [Google Scholar] [CrossRef] [PubMed]
- Nijhof, A.M.; Bodaan, C.; Postigo, M.; Nieuwenhuijs, H.; Opsteegh, M.; Franssen, L.; Jebbink, F.; Jongejan, F. Ticks and associated pathogens collected from domestic animals in The Netherlands. Vector Borne Zoonotic Dis. 2007, 7, 585–596. [Google Scholar] [CrossRef]
- Kooyman, F.N.J.; Zweerus, H.; Nijsse, E.R.; Jongejan, F.; Wagenaar, J.A.; Broens, E.M. Monitoring of ticks and their pathogens from companion animals obtained by the “tekenscanner” application in The Netherlands. Parasitol Res. 2022, 121, 1887–1893. [Google Scholar] [CrossRef]
- Leschnik, M.W.; Khanakah, G.; Duscher, G.; Wille-Piazzai, W.; Hörweg, C.; Joachim, A.; Stanek, G. Species, developmental stage and infection with microbial pathogens of engorged ticks removed from dogs and questing ticks. Med. Vet. Entomol. 2012, 26, 440–446. [Google Scholar] [CrossRef]
- Jenkins, A.; Hvidsten, D.; Matussek, A.; Lindgren, P-E. ; Stuen, S.; Kristiansen, B-E. Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from Norway: evaluation of a PCR test targeting the chromosomal flaB gene. Exp Appl Acarol. 2012, 58, 431–439. [Google Scholar] [CrossRef]
- Schreiber, C.; Krücken, J.; Beck, S.; Maaz, D.; Pachnicke, S.; Krieger, K.; Gross, M.; Kohn, B.; von Samson-Himmelstjerna, G. Pathogens in ticks collected from dogs in Berlin/Brandenburg, Germany. Parasites Vectors 2014, 14, 535. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Al Marai, D.; Andersen, L.O.; Krogfelt, K.A.; Jensen, J.S.; Larsen, K.S.; Nielsen, H.V. Babesia spp. and other pathogens in ticks recovered from domestic dogs in Denmark. Parasites Vectors 2015, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Namina, A.; Capligina,V. ; Seleznova, M.; Krumins, R.; Aleinikova, D.; Kivrane, A.; Akopjana, S.; Lazovska, M.; Berzina, I.; Ranka, R. Tick-borne pathogens in ticks collected from dogs, Latvia, 2011–2016. BMC Vet. Res. 2019, 15, 398. [Google Scholar] [CrossRef] [PubMed]
- Zakham, F.; Korhonen, E.M.; Puonti, P.T.; Castrén, R.S.; Uusitalo, R.; Smura, T.; Kant, R.; Vapalahti, O.; Sironen. ; T., Kinnunenet, P.M. Molecular detection of pathogens from ticks collected from dogs and cats at veterinary clinics in Finland. Parasites Vectors 2023, 16, 327. [Google Scholar] [CrossRef]
- Król, N.; Obiegala, A.; Pfeffer, M.; Lonc, E.; Kiewra, D. Detection of selected pathogens in ticks collected from cats and dogs in the Wroclaw agglomeration, South-West Poland. Parasites Vectors 2016, 9, 351. [Google Scholar] [CrossRef]
- Michalski, M.M.; Kubiak, K.; Szczotko, M.; Chajęcka, M.; Dmitryjuk, M. Molecular detection of Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in ticks collected from dogs in urban areas of north-eastern Poland. Pathogens 2020, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Strnad, M.; Honig, V.; Ruzek, D.; Grubhoffer, L.; Rego, R.O.M. Europe-wide meta-analysis of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks. Appl. Environ. Microbiol. 2017, 83, e00609–17. [Google Scholar] [CrossRef] [PubMed]
- Skotarczak, B. The role of companion animals in the environmental circulation of tick-borne bacterial pathogens. Ann Agric Environ Med. 2018, 25, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Vogt, NA. Lyme borreliosis in animals. In: Merck Veterinary Manual. 2022. Available online: www.merckvetmanual.com/generalized-conditions/lyme-borreliosis/lyme-borreliosis-in-animals (accessed on 31 October 2022).
- Hanincová, K; Schäfer, S.M.; Etti, S.; Sewell, H.S.; Taragelová, V., Ziak, D.; Labuda, M.; Kurtenbach, K. Association of Borrelia afzelii with rodents in Europe. Parasitology 2003, 126, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Schlee, D.B.; Matuschka, F.R. Reservoir competence of various rodents for the Lyme disease spirochete Borrelia spielmanii. Appl. Environ. Microbiol. 2011, 77, 3565–3570. [Google Scholar] [CrossRef] [PubMed]
- Skuballa, J.; Petney, T.; Pfaffle, M.; Oehme, R.; Hartelt, K.; Fingerle, V.; Kimmig, P.; Taraschewski, H. Occurrence of different Borrelia burgdorferi sensu lato genospecies including B. afzelii, B. bavariensis, and B. spielmanii in hedgehogs (Erinaceus spp.) in Europe. Ticks Tick Borne Dis. 2012, 3, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Michalik, J.; Wodecka, B.; Skoracki, M.; Sikora, B.; Stańczak, J. Prevalence of avian-associated Borrelia burgdorferi s. l. genospecies in Ixodes ricinus ticks collected from blackbirds (Turdus merula) and song thrushes (T. philomelos). Int. J. Med. Microbiol. 2008, 298 (Suppl. 1), 129–138. [Google Scholar]
- Richter, D.; Matuschka, FR. Perpetuation of the Lyme disease spirochete Borrelia lusitaniae by lizards. Appl Environ Microbiol. 2006, 72, 4627–4632. [Google Scholar] [CrossRef]
- Onyiche, T.E.; Răileanu, C.; Fischer, S.; Silaghi, C. Global distribution of Babesia species in questing ticks: a systematic review and meta-analysis based on published literature. Pathogens 2021, 10, 230. [Google Scholar] [CrossRef]
- Stańczak, J.; Cieniuch, S.; Lass, A.; Biernat, B.; Racewicz, M. Detection and quantification of Anaplasma phagocytophilum and Babesia spp. in Ixodes ricinus ticks from urban and rural environment, northern Poland, by real-time polymerase chain reaction. Exp. Appl. Acarol. 2015, 66, 63–81. [Google Scholar] [CrossRef]
- Schorn, S.; Pfister, K.; Reulen, H.; Mahling, M.; Silaghi, C. Occurrence of Babesia spp., Rickettsia spp. and Bartonella spp. in Ixodes ricinus in Bavarian public parks, Germany. Parasites Vectors 2011, 4, 135. [Google Scholar] [CrossRef]
- Welc-Falęciak, R.; Bajer, A.; Paziewska-Harris, A.; Baumann-Popczyk, A.; Siński, E. Diversity of Babesia in Ixodes ricinus ticks in Poland. Adv Med Sci. 2012, 57, 364–369. [Google Scholar] [CrossRef]
- Abdullah, S.; Helps, C.; Tasker, S.; Newbury, H. , Wall, R. Prevalence and distribution of Borrelia and Babesia species in ticks feeding on dogs in the UK. Med. Vet. Entomol. 2018, 32, 14–22. [Google Scholar] [CrossRef]
- Asman, M.; Solarz, K.; Cuber, P.; Gasior, T.; Szilman, P.; Szilman, E.; Tondas, E.; Matzullok, A.; Kusion, N.; Florek, K. Detection of protozoans Babesia microti and Toxoplasma gondii and their co-existence in ticks (Acari: Ixodida) collected in Tarnogórski district (Upper Silesia, Poland). Ann Agric Environ. 2015, 22, 80–83. [Google Scholar] [CrossRef]
- Karbowiak, G. Zoonotic reservoir of Babesia microti in Poland. Pol J Microbiol. 2004, 53 (Suppl), 61–65. [Google Scholar]
- Cieniuch, S.; Stańczak, J.; Ruczaj, A. The first detection of Babesia EU1 and Babesia canis canis in Ixodes ricinus ticks (Acari, Ixodidae) collected in urban and rural areas in northern Poland. Pol J Microbiol. 2009, 58, 231–236. [Google Scholar]
- Hamšíková, Z.; Kazimírová, M.; Haruštiaková, Z.; Kazimírová, M.; Haruštiaková, D.; Mahríková, M.; Slovák, M.; Berthová, L.; Kocianová, E.; Schnittger, L. Babesia spp. in ticks and wildlife in different habitat types of Slovakia. Parasites Vectors 2016, 9, 292. [Google Scholar] [CrossRef]
- Rybářová, M.; Honsová, M.; Papoušek, I.; Široký, P. Variability of species of Babesia Starcovici, 1893 in three sympatric ticks (Ixodes ricinus, Dermacentor reticulatus and Haemaphysalis concinna) at the edge of Pannonia in the Czech Republic and Slovakia. Folia Parasitol. 2017, 64, 028. [Google Scholar] [CrossRef]
- Adaszek, Ł.; Winiarczyk, S. Molecular characterization of Babesia canis canis isolates from naturally infected dogs in Poland. Vet. Parasitol. 2008, 52, 235–241. [Google Scholar] [CrossRef]
- Livanova, N.N.; Fomenko, N.V.; Akimov, I.A.; Ivanov, M.J.; Tikunova, N.V.; Armstrong, R.; Konyaev, S.V. 2018. Dog survey in Russian veterinary hospitals: tick identification and molecular detection of tick-borne pathogens. Parasites Vectors 2018, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Zygner, W.; Bąska, P.; Wiśniewski, M.; Wędrychowicz, H. The molecular evidence of Babesia microti in hard ticks removed from dogs in Warsaw. Pol J Mocrobiol. 2010, 59, 95–7. [Google Scholar] [CrossRef]
- Kocoń, A.; Asman, M.; Nowak-Chmura, M.; Witecka, J.; Rączka, G. Exposure of domestic dogs and cats to ticks (Acari: Ixodida) and selected tick-borne diseases in urban and recreational areas in southern Poland. Sci Rep. 2022, 12, 7851. [Google Scholar] [CrossRef] [PubMed]
- Sytykiewicz, H.; Karbowiak, G. , Hapunik, J.; Szpechciński, A.; Supergan-Marwicz, M.; Goławska, S.; Sprawka, I.; Czerniewicz, P. Molecular evidence of Anaplasma phagocytophilum and Babesia microti co-infections in Ixodes ricinus ticks in central-eastern region of Poland. Ann Agric Environ Med. 2012, 19, 45–49. [Google Scholar] [PubMed]
- Asman,M. ; Solarz, K.; Szilman, E.; Szilman, P.; Sikora, B.; Jakubas-Zawalska, J. The occurrence of three tick-borne pathogens in Ixodes ricinus ticks collected from the area of the Kraków-Częstochowa Upland (Southern Poland). Acarologia 2018, 58, 967–975. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Gray, J.; Montero, E. Characteristics of Human Babesiosis in Europe. Pathogens 2023, 12, 323. [Google Scholar] [CrossRef]
- Goethert, H.K. What Babesia microti is now. Pathogens 2021, 10, 1168. [Google Scholar] [CrossRef]
- Moniuszko-Malinowska, A.; Swięcicka, I.; Dunaj, J.; Zajkowska, J.; Czupryna, P.; Zambrowski, G.; Chmielewska-Badora, J.; Żukiewicz-Sobczak, W.; Swierzbińska, R.; Rutkowski, K.; et al. Infection with Babesia microti in humans with non-specific symptoms in North East Poland. Infect. Dis. Lond. Engl. 2016, 48, 537–543. [Google Scholar] [CrossRef]
- Arsuaga, M.; Gonzalez, L.M.; Lobo, C.A.; de la Calle, F.; Bautista, J.M.; Azcárate, I.G.; Puente, S.; Montero, E. First report of Babesia microti-caused babesiosis in Spain. Vector Borne Zoonotic Dis. 2016, 16, 677–679. [Google Scholar] [CrossRef]
- Welc-Falęciak, R.; Pawełczyk, A.; Radkowski, M.; Pancewicz, S.A.; Zajkowska, J.; Siński, E. First report of two asymptomatic cases of human infection with Babesia microti (Franca, 1910) in Poland. Ann. Agric. Environ. Med. 2015, 22, 51–54. [Google Scholar] [CrossRef]
- Pawełczyk, A.; Bednarska, M.; Hamera, A.; Religa, E.; Poryszewska, M.; Mierzejewska, E.J.; Welc-Falęciak, R. Long-term study of Borrelia and Babesia prevalence and co-infection in Ixodes ricinus and Dermacentor recticulatus ticks removed from humans in Poland, 2016–2019. Parasites Vectors 2021, 14, 348. [Google Scholar] [CrossRef]
- Herwaldt, B.L.; Cacció, S.; Gherlinzoni, F.; Aspöck, H.; Slemenda, S.B.; Piccaluga, P.; Martinelli, G.; Edelhofer, R.; Hollenstein, U.; Poletti, G.; Pampiglione, S. , Löschenberger, K., Tura, S.,Pieniazek, N.J. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg. Infect. Dis. 2003, 9, 942–948. [Google Scholar] [CrossRef]
- Rozej-Bielicka, W.; Masny, A.; Gołąb, E. High-resolution melting PCR assay, applicable for diagnostics and screening studies, allowing detection and differentiation of several Babesia spp. infecting humans and animals. Parasitol Res. 2017, 116, 2671–2681. [Google Scholar] [CrossRef]
- Skotarczak, B.; Wodecka, B.; Cichocka, A. Coexistence DNA of Borrelia burgdorferi sensu lato and Babesia microti in Ixodes ricinus ticks from north-western Poland. Ann Agric Environ Med. 2002, 9, 25–8. [Google Scholar]
- Sawczyn-Domańska, A.; Zwoliński, J.; Kloc, A.; Wójcik-Fatla, A. Prevalence of Borrelia, Neoehrlichia mikurensis and Babesia in ticks collected from vegetation in Eastern Poland. Exp. App. Acarol. 2023, 90, 409–428. [Google Scholar] [CrossRef]
- Kubiak, K; Dmitryjuk, M. ; Dziekońska-Rynko, J., Siejwa, P.; Dzika, E. The risk of exposure to ticks and tick-borne pathogens in a spa town in northern Poland. Pathogens 2022, 11, 542. [Google Scholar] [CrossRef]
- Miron, L.D.; Ciuca, L.; Ilie, C.; Potoroaca, A.; Lazăr, C.; Martinescu, G.-V. Co-Infection with Babesia canis and Borrelia burgdorferi s.l. in a dog from Northeastern Romania: A case report. JALSE. 2022, 54, 439–449. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Pauliks, K.; Sachse, S.; Straube, E. Coexistence of Borrelia spp. and Babesia spp. in Ixodes ricinus Ticks in Middle Germany. Vector Borne Zoonotic Dis. 2010, 10, 831–837. [Google Scholar] [CrossRef]
- Meer-Scherrer, L.; Adelson, M.; Mordechai, E.; Lottaz, B.; Tilton Meer-Scherrer, R. Babesia microti Infection in Europe. Curr Microbiol. 2004, 48, 435–437. [Google Scholar] [CrossRef]
- Dunaj, J.; Moniuszko-Malinowska, A.; Swiecicka, I.; Andersson, M.; Czupryna, P.; Rutkowski, K.; Zambrowski, G.; Zajkowska, J.; Grygorczuk, S.; Kondrusik, M.; et al. Tick-borne infections and co-infections in patients with non-specific symptoms in Poland. Adv Med Sci. 2018, 63, 167–172. [Google Scholar] [CrossRef]
- Jabłońska, J.; Żarnowska-Prymek, H.; Stańczak, J.; Kozłowska, J.; Wiercińska-Drapało, A. Symptomatic coinfection with Babesia microti and Borrelia burgdorferi in patient after international exposure; a challenging case in Poland. Ann Agric Environ Med. 2016, 23, 387–389. [Google Scholar] [CrossRef]
- Welc-Falęciak, R.; Hildebrandt, A.; Siński, E. Coinfection with Borrelia species and other tick-borne pathogens in humans: two cases from Poland. Ann Agric Environ Med. 2010, 17, 309–313. [Google Scholar] [PubMed]
- Pańczuk, A.; Tokarska-Rodak, M.; Kozioł-Montewka, M.; Plewik, D. The incidence of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti coinfections among foresters and farmers in eastern Poland. J Vector Borne Dis. 2016, 53, 348–354. [Google Scholar]
- Cosson, J.F.; Michelet, L.; Chotte, J.; Le Naour, E.; Cote, M.; Devillers, E.; Poulle, L.M.; Huet, D.; Galan, M.; Geller, J.; et al. Genetic characterization of the human relapsing fever spirochete Borrelia miyamotoi in vectors and animal reservoirs of Lyme disease spirochetes in France. Parasit. Vectors 2014, 7, 233. [Google Scholar] [CrossRef]
| Females | Males | Nymphs | TOTAL | |
|---|---|---|---|---|
| Borrelia spp. | ||||
| B. afzelii | 10 | 17 | 11 | 38 (3.7) |
| B. garinii | 19 | 8 | 11 | 38 (3.7) |
| B. lusitaniae | 2 | 0 | 7 | 9 (0.9) |
| B. valaisianae | 2 | 1 | 0 | 3 (0.3) |
| Borrelia spp. | 1 | 1 | 0 | 2 (0.2) |
| Total | 34/289 (11.8) | 27/280 (9.6) | 29/460 (6.3) | 90/1029 (8.7) |
| Babesia spp. | ||||
| Babesia microti | 10 | 9 | 8 | 27 (2.6) |
| Babesia canis | 8 | 3 | 3 | 14 (1.4) |
| Babesia venatorum | 3 | 1 | 0 | 4 (0.4) |
| Total | 21/289 (7.3) | 13/280 (4.6) | 11/460 (2.4) | 45/1029 (4.4) |
| co-infections | ||||
| B. afzelii + Ba. canis | 1 | 3 | 0 | 4 (0.4) |
| B. garinii + Ba. canis | 1 | 3 | 0 | 4 (0.4) |
| B. lusitaniae + Ba. venatorum | 2 | 0 | 0 | 2 (0.2) |
| B. lusitaniae + Ba. microti | 1 | 0 | 0 | 1 (0.1) |
| Total | 5 | 6 | 0 | 11/1029 (1.1) |
| Dogs (n= 609) |
Cats (n=117) |
Undefined hosts (n=389) |
TOTAL | |
|---|---|---|---|---|
| Borrelia spp. | ||||
| B. afzelii | 26 (3,7) | 5 (3,3) | 16 (4,0) | 47 (3,7) |
| B. garinii | 4 (0,6) | 0 | 3 (0,7) | 7 (0,6) |
| B. spielmanii | 3 (0,4) | 0 | 0 | 3 (0,2) |
| B. lusitaniae | 1 (0,1) | 0 | 0 | 1 (0,1) |
| B. valaisianae | 0 | 0 | 1 | 1 (0,1) |
| Total | 34/711 (4.8) | 5/153 (3,3) | 20/404 (5,0) | 59/1268 (4,7) |
| Babesia spp. | ||||
| Babesia canis | 23 (3.2) | 1 (0.7) | 11 (2.7) | 35 (2.8) |
| Babesia microti | 18 (2.5) | 2 (1.3) | 8 (2.0) | 28 (2.2) |
| Babesia venatorum | 7 (1.0) | 3 (2.0) | 3 (0.7) | 13 (1.0) |
| Total | 48/711 (6,8) | 6/153 (3,9) | 22/404 (5,7) | 76/1268 (6,0) |
| co-infections | ||||
| B. afzelii + Ba. microti | 5 | 0 | 5 | 10 (0.8) |
| B. spielmanii + Ba. microti | 1 | 0 | 0 | 1 (0.1) |
| B. garinii + Ba. microti | 0 | 0 | 1 | 1 (0.1) |
| B. garinii + Ba. canis | 0 | 1 | 0 | 1 (0.1) |
| Total | 13/1268 (1.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





