1. Introduction
The relationship between smell and pain [
1,
2] is complex, with several intersections at higher levels of central nervous processing [
3]. Studies have found that certain fragrances and smells can help alleviate pain symptoms, while others can exacerbate them. Additionally, exposure to various smells has been shown to have a positive effect on mood, anxiety level, memory, and ability to focus. It's interesting to note that different people may have different reactions to the same odor, depending on their personal experiences and associations [
4,
5].
Olfactory training is an effective tool in the recovery of olfactory function [
6]; moreover it is starting to be considered in the alleviation of symptoms of depression and in the improvement of emotional well-being and cognitive functions [
7] .The underlying mechanism is partially unknown, however, there are strong and verified connections between the structures responsible for the olfactory function, those involved in the perception of emotions (in particular the amygdala and the Orbitofrontal cortex), and those associated with pain perception [
8,
9] , such as the insular cortex, the cingulate gyrus, and the hippocampus. [
10]
[The main aim of this study was to develop a new olfactory rehabilitation protocol with greater interaction and problem-solving components in order to ensure maximum involvement of patients, even the less compliant ones, and to estimate the improvement of painful symptoms, in the numerical form of an improvement in the NRS-11 (numerical rating scale for pain), in patients diagnosed with chronic pain not adequately controlled by pharmacological therapy and/or other forms of non-invasive treatments. The currently available methods of olfactory rehabilitation, none of which are fully validated and are extremely variable between different countries, are unfortunately not supported by instrumental confirmations regarding their effectiveness. The goal of our study is precisely to provide instrumental confirmation to the already hypothesized and empirically demonstrated utility of olfactory rehabilitation, in the context of applications for chronic pain. Moreover, to better understand the neural pathways involved in rehabilitation exercises and the olfactory system, we conducted a study with fMRI in BOLD acquisitions on a healthy, non-hyposmic voluntary subject [
11]. Our goal was to highlight the specific activation of the areas involved in pain perception and emotional elaboration of pain during the first discrimination exercise of mORP.
2. Materials and Methods
2.1. Study Design
This study is structured into four phases to analyze the effects of the Modified Olfactory Rehabilitation Protocol (mORP) on chronic pain patients unresponsive to standard therapies. Phase 1 involved recruiting patients and obtaining informed consent under ethical standards. Phase 2 and 3 included evaluating the patients' compliance and the mORP's effectiveness at 30 and 60 days, respectively, with Phase 3 data used for statistical analysis. The study, now in Phase 4, aims to assess the long-term stability of the mORP benefits. Preliminary results show promise, with plans to expand the sample size to enhance research impact. The study maintains high ethical standards and includes a comprehensive assessment process to ensure patient welfare and data accuracy.
2.2. The modified Olfactory Rehabilitation Protocol (mORP)
The Modified Olfactory Rehabilitation Protocol (mORP) is designed to activate the olfactory systems using specific exercises and essential oils: eucalyptus for the trigeminal olfactory system, lavender for the pure olfactory system, and clove for the mixed system. Unlike traditional methods involving passive exposure, mORP engages patients actively through exercises mirroring those in the Sniffin Sticks protocol:
Discrimination Part I: Distinguish fragrance from water on cotton balls.
Identification: Associate different essential oil fragrances with their names on cotton balls.
Discrimination Part II: Identify a unique fragrance among cotton balls with two different essential oils.
Participants are required to conduct these exercises daily, noting progress, sensations, and any discomfort in a session diary. The initial recruitment included 36 individuals for the Test Group, eventually narrowed down to 34 patients after excluding due to drop-offs like influenza. The age distribution of participants exhibited a normal distribution, as evidenced by the Shapiro-Wilk test (p = 0.6341), indicating no significant deviation from normality. The overall sample consisted of 67 patients, with an average age of 53.55 years (SD = 13.40 years), ranging from 21 to 81 years. When examining the groups separately, the Control Group consisted of 33 patients (25 females and 8 males) with an average age of 52.76 years (SD = 11.86 years), whereas the finale Test Group included 34 patients (24 females and 10 males) with an average age of 54.32 years (SD = 14.89 years).
Exclusion was based on olfactory function and potential trigeminal system impairments, using Sniffin' Sticks Test scores above the 75th percentile as a benchmark [
12] , ensuring no hyposmic patients were included. Statistical analyses focus on the NRS-11 pain scale scores [
13,
14] at 60 days to evaluate the pain relief magnitude, with baseline and follow-up comparisons detailed in
Table 1 and
Table 2. This approach aims to validate mORP's effectiveness without the risk of compromising accuracy, as outlined in the study's methodology . The ongoing study also examines fibromyalgia and lumbar pain correlations with olfactory impairment, referencing previous works [
15] and anticipating further research for substantiation. Comprehensive participant distribution data are documented
Table 1.
2.3. fMRI BOLD 3 Tesla Measurements
The fMRI BOLD 3 Tesla (3T) measurements section of the study highlights the advantages of using 3T MRI over the standard 1.5T, including superior resolution, reduced scan time, and increased sensitivity, particularly beneficial for motor function and somatosensory processing tasks [
16,
17,
18]. The study involved two fMRI sessions on a healthy, normosmic female subject without comorbidities to assess the feasibility of data processing, safety, and the absence of brain alterations that could skew results.
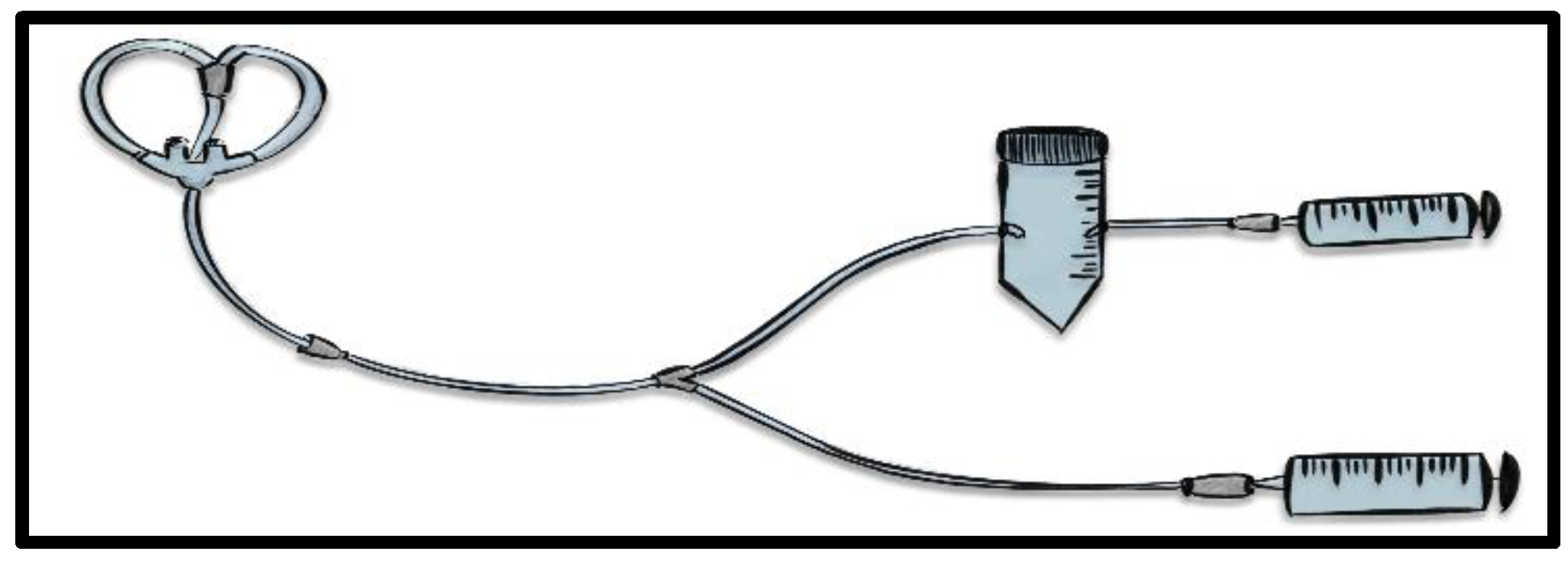
A custom-made circuit was engineered for olfactory stimulus delivery during scans, featuring a nasal cannula for direct odor administration, a three-way connection to a Falcon 50 mL container holding the essential oil on sterile gauze, and a 60 mL syringe for precise control. This setup, detailed in
Figure 1, ensured minimal subject discomfort and high-quality data acquisition compatible with the GE Medical System Signa Premier. The stimulus protocol included odorless air and lavender scent administrations, designed to maintain virtually continuous olfactory stimulation without complete washout, tracked by a chronometer to manage stimulus-recharge cycles. T1-weighted structural acquisitions were obtained in the axial plane (TR, 2260.1 ms; TE, 2.6ms; section thickness, 1 mm; number of sections, 340.) The sequence for functional images were categorized as Ax fMRI (TR, 3000.0 ms; TE, 17.7 ms; section thickness, 2 mm; number of sections, 6480).
The fMRI sessions comprised 18 minutes of scans, divided into structural and functional imaging to capture the brain's baseline activity and responses to olfactory stimuli. T1-weighted structural images and functional images were obtained with specific parameters (TR, TE, section thickness, number of sections) tailored for detailed brain analysis.
Data analysis utilized the CONN software [
19], developed by the McGovern Institute for Brain Research at MIT, for functional connectivity analysis from fMRI data. This included preprocessing steps like kernel smoothing and noise removal, focusing on regions of interest (ROIs) related to olfactory processing such as the olfactory bulbs, Orbitofrontal cortex, medial temporal cortex, and insular cortex [
20].
2.4. Statistical Analysis
To assess the statistical significance of the difference in the change in pain levels (ΔNRS-11) between the Control group and the Test group, we employed the independent samples t-test. The statistics for each analysis are based on the cases in the absence of missing or out-of-range data for each variable of the analysis . The software used for the statistical analysis of the data was JMP ver 17.0.0 for Microsoft Windows..
3. Results
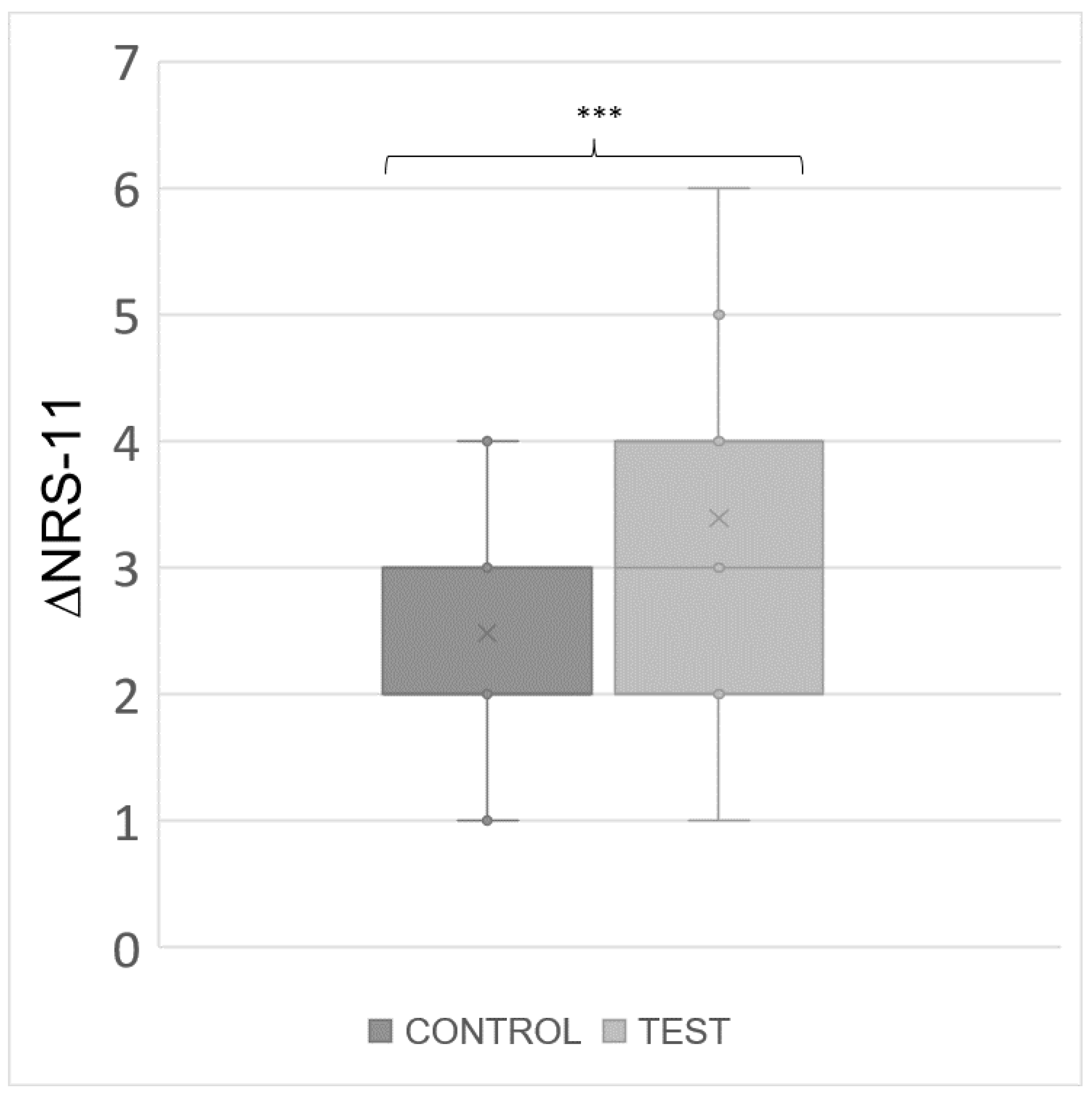
At baseline (t0), the average pain level was consistent between the groups, with Control Group reporting a mean NRS-11 of 8.67 (SD = 0.92) and Test Group reporting a mean NRS-11 of 8.68 (SD = 0.77), indicating a similar starting point for both groups in terms of pain severity. Following the modified Olfactory Rehabilitation Protocol, the mean NRS-11 at t1 demonstrated a divergence between the groups. The Control Group exhibited an average NRS-11 of 6.33 (SD = 1.53), while the Test Group showed a more pronounced reduction in pain, with an average NRS of 5.30 (SD = 1.33). The change in pain levels, represented by ΔNRS-11, further highlights the distinction between the groups' responses to treatment. Control Group experienced an average reduction in pain of 2.33 (SD = 1.14), whereas the Test Group observed a more substantial average decrease of 3.53 (SD = 1.46). In our analysis, the t-test for ΔNRS-11 yielded a t-statistic of -3.73, indicating that the mean change in pain levels for the test group was significantly lower than that for the control group. The p-value associated with this statistic was 0.0004. The descriptive analysis and the t-test results for the ΔNRS-11 are shown in
Table 2. What we obtained in the analysis, comparing the ΔNRS-11 for pain between the two groups, was a significant difference between the two groups, demonstrating the greater excursion of ΔNRS, and consequently greater benefit for patients in Group A. It is important to emphasize that the use of the independent t-test to assess the difference in the variation of pain levels (ΔNRS-11) between the control group and the test group can be interpreted similarly to the paired t-test. This is because the variation of ΔNRS-11 is measured within the same individuals before and after treatment. We opted for the independent t-test to establish that baseline values (
NRS at t0) are equal, and to facilitate further analysis of variations in pain levels under different conditions.
The results obtained from the statistical analysis provide confirmation of a significant difference of
ΔNRS-11 between the Test group and the Control group, as demonstrated by a p-value of 0.0004 for the ordered differences reported with the Student t-Test, thereby leading to the rejection of the null hypothesis. In
Figure 2, a boxplot to highlight the distribution of the ΔNRS-11 values is reported.
3.2. fMRI-BOLD Results and Data Analysis with CONN
The fMRI-BOLD results and data analysis conducted with the CONN software provided significant insights into brain function during resting states and olfactory tasks. Utilising seed-to-voxel and ROI-to-ROI analyses, clear disparities were observed in the activation of networks related to pain perception, smell processing, and their emotional components. Key areas of focus included the anterior and posterior cingulate cortex, insular cortex, prefrontal cortex, thalamus, amygdala, hippocampus, Orbitofrontal cortex, and Temporal Pole, among others. In-depth analysis revealed positive and negative connections across these regions, indicative of the brain's functional organization and its ability to integrate or segregate activities during cognitive and behavioral tasks. Positive connections, highlighting synchronous neuronal activity increases or decreases, were contrasted with negative connections, indicating anti-correlations in neuronal activities between regions. These findings are visualized in in ring connectomes (
Figure 3), showing the intricate networks within the Temporal Pole, a crucial site for smell processing. During the resting state, the Right Temporal Pole showed positive associations with both the right and left amygdala, while the Left Temporal Pole was only positively associated with the ipsilateral amygdala. However, stimulation with odorless air revealed exclusive anti-correlations for both hemispheres. Lavender stimulation resulted in positive correlations between the left Amygdala and both Temporal Poles, with a notable strong positive connection between the left Temporal Pole and the Right Hippocampus, alongside an anti-correlation between the Right Temporal Pole and the Left Hippocampus. This comprehensive analysis, though preliminary and performed on a single subject, underscores the complex neural underpinnings of olfactory processing and its relation to pain and emotional processing circuits.
4. Discussion
Our study centers on the novel application of olfactory therapy in the management of chronic pain, a therapeutic avenue supported by a growing body of evidence in the literature [
21,
22]. The mORP used in our Test Group for 60 days has yielded promising results, substantiated by our statistical analysis. Notably, there was a significant reduction in pain as measured by the ΔNRS-11 [
23] when compared to the Control Group. This outcome, as validated by the Student t-Test, underscores the potential efficacy of olfactory therapy in the clinical setting, and strengthen the already proposed hypothesis [
24] (
Table 2). The success of our protocol is evidenced by the significant pain reduction in the Test Group and the high compliance rate, indicating the protocol's user-friendliness and the clear, concise instructions provided. The patients who were provided with the mORP leaflet appreciated the clear and concise instructions, which made the process easy to understand and follow. The instructions were designed to be easily reproducible, so that patients could continue practicing the exercises at home. The high compliance rate among patients was a testament to the effectiveness of the leaflet and the ease with which the exercises could be integrated into their daily routine. Overall, the mORP leaflet proved to be a valuable tool for patients looking to improve their physical health and wellbeing.
Olfactory stimulation has a wide-reaching impact on neurophysiological pathways
[25,26,27], particularly those tied to pain perception and emotional processing. The challenges we faced in implementing the fMRI experimental setup [
28], notably in engineering a viable alternative to the traditional olfactometer for fMRI sessions, were met with innovative solutions that not only enhanced the study’s execution but also provided a template for future research. fMRI BOLD imaging with 3T MRI technology and analysis with CONN software have provided insight into the complex interactions of these pathways. Specifically, we have focused on the role of the temporal poles, which are crucial for olfactory perception. Within these poles, the piriform area acts as a primary region for scent discrimination, while the amygdala, an anterior temporal pole structure, is essential for the emotional aspects of olfaction, often triggering memories and reactions [
29,
30]. Our analysis has revealed distinct patterns of brain activity during olfactory tasks, characterized by both correlated and anticorrelated neural networks. Notably, we observed a lateralization effect; the left amygdala showed increased activation in response to lavender scent, suggesting a predilection for "emotional" olfactory processing in the left hemisphere—a finding consistent with prior studies
[31,32,33]. Although both the left and right temporal poles are involved in olfactory processing, our findings, along with existing literature, point to a subtle preference for the right temporal pole, though individual differences and external factors like experience and age can influence this bias. Moreover, olfactory perception extends beyond the temporal poles to include areas like the primary olfactory cortex and the orbitofrontal cortex, indicating a vast and interconnected neural network. During the olfactory tasks, particularly with the lavender stimulus, we detected a strong connection from the right temporal pole to the left amygdala, reinforcing the concept of "emotional" activation in the left hemisphere. Conversely, in a resting state or with odorless air, an anticorrelation pattern emerged between the left temporal pole and the ipsilateral amygdala. The successful application of mORP in our study is therefore underscored by the activation patterns observed in the fMRI BOLD imaging. The significant reduction in pain reported by patients following the mORP suggests that olfactory stimulation may have modulated these specific neural circuits traditionally associated with both the perception of odors and the emotional responses they elicit , leading to an altered pain perception. The noted lateralization effect, with increased activation in the left amygdala during olfactory tasks, also indicates that mORP may tap into the hemisphere predominantly involved in processing the emotional components of pain. This alignment of therapeutic intervention with the brain's intrinsic processing of olfactory and emotional stimuli provides a compelling explanation for the mORP's efficacy in mitigating chronic pain symptoms, offering a promising avenue for non-pharmacological pain management.
Despite the promising results of our study investigating mORP, we must acknowledge certain limitations. Firstly, the sample size, though adequate for preliminary analysis, requires expansion for broader generalizability of the findings. Secondly, the study's design as a pilot investigation means the results are exploratory and will benefit from replication in larger, more diverse populations. The use of self-reported measures for pain assessment, while valuable, could introduce subjective bias and would be complemented by objective pain measurement tools in future studies. Additionally, the fMRI analyses, although insightful, were limited to a single subject, which constrains the ability to draw definitive conclusions about the neural mechanisms involved. Lastly, the absence of a long-term follow-up prevents us from understanding the enduring impacts of mORP on chronic pain. Future research should aim to address these limitations by including larger sample sizes, diverse populations, objective pain measurements, broader neural analysis, and extended follow-up periods to fully ascertain the efficacy and potential of mORP in chronic pain management.
5. Conclusions
The results of our pilot study not only support the use of olfactory rehabilitation as a complementary therapy for chronic pain management but also suggest that such interventions may have a foundational role in the treatment regime. As we look to the future, our findings compel us to continue investigating the mechanisms underlying olfactory therapy and to establish a more substantial evidence base that will solidify its place in clinical practice. The potential for olfactory rehabilitation to provide non-pharmacological relief for chronic pain represents a significant advancement in the field, and we are committed to further elucidating this relationship through meticulous research and collaboration.
Author Contributions
Conceptualization, Mariaconsiglia Santantonio and Giulio Cesare Passali; Data curation, Mariaconsiglia Santantonio and Carmela Riso; Formal analysis, Mariaconsiglia Santantonio and Giuseppe Maulucci; Funding acquisition, Giuseppe Maulucci and Giulio Cesare Passali; Investigation, Mariaconsiglia Santantonio, Simona Gaudino and Giulio Cesare Passali; Methodology, Mariaconsiglia Santantonio, Giuseppe Maulucci and Giulio Cesare Passali; Project administration, Giulio Cesare Passali; Resources, Carmela Riso, Simona Gaudino, Alessio Simonetti and Giuseppe Maulucci; Software, Mariaconsiglia Santantonio and Giuseppe Maulucci; Supervision, Giuseppe Maulucci and Giulio Cesare Passali; Validation, Mariaconsiglia Santantonio, Giuseppe Maulucci and Giulio Cesare Passali; Visualization, Simona Gaudino, Giuseppe Varcasia, Francesco Schimperna, Bruno Sergi, Eugenio De Corso, Marco Rossi, Jacopo Galli and Marco De Spirito; Writing – original draft, Mariaconsiglia Santantonio, Giuseppe Maulucci and Giulio Cesare Passali; Writing – review & editing, Mariaconsiglia Santantonio, Giuseppe Maulucci and Giulio Cesare Passali.
Funding
This research received funding from Università Cattolica del Sacro Cuore, Linea D1 2022 (recipient GM).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Università Cattolica del Sacro Cuore in Rome (protocol code 6243 and date of approval 20/12/2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Gossrau, G.; Zaranek, L.; Klimova, A.; Sabatowski, R.; Koch, T.; Richter, M.; Haehner, A. Olfactory training reduces pain sensitivity in children and adolescents with primary headaches. Front Pain Res (Lausanne). 2023, 4, 1091984, PMID: 36860330; PMCID: PMC9968932. [Google Scholar] [CrossRef]
- Garland, E.L. Pain processing in the human nervous system: a selective review of nociceptive and biobehavioral pathways. Prim Care. 2012, 39, 561–571, Epub 2012 Jul 24. PMID: 22958566; PMCID: PMC3438523. [Google Scholar] [CrossRef]
- Hummel, T.; Rissom, K.; Reden, J.; Hähner, A.; Weidenbecher, M.; Hüttenbrink, K.B. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009, 119, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Kronenbuerger, M.; Pilgramm, M. Olfactory Training. 2022 Oct 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. [PubMed]
- Gossrau, G.; Baum, D.; Koch, T.; Sabatowski, R.; Hummel, T.; Haehner, A. Exposure to Odors Increases Pain Threshold in Chronic Low Back Pain Patients. Pain Med. 2020, 21, 2546–2551. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya, G.; Sengul, Y.S. Can olfactory training change the psychosocial aspects of chronic pain? Explore (NY). 2023, 19, 561–564, Epub 2022 Oct 23. PMID: 36307317. [Google Scholar] [CrossRef]
- Kandić, M.; Moliadze, V.; Andoh, J.; Flor, H.; Nees, F. Brain Circuits Involved in the Development of Chronic Musculoskeletal Pain: Evidence From Non-invasive Brain Stimulation. Front Neurol. 2021, 12, 732034, PMID: 34531819, PMCID: PMC8438114. [Google Scholar] [CrossRef]
- Caston, R.M.; Smith, E.H.; Davis, T.S.; Rolston, J.D. The Cerebral Localization of Pain: Anatomical and Functional Considerations for Targeted Electrical Therapies. J Clin Med. 2020, 9, 1945, PMID: 32580436, PMCID: PMC7355617. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.D.; Zubieta, J.K. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005, 9, 463–484, Epub 2005 Jan 21. PMID: 15979027. [Google Scholar] [CrossRef]
- Mueller, S.; Wang, D.; Fox, M.D.; Yeo, B.T.; Sepulcre, J.; Sabuncu, M.R.; Shafee, R.; Lu, J.; Liu, H. Individual variability in functional connectivity architecture of the human brain. Neuron. 2013, 77, 586–595, PMID: 23395382, PMCID: PMC3746075. [Google Scholar] [CrossRef]
- Hinz, A.; Luck, T.; Riedel-Heller, S.G.; Herzberg, P.Y.; Rolffs, C.; Wirkner, K.; Engel, C. Olfactory dysfunction: properties of the Sniffin' Sticks Screening 12 test and associations with quality of life. Eur Arch Otorhinolaryngol. 2019, 276, 389–395, Epub 2018 Nov 20. PMID: 30456541. [Google Scholar] [CrossRef]
- Haefeli, M.; Elfering, A. Pain assessment. Eur Spine J. 2006, 15 (Suppl 1), S17–24, Epub 2005 Dec 1. PMID: 16320034; PMCID: PMC3454549. [Google Scholar] [CrossRef]
- Shibasaki, H. Central mechanisms of pain perception. Suppl Clin Neurophysiol. 2004, 57, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Blanco, S.; Sanromán, L.; Pérez-Calvo, S.; Velasco, L.; Peñacoba, C. Olfactory and cognitive functioning in patients with fibromyalgia. Psychol Health Med. 2019, 24, 530–541, Epub 2018 Nov 19. PMID: 30453770. [Google Scholar] [CrossRef]
- Functional bold MRI: advantages of the 3 T vs. the 1.5 T, Reyes García-Eulate, David García-García, Pablo D Dominguez, Jose J Noguera, Esther De Luis, María C Rodriguez-Oroz, Jose L Zubieta. Clinical Imaging 2011, 35, 236–241. [CrossRef]
- Livshits, I.; Hussein, S.; Kennedy, C.; Weinstock-Guttman, B.; Hojnacki, D.; Zivadinov, R. Comparison of a 1.5T standard vs. 3T optimized protocols in multiple sclerosis patients. Minerva Med. 2012, 103, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Willinek, W.A.; Schild, H.H. Clinical advantages of 3. 0 T MRI over 1.5 T. Eur J Radiol. 2008, 65, 2–14, Epub 2007 Dec 26. PMID: 18162354. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141, Epub 2012 Jul 19. PMID: 22642651. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, H.; Lu, G.; Zhang, Z.; Jiao, Q.; Liu, Y. Detecting functional connectivity in fMRI using PCA and regression analysis. Brain Topogr. 2009, 22, 134–144, Epub 2009 May 1. PMID: 19408112. [Google Scholar] [CrossRef]
- Chen, G.; Chen, G.; Xie, C.; Li, S.J. Negative functional connectivity and its dependence on the shortest path length of positive network in the resting-state human brain. Brain Connect. 2011, 1, 195–206, PMID: 22433048, PMCID: PMC3572722. [Google Scholar] [CrossRef]
- Churchill, N.W.; Madsen, K.; Mørup, M. The Functional Segregation and Integration Model: Mixture Model Representations of Consistent and Variable Group-Level Connectivity in fMRI. Neural Comput. 2016, 28, 2250–2290, Epub 2016 Aug 24. PMID: 27557105. [Google Scholar] [CrossRef]
- Atisook, R.; Euasobhon, P.; Saengsanon, A.; Jensen, M.P. Validity and Utility of Four Pain Intensity Measures for Use in International Research. J Pain Res. 2021, 14, 1129–1139, PMID: 33907460; PMCID: PMC8071079. [Google Scholar] [CrossRef]
- Faria, V.; Dulheuer, J.; Joshi, A.; Wahl, H.; Klimova, A.; Haehner, A.; Gossrau, G. Impact of a 12-week olfactory training programme in women with migraine with aura: protocol for a double-blind, randomised, placebo-controlled trial. BMJ Open. 2023, 13, e071443, PMID: 37419649, PMCID: PMC10335595. [Google Scholar] [CrossRef]
- Zhou, G.; Lane, G.; Cooper, S.L.; Kahnt, T.; Zelano, C. Characterizing functional pathways of the human olfactory system. Elife. 2019, 8, e47177, PMID: 31339489, PMCID: PMC6656430. [Google Scholar] [CrossRef]
- Philpott, C.M.; Bennett, A.; Murty, G.E. A brief history of olfaction and olfactometry. J Laryngol Otol. 2008, 122, 657–662, Epub 2008 Jan 18. PMID: 18201391. [Google Scholar] [CrossRef]
- Weismann, M.; Yousry, I.; Heuberger, E.; Nolte, A.; Ilmberger, J.; Kobal, G.; Yousry, T.A.; Kettenmann, B.; Naidich, T.P. Functional magnetic resonance imaging of human olfaction. Neuroimaging Clin N Am. 2001, 11, 237–250. [Google Scholar] [PubMed]
- Grady, C.L. Meta-analytic and functional connectivity evidence from functional magnetic resonance imaging for an anterior to posterior gradient of function along the hippocampal axis. Hippocampus. 2020, 30, 456–471, Epub 2019 Oct 7. PMID: 31589003. [Google Scholar] [CrossRef]
- Kastrup, A.; Baudewig, J.; Schnaudigel, S.; et al. Behavioral correlates of negative BOLD signal changes in the primary somatosensory cortex. Neuroimage. 2008, 41, 1364–1371. [Google Scholar] [CrossRef]
- Stevens, J.S.; Hamann, S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012, 50, 1578–1593. [Google Scholar] [CrossRef]
- Minnix, J.A.; Versace, F.; Robinson, J.D.; et al. The late positive potential (LPP) in response to varying types of emotional and cigarette stimuli in smokers: a content comparison. Int J Psychophysiol. 2013, 89, 18–25. [Google Scholar] [CrossRef]
- Yalcinkaya, G.; Sengul, Y.S. Can olfactory training change the psychosocial aspects of chronic pain? Explore (NY). 2023, 19, 561–564, Epub 2022 Oct 23. PMID: 36307317. [Google Scholar] [CrossRef]
- Gottfried, J.A. Smell: central nervous processing. Adv Otorhinolaryngol. 2006, 63, 44–69. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).