1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a clinically heterogeneous condition, due to both genetic and environmental factors, highly hereditary, associated with different brain abnormalities and typically complicated by extensive comorbid conditions [
1,
2]
. In Italy, ADHD prevalence is estimated between 1.1% and 3.1% in individuals
aged 5-17 years [
3]. Main symptoms involve inattention, hyperactivity and/or impulsivity and are more frequently observed in males than in females (gender ratios from 3:1 to 9:1)[
4,
5]. A genetic component has been amply documented in the etiology of ADHD. Genes encoding the dopamine receptors (e.g. DRD2, DRD4), the dopamine transporter (DAT1) and the enzymes monoamine oxidase (MAOA) such as catechol-O-methyltransferase (COMT) and dopamine beta-hydroxylase (DBH), are the most studied [
6,
7,
8]. Instead, how genetics influence disease risk needs to be better understood [
9]
.
Children with ADHD can also exhibit hostility or anger and an aggressive behavior. Indeed, i
mpulsive aggression is often described among comorbidities
in individuals with ADHD [
10,
11]
. There are several biologically interesting markers that play a key role in the neurophysiological response to stressful life events[
12]. Among these,
MAOA, which encodes the key enzyme for the degradation of serotonin and catecholamines is the best-documented gene. Human genetic studies associated a higher risk for antisocial and violent behavior both with congenital
MAOA deficiency and low-activity
MAOA variants [
13]. Moreover, a sexual dimorphic effect of MAOA in behavioral traits beyond an increase of its activity levels with age hasbeen detected [
14]. Studies indicated that various
MAOA polymorphisms are involved in ADHD and/or traits of aggressiveness. Most prominently, rs6323 and rs1137070 polymorphisms, respectively located in the exon 8 and exon 14 of the MAOA gene seem to be associated with ADHD and/or personality traits of aggressive behavior, impulsivity, and anti-social behavior[
15,
16,
17,
18,
19]
. Also the
serotonin transporter gene (
SLC6A4) encoding for the serotonin transporter protein (5-HTT) has been associated to impulsive-aggressive behavior although with discordant results that reflect the importance of the environmental influence. Within the promoter region (5-HTTLPR) of the 5-HTT there is a 44-bp insertion/deletion variable-number tandem repeat polymorphism that generates two allelic forms, the long (l) and the short (s) variants; the first one has a more transcriptional activity and higher serotonin uptake than the second one [
20]. Even if some studies highlighted a positive association between the l-allele and violence or aggression [
21,
22], others found an excess of the s-allele among violent males[
23,
24]
. Given the multiple factors that contribute to aggressive behavior development, understanding every gene-contribution to aggressiveness is crucial. Therefore, with the current study, we aim to investigate the influence of MAOA and SLC6A4 genes polymorphisms on aggressive behavior displayed in a group of Italian children with ADHD. Specifically, we provide a novel insight on how to define the risk level of impulsive/aggressive personality traits through typing of a group of genetic polymorphisms
.
2. Materials and Methods
2.1. Population sample and diagnostic assessment
This case-control study was conducted on a total of 80 subjects with ADHD, aged between 6 and 17 years, who were selected among the individuals visited at the Unit of Child Neuropsychiatry at San Salvatore hospital in L'Aquila, Italy. The diagnosis of ADHD (inattentive, impulsive-hyperactive, combined) was determined by DSM-V guided clinical interview and by internal DSM-V-guided symptom checklists. Aggressive behavior was evaluated by the parent-completed Conners’ Rating Scales – III Edition. Exclusion criteria were autism spectrum disorder, intellectual disability or previously diagnosed genetic disorders. Eighty unrelated individuals without ADHD were used for comparison. The study was performed in accordance with the standards of the Ethics Committee (Code 0102550) and the informed consent was obtained from all participants in the study or their legal guardians.
2.2. Genotyping
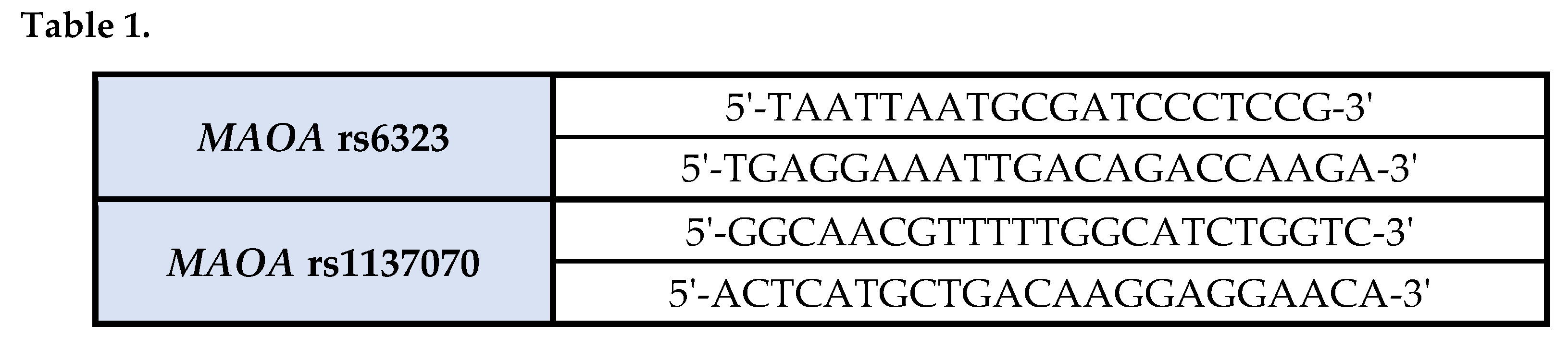
Genomic DNA was obtained from peripheral blood (PB) cells using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany), and stored at -20°C.The genotypes of the two selected exonic SNPs of the MAOA gene (rs6323, Arg297Arg and rs1137070, Asp470Asp) were analyzed by direct sequencing using primers shown in Table 1.
PCR amplifications were performed in 50 μl reaction mixture containing 50ng of genomic DNA, 1X PCR buffer with MgCl2, 0.2 mM each of deoxynucleotides, 0.5 U of Taq polymerase and a 3.2 pmol/μl concentration of each primer. PCR profile consisted of an initial denaturation step at 95 °C for 5’, followed by 35 cycles at 94 °C for 30 seconds, 57 °C for 45 seconds, 72 °C for 30 seconds and a final elongation step at 72 °C for 7 minutes. PCR products were identified on 1.5% agarose gel electrophoresis and subsequently purified by a PCR clean-up reagent (EXOSAP). Sequence reactions were performed using the Big Dye Terminator Chemistry v 1.1 (Applied Biosystems, Foster City, CA), processed on an ABI Prism 3130 Genetic Analyzer (Applied Biosystems) and then purified. Data were collected and typing was obtained based on alignments of the processed sequences with exon 8 and exon 14 sequences of the human MAOA gene retrieved from the Genbank.5-HTTLPR gene polymorphism study was performed with PCR using the following primers: forward 5’-GGCGTTGCCGCTCTGAATGC-3’ and reverse 5’-GAGGGACTGAGCTGGACAACCAC-3’. Cycling conditions were an initial denaturation at 94 °C for 5 minutes, followed by 35 cycles of denaturation at 94 °C for 30 seconds, annealing at 64 °C for 30 seconds, and extension at 72 °C for 60 seconds and a final extension at 72 °C for 7 minutes. The PCR products were subjected to electrophoresis in 2% agarose gels, stained with ethidium bromide. Illumination with ultraviolet light allowed to visualize bands and differentiate the 5HTT long allele (l) consisting of 528 base pair from the short one (s, 484bp).
2.3. Statistical Analysis
Allele and genotype frequencies were obtained by direct counting. Pearson’s chi-square test or Fisher’s Exact test were used to identify allele and genotype frequency differences between ADHD individuals and controls. Bonferroni’s correction (pc) was applied, as appropriate.The Hardy–Weinberg equilibrium (HWE) of alleles was evaluated to test the hypothesis of a random union of gametes. To calculate the risk that a particular genotype or allele can represent for the disease, the odds ratio (OR) and 95% confidence interval (CI) were computed. The level of significance was set at p=0.05. Statistical analysis was carried out using SPSS statistics software.
3. Results
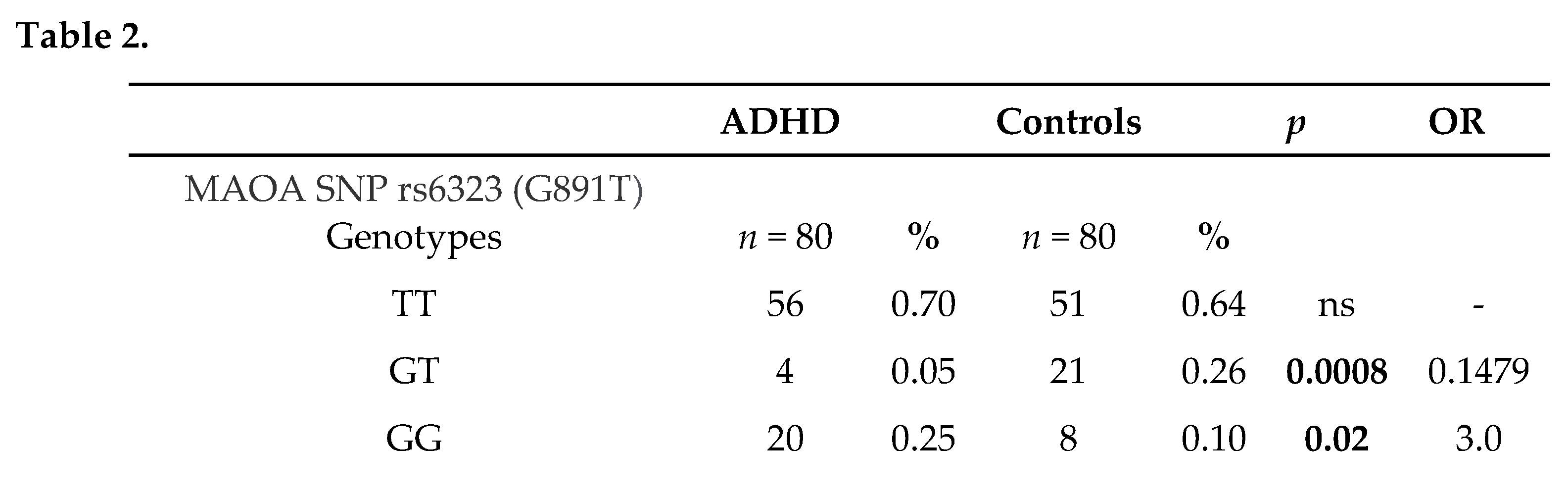
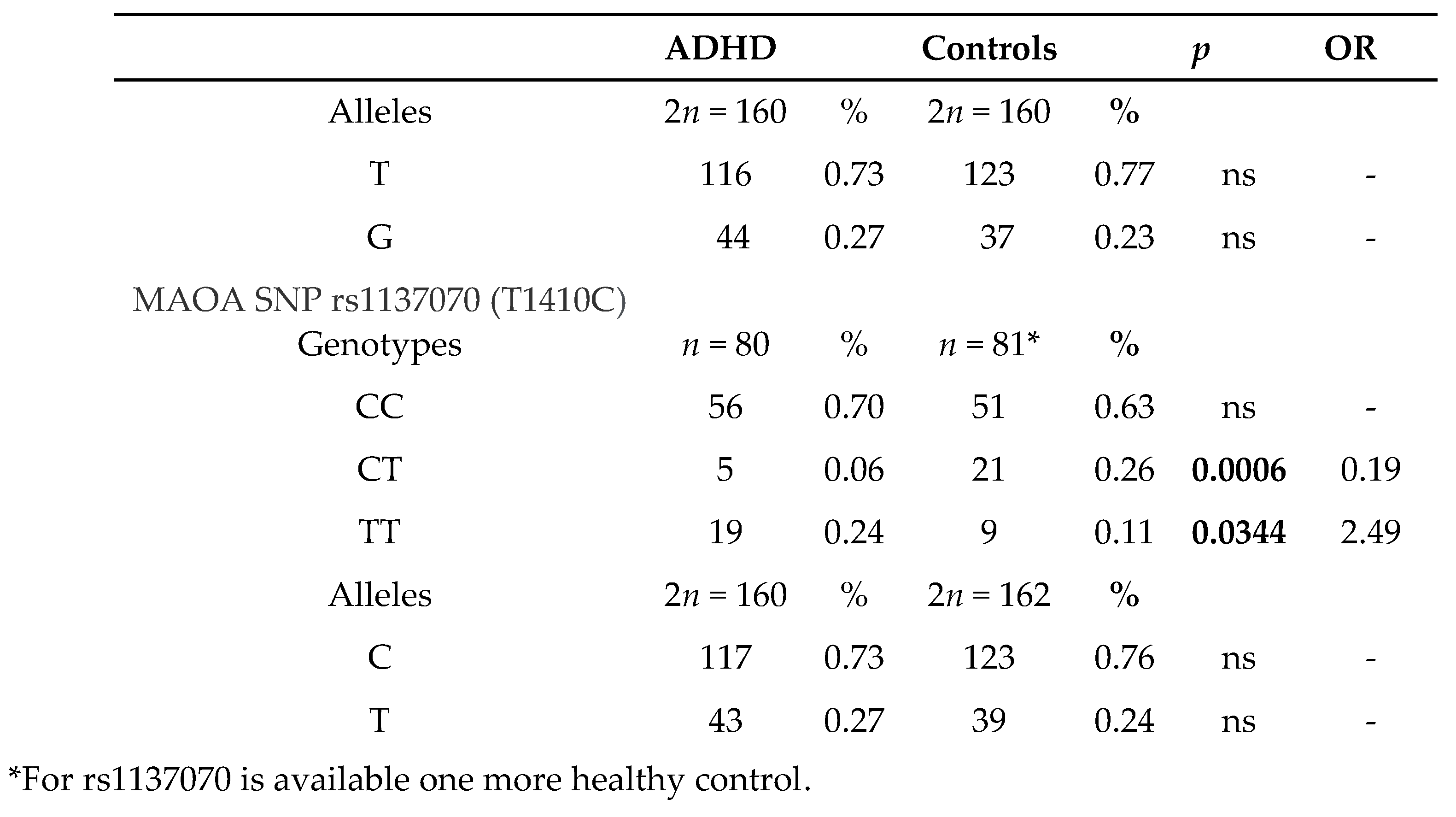
The study group is composed of 80 patients with ADHD (74 males and 6 females with a mean age of 10.8 years), out of whom 3 were predominantly hyperactive-impulsive, 9 were predominantly inattentive and 68 were both inattentive and hyperactive-impulsive (combined, according to DSM-V categorization). The 80 cases included in the study were further stratified in two subtypes based on the aggressive behavior presence (n=32, 40%) or absence (n=48, 60%). Eighty sex and age matched healthy individuals were evaluated as controls. In Table 2 are summarized genotype/allele frequencies of the two SNPs (rs6323 and rs1137070) of MAOA in cases and controls. We found GG genotype frequency of rs6323 was significantly higher in the ADHD group than in the control group (p= 0.02, OR = 3.00, 95% CI: 1.23–7.30). Instead, GT genotype was associated with significantly lower risk of ADHD (p= 0.0002, OR = 0.15, 95% CI: 0.05–0.45). This result has been validated by Bonferroni’s correction for multiple comparison and the p value remained significant (pc=0.0006), suggesting that GT genotype would be a biomarker of resistance to ADHD. Analysis of the rs1137070 polymorphism revealed that the TT genotype was associated with significantly higher risk of ADHD (p= 0.0344, OR = 2.49, 95% CI: 1.05–5.91), while the presence of CT genotype was negatively associated with the development of this disorder (p= 0.0006, OR = 0.19, 95% CI: 0.07–0.53). OR indicates that the presence of this genotype drops the risk for ADHD and p value remained significant (pc= 0.002) also after Bonferroni’s correction.
Interestingly, the positive association with ADHD has been also confirmed when the predisposing genotypes of the two MAOA gene polymorphisms (GG of rs6323 and TT of rs1137030) have been considered together (23.7% (19/80) vs. 10% (8/80) p=0.0238,OR=2.80, CI:1,15 to 6,85). As well as, the presence of both genotypes negatively associated to ADHD (GT + CT) underscored the reduced risk to develop ADHD (5% (4/80) vs. 25% (20/80) p=0.0004, pc=0.0012, OR:0.16, CI=0,05 to 0,49). We therefore additionally investigated if MAOA was associated with the aggressive behavior. Considering the subtype of patients with aggressive behavior, our findings highligted the same trend observed in the total group of patients with a prevalence of TT genotype of rs6323 and CC genotype of rs1137070 (Table 3). However, only a weak negative association was found between both MAOA polymorphisms and aggressiveness; GT genotype of rs6323 and CT genotype of rs1137070 were less frequent in patients with aggressiveness than in controls, so suggesting that these heterozygous genotypes may be protective towards specific changes in behavior, namely aggression (9% vs 26%, p=0.05, OR=0,30, CI: 0,08 to 1,07). Furthermore, we found that individuals carrying GG genotype of rs6323 were significantly increased among patients of subgroup without aggressive component, compared with controls (27% vs 10%, p=0.01, pc=0.03, OR=3.34, CI:1,27 to 8,81) and GT genotype was instead less frequent (2% vs 26%, p=0.0005, pc=0.0015, OR=0.06, CI: 0,01 to 0,46). Additionally, we found that CT genotype of rs1137070 was less frequent in this group of patients than in controls (6.2% vs. 26%, p=0.0055, OR=0.19, CI:0,05 to 0,68).
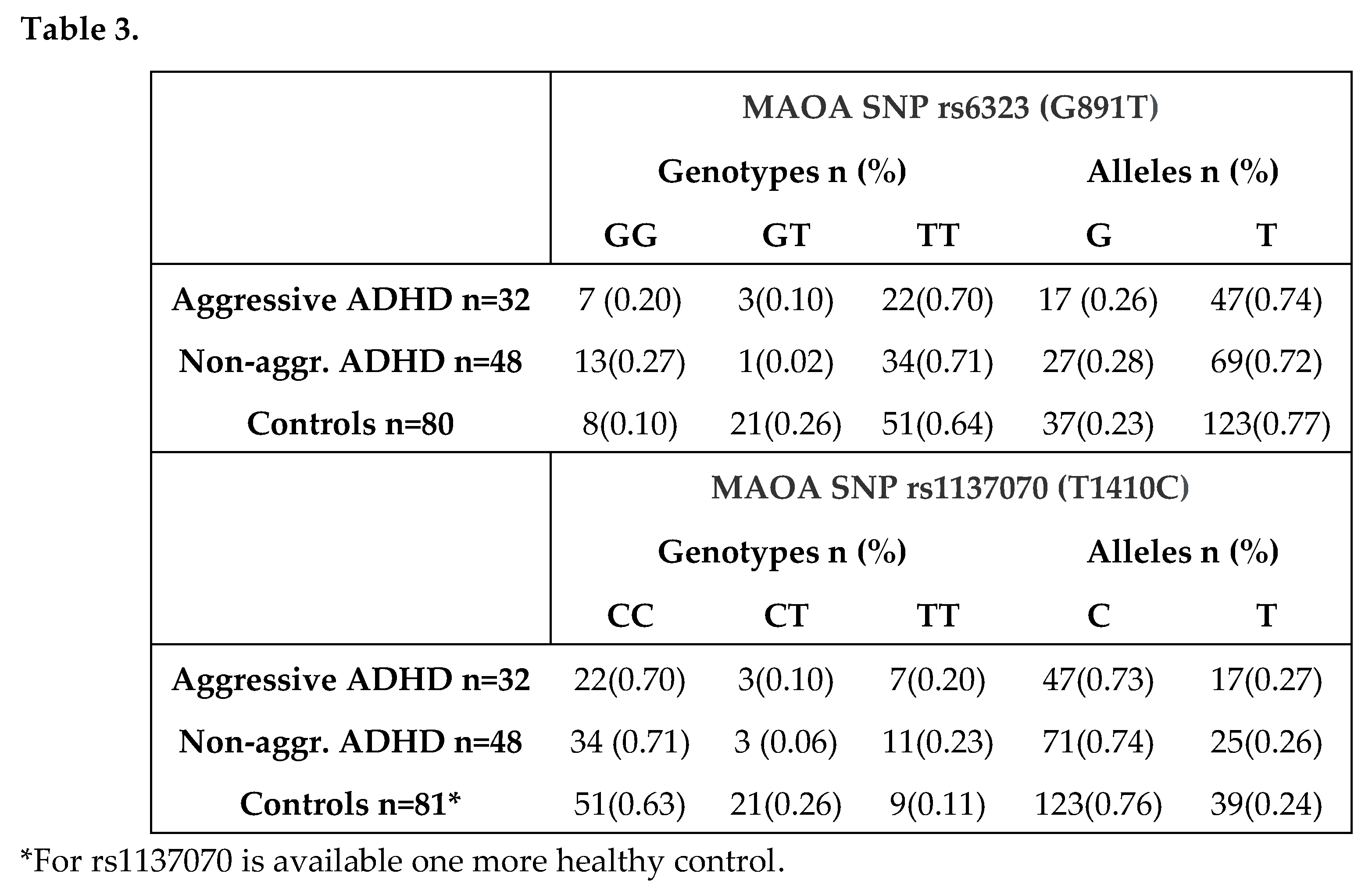
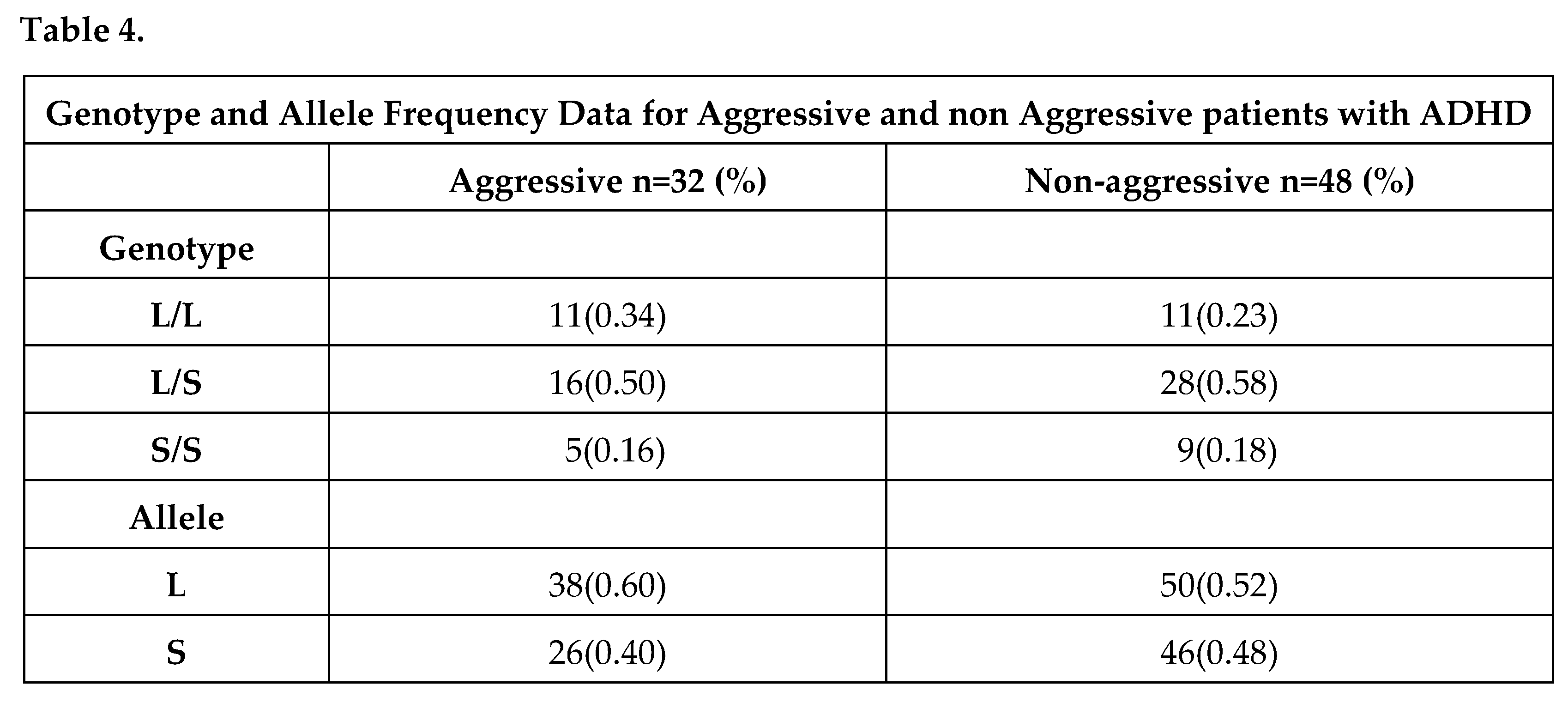
Also, a significantly greater combination of rs6323 GG and rs1137070 TT genotypes was observed in the subtype of patients without aggressive behavior compared to controls (25% (7/32) vs. 10% (8/80) p=0.02, OR=3,00 CI:1,13 to 7,99). Moreover, the 2-marker haplotype analysis indicate a protection profile to ADHD, in which the rs6323 GT and rs1137070 CT genotypes carriers (2% (1/48) vs. 25% (20/80) p=0.0007, OR=0.06, CI: 0.01 to 0.49) have less chance to display an aggressive behavior. No statistically significant differences in MAOA allele frequencies have been detected between patients and healthy individuals. All study participants were also genotyped based on the long and short alleles of the 5HTT gene but no allele and genotype differences have been detected among patients and controls. The distribution of genotypes in the 5-HTT was as follows: 14 patients (17.5%) and 16 controls (20%) had two short alleles (ss), 22 patients (27.5%) and 16 controls (20%) had two long alleles (ll), 44 patients (55%) and 48 controls (60%) had one long allele and one short one (ls).Lastly, no associations between aggressive behavior and the 5-HTTLPR genotypes were found as shown in Table 4.
Both controls and patients were not in Hardy-Weinberg equilibrium at each locus investigated, so, indicating that the population experienced evolutionary changes probably due to factors such as genetic drift, gene flow, natural selection, mutation, and non-random mating.
4. Discussion
Biological, psychological and environmental factors imbalances are at the basis of aggressive behavior onset. Mutations of several genes, certain mental health conditions and/or stress, fear, or a sense of losing control may generate aggression (
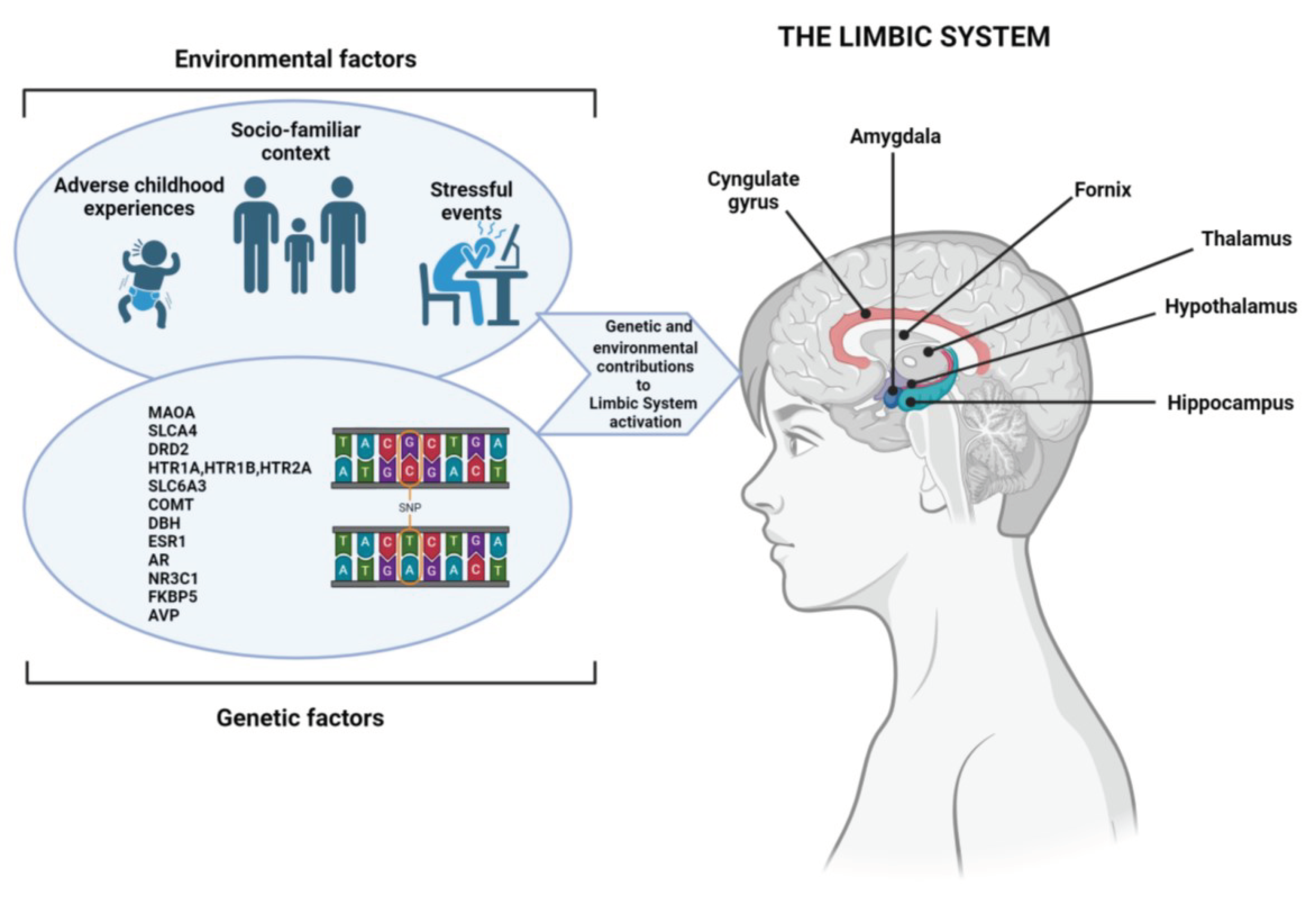
Figure 1) [
25].
Figure 1.
Multiple genetic variants are involved in ADHD development. As in most psychiatric diseases, genetic and environmental factors affect precociously the development of the central nervous system. The interaction between genetic predisposition and the environment, including adverse events that occur in the individual’s life (so-called stressors), may induce certain behaviors or psychological disorders. The relationship between genetic and environmental risk factors that may underlie interindividual variability and susceptibility to ADHD and/or impulsive aggressive behavior are shown here. The Figure was created with Biorender.
Figure 1.
Multiple genetic variants are involved in ADHD development. As in most psychiatric diseases, genetic and environmental factors affect precociously the development of the central nervous system. The interaction between genetic predisposition and the environment, including adverse events that occur in the individual’s life (so-called stressors), may induce certain behaviors or psychological disorders. The relationship between genetic and environmental risk factors that may underlie interindividual variability and susceptibility to ADHD and/or impulsive aggressive behavior are shown here. The Figure was created with Biorender.
In children and adolescents it is also more complex to understand the mechanisms underpinning aggression, given their difficulty of expressing emotions in words to communicate restlessness, anxiety or frustration. Neurodevelopmental disorder such as ADHD can also play a part in aggressive behavior [
10]. In most cases, this behavior is triggered by a particular event as also a pandemic exposure could be [
26]. Aggressiveness can happen as a natural response to pandemic-related stressors. In addition, scientists have found a complex genetic architecture of aggressive behavior in the context of ADHD and several potential risk genes have been identified [
27]. Among these, dopamine and serotonin, key neuromodulators involved in aggressive behavior, have received substantial attention, even if results are unsuccessful [
28,
29]. Anyway, a clear relationship among the MAOA and SLC6A4 genes and aggressiveness has been provided [
30].
Indeed, it is known that MAOs have a key role in the metabolism of neurotransmitters (e.g. serotonin) involved in aggressive behavior [
31,
32]. Many studies investigated the relationship between the aggressive phenotype and the MAO system and have found that specific allelic variations in the genes encoding MAO are associated with aggressive traits [
33]. In 1993, aggressive phenotypes of Brunner syndrome have been related to mutations in the MAOA gene which lead to a complete or partial loss of the MAOA enzyme activity [
34]. More recently, it has been understood that these mutations increase the activity of dopaminergic neurons through upregulation of the N-methyl-D-aspartate receptor (NMDAR) function and this might explain impulsivity and the maladaptive behavior associated to the Brunner syndrome [
34].
Rs6323 variant of MAOA, located in the exonic region, is associated with altered enzyme activity; the G allele is linked with higher levels of the enzyme and the T allele with lower levels [
35]. Some research groups reported that the low-activity MAOA genotype (TT) is associated with a more aggressive behavior [
36], while others have reached the opposite conclusion, identyfing the high-activity MAOA genotype (GG) as the one associated with a more aggressive behavior[
37]. With regard to
rs1137070, it has been shown that
the T variant is associated with higher activity than that of the C allele [
35].
Based on these observations, we investigated genetic variations in MAOA that underlie aggressive phenotypes in the ADHD context. Our study highlighted a different distribution of both MAOA rs6323 and rs1137070 genotypes between ADHD patients and healthy controls. The homozygous genotypes (GG and TT respectively) were significantly associated to ADHD risk. Conversely, heterozygous genotypes (GT and CT) resulted associated to a lower risk of ADHD development. Furthermore, a detailed study of rs6323 and rs1137070 genotype combinations allowed to identify that the combination of rs6323 GG and rs1137070 TT genotypes has an additional predisposing effect to the development of ADHD, while that of heterozygous (GT and CT) genotypes shows a protective association toward the disorder. When we performed subtype analysis (aggressive behavior/non-aggressive behavior) of ADHD, we found a protective association between heterozygous genotypes of both SNPs of the MAOA gene and aggressive subtype. Moreover, the GG genotype of rs6323 was a risk factor for the development of aggressive behavior in the non-aggressive patients. Instead, GT genotype of rs6323 and CT genotype of rs1137070 resulted protective toward aggressive symptoms of ADHD. This finding could appear opposite to the expected one; anyway, in support of this, Zhao et al. demonstrated that individuals carrying G allele seem more easily affected by environment than those carrying T allele. They also reported that adolescents with G genotype may be more sensitive to certain environmental situations because they recall emotion-related brain regions and neurophysiological over activation [
38]. Furthermore, Johnson et al. suggested that the exposure to different environments alters the genetic vulnerability of adolescents by influencing the expression of genetic factors [
39]. In this regard, it is of interest that a significantly positive relationship between the exposure to COVID-19 and an increased risk of stress has been detected and that this can lead to emotional/behavioral outcomes, such as anxiety, which might represent a mediating factor for aggressive behavior development [
40].
In addition, since an interactive effect of MAOA and 5-HTT gene polymorphisms on the brain activity has been previously reported [
41] and given the strong allele-allele interaction in the anterior cingulate cortex (implicated in the pathophysiology of impulsive/aggressive behavior), we also wanted to investigate if the polymorphism in the promoter region of the serotonin transporter (5HTT) was implicated to impulsive/aggressive behavior in our study group. Since no differences in 5-HTT genotype/allele frequencies among patients and controls have been found, at the moment we can't confirm this hypothesis.
5. Conclusions
The complex etiology of ADHD is still in part unknown. Sometimes, ADHD symptoms are linked with physical comorbidities as asthma in early childhood, injuries, sleep disturbances, epilepsy, and excess weight that can mask the early diagnosis. It follows that a late diagnosis of cognitive and functional defects associated with ADHD can lead to adults with social and interpersonal difficulties.
Understanding how patients with ADHD respond to specific environmental changes and identifying genetic markers associated with phenotypic plasticity might help to create and validate new tools as the algorithms [
42] able to identify ADHD children, classify them and distinguish those who are at highest risk for aggressive behavior.
Author Contributions
Conceptualization, L.N. and A.A.; methodology and investigation P.S., T.D.B., A.C.; validation, B.M., L.N., and A.A.; formal analysis and visualization, A.A.; resources, A.A.; data curation, L.N. and A.A.; writing—original draft preparation, A.A.; writing—review and editing, L.N., B.M. and A.A.; supervision, M.P.L.; project administration and funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by National Research Council ref. DSB.AD007.228.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Local Ethical Committee of the Abruzzo Region (Code 0102550).
Informed Consent Statement
Written informed consent has been obtained from all subjects involved in the study.
Data Availability Statement
All data, code, and materials used in the analysis are available to any researcher for purposes of reproducing or extending the analysis and are available in the main text.
Acknowledgments
The authors thank Dr. Sandra Gessani for helpful discussion during the preparation of the manuscript, Dr. Alessandro Giovannelli for statistical consulting and Mrs. Nunziatina Cherubini for administrative support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gnanavel, S.; Sharma, P.; Kaushal, P.; Hussain, S. Attention deficit hyperactivity disorder and comorbidity: A review of literature. World J. Clin. Cases 2019, 7, 2420–2426. [Google Scholar] [CrossRef] [PubMed]
- Curatolo, P.; Paloscia, C.; D'Agati, E.; Moavero, R.; Pasini, A. The neurobiology of attention deficit/hyperactivity disorder. Eur. J. Paediatr. Neurol. 2009, 13, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Reale, L.; Bonati, M. ADHD prevalence estimates in Italian children and adolescents: a methodological issue. Ital. J. Pediatr. 2018, 44, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.E. Sex differences in ADHD: conference summary. J. Abnorm. Child Psychol. 1996, 24, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Gaub, M.; Carlson, C.L. Gender differences in ADHD: a meta-analysis and critical review. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 1036–45. [Google Scholar] [CrossRef] [PubMed]
- Kirley, A.; Hawi, Z. ; Daly, G.; McCarron, M.; Mullins, C.; Millar, N.; Waldman, I.; Fitzgerald, M.; Gill,M. Dopaminergic System Genes in ADHD: Toward a Biological Hypothesis. Neuropsychopharmacology 2002, 27, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Barr, C.R.; Misener, V.L. Dopamine system genes and ADHD: a review of the evidence. Future Neurol. 2008, 3, 705–728. [Google Scholar] [CrossRef]
- Faraone, S.V.; Larsson, H. Genetics of attention deficit hyperactivity disorder. Mol. Psychiatry 2019, 24, 562–575. [Google Scholar] [CrossRef]
- Balogh, L.; Pulay, A.J.; Réthelyim, J.M. Genetics in the ADHD Clinic: How Can Genetic Testing Support the Current Clinical Practice? Front. Psychol. 2022, 13, 751041–751058. [Google Scholar] [CrossRef]
- Saylor, K.E.; Amann, B.H. Impulsive aggression as a comorbidity of attention-deficit/hyperactivity disorder in children and adolescents. J. Child Adolesc. Psychopharmacol. 2016, 26, 19–25. [Google Scholar] [CrossRef]
- Drechsler, R.; Brem, S.; Brandeis, D.; Grünblatt, E.; Berger, G.; Walitza, S. ADHD: Current Concepts and Treatments in Children and Adolescents. Neuropediatrics 2020, 51, 315–335. [Google Scholar] [CrossRef]
- Brevik, E.J.; van Donkelaar, M.M.; Weber, H.; Sánchez-Mora, C.; Jacob, C.; Rivero, O.; Kittel-Schneider, S.; Garcia-Martínez, I.; Aebi, M.; van Hulzen, K.; Cormand, B.; Ramos-Quiroga, J.A.; IMAGE, Consortium; Lesch, K.P.; Reif, A.; Ribasés, M.; Franke, B.; Posserud, M.B.; Johansson, S.; Lundervold, A.J.; Haavik, J.; Zayats, T. Genome-wide analyses of aggressiveness in attention-deficit hyperactivity disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016, 171, 733–747. [Google Scholar] [CrossRef]
- Kolla, N.J.; Bortolato, M. The role of monoamine oxidase A in the neurobiology of aggressive, antisocial, and violent behavior: A tale of mice and men. Prog. Neurobiol. 2020, 194, 101875–101898. [Google Scholar] [CrossRef]
- Xu, M.K.; Gaysina, D.; Tsonaka, R.; Morin, A.J.S.; Croudace, T.J.; Barnett, J.H.; Houwing-Duistermaat, J.; Richards, M.; Jones, P.B.; LHA Genetics Group. Monoamine Oxidase A (MAOA) Gene and Personality Traits from Late Adolescence through Early Adulthood: A Latent Variable Investigation. Front. Psychol. 2017, 8, 1736–1748. [Google Scholar] [CrossRef] [PubMed]
- Manuck, S.B.; Flory, J.D.; Ferrell, R.E.; Mann, J.J.; Muldoon, M.F. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res. 2000, 95, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Volavka, J.; Bilder, R.; Nolan, K. Catecholamines and aggression: the role of COMT and MAO polymorphisms. Ann. N. Y. Acad. Sci. 2004, 1036, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, D.; Haberstick, B.C.; Smolen, A.; Menard, S.; Young, S.E.; Corley, R.P.; Stalling, M.C.; Grotpeter, J.; Hewitt, J.K. Childhood maltreatment, subsequent antisocial behavior, and the role of monoamine oxidase A genotype. Biol. Psychiatry 2006, 60, 677–683. [Google Scholar] [CrossRef]
- Sjöberg, R.L.; Ducci, F. , Barr, C.S.; Newman, T.K.; Dell'osso, L.; Virkkunen, M.; Goldman, D.A. Non-additive interaction of a functional MAO-A VNTR and testosterone predicts antisocial behavior. Neuropsychopharmacology 2008, 33, 425–430. [Google Scholar] [CrossRef]
- Shah, S.S.; Mohyuddin, A.; Colonna, V.; Mehdi, S.Q.; Ayub, Q. Monoamine Oxidase A gene polymorphisms and self reported aggressive behaviour in a Pakistani ethnic group. J. Pak. Med. Assoc. 2015, 65, 818–824. [Google Scholar] [PubMed]
- Eun, T.K.; Jeong, S.H.; Lee, K.Y.; Kim, S.H.; Ahn, Y.M.; Bang, Y.W.; Joo, E.J. Association between the 5-HTTLPR Genotype and Childhood Characteristics in Mood Disorders. Clin. Psychopharmacol. Neurosci. 2016, 14, 88–95. [Google Scholar] [CrossRef]
- Twitchell, G.R.; Hanna, G.L.; Cook, E.H.; Stoltenberg, S.F.; Fitzgerald, H.E.; Zucker, R.A. Serotonin transporter promoter polymorphism genotype is associated with behavioral disinhibition and negative affect in children of alcoholics. Alcohol Clin. Exp. Res. 2001, 25, 953–959. [Google Scholar] [CrossRef]
- Zalsman, G.; Frisch, A.; Bromberg, M.; Gelernter, J.; Michaelovsky, E.; Campino, A.; Erlich, Z.; Tyano, S.; Apter, A.; Weizman, A. Family-based association study of serotonin transporter promoter in suicidal adolescents: No association with suicidality but possible role in violence traits. Am. J. Med. Genet. 2001, 105, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.L.; Hong, C.J.; Shih, H.L.; Tsai, S.J. Possible association between serotonin transporter promoter region polymorphism and extremely violent crime in Chinese males. Biol. Psychiatry 2004, 50, 284–287. [Google Scholar] [CrossRef]
- Gerra, G.; Garofano, L.; Santoro, G.; Bosari, S.; Pellegrini, C.; Zaimovic, A.; Moi, G.; Bussandri, M.; Moi, A.; Brambilla, F. Donnini, C. Association between low-activity serotonin transporter genotype and heroin dependence: Behavioral and personality correlates. Am. J. Med. Genet. (Neuropsychiatr. Genet.) Part B 2004, 126B, 37–42. [Google Scholar] [CrossRef]
- Kuzhiyengal Mambra, A.J.; Kotian, S. Understanding Aggressive Behavior: a Comprehensive Review of Research. IJNRD | 2023, 8, 144–149. [Google Scholar]
- Zhen, B.; Yao, B.; Zhou, X. Pandemic Exposure, Post-traumatic Stress Disorder, Conflict Behaviors, and Online Aggressive Behaviors Among College Students During the COVID-19 Pandemic: Examining the Moderating Role of Gender. Front. Psychiatry 2022, 13, 809173–809180. [Google Scholar] [CrossRef] [PubMed]
- Demontis, D.; Walters, R.K.; Rajagopal, V.M.; Waldman, I.D.; Grove, J.; Als, T.D.; Dalsgaard, S.; Ribases, M.; Bybjerg Grauholm, J.; Baekvad Hansen, M.; Werge, T.; Nordentoft, M.; Nors, O.; Mortensen, P.B.; ADHD Working Group of the Psychiatric Genomics Consortium (PGC), Cormand, B.; Hougaard, D.M.; Neale, BM.; Franke, B.; Faraone, S.V.; Borglum, A.D. Author Correction: Risk variants and polygenic architecture of disruptive behavior disorders in the context of attention-deficit/hyperactivity disorder. Nat. Commun. 2021, 12, 1166–1177. [Google Scholar] [CrossRef]
- Seo, D.; Patrick, C.J.; Kennealy, P.J. Role of Serotonin and Dopamine System Interactions in the Neurobiology of Impulsive Aggression and its Comorbidity with other Clinical Disorders. Aggress Violent Behav. 2008, 13, 383–395. [Google Scholar] [CrossRef]
- Narvaes, R.; Martins de Almeida, R.M. Aggressive behavior and three neurotransmitters: dopamine, GABA, and serotonin-A review of the last 10 years. Psychol. Neurosci. 2014, 7, 601–607. [Google Scholar] [CrossRef]
- Palumbo, S.; Mariotti, V.; Iofrida, C.; Pellegrini, S. Genes and Aggressive Behavior: Epigenetic Mechanisms Underlying Individual Susceptibility to Aversive Environments. Front. Behav. Neurosci. 2018, 12, 117–125. [Google Scholar] [CrossRef]
- Godar, S.C.; Fite, P.J.; McFarlin, K.M.; Bortolato, M. The role of monoamine oxidase A in aggression: Current translational developments and future challenges. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 69, 90–100. [Google Scholar] [CrossRef]
- Popova, N.K. From genes to aggressive behavior: the role of serotonergic system. Bioessays 2006, 28, 495–503. [Google Scholar] [CrossRef]
- Mentis, A.F.A.; Dardiotis, E.; Katsouni, E.; Chrousos, G.P. Fromwarrior genesto translational solutions: novel insights into monoamine oxidases (MAOs) and aggression. Transl. Psychiatry 2021, 11, 130–140. [Google Scholar] [CrossRef]
- van Rhijn, J.R.; Shi, Y. , Bormann, M.; Mossink, B.; Frega, M.; Recaioglu, H.; Hakobjan, M.; Gunnewiek, T.K..; Schoenmaker, C.; Palmer, E.; Faivre, L.; Kittel-Schneider, S.; Schubert, D.; Brunner, H.; Franke, B.; Nadif Kasri, N. Brunner syndrome associated MAOA mutations result in NMDAR hyperfunction and increased network activity in human dopaminergic neurons. Neurobiol Dis. 2022, 163, 105587–105600. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Breakefield, X.O. Human monoamine oxidase A gene determines levels of enzyme activity. Am. J. Hum. Genet. 1991, 49, 383–392. [Google Scholar] [PubMed]
- Wang, M.; Li, H.; Deater-Deckard, K.; Zhang, W. Interacting Effect of Catechol-O-Methyltransferase (COMT) and Monoamine Oxidase A (MAOA) Gene Polymorphisms, and Stressful Life Events on Aggressive Behavior in Chinese Male Adolescents. Front Psychol. 2018, 9, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Antypa, N.; Giegling, I.; Calati, R.; Schneider, B.; Hartmann, A.M.; Friedl, M.; Konte, B.; Lia, L.; De Ronchi, D.; Serretti, A.; Rujescu, D. MAOA and MAOB polymorphisms and anger-related traits in suicidal participants and controls. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Cao, Y.; Zhang, L.; Zhang, W. Parenting Practices and Adolescent Effortful Control: MAOAT941G Gene Polymorphism as a Moderator. Front. Psychol. 2020, 11, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W. Genetic and environmental influences on behavior: capturing all the interplay. Psycholog. Rev. 2007, 114, 423–440. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, B.; Ma, T.; Feng, Z.; Chen, X.; Huang, D.; Liu, L. Exposure to COVID-19 and aggression: the mediating role of anxiety and the moderating role of rumination. Curr Psychol. 2023, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, L.; Cerasa, A.; Gioia, M.C.; Magariello, A.; Muglia, M.; Quattrone, A.; Fera, F. Genetically dependent modulation of serotonergic inactivation in the human prefrontal cortex. Neuroimage 2008, 40, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Slaby, I.; Hain, H.S.; Abrams, D.; Mentch, F.D.; Glessner, J.T.; Sleiman, P.M.A.; Hakonarson, H. An electronic health record (EHR) phenotype algorithm to identify patients with attention deficit hyperactivity disorders (ADHD) and psychiatric comorbidities. J. Neurodev. Disord. 2022, 14, 37–46. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).