Submitted:
01 March 2024
Posted:
01 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Aqueous Polymer Solutions and Rheology Study
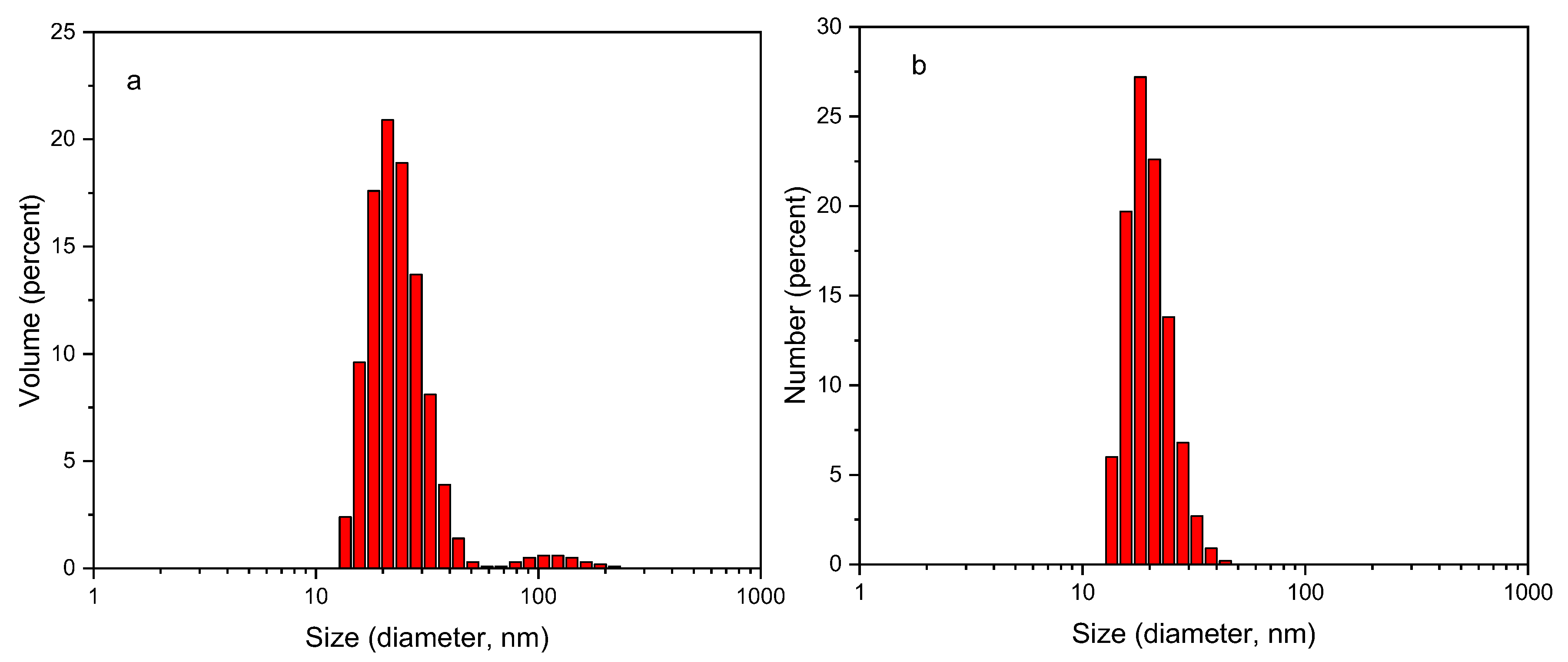
2.3. Loading and Release of Nile Red from P2VP-b-PEO micelles
2.4. Release of Nile Red from ALG-g-HG/P2VP-b-PEO hydrogel Composite System
2.5. Techniques
3. Results and Discussion
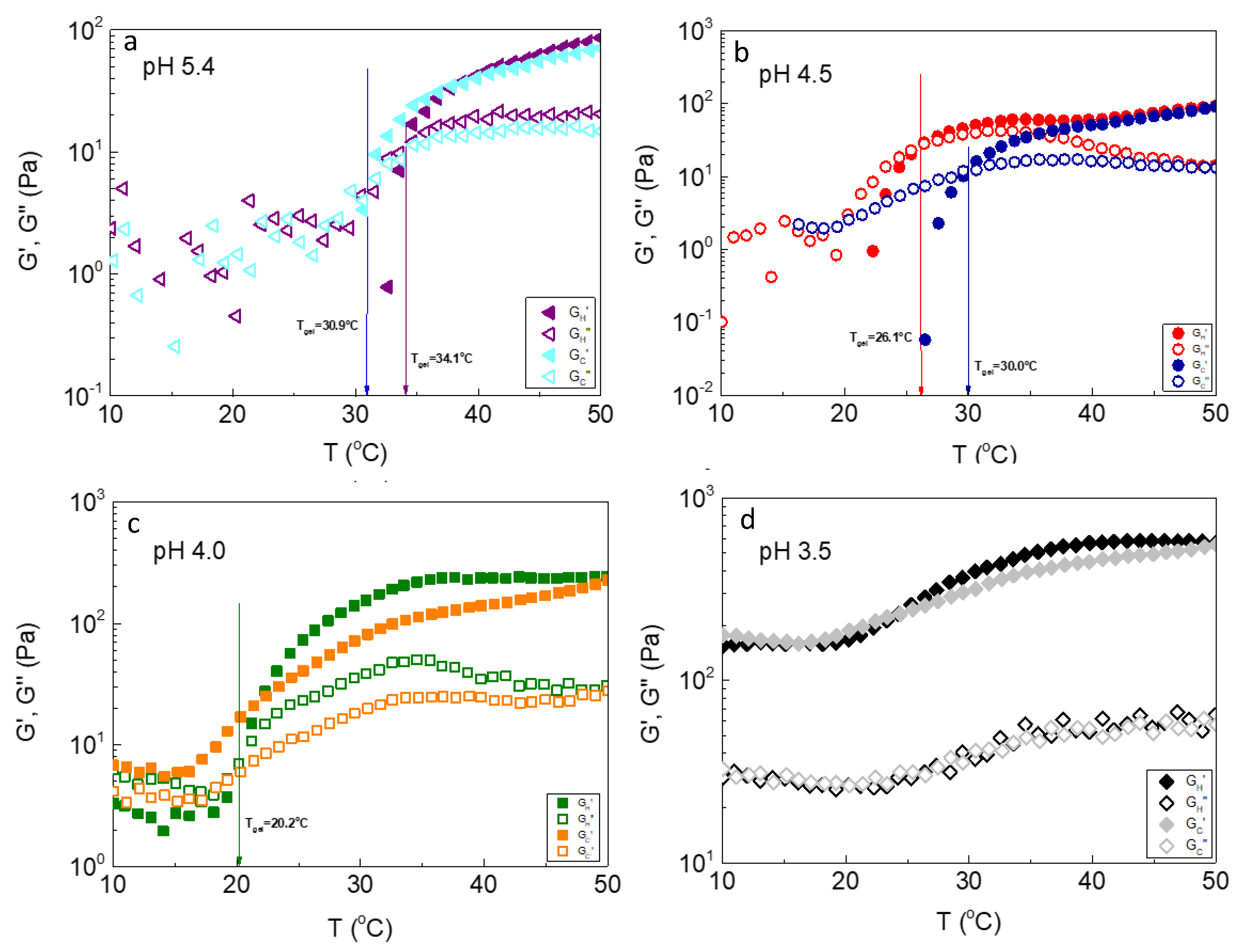
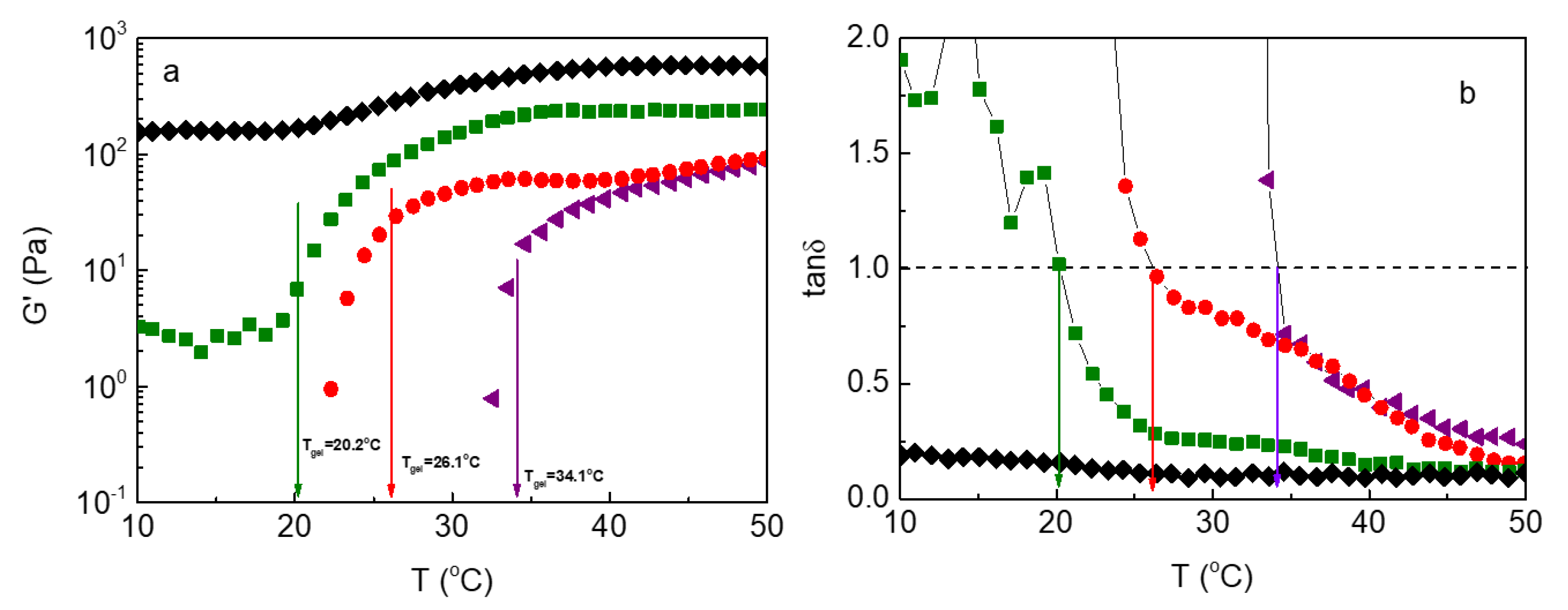
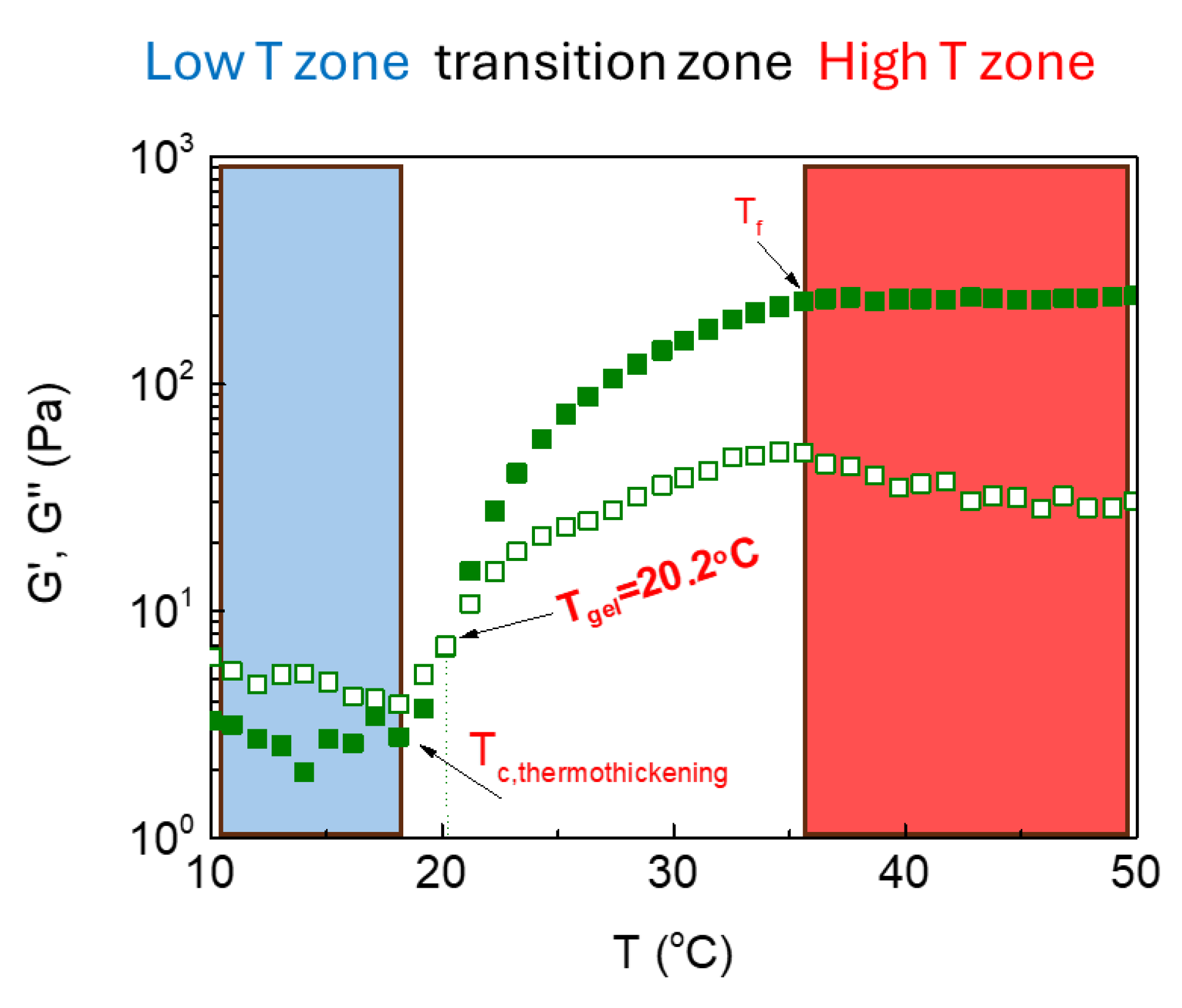
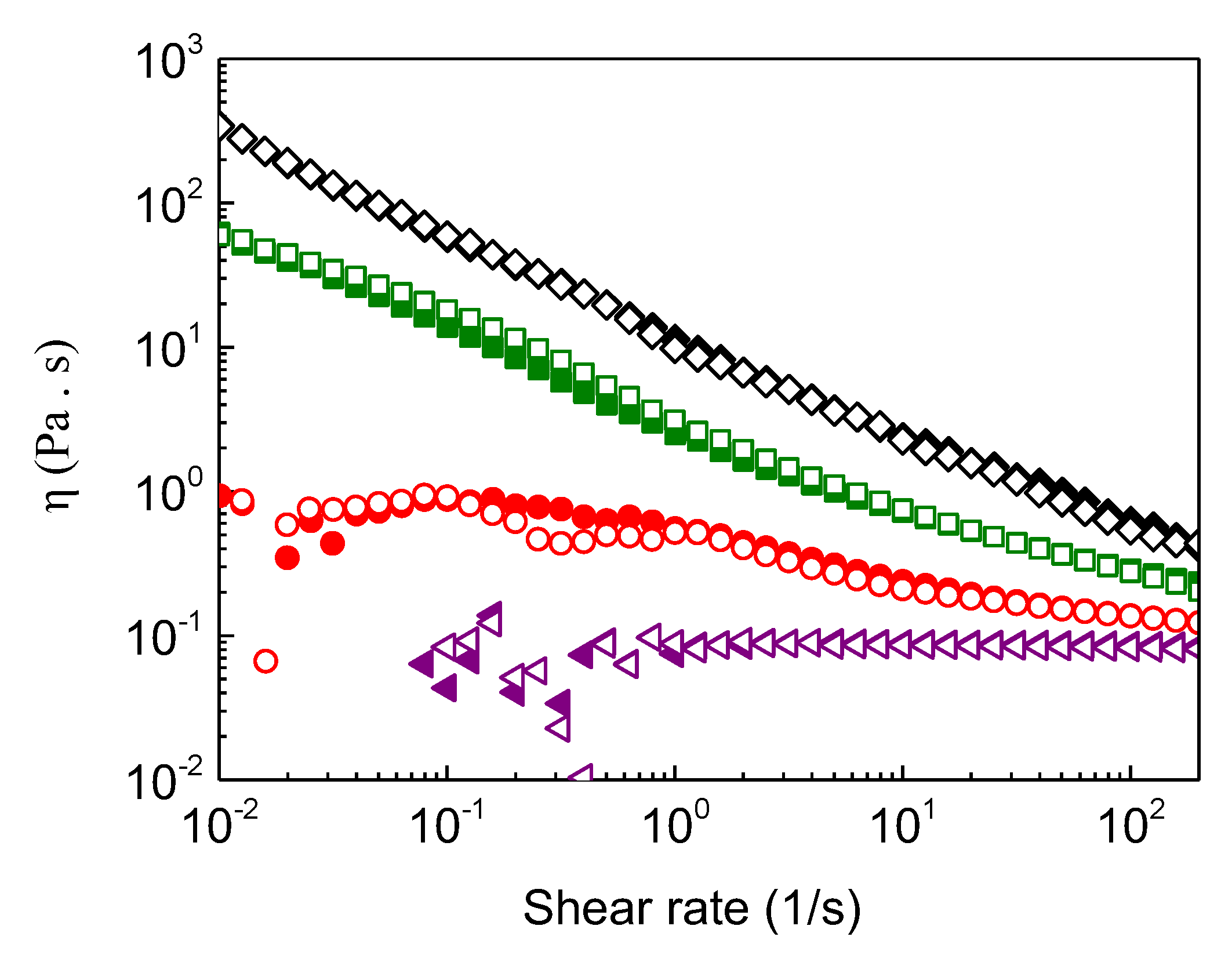
3.1. Rheological investigation at various pH
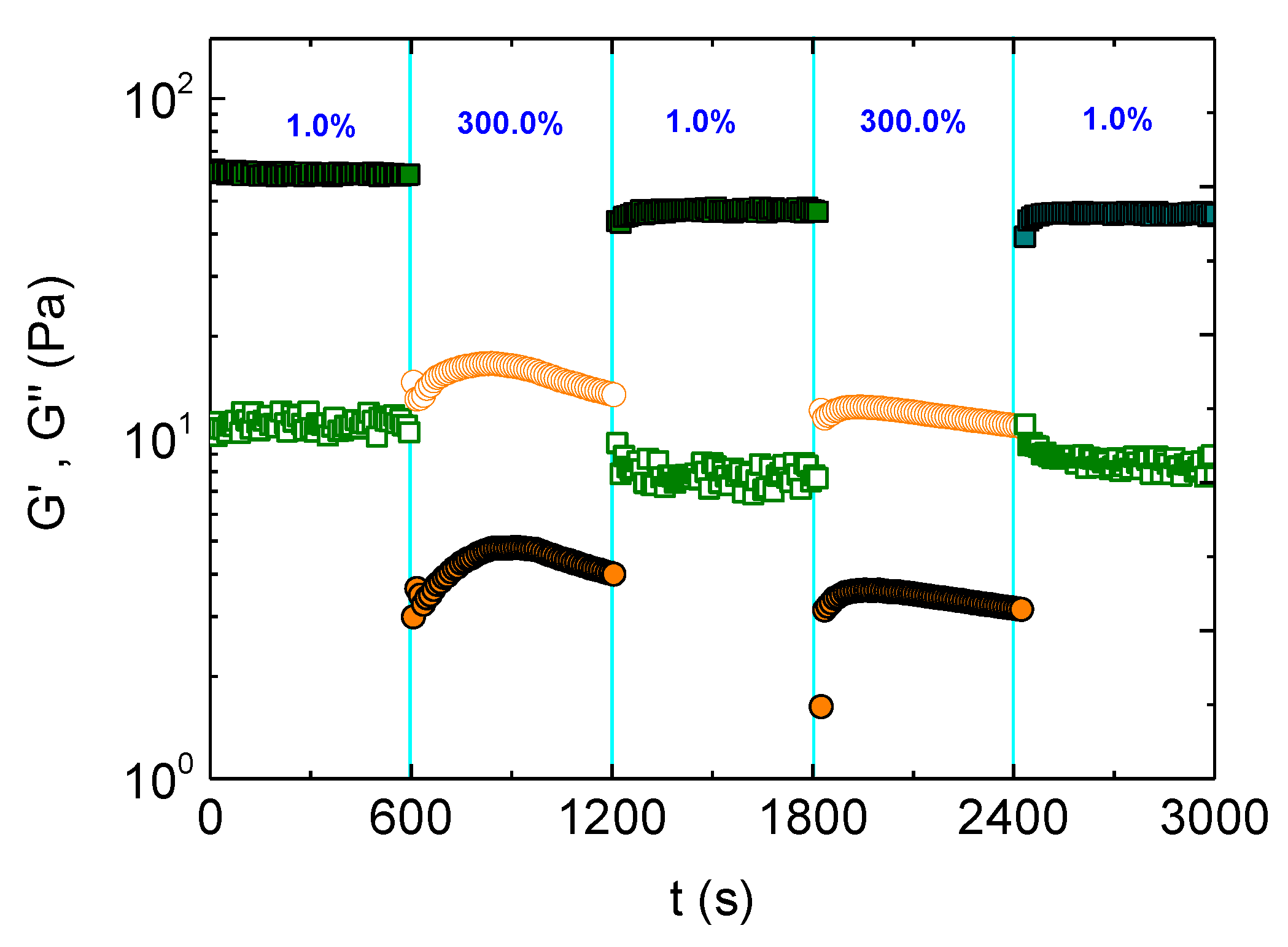
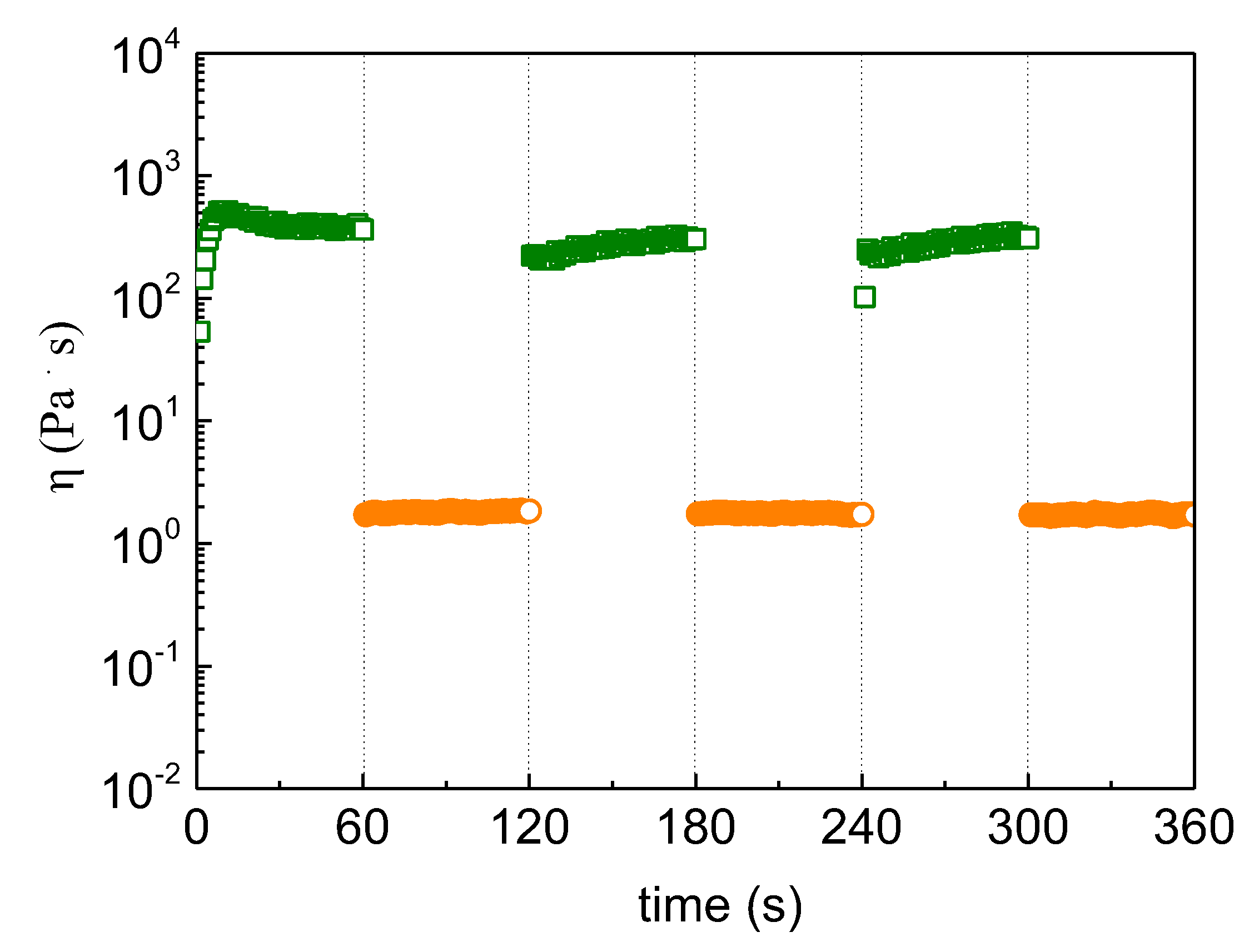
3.2. Self-healing and Injectability
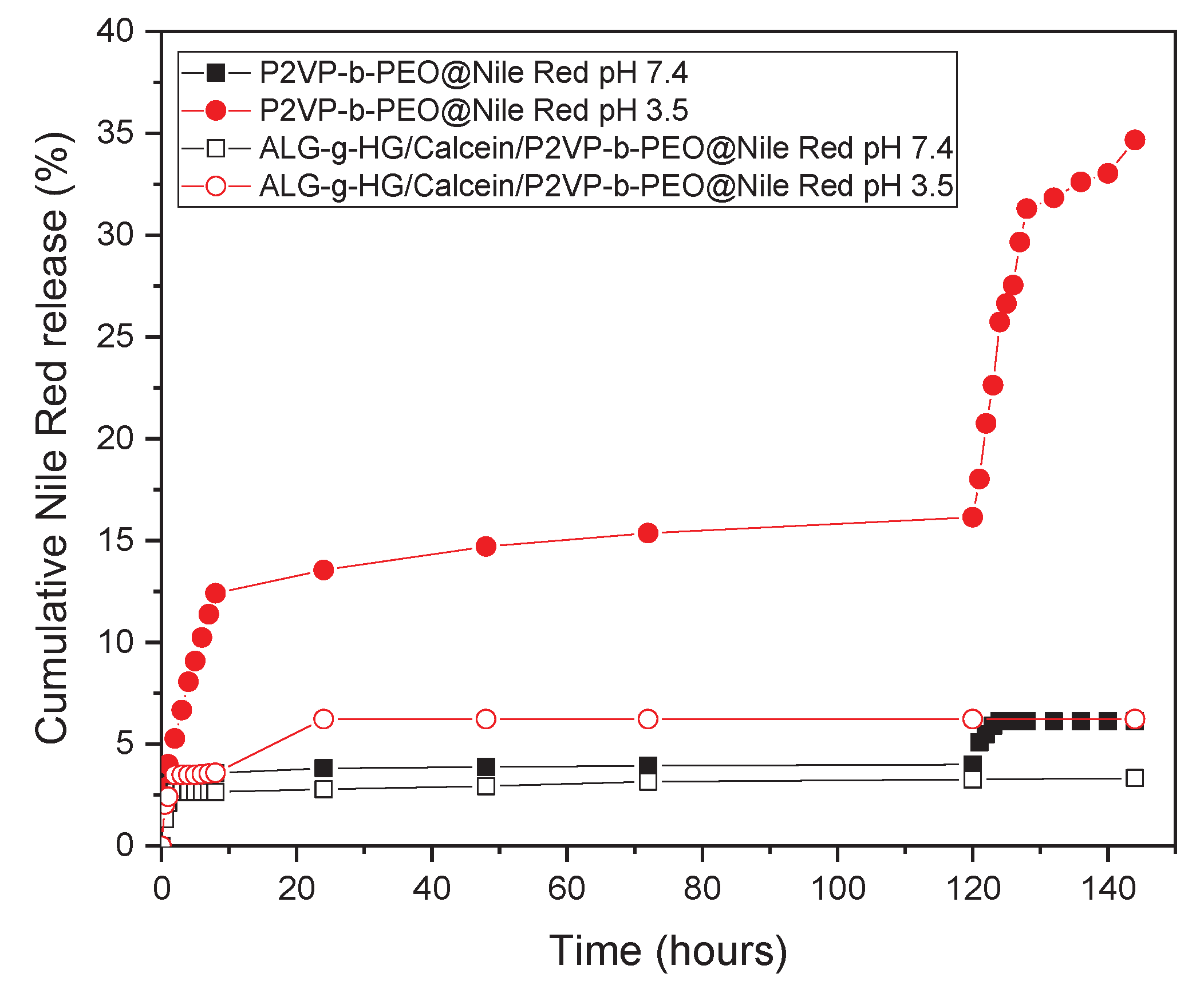
3.3. Evaluation as drug delivery system
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chassenieux, C.; Tsitsilianis, C. Recent trends on pH/thermo-responsive self assembling hydrogels: From polyions to peptide-based polymeric gelators. Soft Matter 2016, 12, 1344–1359. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Choudhury, A.R. Stimuli-Responsive Polysaccharide-Based Smart Hydrogels and Their Emerging Applications. Ind. Eng. Chem. Res. 2023, 62, 841–866. [Google Scholar] [CrossRef]
- Trivedi, J.; Chourasia, A. Sodium Salt of Partially Carboxymethylated Sodium Alginate-Graft-Poly(Acrylonitrile): II Superabsorbency, Salt Sensitivity and Swelling Kinetics of Hydrogel, H-Na-PCMSA-g-PAN. Gels 2023, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Lupu, A.; Gradinaru, L.M.; Rusu, D.; Bercea, M. Self-Healing of Pluronic® F127 Hydrogels in the Presence of Various Polysaccharides. Gels 2023, 9, 719. [Google Scholar] [CrossRef] [PubMed]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Engin. R 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Manna, K.; Pal, S. Recent Advances in Various Stimuli-Responsive Hydrogels: From Synthetic Designs to Emerging Healthcare Applications. Mater. Chem. Front. 2022, 6, 2338–2385. [Google Scholar] [CrossRef]
- Afzal, S.; Maswal, M.; Ahmad Dar, A. Rheological behavior of pH responsive composite hydrogels ofchitosan and alginate: Characterization and its use in encapsulation of citral. Colloids Surf. B Biointerfaces 2018, 169, 99–106. [Google Scholar] [CrossRef]
- Diego, S.; Lima, D.S.; Tenório-Neto, E.T.; Lima-Tenório, M.K.; Guilherme, M.R.; Scariot, D.B.; Nakamura, C.V.; Muniz, E.C.; Rubira, A.F. pH-responsive alginate-based hydrogels for protein delivery. J. Mol. Liq. 2018, 262, 29–36. [Google Scholar] [CrossRef]
- Lin, X.; Guan, X.; Wu, Y.; Zhuang, S.; Wu, Y.; Du, L.; Zhao, J.; Rong, J.; Zhao, J.; Tu, M. An Alginate/Poly(N-Isopropylacrylamide)-Based Composite Hydrogel Dressing with Stepwise Delivery of Drug and Growth Factor for Wound Repair. Mater. Sci. Eng. C 2020, 115, 111123. [Google Scholar] [CrossRef]
- Ilgin, P.; Ozay, H.; Ozay, O. Synthesis and characterization of pH responsive alginate based-hydrogels as oral drug delivery carrier. J. Polym. Res. 2020, 27, 251. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chi-leung Hui, P. Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers 2021, 13, 2086. [Google Scholar] [CrossRef] [PubMed]
- Santhamoorthy, M.; Vy Phan, T.T.; Ramkumar, V.; Raorane, C.J.; Thirupathi, K.; Kim, S.-C. Thermo-sensitive Poly (N-Isopropylacrylamide-Co-Polyacrylamide) Hydrogel for PH-Responsive Therapeutic Delivery. Polymers 2022, 14, 4128. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.; Gani, A. Alginate-Based pH-Sensitive Hydrogels Encoated with Chitosan as a Bioactive Cargo Carrier with Caffeic Acid as a Model Biomolecule. ACS Food Sci. Technol. 2022, 2, 667–672. [Google Scholar] [CrossRef]
- Suhail, M.; Ullah, H.; Vu, Q.L.; Khan, A.; Tsai, M.-J.; Wu, P.-C. Preparation of pH-Responsive Hydrogels Based on Chondroitin Sulfate/Alginate for Oral Drug Delivery. Pharmaceutics 2022, 14, 2110. [Google Scholar] [CrossRef]
- Peng, X.; Peng, Q.; Wu, M.; Wang, W.; Gao, Y.; Liu, X.; Sun, Y.; Yang, D.; Peng, Q.; Wang, T.; Chen, X.-Z.; Liu, J.; Zhang, H.; Zeng, H. A PH and Temperature Dual-Responsive Microgel-Embedded, Adhesive, and Tough Hydrogel for Drug Delivery and Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 19560–19573. [Google Scholar] [CrossRef] [PubMed]
- Aleena Mir, A.; Kumar, A.; Alam, J.; Riaz, U. Synthesis and characterization of pH-responsive conducting polymer/ Na-alginate/gelatin based composite hydrogels for sustained release of amoxicillin drug. Int. J. Biol. Macromol. 2023, 252, 126015. [Google Scholar] [CrossRef]
- Murab, S.; Gupta, A.; Włodarczyk-Biegun, M.K.; Kumar, A.; van Rijn, P.; Whitlock, P.; Han, S.S.; Agrawal, G. Alginate Based Hydrogel Inks for 3D Bioprinting of Engineered Orthopedic Tissues. Carbohydr. Polym. 2022, 296, 119964. [Google Scholar] [CrossRef]
- Saravanou, S.F.; Ioannidis, K.; Dimopoulos, A.; Paxinou, A.; Kounelaki, F.; Varsami, S.M.; Tsitsilianis, C.; Papantoniou, I.; Pasparakis, G. Dually Crosslinked Injectable Alginate-Based Graft Copolymer Thermoresponsive Hydrogels as 3D Printing Bioinks for Cell Spheroid Growth and Release. Carbohydr. Polym. 2023, 312, 120790. [Google Scholar] [CrossRef]
- Saravanou, S.F.; Tsitsilianis, C.; Pasparakis, G. Harnessing the Interplay of Triple Cross-Linked Hydrogels toward Multiresponsive Alginate-Based Injectable Gels for 3D Printing Bioapplications. ACS Macro Lett. 2023, 12, 1614–1622. [Google Scholar] [CrossRef]
- Gialouri, A.; Saravanou, S.F.; Loukelis, K.; Chatzinikolaidou, M.; Pasparakis, G.; Bouropoulos, N. Thermoresponsive Alginate-GraftpNIPAM/Methyl Cellulose 3D-Printed Scaffolds Promote Osteogenesis In Vitro. Gels 2023, 9, 984. [Google Scholar] [CrossRef]
- Knipe, J.M.; Peppas, N.A. Multi-Responsive Hydrogels for Drug Delivery and Tissue Engineering Applications. Regen. Biomater. 2014, 1, 57–65. [Google Scholar] [CrossRef]
- Kumar, A.; I Matari, I.A.; Han, S.S. 3D Printable Carboxylated Cellulose Nanocrystal-Reinforced Hydrogel Inks for Tissue Engineering. Biofabrication 2020, 12, 025029. [Google Scholar] [CrossRef]
- Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P. Injectable thermoresponsive hydrogels as drug delivery system for the treatment of central nervous system disorders: A review. J. Control. Release 2021, 329, 16–35. [Google Scholar] [CrossRef]
- Ghandforoushan, P.; Alehosseini, M.; Golafshan, N.; Castilho, M.; Dolatshahi-Pirouz, A.; Hanaee, J.; Davaran, S.; Orive, G. Injectable hydrogels for cartilage and bone tissue regeneration: A review. Int. J. Biol. Macromol. 2023, 246, 125674. [Google Scholar] [CrossRef]
- Qian Huang, Q.; Zhou1, Y.; Fu, Z.; Zhu, J. Preparation of an injectable hydrogel reinforced by graphene oxide and its application in dye wastewater treatment. J. Mater. Sci. 2023, 58, 3117–3133. [Google Scholar] [CrossRef]
- Tanga, S.; Aucamp, M.; Ramburrun, P. Injectable Thermoresponsive Hydrogels for Cancer Therapy: Challenges and Prospects. Gels 2023, 9, 418. [Google Scholar] [CrossRef]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, W.; Li, P.; Liao, Q.; Xia, A.; Zhu, X.; Zhu, X. Poly(N-isopropylacrylamide-co-N-tert-butylacrylamide)-graph-polydopamine as a Thermoresponsive Surface with Adjustable Transformation Temperature for Efficient Microalgal Cell Adhesion and Detachment. ACS Appl. Polym. Mater. 2022, 4, 8180–8192. [Google Scholar] [CrossRef]
- Barbier, L.; Protat, M.; Pipart, P.; Marcellan, A.; Tran, Y.; Hourdet, D. Sol/gel transition of thermoresponsive Hyaluronan: From liquids to elastic and sticky materials. Carbohydr. Polym. 2023, 310, 120715. [Google Scholar] [CrossRef]
- Eskens, O.; Villani, G.; Amin, S. Rheological Investigation of Thermoresponsive Alginate-Methylcellulose Gels for Epidermal Growth Factor Formulation. Cosmetics 2021, 8, 3. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution Properties of Poly(N–isopropylacrylamide). J. Macromol. Sci. Part A – Chem. 1969, 2, 1441–1455. [Google Scholar] [CrossRef]
- Iatridi, Z.; Saravanou, S.F.; Tsitsilianis, C. Injectable self-assembling hydrogel from alginate grafted by P(N-isopropylacrylamide-co-N-tert-butylacrylamide) random copolymers. Carbohydr. Polym. 2019, 219, 344–352. [Google Scholar] [CrossRef]
- Safakas, K.; Saravanou, S.-F.; Iatridi, Z.; Tsitsilianis, C. Alginate-g-PNIPAM-based Thermo/shear-responsive Injectable Hydrogels: Tailoring the Rheological Properties by Adjusting the LCST of the Grafting Chains. Int. J. Mol. Sci. 2021, 22, 3824. [Google Scholar] [CrossRef]
- Durand, A.; Hourdet, D. Thermoassociative graft copolymers based on poly(N-isopropylacrylamide): Effect of added co-solutes on the rheological behaviour. Polymer 2000, 41, 545–557. [Google Scholar] [CrossRef]
- Leal, D.; De Borggraeve, W.; Encinas, M.V.; Matsuhiro, B.; Muller, R. Preparation and characterization of hydrogels based on homopolymeric fractions of sodium alginate and PNIPAAm. Carbohydr. Polym. 2013, 92, 157–166. [Google Scholar] [CrossRef]
- Martinez-Gomez, F.; Encinas, M.V.; Matsuhiro, B.; Pavez, J. Preparation and swelling properties of homopolymeric alginic acid fractions/poly(N-isopropyl acrylamide) graft copolymers. J. Appl. Polym. Sci. 2015, 132, 42398. [Google Scholar] [CrossRef]
- Lencina, S.M.M.; Ciolino, A.E.; Andreucetti, N.A.; Villar, M.A. Thermoresponsive hydrogels based on alginate-g-poly(N-isopropylacrylamide) copolymers obtained by low doses of gamma radiation. Eur. Polym. J. 2015, 68, 641–649. [Google Scholar] [CrossRef]
- Liu, M.; Song, X.; Wen, Y.; Zhu, J.-L.; Li, J. Injectable Thermoresponsive Hydrogel Formed by Alginate-g-Poly(N-isopropylacrylamide) Releasing Doxorubicin-Encapsulated Micelles as Smart Drug Delivery System. ACS Appl. Mater. Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef]
- Guo, H.; de Magalhaes Goncalves, M.; Ducouret, G.; Hourdet, D. Cold and Hot Gelling of Alginate-graft-PNIPAM: A Schizophrenic Behavior Induced by Potassium Salts. Biomacromolecules 2018, 12, 576–587. [Google Scholar] [CrossRef]
- Chalanqui, M.J.; Pentlavalli, S.; McCrudden, C.; Chambers, P.; Ziminska, M.; Dunne, N.; McCarthy, H.O. Influence of alginate backbone on efficacy of thermo-responsive alginate-g-P(NIPAAm) hydrogel as a vehicle for sustained and controlled gene delivery. Mater. Sci. Eng. C 2019, 95, 409–421. [Google Scholar] [CrossRef]
- Lencina, M.M.S.; Iatridi, Z.; Villar, M.A.; Tsitsilianis, C. Thermoresponsive hydrogels from alginate-based graft copolymers. Eur. Polym. J. 2014, 61, 33–44. [Google Scholar] [CrossRef]
- Safakas, K.; Saravanou, S.-F.; Iatridi, Z.; Tsitsilianis, C. Thermo-Responsive Injectable Hydrogels Formed by Self-Assembly of Alginate-Based Heterograft Copolymers. Gels 2023, 9, 236. [Google Scholar] [CrossRef]
- Wei, L.; Cai, C.; Lin, J.; Chen, T. Dual-drug delivery system based on hydrogel/micelle composites. Biomaterials 2009, 30, 2606–2613. [Google Scholar] [CrossRef]
- Lu, C.; Mikhail, A.S.; Wang, X.; Brook, M.A.; Allen, C. Hydrogels Containing Core Cross-Linked Block Co-Polymer Micelles. J. Biomater. Sci. Polym. Ed. 2012, 23, 1069–1090. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Parvathy, J.; Nair, A.S. A novel composite matrix based on polymeric micelle and hydrogel asa drug carrier for the controlled release of dual drugs. Carbohydr. Polym. 2016, 136, 1118–1127. [Google Scholar] [CrossRef]
- Theodorakis, N.; Saravanou, S.-F.; Kouli, N.-P.; Iatridi, Z.; Tsitsilianis, C. pH/Thermo-Responsive Grafted Alginate-Based SiO2 Hybrid Nanocarrier/Hydrogel Drug Delivery Systems. Polymers 2021, 13, 1228. [Google Scholar] [CrossRef]
- Ertugral-Samgar, E.G.; Ozmen, A.M.; Gok, O. Thermo-Responsive Hydrogels Encapsulating Targeted Core–Shell Nanoparticles as Injectable Drug Delivery Systems. Pharmaceutics 2023, 15, 2358. [Google Scholar] [CrossRef]
- Karanikolas, A.; Tsolakis, P.; Bokias, G.; Tsitsilianis, C. Stimuli-responsive poly(ethylene oxide)-b-poly(2-vinylpyridine)-b- poly(ethylene oxide) triblock copolymers and complexation with poly(acrylic acid) at low pH. Europ. Phys. J. E. 2008, 27, 335–343. [Google Scholar] [CrossRef]
- Martin, T.J.; Prochazka, K.; Munk, P.; Webber, S.E. pH-Dependent Micellization of Poly(2-vinylpyridine)-block-poly(ethylene oxide). Macromolecules 1996, 29, 6071. [Google Scholar] [CrossRef]
- Förster, S.; Abetz, V.; Muller, A.H.E. Polyelectrolyte Block Copolymer Micelles. In: Schmidt, M. (Eds.), Polyelectrolytes with Defined Molecular Architecture II. Adv. Polym. Sci. 2004, 166, 173. [Google Scholar] [CrossRef]
- Vamvakaki, M.; Papoutsakis, L.; Katsamanis, V.; Afchoudia, T.; Fragouli, P.G.; Iatrou, H.; Hadjichristidis, N.; Armes, S.P.; Sidorov, S.; Zhirov, D.; Zhirov, V.; Kosylev, M.; Bronstein, L.; Anastasiadis, S.H. Micellization in pH-sensitive amphiphilic block copolymers in aqueous media and the formation of metal nanoparticles. Faraday Discuss. 2005, 128, 129. [Google Scholar] [CrossRef]
- Gohy, J.-F.; Mores, S.; Varshney, S.K.; Zhang, J.-X.; Jérôme, R. pH-Dependence of the morphology of micelles formed by poly(2-vinylpyridine)-block-poly(ethylene oxide) copolymers in water. e-Polymers 2002, 2, 290–297. [Google Scholar] [CrossRef]
- Borchert, U.; Lipprandt, U.; Bilang, M.; Kimpfler, A.; Rank, A.; Peschka-Süss, R.; Schubert, R.; Lindner, P.; Förster, S. pH-Induced Release from P2VP-PEO Block Copolymer Vesicles. Langmuir 2006, 22, 5843–5847. [Google Scholar] [CrossRef] [PubMed]
- Brewer, K.; Bai, F.; Blencowe, A. pH-Responsive Poly(ethylene glycol)-b-poly(2-vinylpyridine) Micelles for the Triggered Release of Therapeutics. Pharmaceutics 2023, 15, 977. [Google Scholar] [CrossRef] [PubMed]
- Chroni, A.; Mavromoustakos, T.; Pispas, S. Curcumin-Loaded PnBA-b-POEGA Nanoformulations: A Study of Drug-Polymer Interactions and Release Behavior. Int. J. Mol. Sci. 2023, 24, 4621. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.-T.; Tsitsilianis, C. Controlled Delivery of Functionalized Gold Nanoparticles by pH-Sensitive Polymersomes. ACS Macro Lett. 2013, 2, 222–225. [Google Scholar] [CrossRef]

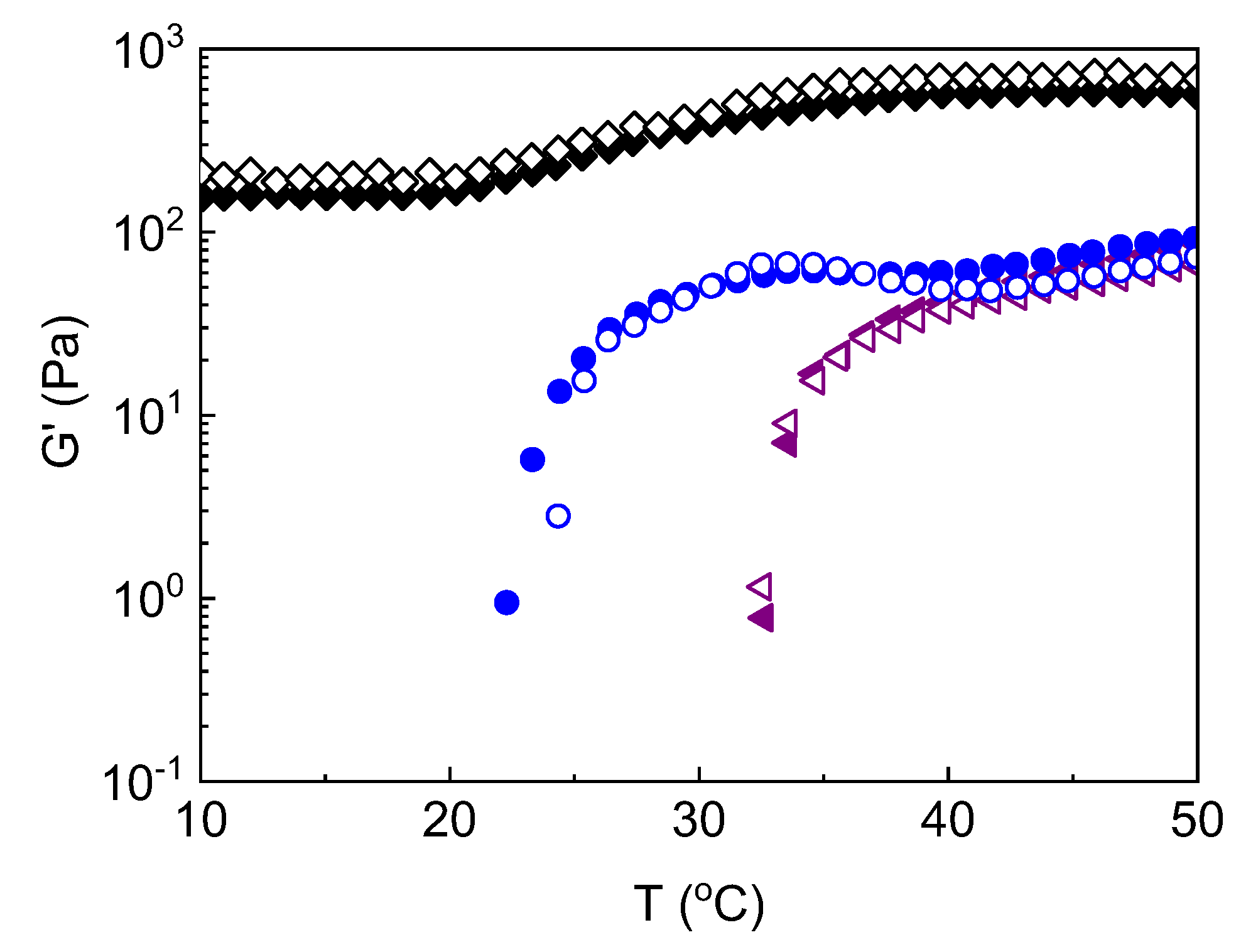
| ALG-g-HG/P2VP-b-PEO | |||||
|---|---|---|---|---|---|
| pH | Tc,thermothickening (°C) | Tgel (°C) | Tf (°C) | ΔT=Tf-Tc, thermothickening (°C) | G’(T=50)/G’(Tc, thermothickening) |
| 5.4 | 32.6 | 34.1 | 41.7 | 9.4 | 86.2/0.78=110.5 |
| 4.5 | 22.3 | 26.1 | 40.8 | 18.5 | 92.3/0.95=97.2 |
| 4.0 | 18.1 | 20.2 | 36.6 | 18.5 | 244.6/2.8=87.3 |
| 3.5 | 19.2 | -- | 38.7 | 19.5 | 565.2/160.7=3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





