Submitted:
29 February 2024
Posted:
04 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiology and Social Impact of ALD
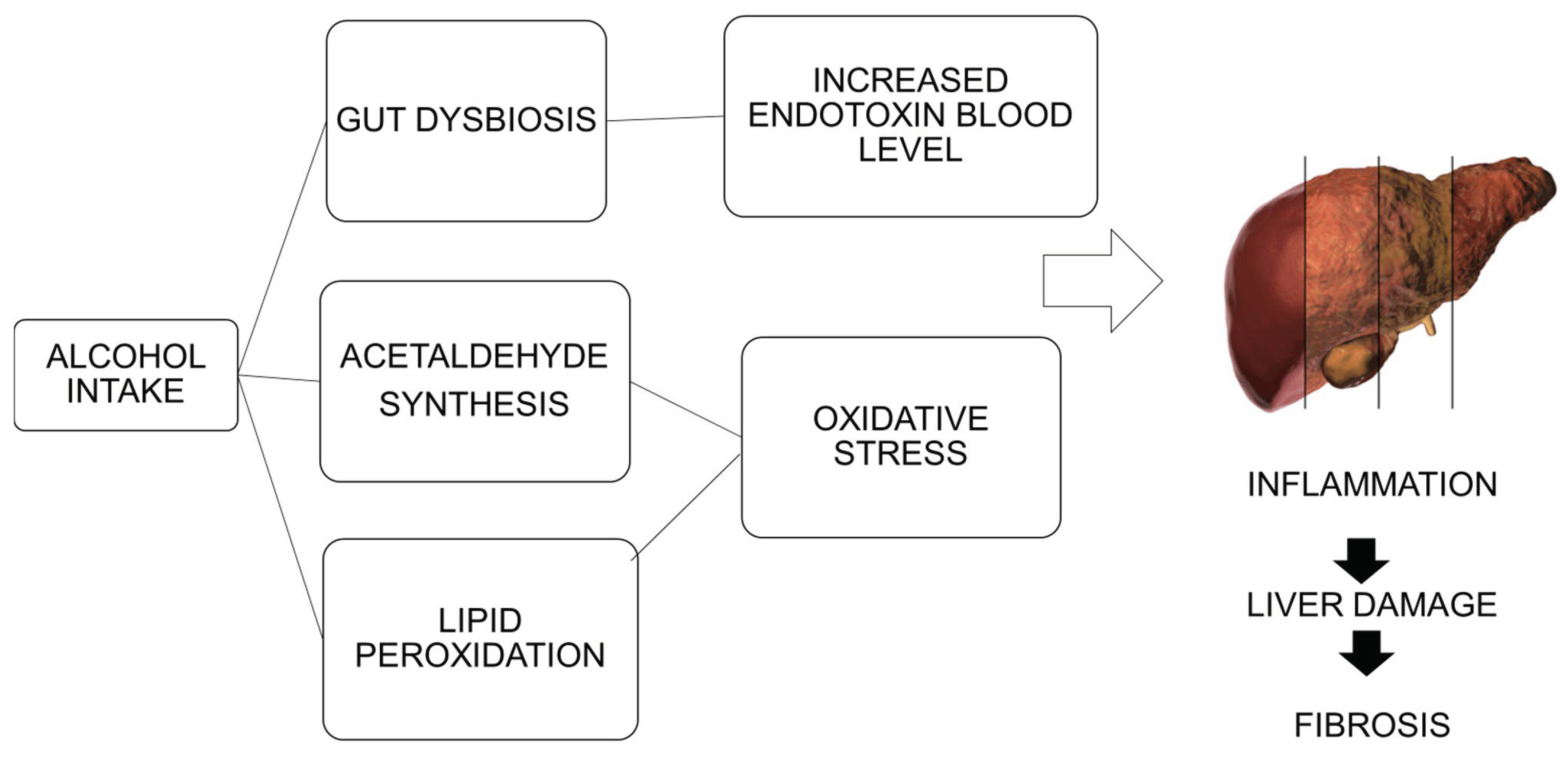
3. ALD Pathogenesis
4. Role of Pro-Inflammatory Cytokines
4.1. TNF-α
4.2. IL-8 and CXCL1
4.3. IL-1β
5. Role of Anti-Inflammatory Cytokines
5.1. IL-6 and IL-10
5.2. IL-22
6. New Therapeutic Approaches
6.1. Biological Drugs
6.2. Gut Microbiota Modulation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADH | aldehyde dehydrogenase |
| AFL | alcoholic fatty liver |
| AH | alcoholic hepatitis |
| ALD | alcoholic liver disease |
| ASH | alcoholic steatohepatitis |
| AUD | alcohol use disorder |
| CXCL1 | chemokine CXC motif chemokine ligand 1 |
| CYP2E1 | cytochrome P450 2E1 |
| ERK1/2 | extracellular receptor-activated kinase 1/2 |
| HCC | hepatocellular carcinoma |
| HSCs | hepatic stellate cells |
| IgG | immunoglobulin G |
| IL | interleukin |
| iNKT | invariant natural-killer T lymphocytes |
| KS | Korsakoff’s syndrome |
| LPS | lipopolysaccharide |
| NF-κB | nuclear factor κB |
| NLRP3 | NLR family pyrin domain containing 3 |
| NOX | NADPH oxidase |
| PAMPs | pathogen-associated molecular patterns |
| REG3G | antimicrobial C-type lectin regenerating islet-derived 3 gamma |
| ROS | reactive oxygen species |
| STAT3 | signal transducer and activator of transcription 3 |
| TGF-β | transforming growth factor-β |
| TLRs | toll-like receptors |
| TNF-α | tumor necrosis factor-alpha |
| WE | Wernicke encephalopathy |
References
- Singal, A.K.; Bataller, R.; Ahn, J.; Kamath, P.S.; Shah, V.H. ACG Clinical Guideline: Alcoholic Liver Disease. Am. J. Gastroenterol. 2018, 113, 175–194. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J. Hepatol. 2018, 69, 154–181. [Google Scholar] [CrossRef] [PubMed]
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef]
- Abenavoli, L.; Spagnuolo, R.; Scarlata, G.G.M.; Scarpellini, E.; Boccuto, L.; Luzza, F. Ultrasound Prevalence and Clinical Features of Nonalcoholic Fatty Liver Disease in Patients with Inflammatory Bowel Diseases: A Real-Life Cross-Sectional Study. Medicina 2023, 59, 1935. [Google Scholar] [CrossRef]
- Singal, A.K.; Bataller, R.; Ahn, J.; Kamath, P.S.; Shah, V.H. ACG Clinical Guideline: Alcoholic Liver Disease. Am. J. Gastroenterol. 2018, 113, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Crabb, D.W.; Bataller, R.; Chalasani, N.P.; Kamath, P.S.; Lucey, M.; Mathurin, P.; McClain, C.; McCullough, A.; Mitchell, M.C.; Morgan, T.R.; et al. Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 2016, 150, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.E.; Ding, W.X.; Cresci, G.; Saikia, P.; Shah, V.H. Linking Pathogenic Mechanisms of Alcoholic Liver Disease With Clinical Phenotypes. Gastroenterology 2016, 150, 1756–1768. [Google Scholar] [CrossRef]
- Argemi, J.; Ventura-Cots, M.; Rachakonda, V.; Bataller, R. Alcoholic-related liver disease: Pathogenesis, management and future therapeutic developments. Rev. Esp. Enferm. Dig. 2020, 112, 869–878. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- World Health Organization. Global status report on alcohol and health 2018. Available online: https://www.who.int/publications/i/item/9789241565639 (accessed on 19 February 2024).
- Stein, E.; Cruz-Lemini, M.; Altamirano, J.; Ndugga, N.; Couper, D.; Abraldes, J.G.; Bataller, R. Heavy daily alcohol intake at the population level predicts the weight of alcohol in cirrhosis burden worldwide. J. Hepatol. 2016, 65, 998–1005. [Google Scholar] [CrossRef]
- Aslam, A.; Kwo, P.Y. Epidemiology and Disease Burden of Alcohol Associated Liver Disease. J. Clin. Exp. Hepatol. 2023, 13, 88–102. [Google Scholar] [CrossRef]
- Singal, A.K.; Arsalan, A.; Dunn, W.; Arab, J.P.; Wong, R.J.; Kuo, Y.F.; Kamath, P.S.; Shah, V.H. Alcohol-associated liver disease in the United States is associated with severe forms of disease among young, females and Hispanics. Aliment. Pharmacol. Ther. 2021, 54, 451–446. [Google Scholar] [CrossRef]
- Hagström, H.; Hemmingsson, T.; Discacciati, A.; Andreasson, A. Alcohol consumption in late adolescence is associated with an increased risk of severe liver disease later in life. J. Hepatol. 2018, 68, 505–510. [Google Scholar] [CrossRef]
- Hagström, H.; Thiele, M.; Roelstraete, B.; Söderling, J.; Ludvigsson, J.F. Mortality in biopsy-proven alcohol-related liver disease: A population-based nationwide cohort study of 3453 patients. Gut 2021, 70, 170–179. [Google Scholar] [CrossRef]
- Jyani, G.; Prinja, S.; Ambekar, A.; Bahuguna, P.; Kumar, R. Health impact and economic burden of alcohol consumption in India. Int. J. Drug Policy 2019, 69, 34–42. [Google Scholar] [CrossRef]
- Díaz, L.A.; Idalsoaga, F.; Fuentes-López, E.; Márquez-Lomas, A.; Ramírez, C.A.; Roblero, J.P.; Araujo, R.C.; Higuera-de-la-Tijera, F.; Toro, LG.; Pazmiño, G.; et al. Impact of Public Health Policies on Alcohol-Associated Liver Disease in Latin America: An Ecological Multinational Study. Hepatology 2021, 74, 2478–2490. [Google Scholar] [CrossRef]
- White, A.M.; Castle, I.P.; Powell, P.A.; Hingson, R.W; Koob, G.F. Alcohol-Related Deaths During the COVID-19 Pandemic. JAMA 2022, 327, 1704–1706. [Google Scholar] [CrossRef]
- Rehm, J.; Shield, K.D. Global Burden of Alcohol Use Disorders and Alcohol Liver Disease. Biomedicines 2019, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Martinson, N.; Martinson, M. Mortality and costs associated with alcoholic hepatitis: A claims analysis of a commercially insured population. Alcohol. 2018, 71, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Qian, A.S.; Nguyen, N.H.; Stukalin, I.; Congly, S.E.; Shaheen, A.A.; Swain, M.G.; Teriaky, A.; Asrani, S.K.; Singh, S. Trends in the Economic Burden of Chronic Liver Diseases and Cirrhosis in the United States: 1996-2016. Am. J. Gastroenterol. 2021, 116, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.F.; Goldstein, R.B.; Saha, T.D.; Chou, S.P.; Jung, J.; Zhang, H.; Pickering, R.P.; Ruan, W.J.; Smith, S.M.; Huang, B.; et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015, 72, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Lucey, M.R.; Mathurin, P.; Morgan, T.R. Alcoholic hepatitis. N. Engl. J. Med. 2009, 360, 2758–2769. [Google Scholar] [CrossRef] [PubMed]
- Styskel, B.; Natarajan, Y.; Kanwal, F. Nutrition in Alcoholic Liver Disease: An Update. Clin. Liver Dis. 2019, 23, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S. Nutrition and Alcoholic Liver Disease: Effects of Alcoholism on Nutrition, Effects of Nutrition on Alcoholic Liver Disease, and Nutritional Therapies for Alcoholic Liver Disease. Clin. Liver Dis. 2016, 20, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Medici, V.; Halsted, C.H. Folate, alcohol, and liver disease. Mol. Nutr. Food Res. 2013, 57, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.M.; Johnson, C.L.; Jain, R.B.; Yetley, E.A.; Picciano, M.F.; Rader, J.I.; Fisher, K.D. Mulinare J, Osterloh JD. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988 2004. Am J Clin Nutr 2007, 86, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, J.A.; Halsted, C.H. Hepatic transmethylation reactions in micropigs with alcoholic liver disease. Hepatology 2004, 39, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.M.; Robinson, G.; Pinner, A.; Chamlee, L.; Ulasova, E.; Pompilius, M.; Page, G.P.; Chhieng, D.; Jhala, N.; Landar, A.; et al. S-adenosylmethionine prevents chronic alcohol-induced mitochondrial dysfunction in the rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G857–G867. [Google Scholar] [CrossRef]
- Sinha, S.; Kataria, A.; Kolla, B.P.; Thusius, N.; Loukianova, L.L. Wernicke Encephalopathy-Clinical Pearls. Mayo Clin. Proc. 2019, 94, 1065–1072. [Google Scholar] [CrossRef]
- Wilson, E.C.; Stanley, G.; Mirza, Z. The Long-Term Cost to the UK NHS and Social Services of Different Durations of IV Thiamine (Vitamin B1) for Chronic Alcohol Misusers with Symptoms of Wernicke’s Encephalopathy Presenting at the Emergency Department. Appl. Health Econ. Health Policy 2016, 14, 205–215. [Google Scholar] [CrossRef]
- Thompson, J.A.; Martinson, N.; Martinson, M. Mortality and costs associated with alcoholic hepatitis: A claims analysis of a commercially insured population. Alcohol. 2018, 71, 57–63. [Google Scholar] [CrossRef]
- Teschke, R. Alcoholic Liver Disease: Alcohol Metabolism, Cascade of Molecular Mechanisms, Cellular Targets, and Clinical Aspects. Biomedicines 2018, 6, 106. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Rametta, R.; Dongiovanni, P. Genetic and Epigenetic Modifiers of Alcoholic Liver Disease. Int. J. Mol. Sci. 2018, 19, 3857. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, T.; Kusumanchi, P.; Han, S.; Yang, Z.; Liangpunsakul, S. Alcohol Metabolizing Enzymes, Microsomal Ethanol Oxidizing System, Cytochrome P450 2E1, Catalase, and Aldehyde Dehydrogenase in Alcohol-Associated Liver Disease. Biomedicines 2020, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.Z.; Chandimali, N.; Han, Y.H.; Lee, D.H.; Kim, J.S.; Kim, S.U.; Kim, T.D.; Jeong, D.K.; Sun, H.N.; Lee, D.S.; et al. Pathogenesis, Early Diagnosis, and Therapeutic Management of Alcoholic Liver Disease. Int. J. Mol. Sci. 2019, 20, 2712. [Google Scholar] [CrossRef] [PubMed]
- Ceni, E.; Mello, T.; Galli, A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J. Gastroenterol. 2014, 20, 17756–17772. [Google Scholar] [CrossRef] [PubMed]
- Boccuto, L.; Abenavoli, L. Genetic and Epigenetic Profile of Patients With Alcoholic Liver Disease. Ann. Hepatol. 2017, 16, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.K.; Yates, E.; Lilly, K.; Dhanda, A.D. Oxidative stress in alcohol-related liver disease. World J. Hepatol. 2020, 12, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Niederreiter, L.; Tilg, H. Cytokines and fatty liver diseases. Liver Res. 2018, 2, 14–20. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarlata, G.G.M.; Paravati, M.R.; Boccuto, L.; Luzza, F.; Scarpellini, E. Gut Microbiota and Liver Transplantation: Immune Mechanisms behind the Rejection. Biomedicines 2023, 11, 1792. [Google Scholar] [CrossRef]
- Cassard, A.M.; Ciocan, D. Microbiota, a key player in alcoholic liver disease. Clin. Mol. Hepatol. 2018, 24, 100–107. [Google Scholar] [CrossRef]
- Yan, AW.; Fouts, D.E.; Brandl, J.; Stärkel, P.; Torralba, M.; Schott, E.; Tsukamoto, H.; Nelson, K.E.; Brenner, D.A.; Schnabl, B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 2011, 53, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.A.; Gillevet, P.M.; Rangwala, H.; Sikaroodi, M.; Naqvi, A.; Engen, P.A.; Kwasny, M.; Lau, C.K.; Keshavarzian, A. Colonic microbiome is altered in alcoholism. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G966–G978. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G. Gut-liver axis in alcoholic liver disease. Gastroenterology 2015, 148, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Zhong, W.; Zheng, X.; Li, Q.; Qiu, Y.; Li, H.; Chen, H.; Zhou, Z.; Jia, W. Chronic ethanol consumption alters mammalian gastrointestinal content metabolites. J. Proteome Res. 2013, 12, 3297–3306. [Google Scholar] [CrossRef]
- Dubinkina, V.B.; Tyakht, A.V.; Odintsova, V.Y.; Yarygin, K.S.; Kovarsky, B.A.; Pavlenko, A.V.; Ischenko, D.S.; Popenko, A.S.; Alexeev, D.G.; Taraskina, A.Y.; et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 2017, 5, 141. [Google Scholar] [CrossRef]
- Michelena, J.; Altamirano, J.; Abraldes, J.G.; Affò, S.; Morales-Ibanez, O.; Sancho-Bru, P.; Dominguez, M.; García-Pagán, J.C.; Fernández, J.; Arroyo, V.; Ginès, P.; et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology 2015, 62, 762–772. [Google Scholar] [CrossRef]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef]
- Mandrekar, P.; Szabo, G. Signalling pathways in alcohol-induced liver inflammation. J. Hepatol. 2009, 50, 1258–1266. [Google Scholar] [CrossRef]
- Shasthry, S.M.; Sarin, S.K. New treatment options for alcoholic hepatitis. World J. Gastroenterol. 2016, 22, 3892–3906. [Google Scholar] [CrossRef]
- Thakur, V.; Pritchard, M.T.; McMullen, M.R.; Wang, Q.; Nagy, L.E. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: Role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J. Leukoc. Biol. 2006, 79, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Affò, S.; Dominguez, M.; Lozano, J.J.; Sancho-Bru, P.; Rodrigo-Torres, D.; Morales-Ibanez, O.; Moreno, M.; Millán, C.; Loaeza-del-Castillo, A.; Altamirano, J.; et al. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut 2013, 62, 452–460. [Google Scholar] [CrossRef]
- Ciećko-Michalska, I.; Szczepanek, M.; Cibor, D.; Owczarek, D.; Skulina, D.; Szczepański, W.; Michalski, M. Zastosowanie oznaczania cytokin w ocenie rokowania u chorych z alkoholowa choroba watroby [Serum cytokine concentration as prognostic factor in patients with alcoholic liver disease]. Przegl Lek. 2006, 63, 249–252. [Google Scholar]
- Gonzalez-Quintela, A.; Campos, J.; Loidi, L.; Quinteiro, C.; Perez, L.F.; Gude, F. Serum TNF-alpha levels in relation to alcohol consumption and common TNF gene polymorphisms. Alcohol. 2008, 42, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, P.; Bala, S.; Catalano, D.; Kodys, K.; Szabo, G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J. Immunol. 2009, 183, 1320–1327. [Google Scholar] [CrossRef]
- Mandrekar, P.; Catalano, D.; White, B.; Szabo, G. Moderate alcohol intake in humans attenuates monocyte inflammatory responses: Inhibition of nuclear regulatory factor kappa B and induction of interleukin 10. Alcohol. Clin. Exp. Res. 2006, 30, 135–139. [Google Scholar] [CrossRef]
- Mookerjee, R.P.; Sen, S.; Davies, N.A.; Hodges, S.J.; Williams, R.; Jalan, R. Tumour necrosis factor alpha is an important mediator of portal and systemic haemodynamic derangements in alcoholic hepatitis. Gut 2003, 52, 1182–1187. [Google Scholar] [CrossRef]
- Diehl, A.M. Cytokine regulation of liver injury and repair. Immunol. Rev. 2000, 174, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Yagi, S.; Hirata, M.; Miyachi, Y.; Uemoto, S. Liver Regeneration after Hepatectomy and Partial Liver Transplantation. Int. J. Mol. Sci. 2020, 21, 8414. [Google Scholar] [CrossRef]
- Jaeschke, H. Neutrophil-mediated tissue injury in alcoholic hepatitis. Alcohol. 2002, 27, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Tsukamoto, H. Inflammation in Alcoholic and Nonalcoholic Fatty Liver Disease: Friend or Foe? Gastroenterology 2016, 150, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Patel, O.P.; Noor, M.T.; Kumar, R.; Thakur, B.S. Serum interleukin 8 and 12 levels predict severity and mortality in patients with alcoholic hepatitis. Indian. J. Gastroenterol. 2015, 34, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Wieser, V.; Adolph, T.E.; Enrich, B.; Kuliopulos, A.; Kaser, A.; Tilg, H.; Kaneider, N.C. Reversal of murine alcoholic steatohepatitis by pepducin-based functional blockade of interleukin-8 receptors. Gut 2017, 66, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Nischalke, H.D.; Berger, C.; Lutz, P.; Langhans, B.; Wolter, F.; Eisenhardt, M.; Krämer, B.; Kokordelis, P.; Glässner, A.; Müller, T.; et al. Influence of the CXCL1 rs4074 A allele on alcohol induced cirrhosis and HCC in patients of European descent. PLoS ONE 2013, 8, e80848. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Xu, M.J.; Zhou, Z.; Cai, Y.; Li, M.; Wang, W.; Feng, D.; Bertola, A.; Wang, H.; Kunos, G.; et al. Short- or long-term high-fat diet feeding plus acute ethanol binge synergistically induce acute liver injury in mice: An important role for CXCL1. Hepatology 2015, 62, 1070–1085. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.S.; Zhang, B.; Loomba, R.; Seki, E. TLR2 and TLR9 contribute to alcohol-mediated liver injury through induction of CXCL1 and neutrophil infiltration. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G30–G41. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Szabo, G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2016, 64, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Knorr, J.; Wree, A.; Tacke, F.; Feldstein, A.E. The NLRP3 Inflammasome in Alcoholic and Nonalcoholic Steatohepatitis. Semin. Liver Dis. 2020, 40, 298–306. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Petrasek, J.; Bala, S.; Csak, T.; Lippai, D.; Kodys, K.; Menashy, V.; Barrieau, M.; Min, S.Y.; Kurt-Jones, E.A.; Szabo, G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Invest. 2012, 122, 3476–3489. [Google Scholar] [CrossRef]
- Cui, K.; Yan, G.; Xu, C.; Chen, Y.; Wang, J.; Zhou, R.; Bai, L.; Lian, Z.; Wei, H.; Sun, R.; et al. Invariant NKT cells promote alcohol-induced steatohepatitis through interleukin-1β in mice. J. Hepatol. 2015, 62, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Voican, C.S.; Njiké-Nakseu, M.; Boujedidi, H.; Barri-Ova, N.; Bouchet-Delbos, L.; Agostini, H.; Maitre, S.; Prévot, S.; Cassard-Doulcier, A.M.; Naveau, S.; et al. Alcohol withdrawal alleviates adipose tissue inflammation in patients with alcoholic liver disease. Liver Int. 2015, 35, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Alegre, F.; Pelegrin, P.; Feldstein, A.E. Inflammasomes in Liver Fibrosis. Semin. Liver Dis. 2017, 37, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. NKT-cell subsets: Promoters and protectors in inflammatory liver disease. J. Hepatol. 2013, 59, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Gao, B.; Zakhari, S.; Nagy, L.E. Inflammation in alcoholic liver disease. Annu. Rev. Nutr. 2012, 32, 343–368. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.; Feng, D.; Maricic, I.; Ju, C.; Kumar, V.; Gao, B. Invariant natural killer T cells contribute to chronic-plus-binge ethanol-mediated liver injury by promoting hepatic neutrophil infiltration. Cell Mol. Immunol. 2016, 13, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Iracheta-Vellve, A.; Petrasek, J.; Gyogyosi, B.; Bala, S.; Csak, T.; Kodys, K.; Szabo, G. Interleukin-1 inhibition facilitates recovery from liver injury and promotes regeneration of hepatocytes in alcoholic hepatitis in mice. Liver Int. 2017, 37, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lafdil, F.; Kong, X.; Gao, B. Signal transducer and activator of transcription 3 in liver diseases: A novel therapeutic target. Int. J. Biol. Sci. 2011, 7, 536–550. [Google Scholar] [CrossRef]
- Ansari, R.A.; Husain, K.; Rizvi, S.A. Role of Transcription Factors in Steatohepatitis and Hypertension after Ethanol: The Epicenter of Metabolism. Biomolecules 2016, 6, 29. [Google Scholar] [CrossRef]
- Hong, F.; Kim, W.H.; Tian, Z.; Jaruga, B.; Ishac, E.; Shen, X.; Gao, B. Elevated interleukin-6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol-induced apoptosis in the liver: Involvement of induction of Bcl-2 and Bcl-x(L) proteins. Oncogene 2002, 21, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tachibana, S.; Wang, H.; Hisada, M.; Williams, G.M.; Gao, B.; Sun, Z. Interleukin-6 is an important mediator for mitochondrial DNA repair after alcoholic liver injury in mice. Hepatology 2010, 52, 2137–2147. [Google Scholar] [CrossRef]
- Kawaratani, H.; Moriya, K.; Namisaki, T.; Uejima, M.; Kitade, M.; Takeda, K.; Okura, Y.; Kaji, K.; Takaya, H.; Nishimura, N.; et al. Therapeutic strategies for alcoholic liver disease: Focusing on inflammation and fibrosis (Review). Int. J. Mol. Med. 2017, 40, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Klein, A.S.; Radaeva, S.; Hong, F.; El-Assal, O.; Pan, H.N.; Jaruga, B.; Batkai, S.; Hoshino, S.; Tian, Z.; et al. In vitro interleukin-6 treatment prevents mortality associated with fatty liver transplants in rats. Gastroenterology 2003, 125, 202–215. [Google Scholar] [CrossRef] [PubMed]
- El-Assal, O.; Hong, F.; Kim, W.H.; Radaeva, S.; Gao, B. IL-6-deficient mice are susceptible to ethanol-induced hepatic steatosis: IL-6 protects against ethanol-induced oxidative stress and mitochondrial permeability transition in the liver. Cell Mol. Immunol. 2004, 1, 205–211. [Google Scholar] [PubMed]
- Miller, A.M.; Wang, H.; Bertola, A.; Park, O.; Horiguchi, N.; Ki, SH.; Yin, S.; Lafdil, F.; Gao, B. Inflammation-associated interleukin-6/signal transducer and activator of transcription 3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in interleukin-10-deficient mice. Hepatology 2011, 54, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.S.; Suh, Y.G.; Yi, H.S.; Lee, Y.S.; Jeong, W.I. Activation of toll-like receptor 3 attenuates alcoholic liver injury by stimulating Kupffer cells and stellate cells to produce interleukin-10 in mice. J. Hepatol. 2013, 58, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Wang, H.; Park, O.; Wei, W.; Shen, J.; Gao, B. Enhanced liver regeneration in IL-10-deficient mice after partial hepatectomy via stimulating inflammatory response and activating hepatocyte STAT3. Am. J. Pathol. 2011, 178, 1614–1621. [Google Scholar] [CrossRef]
- Yang, A.M.; Wen, L.L.; Yang, C.S.; Wang, S.C.; Chen, C.S.; Bair, M.J. Interleukin 10 promoter haplotype is associated with alcoholic liver cirrhosis in Taiwanese patients. Kaohsiung J. Med. Sci. 2014, 30, 291–298. [Google Scholar] [CrossRef]
- Dudakov, J.A.; Hanash, A.M.; van den Brink, M.R. Interleukin-22: Immunobiology and pathology. Annu. Rev. Immunol. 2015, 33, 747–785. [Google Scholar] [CrossRef]
- Gao, B.; Xiang, X. Interleukin-22 from bench to bedside: A promising drug for epithelial repair. Cell Mol. Immunol. 2019, 16, 666–667. [Google Scholar] [CrossRef] [PubMed]
- Park, O.; Wang, H.; Weng, H.; Feigenbaum, L.; Li, H.; Yin, S.; Ki, S.H.; Yoo, S.H.; Dooley, S.; Wang, F.S.; et al. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology 2011, 54, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.X.; Zhao, R.; Huo, L.J. Interleukin-22 alleviates alcohol-associated hepatic fibrosis, inhibits autophagy, and suppresses the PI3K/AKT/mTOR pathway in mice. Alcohol. Clin. Exp. Res. (Hoboken) 2023, 47, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Sagaram, M.; Frimodig, J.; Jayanty, D.; Hu, H.; Royer, A.J.; Bruner, R.; Kong, M.; Schwandt, M.L.; Vatsalya, V. One-month assessment of Th-cell axis related inflammatory cytokines, IL-17 and IL-22 and their role in alcohol-associated liver disease. Front. Immunol. 2023, 14, 1202267. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, J.; Li, Q.; Liang, Y.; Gao, J.; Meng, Z.; Li, P.; Yao, M.; Gu, J.; Tu, H.; et al. Environmental eustress promotes liver regeneration through the sympathetic regulation of type 1 innate lymphoid cells to increase IL-22 in mice. Hepatology 2023, 78, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Hendrikx, T.; Duan, Y.; Wang, Y.; Oh, J.H.; Alexander, L.M.; Huang, W.; Stärkel, P.; Ho, S.B.; Gao, B.; Fiehn, O.; et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut 2019, 68, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, J.; Aiglová, K.; Urban, O.; Cveková, S.; Dvoran, P. Alcohol-related liver diseases (ALD). Vnitr. Lek. 2020, 66, 39–51. [Google Scholar] [CrossRef]
- Burnette, E.M.; Nieto, S.J.; Grodin, E.N.; Meredith, L.R.; Hurley, B.; Miotto, K.; Gillis, A.J.; Ray, L.A. Novel Agents for the Pharmacological Treatment of Alcohol Use Disorder. Drugs 2022, 82, 251–274. [Google Scholar] [CrossRef]
- Leggio, L.; Litten, R.Z. The GABA-B receptor agonist baclofen helps patients with alcohol use disorder: Why these findings matter. Neuropsychopharmacology 2021, 46, 2228–2229. [Google Scholar] [CrossRef]
- Lieber, S.R.; Rice, J.P.; Lucey, M.R.; Bataller, R. Controversies in clinical trials for alcoholic hepatitis. J. Hepatol. 2018, 68, 586–592. [Google Scholar] [CrossRef]
- Louvet, A.; Thursz, M.R.; Kim, D.J.; Labreuche, J.; Atkinson, S.R.; Sidhu, S.S.; O’Grady, J.G.; Akriviadis, E.; Sinakos, E.; Carithers, R.L. Jr.; et al. Corticosteroids Reduce Risk of Death Within 28 Days for Patients With Severe Alcoholic Hepatitis, Compared With Pentoxifylline or Placebo-a Meta-analysis of Individual Data From Controlled Trials. Gastroenterology 2018, 155, 458–468. [Google Scholar] [CrossRef]
- Dao, A.; Rangnekar, A.S. Steroids for Severe Alcoholic Hepatitis: More Risk Than Reward? Clin Liver Dis (Hoboken) 2019, 12, 151–153. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, H.J.; Kim, J.H.; Yoo, Y.J.; Kim, T.S.; Kang, S.H.; Suh, S.J.; Joo, M.K.; Jung, Y.K.; Lee, B.J.; et al. Treatment of Severe Alcoholic Hepatitis With Corticosteroid, Pentoxifylline, or Dual Therapy: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2017, 51, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Thursz, M.R.; Richardson, P.; Allison, M.; Austin, A.; Bowers, M.; Day, C.P.; Downs, N.; Gleeson, D.; MacGilchrist, A.; Grant, A.; et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N. Engl. J. Med. 2015, 372, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Naveau, S.; Chollet-Martin, S.; Dharancy, S.; Mathurin, P.; Jouet, P.; Piquet, M.A.; Davion, T.; Oberti, F.; Broët, P.; Emilie, D.; et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004, 39, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, A.; Sharma, B.C.; Sarin, S.K. Infliximab monotherapy for severe alcoholic hepatitis and predictors of survival: An open label trial. J. Hepatol. 2009, 50, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Mackowiak, B.; Fu, Y.; Maccioni, L.; Gao, B. Alcohol-associated liver disease. J. Clin. Invest. 2024, 134, e176345. [Google Scholar] [CrossRef] [PubMed]
- Boetticher, N.C.; Peine, C.J.; Kwo, P.; Abrams, G.A.; Patel, T.; Aqel, B.; Boardman, L.; Gores, G.J.; Harmsen, W.S.; McClain, C.J.; et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology 2008, 135, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, P.; Bachmann, M.; Goren, I.; Zwissler, B.; Pfeilschifter, J.; Mühl, H. Application of interleukin-22 mediates protection in experimental acetaminophen-induced acute liver injury. Am. J. Pathol. 2013, 182, 1107–1113. [Google Scholar] [CrossRef]
- Xiang, X.; Feng, D.; Hwang, S.; Ren, T.; Wang, X.; Trojnar, E.; Matyas, C.; Mo, R.; Shang, D.; He, Y.; et al. Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming impaired regeneration pathways in mice. J. Hepatol. 2020, 72, 736–745. [Google Scholar] [CrossRef]
- Tang, K.Y.; Lickliter, J.; Huang, Z.H.; Xian, Z.S.; Chen, H.Y.; Huang, C.; Xiao, C.; Wang, Y.P.; Tan, Y.; Xu, L.F.; et al. Safety, pharmacokinetics, and biomarkers of F-652, a recombinant human interleukin-22 dimer, in healthy subjects. Cell Mol. Immunol. 2019, 16, 473–482. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; Wang, Y.; Lekkerkerker, A.; Danilenko, D.M.; Maciuca, R.; Erickson, R.; Herman, A.; Stefanich, E.; Lu, T.T. Randomized Phase I Healthy Volunteer Study of UTTR1147A (IL-22Fc): A Potential Therapy for Epithelial Injury. Clin. Pharmacol. Ther. 2019, 105, 177–189. [Google Scholar] [CrossRef]
- Arab, J.P.; Sehrawat, T.S.; Simonetto, D.A.; Verma, V.K.; Feng, D.; Tang, T.; Dreyer, K.; Yan, X.; Daley, W.L.; Sanyal, A.; et al. An Open-Label, Dose-Escalation Study to Assess the Safety and Efficacy of IL-22 Agonist F-652 in Patients With Alcohol-associated Hepatitis. Hepatology 2020, 72, 441–453. [Google Scholar] [CrossRef]
- Jiang, R.; Tan, Z.; Deng, L.; Chen, Y.; Xia, Y.; Gao, Y.; Wang, X.; Sun, B. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology 2011, 54, 900–909. [Google Scholar] [CrossRef]
- Park, O.; Wang, H.; Weng, H.; Feigenbaum, L.; Li, H.; Yin, S.; Ki, S.H.; Yoo, S.H.; Dooley, S.; Wang, F.S.; et al. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology 2011, 54, 252–261. [Google Scholar] [CrossRef]
- Vergis, N.; Patel, V.; Bogdanowicz, K.; Czyzewska-Khan, J.; Fiorentino, F.; Day, E.; Cross, M.; Foster, N.; Lord, E.; Goldin, R.; et al. IL-1 Signal Inhibition In Alcoholic Hepatitis (ISAIAH): A study protocol for a multicentre, randomised, placebo-controlled trial to explore the potential benefits of canakinumab in the treatment of alcoholic hepatitis. Trials 2021, 22, 792. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Gawrieh, S.; Dasarathy, S.; Mitchell, M.C.; Simonetto, D.A.; Patidar, K.R.; McClain, C.J.; Bataller, R.; Szabo, G.; Tang, Q.; et al. Design of a multicenter randomized clinical trial for treatment of Alcohol-Associated Hepatitis. Contemp. Clin. Trials Commun. 2023, 32, 101074. [Google Scholar] [CrossRef] [PubMed]
- Urien, S.; Bardin, C.; Bader-Meunier, B.; Mouy, R.; Compeyrot-Lacassagne, S.; Foissac, F.; Florkin, B.; Wouters, C.; Neven, B.; Treluyer, J.M.; et al. Anakinra pharmacokinetics in children and adolescents with systemic-onset juvenile idiopathic arthritis and autoinflammatory syndromes. BMC Pharmacol. Toxicol. 2013, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Z.; Bai, F. Roles of Gut Microbiota in Alcoholic Liver Disease. Int. J. Gen. Med. 2023, 16, 3735–3746. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, S.; Huo, J.; Dong, K.; Ding, Y.; Man, C.; Zhang, Y.; Jiang, Y. Lactobacillus plantarum J26 Alleviating Alcohol-Induced Liver Inflammation by Maintaining the Intestinal Barrier and Regulating MAPK Signaling Pathways. Nutrients 2022, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Cao, F.; Lai, S.; Zhuge, H.; Chang, K.; Valencak, T.G.; Liu, J.; Li, S.; Ren, D. Lactobacillus plantarum ZY08 relieves chronic alcohol-induced hepatic steatosis and liver injury in mice via restoring intestinal flora homeostasis. Food Res. Int. 2022, 157, 111259. [Google Scholar] [CrossRef]
- Vatsalya, V.; Feng, W.; Kong, M.; Hu, H.; Szabo, G.; McCullough, A.; Dasarathy, S.; Nagy, L.E.; Radaeva, S.; Barton, B.; et al. The Beneficial Effects of Lactobacillus GG Therapy on Liver and Drinking Assessments in Patients with Moderate Alcohol-Associated Hepatitis. Am. J. Gastroenterol. 2023, 118, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.S.; Dusseja, A.; Shalimar; Nijhawan, S.; Kapoor, D.; Goyal, O.; Kishore, H. A multicenter double-blind, placebo-controlled randomized trial to evaluate the safety and efficacy of bovine colostrum in the treatment of severe alcoholic hepatitis (SAH). Trials 2023, 24, 515. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Scarlata, G.G.; Scarpellini, E.; Procopio, A.C.; Ponziani, F.R.; Boccuto, L.; Cetkovic, N.; Luzza, F. Therapeutic success in primary biliary cholangitis and gut microbiota: A safe highway? Minerva Gastroenterol (Torino) 2024. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Montori, M.; Svegliati Baroni, G.; Argenziano, M.E.; Giorgi, F.; Scarlata, G.G.M.; Ponziani, F.; Scarpellini, E. Perspective on the Role of Gut Microbiome in the Treatment of Hepatocellular Carcinoma with Immune Checkpoint Inhibitors. Medicina (Kaunas) 2023, 59, 1427. [Google Scholar] [CrossRef]
| Cytokine evaluated | Reference | Study design | Outcome |
|---|---|---|---|
| TNF-α | Thakur et al., 2006 [53] | Pre-clinical study | Reduction of ERK1/2 activation and inhibition of NOX4 resulting in lower ROS and TNF-α production after the use of diphenyl-iodonium and dilinoleoyl-phosphatidylcholine in Kupffer cells of mice models |
| Ciećko-Michalska et al., 2006 [55] | Case-control study | Higher concentrations of TNF-α in ALD patients were correlated with poor prognosis | |
| Mandrekar et al., 2006 [58] | Pre-clinical study | Significant reduction in monocyte production of TNF-α in response to LPS or staphylococcal enterotoxin B stimulation eighteen hours after moderate alcohol consumption | |
| Gonzales-Quintela et al., 2008 [56] | Case-control study | TNF-α levels were almost similar in the general population, in teetotalers and alcohol drinkers, while elevated in chronic alcoholics | |
| Affò et al., 2013 [54] | Translational study | TNF superfamily receptors are overexpressed in AH humans and mice models | |
| IL-8 | Patel et al., 2015 [64] | Case-control study | Serum IL-8 predicts severity and mortality in patients with AH |
| Wieser et al., 2017 [65] | Pre-clinical study | Blockade of IL-8 receptors with pepducin reduced liver inflammation, weight loss and mortality associated with ALD in mice models | |
| CXCL1 | Nischalke et al., 2013 [66] | Case-control study | CXCL1 rs4074 single nucleotide polymorphism was associated with increased blood levels of CXCL1 and an increased risk of developing liver cirrhosis and HCC |
| Chang et al., 2015 [67] | Pre-clinical study | Mice models treated with a high-fat and ethanol-rich diet markedly upregulated the hepatic expression of CXCL1, with a reduction of the infiltration and hepatic damage of neutrophils | |
| Roh et al., 2015 [68] | Pre-clinical study | Ethanol-treated wild-type mice showed an increased hepatic expression of CXCL1 and serum level of CXCL1, while TLR2- and TLR9-deficient mice showed significantly lower levels | |
| IL-1β | Petrasek et al., 2012 [72] | Pre-clinical study | IL-1β levels were significantly increased in the liver of chronic alcohol-fed mice compared to controls |
| Cui et al., 2015 [73] | Pre-clinical study | Up-regulation of NLRP3 inflammasome components and IL-1β in Kupffer cells of ethanol-fed mice | |
| Voican et al., 2015 [74] | Prospective cohort study | Significantly increased levels of IL-1β in ALD patients after one week of alcohol withdrawal | |
| Mathews et al., 2016 [78] | Pre-clinical study | A loss of function of IL-1β protected mice models from hepatic infiltration of neutrophils and liver damage induced by chronic ethanol binges |
| Cytokine evaluated | Reference | Study design | Outcome |
|---|---|---|---|
| IL-6 | Sun et al., 2003 [85] | Pre-clinical study | Pre-treatment with IL-6 induces hepatoprotection of steatotic liver isotransplants by preventing the apoptosis of sinusoidal endothelial cells, improving hepatic microcirculation and protecting against hepatocyte death in mice models |
| El-Assal et al., 2004 [86] | Pre-clinical study | IL-6 knockout mice fed with alcohol exhibited increased liver fat accumulation, lipid peroxidation, mitochondrial DNA damage and hepatocyte sensitization to TNFα-induced apoptosis | |
| Zhang et al., 2010 [83] | Pre-clinical study | IL-6 activates enzymes to repair mitochondrial DNA in hepatocytes damaged by chronic alcohol consumption in mice models | |
| Miller et al., 2011 [87] | Pre-clinical study | IL-10 knockout mice showed a more severe hepatic inflammatory response with higher levels of IL-6 and STAT3 activation, compared to wild-type mice, but lower liver steatosis severity and hepatocellular damage after higher alcohol levels or fatty dietary regimen | |
| IL-10 | Byun et al., 2013 [88] | Pre-clinical study | Treatment with polyinosinic:polycytidylic acid in vitro stimulates IL-10 production in HSCs through TLR-3 activation with reduction of alcoholic liver damage |
| Yang et al., 2014 [90] | Case-control study | IL-10 promoter polymorphisms allow the development of severe forms of alcoholic liver cirrhosis | |
| IL-22 | Meng et al., 2023 [94] | Pre-clinical study | Four-week treatment with IL-22 reduced liver fibrosis in mice models with ALD |
| Sagaram et al., 2023 [95] | Prospective cohort study | IL-22 levels were significantly increased in alcohol-associated non-severe hepatitis and alcohol-associated severe hepatitis patients compared to controls | |
| Liu et al., 2023 [96] | Pre-clinical study | IL-22 levels were significantly increased in murine models following liver regeneration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





