1. Introduction
Spinal metastases are relatively common among patients with metastatic disease, particularly in those with lung cancer, breast cancer, prostate cancer, myeloma, or renal cell carcinoma [
1,
2]. For many of these patients, spinal metastases are associated with metastatic epidural spinal cord compression (MESCC) and consequently motor and sensory deficits. Select patients with a good performance status, an estimated survival time of at least 3 months, involvement of only one spinal segment by MESCC, and not overly radiosensitive tumors were demonstrated to benefit from the addition of upfront decompressive surgery to radiotherapy [
3]. However, many patients with MESCC receive radiotherapy alone. For these patients, several dose-fractionation regimens are available [
1,
2,
4]. These include single-fraction, multi-fraction short-course (overall treatment time approximately one week) and multi-fraction long-course (overall treatment time 2-4 weeks) programs. It is generally agreed that patients with poor to intermediate survival prognoses should be treated with short-course or, in case of very poor prognoses, single-fraction radiotherapy [
1,
2,
4,
5,
6,
7].
However, many patients irradiated for MESCC have more favorable survival prognoses. These patients were shown to benefit from longer-course radiotherapy, particularly in terms of better local control (LC) and local progression-free survival (LPFS) of MESCC, when compared to shorter programs [
4,
8]. The most common longer-course program used for MESCC worldwide is 10 x 3.0 Gy over 2 weeks [
1,
2]. In a retrospective matched-pair study of 382 patients with MESCC and favorable survival prognoses, longer-course radiotherapy with higher doses, namely 15 x 2.5 Gy [equivalent dose in 2 Gy-fractions (EQD2) = 39.1 Gy
10] and 20 x 2 Gy (EQD2 = 40.0 Gy
10), resulted in significantly better long-term LC, LPFS, and overall survival (OS) when compared to 10 x 3.0 Gy (EQD2 = 32.5 Gy
10) [
9,
10,
11]. These findings led to the current RAMSES-01 trial which investigated whether further incremental dose escalation could improve outcomes. Patients included in this phase 2 trial had favorable survival prognoses and were scheduled for highly-conformal radiotherapy with 18 x 2.333 Gy (EQD2 = 43.2 Gy
10) or 15 x 2.633 Gy (EQD2 = 41.6 Gy
10). In addition, patients of the RAMSES-01 cohort were compared to a historical control group treated with 10 x 3.0 Gy of conventional radiotherapy. The major goal of this study was to show that highly-conformal radiotherapy with increased doses results in better LPFS when compared to the commonly used regimen 10 x 3.0 Gy.
2. Materials and Methods
In this multi-center phase 2 trial (RAMSES-01), the outcomes of highly-conformal radiotherapy with increased doses were investigated in patients with motor deficits due to MESCC and favorable survival prognoses assigned to radiotherapy alone without upfront surgery [
12]. Highly-conformal radiotherapy included volumetric modulated arc therapy (VMAT) and intensity-modulated radiation therapy (IMRT). Favorable prognosis was defined as ≥36 points on a validated survival score that incorporates six independent prognostic factors including type of primary tumor, other bone metastases or visceral metastases, interval between tumor diagnosis and MESCC, pre-radiotherapy ambulatory status, and time developing motor deficits [
13]. The study was approved by local ethic committees (leading committee: University of Lübeck, file: 18-360), performed in accordance with the Helsinki Declaration, and registered at clinicaltrials.gov (identifier NCT04043156).
The initial dose-fractionation regimen of the RAMSES-01 trial was 18 x 2.333 Gy, representing an EQD2 of 43.2 Gy
10 for tumor cell kill (α/β 10 Gy). This represented an increase of the EQD2 by 33% compared to 10 x 3.0 Gy (EQD2 = 32.5 Gy
10) [
10,
11]. When the Covid-19 pandemic started, the question arose of whether it was reasonably possible to reduce the number of radiotherapy sessions. It was decided to offer 15 x 2.633 Gy as alternative, which represents an EQD2 of 41.6 Gy
10 and an increase of the EQD2 by 28% compared to 10 x 3.0 Gy [
9,
10]. The maximum relative doses allowed to the spinal cord were 101.5 % of the prescribed dose for 18 x 2.333 Gy and 101.2% for 15 x 2.633 Gy. Both doses represented an EQD2 of 46.6 Gy
2 for myelopathy (α/β 2 Gy) [
9,
10].
Patients included must have had motor deficits of the lower extremities due to MESCC of the thoracic or lumbar spine lasting no longer than 30 days. MESCC was confirmed by magnetic resonance imaging or, if not possible, by computed tomography. Prior to radiotherapy, patients were presented to a surgeon to assess the necessity for decompressive surgery. Those operated on were ineligible. During the radiotherapy course patients were recommended to receive concomitant treatment with dexamethasone. Primary endpoint of the RAMSES-01 trial was LPFS at 12 months following radiotherapy. LPFS was defined as no deterioration of motor function during and no in-field recurrence of MESCC following radiotherapy. Secondary endpoints included improvement of motor and sensory functions, post-radiotherapy ambulatory status, relief of pain and distress, toxicities, and OS. Motor function was assessed using a 5-point scale (0 = normal strength, 1 = ambulatory without aid, 2 = ambulatory with aid, 3 = not ambulatory, 4 = complete paraplegia), sensory function impaired vs. normal [
14,
15]. Improvement and deterioration of motor deficits were defined as a change of motor function by ≥1 point [
14]. Intensity of pain was rated with a numeric self-assessment scale, ranging between 0 points (no pain) and 10 points (maximum pain) [
16]. Partial pain relief was defined as an improvement by ≥2 points from baseline without an increase of daily opioid analgesics or a reduction of opioid analgesics by at least 25% without an increase of pain [
16]. Complete pain relief was defined as a decrease of pain to 0. Distress was evaluated using the distress thermometer, also ranging between 0 points (no distress) and 10 points (maximum distress) [
17]. Relief of distress was defined as a decrease by ≥2 points from baseline. The Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 was used to grade toxicities [
18]. These endpoints were assessed directly and at 1, 3, 6, 9, and 12 months following radiotherapy. In case of an out-field recurrence of MESCC, patients were censored for LPFS. Further details of the study protocol were reported previously [
12].
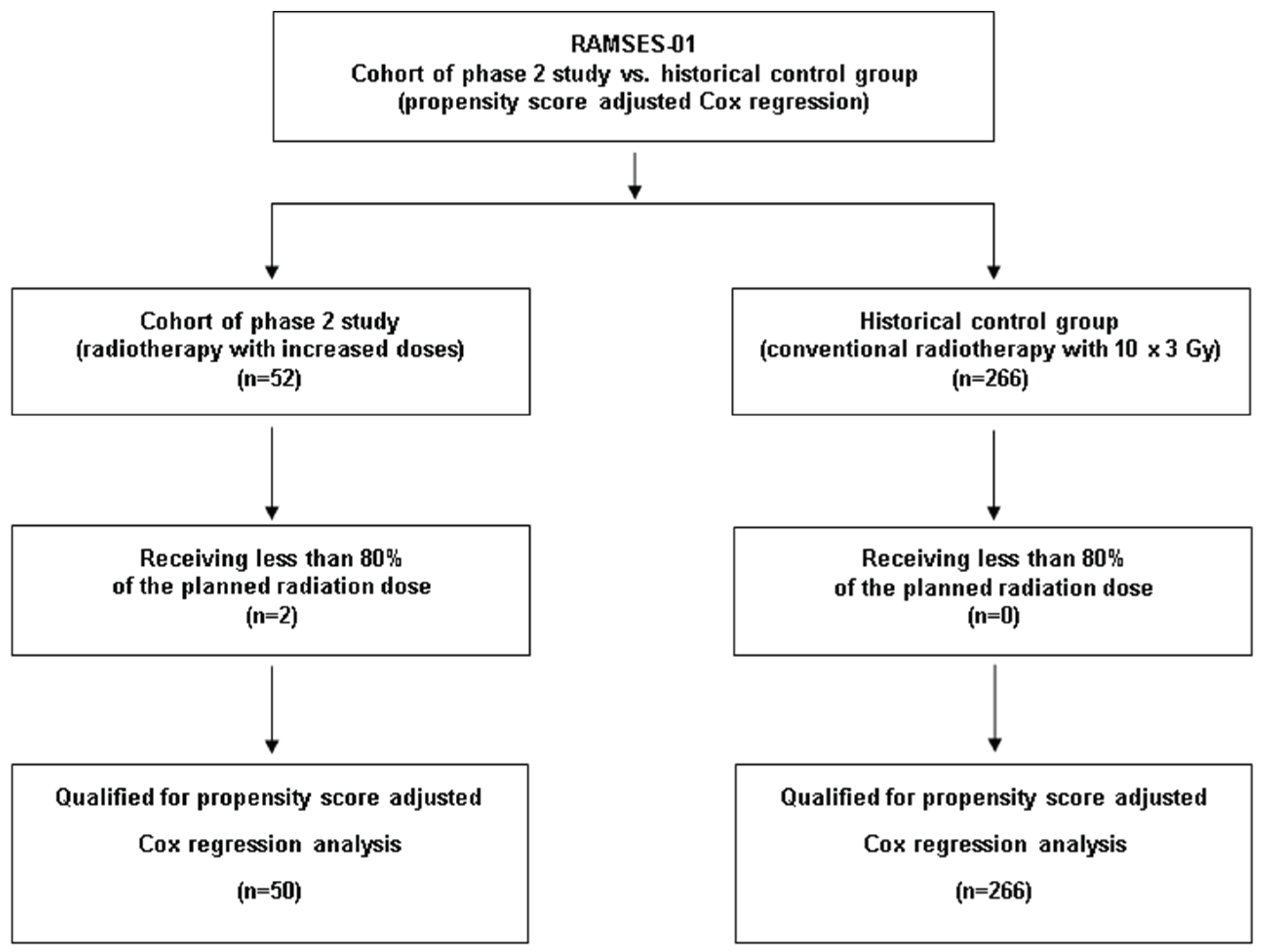
Patients of the RAMSES-01 trial were compared to a historical control group from multiple institutions with motor deficits due to MESCC and favorable survival prognoses who were irradiated with 10 x 3.0 Gy of conventional radiotherapy, using propensity score adjusted analyses. The baseline characteristics of both treatment groups are summarized in
Table 1. Both groups were compared with respect to LPFS, OS, improvement of motor function, and post-radiotherapy ambulatory status. In order to avoid a bias due to different length of follow up, the follow up period in the historical control group was limited to 12 months.
Statistical Considerations
For the RAMSES-01 trial, a required sample size of n=62 was calculated [
12]. Assuming that 5% of the enrolled patients would not be eligible, recruitment of 65 patients was planned. The evaluation was performed in those patients, who were available for assessment of the primary endpoint and received at least 80% of the planned radiation dose, i.e., 15 of 18 fractions with 2.333 Gy and 12 of 15 fractions with 2.633 Gy, respectively. LPFS and OS rates were calculated with the Kaplan-Meier-method. For the comparison of the RAMSES-01 cohort and the historical control group, the propensities were estimated using several baseline characteristics, namely age (≤64 vs. ≥65 years), gender, type of primary tumor (breast cancer vs. prostate cancer vs. myeloma/lymphoma vs. lung cancer vs. other tumors), interval between tumor diagnosis and MESCC (≤15 vs. >15 months), number of involved vertebrae (1-2 vs. ≥3), other bone metastases (no vs. yes), visceral metastases (no vs. yes), time developing motor deficits prior to radiotherapy (0-7 vs. 8-14 vs. >14 days), pre-radiotherapy ambulatory status (no vs. yes), and Eastern Cooperative Oncology Group (ECOG) performance score (0-2 vs. 3-4) [
12]. To obtain valid maximum likelihood estimates, time developing motor deficits was aggregated and included in the respective logistic regression model as a binary variable with values ≤14 days and >14 days. The Hosmer-Lemeshow Goodness-of-Fit Test was
p = 0.54).
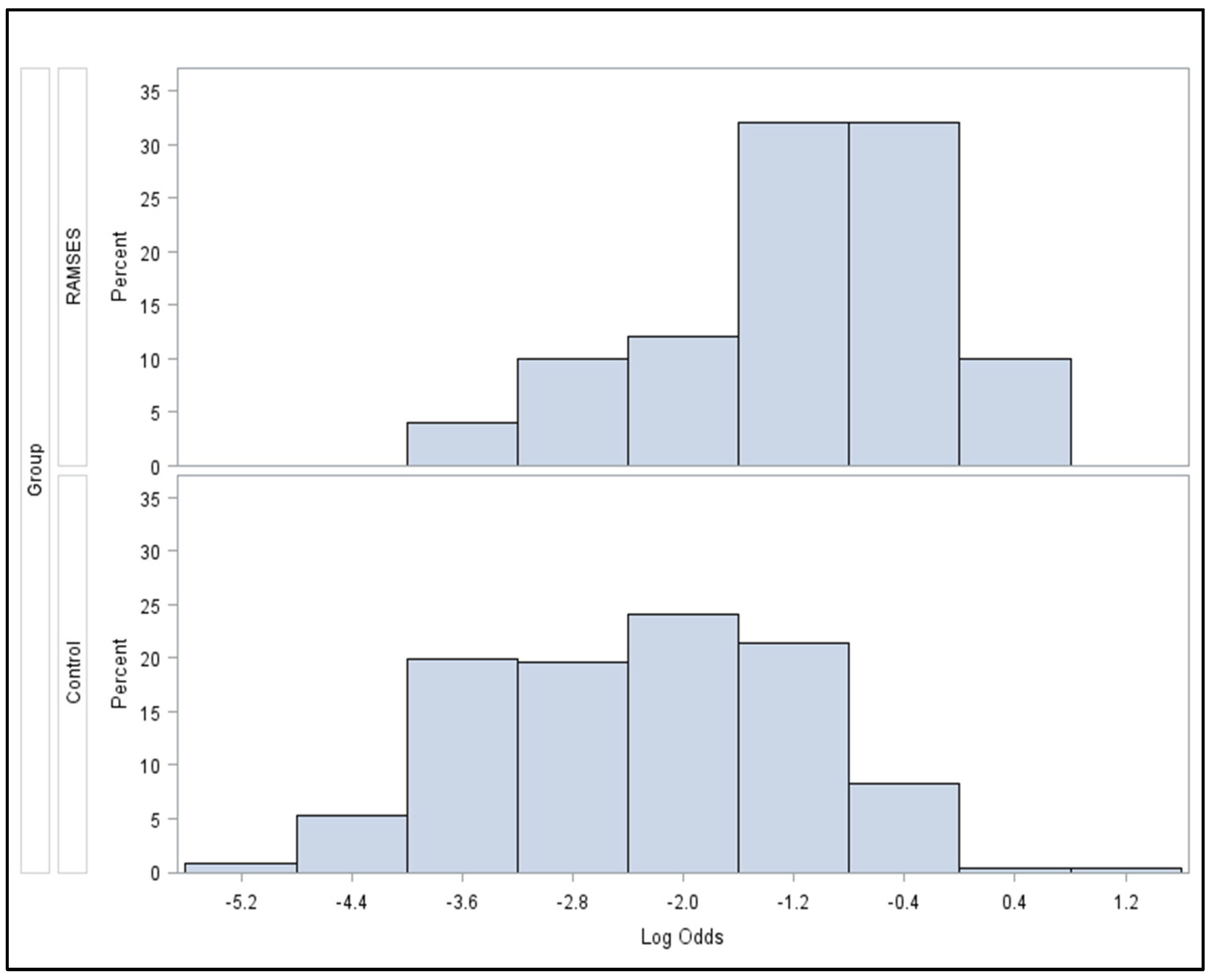
After the propensity scores were estimated, several methods and their variations existed to create balance between the RAMSES-01 cohort and the prospective and historical control group. However, due to low number of expected events, the commonly used approaches such as one-to-one matching, stratification, and inverse probability of treatment weighting were not recommended. Instead, regression on the propensity score by estimating a Cox-proportional hazards model for the outcome of interest with independent variables for treatment and the logit of the propensity scores was applied. The regression model was chosen to allow for a non-linear association between the propensity score and the outcome link function by means of the ‘one-spline’ approach as described by Franklin et al. [
19]. To reduce any potential bias, this modeling approach was already pre-specified in the study protocol. In particular, a restricted cubic spline transformation was used consisting of cubic functions between the knots and linear function in the tails, which allowed for many possible complex forms. Five knots were placed at equally spaced percentiles of the log odds. To compare the treatment groups with respect to their effect on improvement of motor function (yes vs. no) and post-radiotherapy ambulatory status (yes vs. no), a logistic regression model was applied. For adjustment the same propensities as above were used. The statistical analyses were performed with the SAS software (version 9.4; SAS, Cary, NC, USA).
3. Results
RAMSES-01 Trial
For the RAMSES-01 trial, 52 of 65 planned patients were recruited between 08/2019 and 11/2021. Since during the study, OS was found to be worse than expected, a new survival score was developed, which was more precise in predicting OS than the tool used for the RAMSES-01 trial [
20]. As a consequence, the RAMSES-01 trial was terminated. Two of the 52 enrolled patients died during the radiotherapy course after 6 of 18 fractions (sepsis) and 12 of 18 fractions (acute decompensated heart failure), respectively, and were not evaluable for LPFS. In accordance with the study protocol, these patients were not included in the analyses. Of the remaining 50 patients, 41 patients (82%) received VMAT and 9 patients (18%) IMRT, respectively. Of 13 patients treated with 18 fractions, 12 patients received 18 x 2.333 Gy, and one patient treated with concurrent immunotherapy received 15 x 2.333 + 3 x 2.0 Gy (EQD2 = 42.0 Gy
10, increase by 29% over 10 x 3.0 Gy). Of 37 patients treated with 15 fractions, 33 patients received 15 x 2.633 Gy, three patients treated with concurrent immunotherapy received 12 x 2.633 + 3 x 2.333 Gy (EQD2 = 40.5 Gy
10, increase by 25%), and one emergency patient who started with 3.0 Gy received 1 x 3.0 Gy + 12 x 2.633 + 2 x 2.333 (EQD2 = 41.4 Gy
10, increase by 27%), respectively. Twenty-eight patients received concurrent dexamethasone (median 8 mg/day, range: 4-16 mg/day). The other patients had contraindications, refused to take corticosteroids, or had very mild motor impairment. Since all 50 patients received at least 80% of the planned dose per protocol, they were eligible for the analyses.
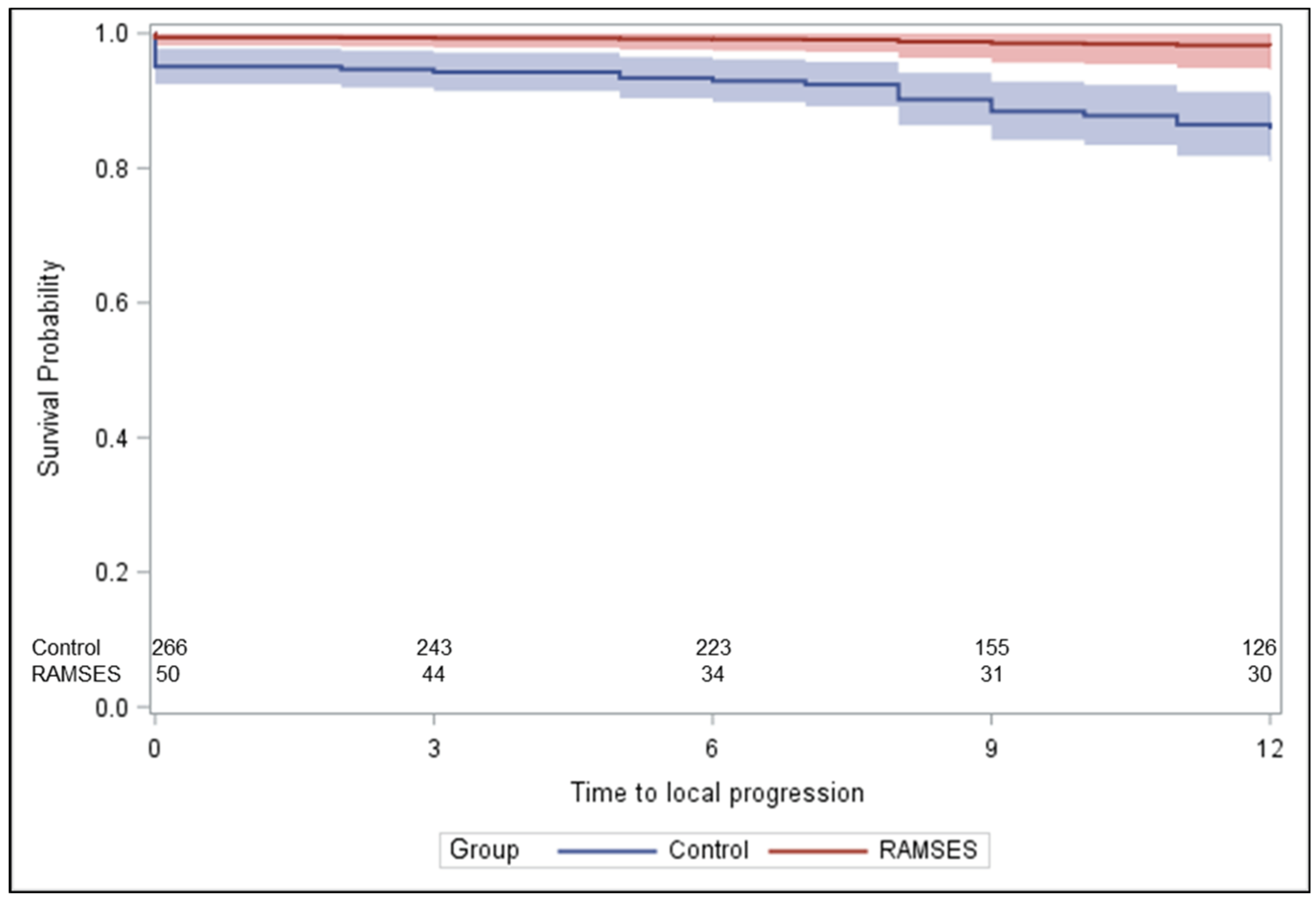
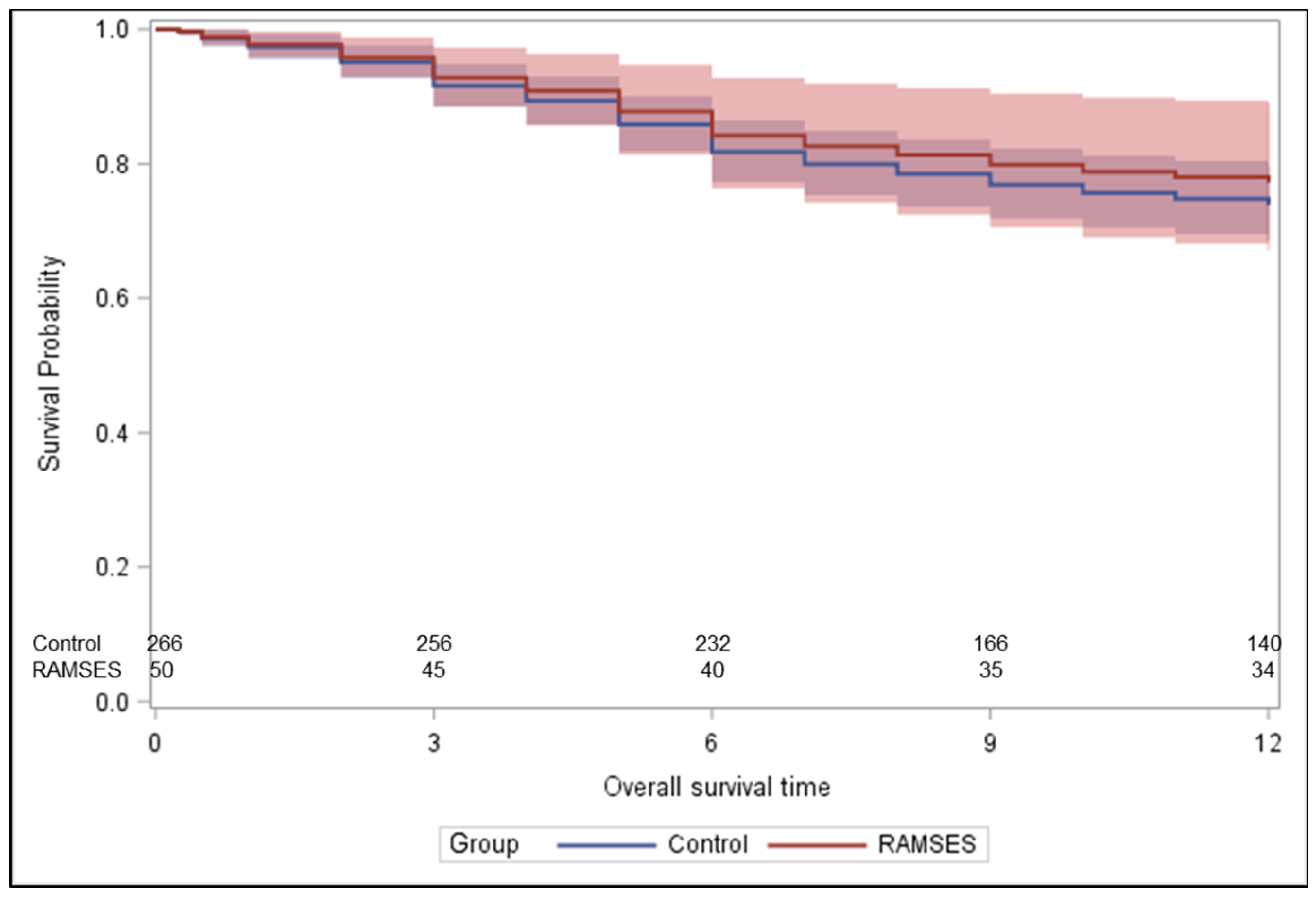
In these 50 patients, 12-month rates of LPFS and OS were 96.8% [95% CI: (79.2%; 99.5%)] and 69.9% [95% CI: (55.1%; 80.6%)], respectively. LPFS rates at 3, 6, and 9 months following radiotherapy were 100%, 100% and 96.8%, respectively, and OS rates were 88%, 74% and 72%, respectively. During the first 6 months following radiotherapy, improvement of motor function occurred in 28 patients (56.0%). Two additional patients (4.0%) improved until 9 months. Forty-seven patients (94.0%) were ambulatory following radiotherapy, including 45 patients who were ambulatory prior to radiotherapy and two non-ambulatory patients. Of the 27 patients being ambulatory with aid prior to radiotherapy, 8 patients (29.6%) regained normal strength and another 11 patients (40.7%) became ambulatory without aid.
Within 6 months following radiotherapy, 12 of 21 patients (57.2%) with pre-radiotherapy sensory deficits improved. One additional patient (4.8%) improved until 9 months. Thirty-eight of 45 patients with pre-radiotherapy pain (84.4%) experienced at least partial relief (best response) within 6 months following radiotherapy, of whom 10 patients (22.2%) achieved complete relief. Forty-one patients (82.0%) reported relief of distress within 6 months following radiotherapy. Grade 2 toxicities (mainly esophagitis/dysphagia) occurred in 10 patients (20.0%), grade 3 toxicities in another two patients (1 diarrhea, 1 esophagitis). Late sequelae such as myelopathy or vertebral fractures did not occur.
Comparison to the Historical Control Group
Two-hundred-sixty-six patients met the criteria for the control group including reception of ≥80% of the planned dose of 10 x 3.0 Gy (
Figure 1). These patients received concurrent dexamethasone with doses of 4-32 mg/day.
Figure 2 demonstrates the statistically significant heterogeneity of the propensity score distributions between the groups and the need for adjustment. After propensity score adjustment, patients of the RAMSES-01 trial showed significantly better LPFS than patients of the control group (hazard ratio 0.116,
p = 0.039,
Figure 3) and a trend for improvement of motor function (odds ratio 1.943,
p = 0.057). Post-radiotherapy ambulatory rates (odds ratio 1.484,
p = 0.56) and OS rates (hazard ratio 0.851,
p = 0.62,
Figure 4) were not significantly different. The results of the unadjusted/crude and propensity score adjusted analyses regarding LPFS, improvement of motor function, post-radiotherapy ambulatory status, and OS are shown in
Table 2.
3. Discussion
In 2011, a retrospective matched-pair study was performed in patients with favorable survival prognoses receiving conventional radiotherapy for motor deficits due to MESCC [
11]. This study compared 191 patients receiving 10 x 3.0 Gy (EQD2 = 32.5 Gy
10) to 191 patients treated with 15 x 2.5 Gy (EQD2 = 39.1 Gy
10) or 20 x 2.0 Gy (EQD2 = 40.0 Gy
10). The higher doses were associated with significantly improved LC of MESCC (92% vs. 87% at 12 months, p = 0.012), LPFS (90% vs. 84%,
p = 0.013), and OS (81% vs. 76%,
p = 0.032) [
11]. Since highly-conformal radiotherapy techniques such as VMAT and IMRT provide better sparing of normal tissues including the spinal cord, these techniques allow an increase of the EQD2 beyond 40.0 Gy without exceeding the tolerance dose of the spinal cord of 45-50 Gy [
21,
22]. In the RAMSES-01 trial, patients received VMAT or IMRT with an EQD2 of 40.5 Gy
10 to 43.2 Gy
10 for tumor cell kill (≥41.4 Gy
10 in 94% of patients) and a maximum EQD2 of 46.6 Gy
2 for myelopathy [
9,
10]. Patients of the RAMSES-01 cohort were compared to a historical control group treated with 10 x 3.0 Gy, the most common longer-course regimen for MESCC.
During the phase 2 trial, we realized that the survival of the patients was worse than expected. This finding may be explained by the fact that, since the Patchell trial was published, many patients with longer estimated survival times receive upfront surgery and those assigned to radiotherapy alone have comparably less favorable prognoses [
3]. Therefore, we decided to develop a new survival score based on the data of patients treated with radiotherapy alone within prospective trials [
20]. The new scoring tool was based on three prognostic factors, namely type of primary tumor, pre-radiotherapy ambulatory status, and visceral metastases. It was more accurate in identifying patients who live 6 months or longer when compared to the previous score [
13,
20]. Corresponding positive predictive values were 90% and 64%, respectively. Since the RAMSES-01 trial was based on the previous survival score, the decision was made to stop the recruitment. At that time, 52 patients (80% of the planned sample size) had already been enrolled, of whom 50 patients were evaluable for the primary endpoint LPFS and, therefore, eligible for the planned analyses.
The outcomes of these 50 patients were very promising with respect to LPFS (96.8%), improvement of motor function (56.0%), improvement of sensory function (57.2%), post-radiotherapy ambulatory status (94.0%), pain relief (84.4%), and relief of distress (78.0%). Moreover, the dose-fractionation regimens used in the phase 2 part of our study were sufficiently well tolerated with grade 3 toxicities occurring in only two patients (4%). Furthermore, late radiation-related toxicity was not observed during the period of follow-up. When compared to the results of the previous matched-pair study, the 12-month LPFS rate in the RAMSES-01 cohort was higher [
11]. This applied to both patients receiving 10 x 3.0 Gy (96.8% vs. 84%) and patients receiving 15 x 2.5 Gy or 20 x 2 Gy (96.8% vs. 90%). In addition, improvement of motor function was more frequent in the RAMSES-01 cohort than in the treatment groups of the matched-pair study (56.0% vs. 41% and 40%, respectively). Moreover, the rate of pain relief was higher than the rates of 58-81% reported in previous prospective trials for patients irradiated for painful bone metastases [
23,
24,
25,
26,
27]. The favorable outcomes of the dose-fractionation regimens used in the RAMSES-01 trial were confirmed in the second part of the present study, the comparison to the historic control group treated with 10 x 3.0 Gy. The RAMSES-01 regimens resulted in significantly better LPFS and showed a strong trend for higher rate of improvement of motor function.
Although dose-fractionation regimens of the RAMSES-01 trial appear preferable for patients with MESCC and favorable survival prognoses, the limitations of this study should be considered. Its major limitation is the fact that the study had to be closed after inclusion of 80% of the planned sample size. On the other hand, the main study objective, demonstrating that the RAMSES-01 regimens lead to improved LPFS, has been achieved. Further limitations of this study include the fact that four patients (8%) received an EQD2 of less than 41.6 Gy
10, the EQD2 of 15 x 2.633 Gy, and the retrospective nature of the historical control group. Despite the procedure of propensity score adjusted Cox regression, a hidden selection bias cannot be entirely eliminated. Moreover, selected patients with single or very few lesions may be candidates for other highly-conformal techniques such as stereotactic body radiation therapy (SBRT) [
28,
29,
30].
5. Conclusions
Highly-conformal radiotherapy with 15 x 2.633 Gy or 18 x 2.333 Gy was sufficiently well tolerated and resulted in significantly better long-term LPFS than 10 x 3.0 Gy in patients with MESCC and favorable survival prognoses. Given the limitations of the present study, the dose-fractionation regimens of the RAMSES-01 trial appear preferable for these patients, if they are candidates for radiotherapy alone without upfront surgery. Additional prospective trials are required to define the optimal dose-fractionation regimen of radiotherapy for MESCC in patients with favorable survival prognoses.
Author Contributions
Conceptualization, D.R., D.L., N.J., F.L.C., A.N.-M., B.S., B.G., C.S., C.K., K.D., S.E.S. and J.C.; methodology, D.R., D.L., N.J., F.L.C., A.N.-M., B.S., B.G., C.S., C.K., K.D., S.E.S. and J.C.; validation, D.R. (supported by a professional statistician); formal analysis, D.R. (supported by a professional statistician); investigation, D.R., D.L., N.J., F.L.C., A.N.-M., B.S., B.G., C.S., C.K., K.D. and J.C.; resources, D.R., D.L., N.J., F.L.C., A.N.-M., B.S., B.G., C.S., C.K., K.D. and J.C.; data curation, D.R. (supported by a professional statistician); writing—original draft preparation, D.R., K.D. and S.E.S.; writing—review and editing, D.R., D.L., N.J., F.L.C., A.N.-M., B.S., B.G., C.S., C.K., K.D., S.E.S. and J.C.; visualization, D.R., D.L., N.J., F.L.C., A.N.-M., B.S., B.G., C.S., C.K., K.D., S.E.S. and J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was performed within the project Changing Cancer Care, which received funding from the European Regional Development Fund through the Interreg Deutschland-Danmark program. The study itself has not received specific external funding.
Institutional Review Board Statement
The study was approved by local ethic committees (leading committee: University of Lübeck, file: 18-360) on 19-MAR-2019.
Informed Consent Statement
Written informed consent has been obtained from the patients for participation in the prospective RAMSES-01 trial. The data of the historical control group were obtained from an existing anonymized database.
Data Availability Statement
Data of the RAMSES-01 trial are available at clinicaltrials.gov (identifier NCT04043156). Otherwise, the data analyzed for this paper cannot be shared due to data protection regulations.
Conflicts of Interest
The authors declare no conflict of interest related to this study.
References
- Lawton, A.J.; Lee, K.A.; Cheville, A.L.; Ferrone, M.L.; Rades, D.; Balboni, T.A.; Abrahm, J.L. Assessment and management of patients with metastatic spinal cord compression: A multidisciplinary review. J. Clin. Oncol. 2019, 37, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Prasad, D.; Schiff, D. Malignant spinal cord compression. Lancet Oncol. 2005, 6, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005, 366, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Stalpers, L.J.A.; Veninga, T.; Schulte, R.; Hoskin, P.J.; Obralic, N.; Bajrovic, A.; Rudat, V.; Schwarz, R.; Hulshof, M.C.; et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression in a series of 1304 patients. J. Clin. Oncol. 2005, 23, 3366–3375. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Hopkins, K.; Misra, V.; Holt, T.; McMenemin, R.; Dubois, D.; McKinna, F.; Foran, B.; Madhavan, K.; MacGregor, C.; et al. Effect of single-fraction vs multifraction radiotherapy on ambulatory status among patients with spinal canal compression from metastatic cancer: The SCORAD randomized clinical trial. JAMA 2019, 322, 2084–2094. [Google Scholar] [CrossRef] [PubMed]
- Thirion, P.G.; Dunne, M.T.; Kelly, P.J.; Flavin, A.; O’Sullivan, J.M.; Hacking, D.; Sasiadek, W.; Small, C.; Pomeroy, M.M.; Martin, J.; et al. Non-inferiority randomised phase 3 trial comparing two radiation schedules (single vs. five fractions) in malignant spinal cord compression. Br. J. Cancer, 2020; 122, 1315–1323. [Google Scholar] [CrossRef]
- Rades, D.; Šegedin, B.; Conde-Moreno, A.J.; Garcia, R.; Perpar, A.; Metz, M.; Badakhshi, H.; Schreiber, A.; Nitsche, M.; Hipp., P; et al. Radiotherapy with 4 Gy × 5 versus 3 Gy × 10 for metastatic epidural spinal cord compression: Final results of the SCORE-2 trial (ARO 2009/01). J. Clin. Oncol. 2016, 34, 597–602. [Google Scholar] [CrossRef]
- Rades, D.; Lange, M.; Veninga, T.; Stalpers, L.J.; Bajrovic, A.; Adamietz, I.A.; Rudat, V.; Schild, S.E. Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 524–530. [Google Scholar] [CrossRef]
- Barendsen, G.W. Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 1981–1997. [Google Scholar] [CrossRef]
- Joiner, M.C.; Van der Kogel, A.J. The linear-quadratic approach to fractionation and calculation of isoeffect relationships. In Steel, G.G. (Ed.), Basic clinical radiobiology, Oxford University Press, New York; 1997, pp. 106-112.
- Rades, D.; Panzner, A.; Rudat, V.; Karstens, J.H.; Schild, S.E. Dose escalation of radiotherapy for metastatic spinal cord compression (MSCC) in patients with relatively favorable survival prognosis. Strahlenther. Onkol. 2011, 187, 729–735. [Google Scholar] [CrossRef]
- Rades, D.; Hansen, O.; Jensen, L.H.; Dziggel, L.; Staackmann, C.; Doemer, C.; Cacicedo, J.; Conde-Moreno, A.J.; Segedin, B.; Ciervide-Jurio, R.; et al. Radiotherapy for metastatic spinal cord compression with increased radiation doses (RAMSES-01): A prospective multicenter study. BMC Cancer 2019, 19, 1163. [Google Scholar] [CrossRef]
- Rades, D.; Dunst, J.; Schild, S.E. The first score predicting overall survival in patients with metastatic spinal cord compression. Cancer 2008, 112, 157–161. [Google Scholar] [CrossRef]
- Tomita, T.; Galicich, J.H.; Sundaresan, N. Radiation therapy for spinal epidural metastases with complete block. Acta Radiol. Oncol. 1983, 22, 135–143. [Google Scholar] [CrossRef]
- Baskin, D.S. Spinal cord injury. In Evans, R.W. (Ed.), Neurology and trauma, WB Saunders, Philadelphia; 1996, pp. 276–299.
- Chow, E.; Hoskin, P.; Mitera, G.; Zeng, L.; Lutz, S.; Roos, D.; Hahn, C.; van der Linden, Y.; Hartsell, W.; Kumar, E.; International Bone Metastases Consensus Working Party. Update of the international consenus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1730–1737. [Google Scholar] [CrossRef]
- Holland, J.C; Andersen, B.; Breitbart, W.S.; Buchmann, L.O.; Compas, B.; Deshields, T.L.; Dudley, M.M.; Fleishman, S.; Fulcher, C.D.; Greenberg, D.B.; et al. National Comprehensive Cancer Network. Distress management clinical practice guidelines in oncology. J. Natl. Comp. Cancer Network 2013, 11, 190–209. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health/National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. National Institutes of Health/National Cancer Institute 2010.
- Franklin, J.M.; Eddings, W.; Austin, P.C.; Stuart, E.A.; Schneeweiss, S. Comparing the performance of propensity score methods in healthcare database studies with rare outcomes. Stat. Med. 2017, 36, 1946–1963. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Cacicedo, J.; Lomidze, D.; Al-Salool, A.; Segedin, B.; Groselj, B.; Jankarashvili, N.; Conde-Moreno, A.J.; Schild, S.E. A new and easy-to-use survival score for patients irradiated for metastatic epidural spinal cord compression. Pract. Radiat. Oncol. 2022, 12, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.B.; Yorke, E.D.; Jackson, A.; Ten Haken, R.K.; Constine, L.S.; Eisbruch, A.; Bentzen, S.M.; Nam, J.; Deasy, J.O. Use of normal tissue complication probability models in the clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010, 6(3 Suppl), S10–S19. [Google Scholar] [CrossRef]
- Emami, B. Tolerance of the normal tissue to therapeutic irradiation. Rep. Radiother. Oncol. 2013, 1, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Steenland, E.; Leer, J.W.; van Houwelingen, H.; Post, W.J.; van den Hout, W.B.; Kievit, J.; de Haes, H.; Martijn, H.; Oei, B.; Vonk, E.; et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: A global analysis of the Dutch Bone Metastasis Study. Radiother. Oncol. 1999, 52, 101–109. [Google Scholar] [CrossRef]
- Bone Pain Trial Working Party. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain (randomised comparison with a multifraction schedule over 12 months of patient follow-up). Radiother. Oncol. 1999, 52, 111–121. [CrossRef]
- Hartsell, WE.; Scott, C.B.; Bruner, D.W.; Scarantino, C.W.; Ivker, R.A.; Roach, M. 3rd; Suh, J.H.; Demas, W.F.; Movsas, B.; Petersen, I.A.; et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J. Natl. Cancer Inst. 2005, 97, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Roos, D.E.; Turner, S.L.; O’Brien, PC.; Smith, J.G.; Spry, N.A.; Burmeister, B.H.; Hoskin, P.J.; Ball, D.L; Trans-Tasman Radiation Oncology Group, TROG 96.05. Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases (Trans-Tasman Radiation Oncology Group, TROG 96.05). Radiother. Oncol. 2005, 75, 54-63. [CrossRef]
- Foro Arnalot, P.; Fontanals, A.V.; Galcerán, J.C.; Lynd, F.; Latiesas, X.S.; de Dios, N.R.; Castillejo, A.R.; Bassols, M.L.; Galán, J.L.; Conejo, I.M.; et al. Randomized clinical trial with two palliative radiotherapy regimens in painful bone metastases: 30 Gy in 10 fractions compared with 8 Gy in single fraction. Radiother. Oncol. 2008, 89, 150–155. [Google Scholar] [CrossRef]
- Guckenberger, M.; Mantel, F.; Gerszten, P.C.; Flickinger, J.C.; Sahgal, A.; Létourneau, D.; Grills, I.S.; Jawad, M.; Fahim, D.K.; Shin, J.H.; et al. Safety and efficacy of stereotactic body radiotherapy as primary treatment for vertebral metastases: A multi-institutional analysis. Radiat. Oncol. 2014, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.C.Y.; Lee, S.F.; Chan, A.W.; Caini, S.; Hoskin, P.; Simone, C.B. 2nd; Johnstone, P.; van der Linden, Y.; van der Velden, J.M.; Martin, E.; et al. Stereotactic body radiation therapy versus conventional external beam radiotherapy for spinal metastases: A systematic review and meta-analysis of randomized controlled trials. Radiother. Oncol. 2023, 189, 109914. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Dahele, M.; Ong, W.L.; Sahgal, A. Stereotactic body radiation therapy for spinal metastases: Benefits and limitations. Semin. Radiat. Oncol. 2023, 33, 159-171. Radiat. Oncol. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).