Submitted:
24 February 2024
Posted:
01 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experiment 1: Temporal Attention and Endogenous Spatial Attention
2.1. Participants
2.2. Experimental Design
2.3. Experimental Procedure
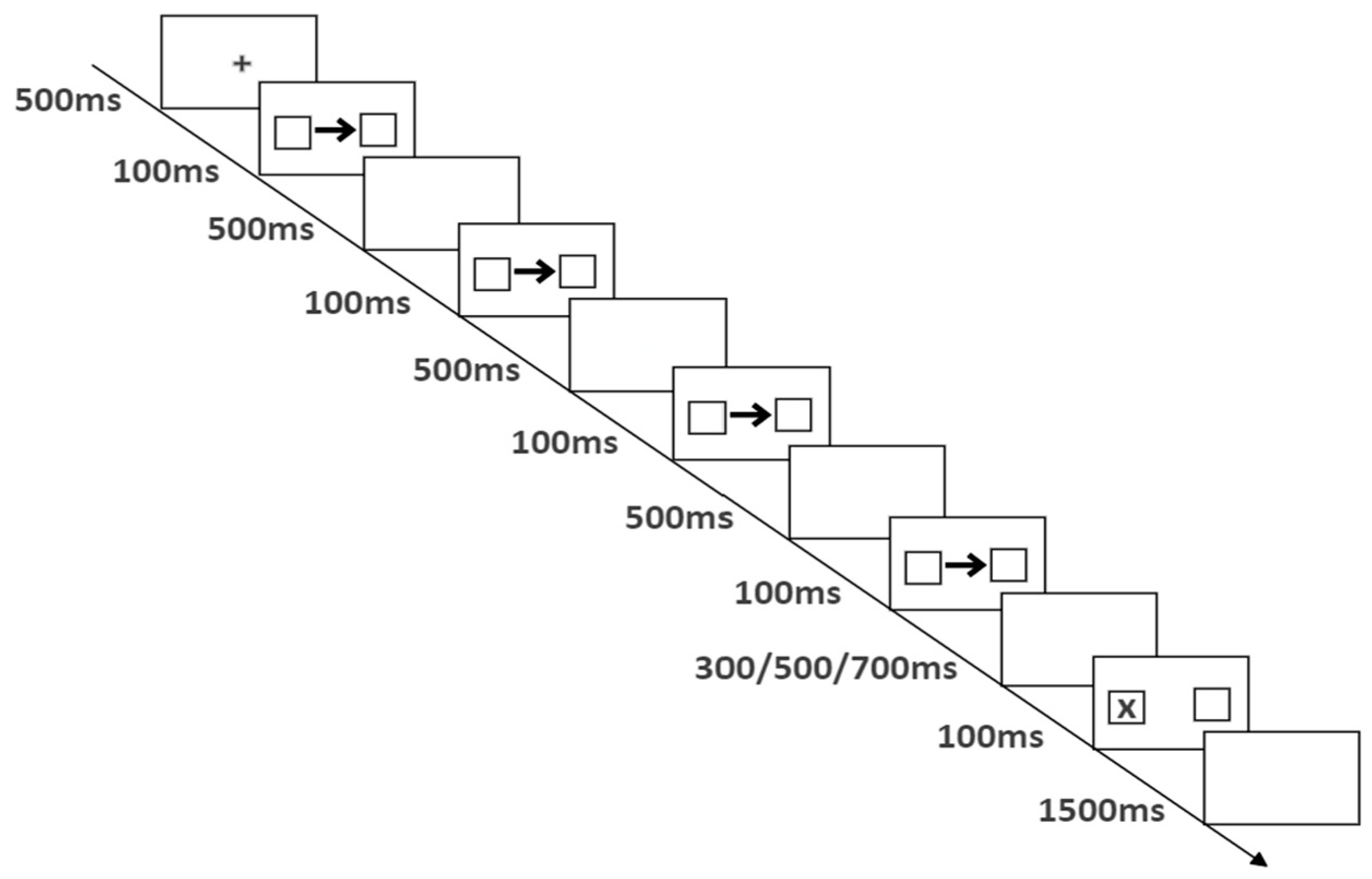
2.4. Data Analysis
2.5. Experimental Results
2.5.1. Accuracy
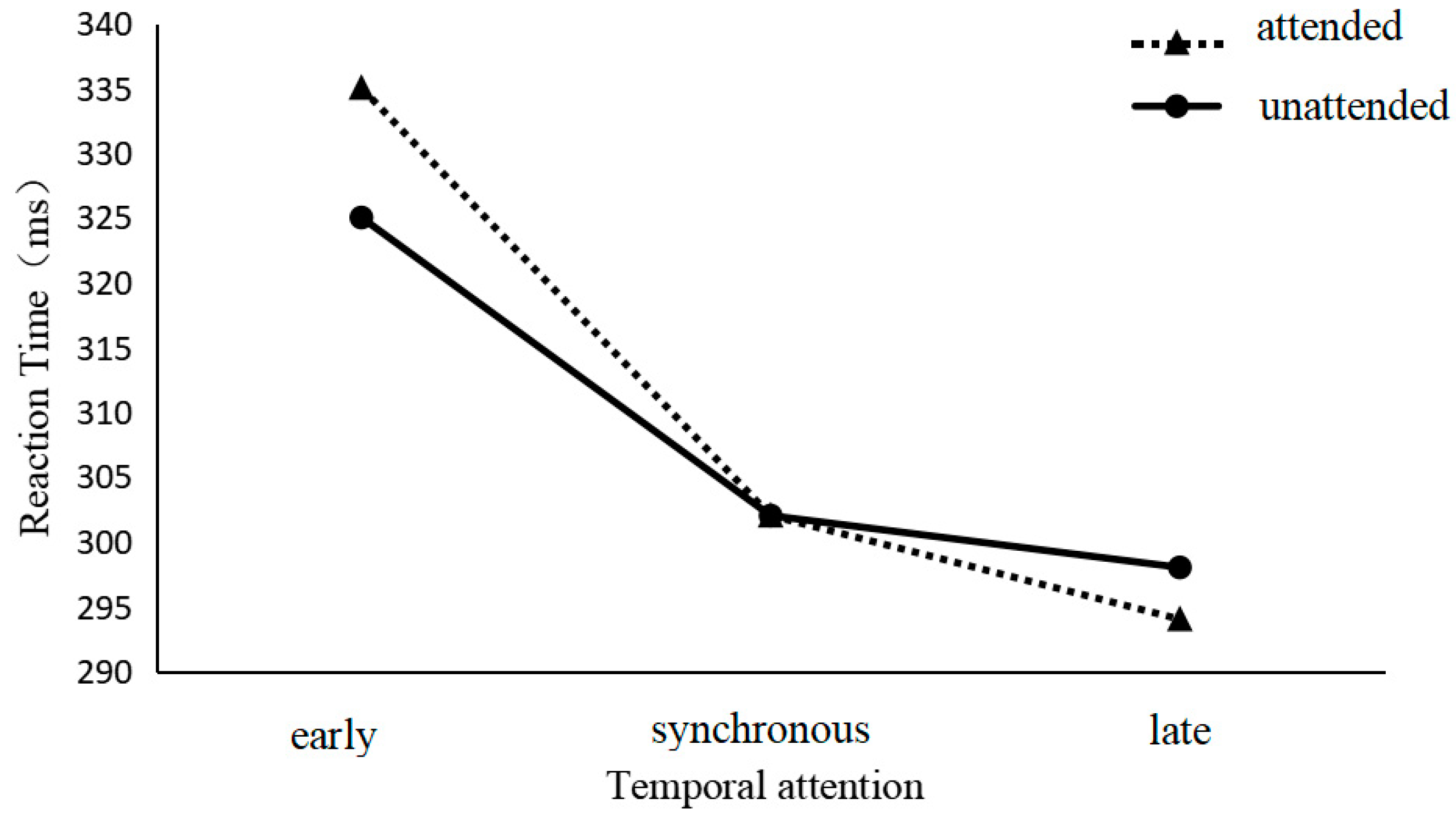
2.5.2. Reaction Time
| spatial attented condition | spatial unattented condition | |||
| 4 | 5 | 4 | 5 | |
| early | 330±70 | 299±69 | 348±80 | 318±71 |
| synchronous | 309±71 | 278±62 | 327±69 | 296±72 |
| late | 280±67 | 269±71 | 302±74 | 289±66 |
3. Experiment 2: Temporal Attention and Exogenous Spatial Attention
3.1. Participants
3.2. Experimental Design
3.3. Experimental Procedure
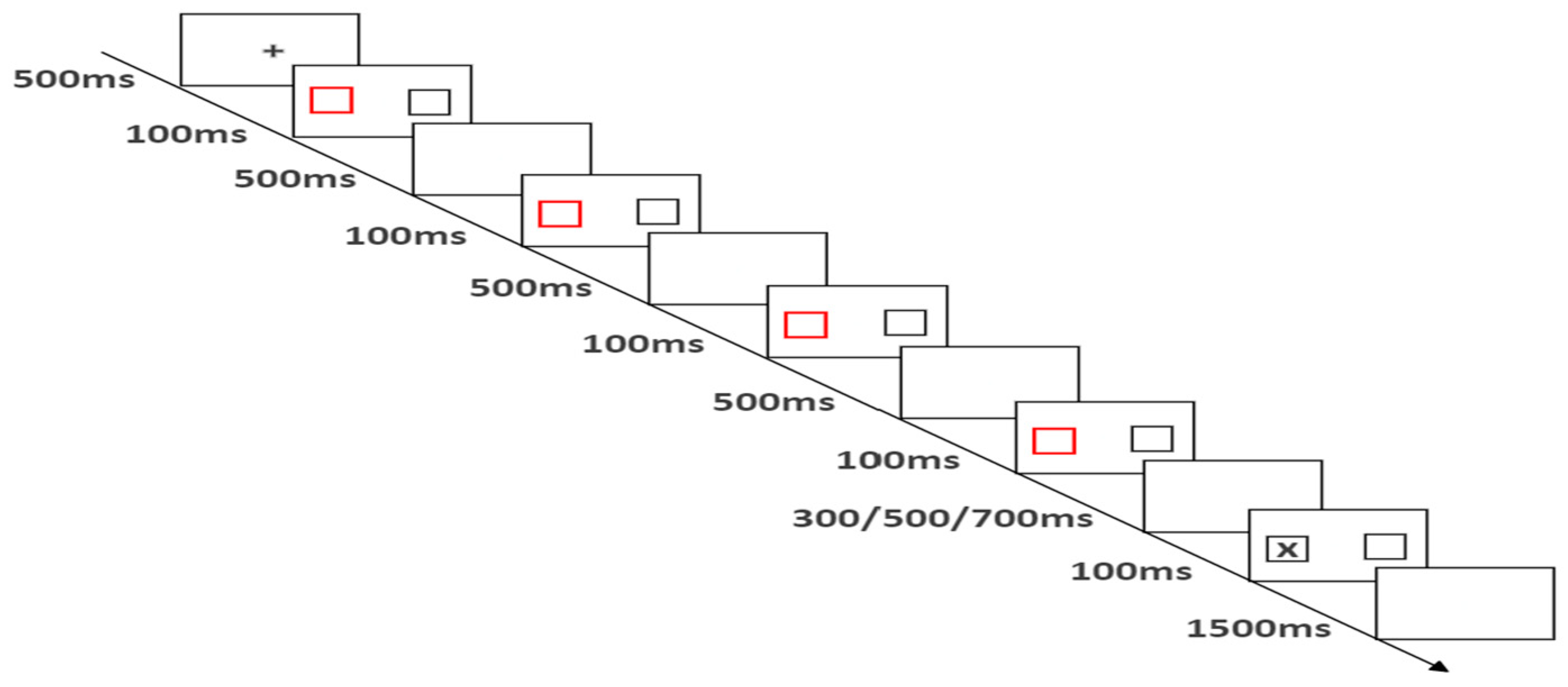
3.4. Data Analysis
3.5. Experimental Results
3.5.1. Accuracy
3.5.2. Reaction Time
| spatial attented condition | spatial unattented condition | |||
| 4 | 5 | 4 | 5 | |
| early | 362±61 | 307±43 | 345±45 | 306±41 |
| synchronous | 315±36 | 289±37 | 319±40 | 285±34 |
| late | 296±38 | 291±36 | 302±46 | 294±45 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meehan, C.E.; Wiesman, A.I.; Spooner, R.K.; Schantell, M.; Eastman, J.A.; Wilson, T.W. Differences in rhythmic neural activity supporting the temporal and spatial cueing of attention. Cereb. Cortex. 2021, 31, 4933–4944. [Google Scholar] [CrossRef] [PubMed]
- Nobre, A.C.; Rohenkohl, G. Time for the fourth dimension in attention. In: The Oxford Handbook of Attention (Nobre, A.C.; Kastner, S. eds), 2014; pp. 676–724. Oxford: Oxford UP.
- Posner, M.A. Orienting of attention. Q. J. Exp. Psychol. 1980, 32, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, M. Spatial covert attention: Perceptual modulation. In K. Nobre (Ed.), The Oxford Handbook of Attention, 2014, Oxford University Press.
- Klein, R.M. Inhibition of return. Trends. Cogn. Sci. 2000, 4, 138–147. [Google Scholar] [CrossRef]
- Chao, H.F.; Hsiao, F.S. Location-response binding and inhibition of return in a detection task. Atten. Percept. Psychophys. 2021, 83, 1992–2001. [Google Scholar] [CrossRef]
- Jones, A.; Forster, B. Reflexive attention in touch: An investigation of event related potentials and behavioural responses. Bio. Psychol. 2012, 89, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, M.; Wu, L.; Zhang, Q.; Wei, P. Neural mechanisms of reward-by-cueing interactions: ERP evidence. Front. Hum. Neurosci. 2021, 15, 608427. [Google Scholar] [CrossRef]
- Martín-Arévalo, E.; Lupiáñez, J.; Narganes-Pineda, C.; Marino, G.; Colás, I.; Chica, A.B. The Causal Role of the Left Parietal Lobe in Facilitation and Inhibition of Return. Cortex. 2019, 117, 311–322. [Google Scholar] [CrossRef]
- Nobre, A.C.; van Ede, F. Anticipated moments: Temporal structure in attention. Nat. Rev. Neurosc. 2018, 19, 34–48. [Google Scholar] [CrossRef]
- Jones, A. Temporal expectancies and rhythmic cueing in touch: The influence of spatial attention. Cognition. 2019, 182, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Nozaradan, S.; Zerouali, Y.; Peretz, I.; Mouraux, A. Capturing with EEG the neural entrainment and coupling underlying sensorimotor synchronization to the beat. Cereb. Cortex. 2015, 25, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Ongchoco, J.D.K.; Scholl, B.J. Hallucinating visual structure: Individual differences in ‘scaffolded attention’. Cognition. 2022, 225. [Google Scholar] [CrossRef]
- Jones, A. Independent effects of bottom-up temporal expectancy and top-down spatial attention. An audiovisual study using rhythmic cueing. Front. Integr. Neurosci. 2015, 8. [Google Scholar] [CrossRef]
- Mathewson, K.E.; Fabiani, M.; Gratton, G.; Beck, D.M.; Lleras, A. Rescuing stimuli from invisibility: Inducing a momentary release from visual masking with pre-target entrainment. Cognition. 2010, 115, 186–191. [Google Scholar] [CrossRef]
- Rohenkohl, G.; Coull, J.T.; Nobre, A.C. Behavioural dissociation between exogenous and endogenous temporal orienting of attention. PLoS. One. 2011, 6, e14620. [Google Scholar] [CrossRef]
- Pomper, U.; Keil, J.; Foxe, J.J.; Senkowski, D. Intersensory selective attention and temporal orienting operate in parallel and are instantiated in spatially distinct sensory and motor cortices. Hum. Brain. Mapp. 2015, 36, 3246–3259. [Google Scholar] [CrossRef]
- Rohenkohl, G.; Nobre, A.C. Alpha Oscillations Related to Anticipatory Attention Follow Temporal Expectations. J. Neurosci. 2011, 31, 14076–14084. [Google Scholar] [CrossRef]
- Lawrence, M.A.; Klein, R.M. Isolating exogenous and endogenous modes of temporal attention. J. Exp. Psychol. Gen. 2013, 142, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Ball, F.; Michels, L.E.; Thiele, C.; Noesselt, T. The role of multisensory interplay in enabling temporal expectations. Cognition. 2018, 170, 130–146. [Google Scholar] [CrossRef]
- Large, E.W.; Jones, M.R. The dynamics of attending: How people track time-varying events. Psychol. Rev. 1999, 106, 119–159. [Google Scholar] [CrossRef]
- Nobre, A.C.; Correa, A.; Coull, J.T. The hazards of time. Curr. Opin. Neurobiol. 2007, 17, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Hsu, Y.F.; Granjon, L.; Waszak, F. Temporal expectancies driven by self- and externally generated rhythms. Neuroimage. 2017, 156, 352–362. [Google Scholar] [CrossRef]
- Jones, M.R.; Moynihan, H.; Mackenzie, N.; Puente, J. Temporal Aspects of Stimulus-Driven Attending in Dynamic Arrays. Psychol. Sci. 2002, 13, 313–319. [Google Scholar] [CrossRef]
- Herrmann, B.; Henry, M.J.; Haegens, S.; Obleser, J. Temporal expectations and neural amplitude fluctuations in auditory cortex interactively influence perception. NeuroImage. 2016, 124, 487–497. [Google Scholar] [CrossRef]
- MacKay, A.; Juola, J.F. Are spatial and temporal attention independent? Percept. Psychophys. 2007, 69, 972–979. [Google Scholar] [CrossRef]
- Nobre, A.C. How can temporal expectations bias perception and action. In: Attention and Time, eds A. C. Nobre and J. T. Coull (Oxford, UK: Oxford University Press), 2010; pp. 137–330.
- Sharp, P.; Melcher, D.; Hickey, C. Different effects of spatial and temporal attention on the integration and segregation of stimuli in time. Atten. Percept. Psychophys. 2019, 81, 433–441. [Google Scholar] [CrossRef]
- Doherty, J.R.; Rao, A.; Mesulam, M.M.; Nobre, A.C. Synergistic Effect of Combined Temporal and Spatial Expectations on Visual Attention. J. Neurosci. 2005, 25, 8259–8266. [Google Scholar] [CrossRef] [PubMed]
- Rohenkohl, G.; Gould, I.C. , Pessoa, J.; Nobre, A.C. Combining spatial and temporal expectations to improve visual perception. J. Vis. 2014, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Ward, E.V.; Csiszer, E.L.; Szymczak, J. Temporal Expectation Improves Recognition Memory for Spatially Attended Objects. J. Cogn. Neurosci. 2022, 34, 1616–1629. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Forster, B. Neural correlates of endogenous attention, exogenous attention and inhibition of return in touch. Eur. J. Neurosci. 2014, 40, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Peelen, M.V.; Heslenfeld, D.J.; Theeuwes, J. Endogenous and exogenous attention shifts are mediated by the same large-scale neural network. NeuroImage. 2004, 22, 822–830. [Google Scholar] [CrossRef]
- Cravo, A.M.; Rohenkohl, G.; Wyart, V.; Nobre, A.C. Endogenous modulation of low frequency oscillations by temporal expectations. J. Neurophysiol. 2011, 106, 2964–2972. [Google Scholar] [CrossRef]
- van Ede, F.; de Lange, F.; Jensen, O.; Maris, E. Orienting attention to an upcoming tactile event involves a spatially and temporally specific modulation of sensorimotor alpha- and beta-band oscillations. J. Neurosci. 2011, 31, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Johnston, H.M.; Puente, J. Effects of auditory pattern structure on anticipatory and reactive attending. Cogn. Psychol. 2006, 53, 59–96. [Google Scholar] [CrossRef]
- Lawrance, E.L.A.; Harper, N.S.; Cooke, J.E.; Schnupp, J.W.H. Temporal predictability enhances auditory detection. J. Acoust. Soc. Am. 2014, 135, EL357–EL363. [Google Scholar] [CrossRef]
- Rimmele, J.; Jolsvai, H.; Sussman, E. Auditory Target Detection Is Affected by Implicit Temporal and Spatial Expectations. J. Cogn. Neurosci. 2011, 23, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Weinbach, N.; Shofty, I.; Gabay, S.; Henik, A. Endogenous temporal and spatial orienting: Evidence for two distinct attentional mechanisms. Psychon. Bull. Rev. 2015, 22, 967–973. [Google Scholar] [CrossRef]
- Coull, J.T.; Cotti, J.; Vidal, F. Differential roles for parietal and frontal cortices in fixed versus evolving temporal expectations: Dissociating prior from posterior temporal probabilities with fMRI. NeuroImage. 2016, 141, 40–51. [Google Scholar] [CrossRef]
- Kizuk, S.A.D.; Mathewson, K.E. Power and Phase of Alpha Oscillations Reveal an Interaction between Spatial and Temporal Visual Attention. J. Cogn. Neurosci. 2017, 29, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Henderson, J.M. Facilitation of return during scene viewing. Vis. Cogn. 2009, 17, 1083–1108. [Google Scholar] [CrossRef]
- Tang, X.; Gao, Y.; Yang, W.; Ren, Y.; Wu, J.; Zhang, M.; Wu, Q. Bimodal-divided attention attenuates visually induced inhibition of return with audiovisual targets. Exp. Brain. Res. 2019, 237, 1093–1107. [Google Scholar] [CrossRef]
- Tang, X.; Wang, X.; Peng, X.; Li, Q.; Zhang, C.; Wang, A.; Zhang, M. Electrophysiological evidence of different neural processing between visual and audiovisual inhibition of return. Sci. Rep. 2021, 11, 8056. [Google Scholar] [CrossRef] [PubMed]
- Muller-Gethmann, H.; Ulrich, R.; Rinkenauer, G. Locus of the effect of temporal preparation: Evidence from the lateralized readiness potential. Psychophysiology. 2003, 40, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Schroter, H.; Birngruber, T.; Bratzke, D.; Miller, J.; Ulrich, R. Task predictability influences the variable foreperiod effect: Evidence of task-specific temporal preparation. Psychol. Res. 2015, 79, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Jones, M.R. Rhythmic context modulates foreperiod effects. Atten. Percept. Psychophys. 2010, 72, 2274–2288. [Google Scholar] [CrossRef] [PubMed]
- Seibold, V.C.; Stepper, M.Y.; Rolke, B. Temporal attention boosts perceptual effects of spatial attention and feature-based attention. Brain. Cogn. 2020, 142, 105570. [Google Scholar] [CrossRef] [PubMed]
- Krauzlis. R.J.; Lovejoy, L.P.; Zenon, A. Superior colliculus and visual spatial attention. Ann. Rev. Neurosci. 2013, 36, 165–182. [Google Scholar] [CrossRef]
- Serences, J.T.; Yantis, S. Selective visual attention and perceptual coherence. Trends. Cogn. Sci. 2006, 10, 38–45. [Google Scholar] [CrossRef]
- Zhang, X.; Japee, S.; Safiullah, Z.; Mlynaryk, N.; Ungerleider, L.G. A normalization framework for emotional attention. PloS. Biol. 2016, 14. [Google Scholar] [CrossRef]
- Anton-Erxleben, K.; Carrasco, M. Attentional enhancement of spatial resolution: Linking behavioural and neurophysiological evidence. Nat. Rev. Neurosci. 2013, 14, 188–200. [Google Scholar] [CrossRef]
- Hickok, G.; Farahbod, H.; Saberi, K. The rhythm of perception: Entrainment to acoustic rhythms induces subsequent perceptual oscillation. Psychol. Sci. 2015, 26, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Spaak, E.; de Lange, F.P.; Jensen, O. Local entrainment of alpha oscillations by visual stimuli causes cyclic modulation of perception. J. Neurosci. 2014, 34, 3536–3544. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.K.R.; Jaeger, M.; Thorne, J.D.; Bendixen, A.; Debener, S. The auditory dynamic attending theory revisited: A closer look at the pitch comparison task. Brain. Res. 2015, 1626, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Dukewich, K.R. Reconceptualizing inhibition of return as habituation of the orienting response. Psychon. Bull. Rev. 2009, 16, 238–251. [Google Scholar] [CrossRef]
- McAuley, J.D.; Fromboluti, E.K. Attentional entrainment and perceived event duration. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2014, 369, 20130401. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




