Submitted:
02 March 2024
Posted:
04 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. ASD – Genetics
2.1. Epigenetics
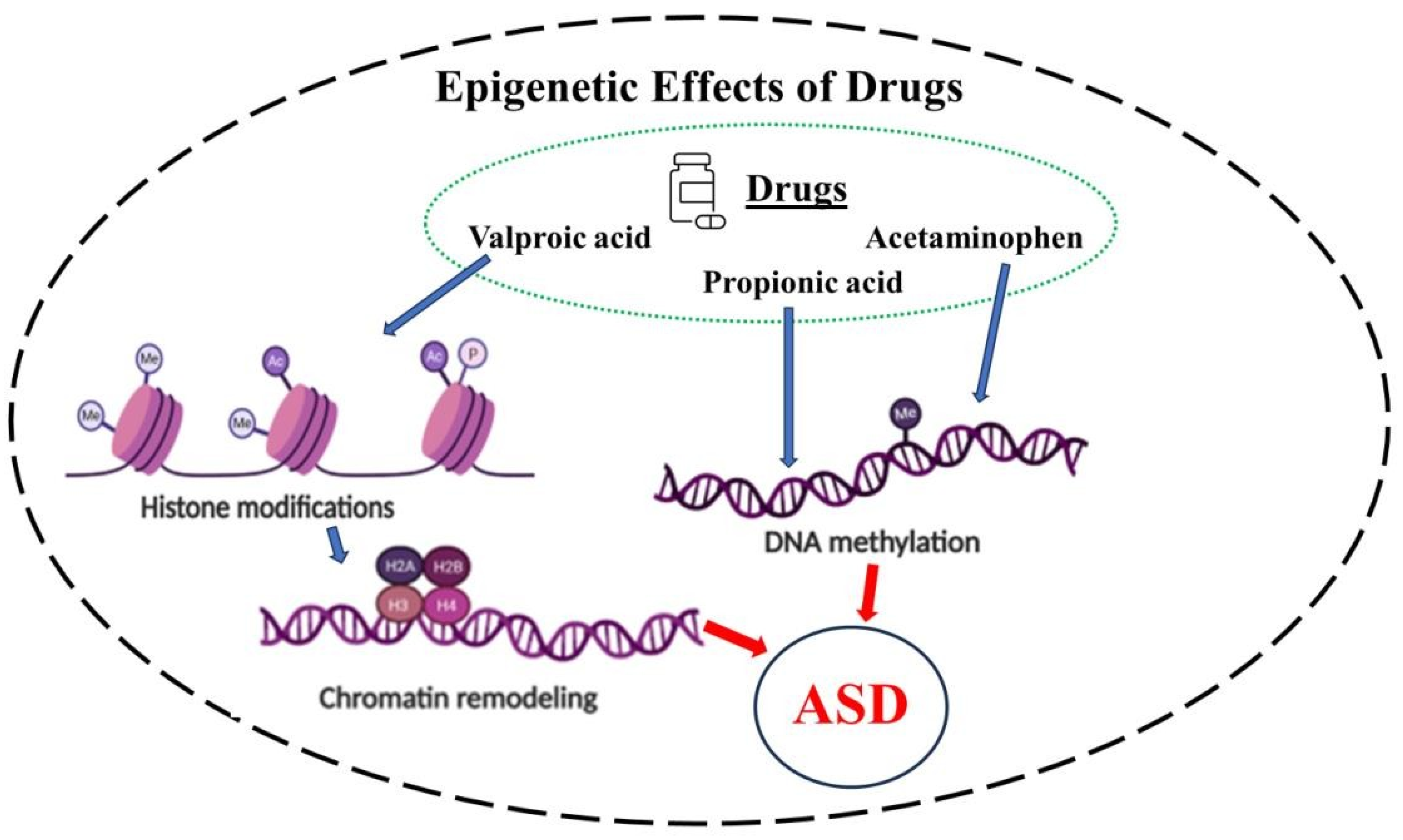
- Modifications to histones that either make the chromatin available (euchromatin state) or unavailable (heterochromatin) for transcriptional processes [24,35,36]. In this context, three different mechanisms have been described. First, is histone methylation that usually silences DNA expression. Second is histone acetylation that relaxes DNA coiling, increasing its transcription. Third is the reverse process, histone deacetylation that removes an acetyl group and further tightens DNA coiling, thus decreasing gene expression.
- DNA methylation [37,38], is a reversible mechanism wherein methyl groups (–CH3) are delivered to cytosines positioned in CpG (5′ -cytosine-phosphate-guanosine-3′ ) nucleotides turning these cytosines into 5-methyl cytosines (5mC) [39]. When methylation occurs in cytosine-phosphate-guanine (CpG) islands in the gene promoter, interaction between the DNA and transcription factors is reduced and the gene is silenced [3,40]. In neural cells, either hypermethylation or hypomethylation of DNA can affect learning or memory. Indeed, dysregulated methylation has been linked to neurodevelopmental disorders such as ASD [39].
- Gene silencing may also occur via non-coding RNA (ncRNA), referring to RNA sequences that are transcribed but not translated, hence not leading to protein synthesis [41]. More than 89% of non-ribosomal RNA transcripts are non-coding [41]. After years of being considered as junk RNA, recent studies emphasize the crucial role of ncRNA in modulating the expression of the genome [42].
2.2. Epigenetics – ASD
2.3. Glial Cells -ASD
3. Valproic Acid (VPA)
3.1. VPA Action in Glial Cells
3.2. VPA and Pregnancy
3.3. VPA and ASD
4. Acetaminophen (APAP)
4.1. APAP Action in Glial Cells
4.2. APAP and Pregnancy
4.3. APAP and ASD
5. Propionic Acid
5.1. PPA–Glial Cells–ASD
5.2. Epigenetic Mechanisms in ASD
5.3. Epigenetics-Glial Cells-ASD
5.4. VPA–Epigenetics–ASD
5.5. APAP–Epigenetics–ASD
5.6. PPA-Epigenetics-ASD
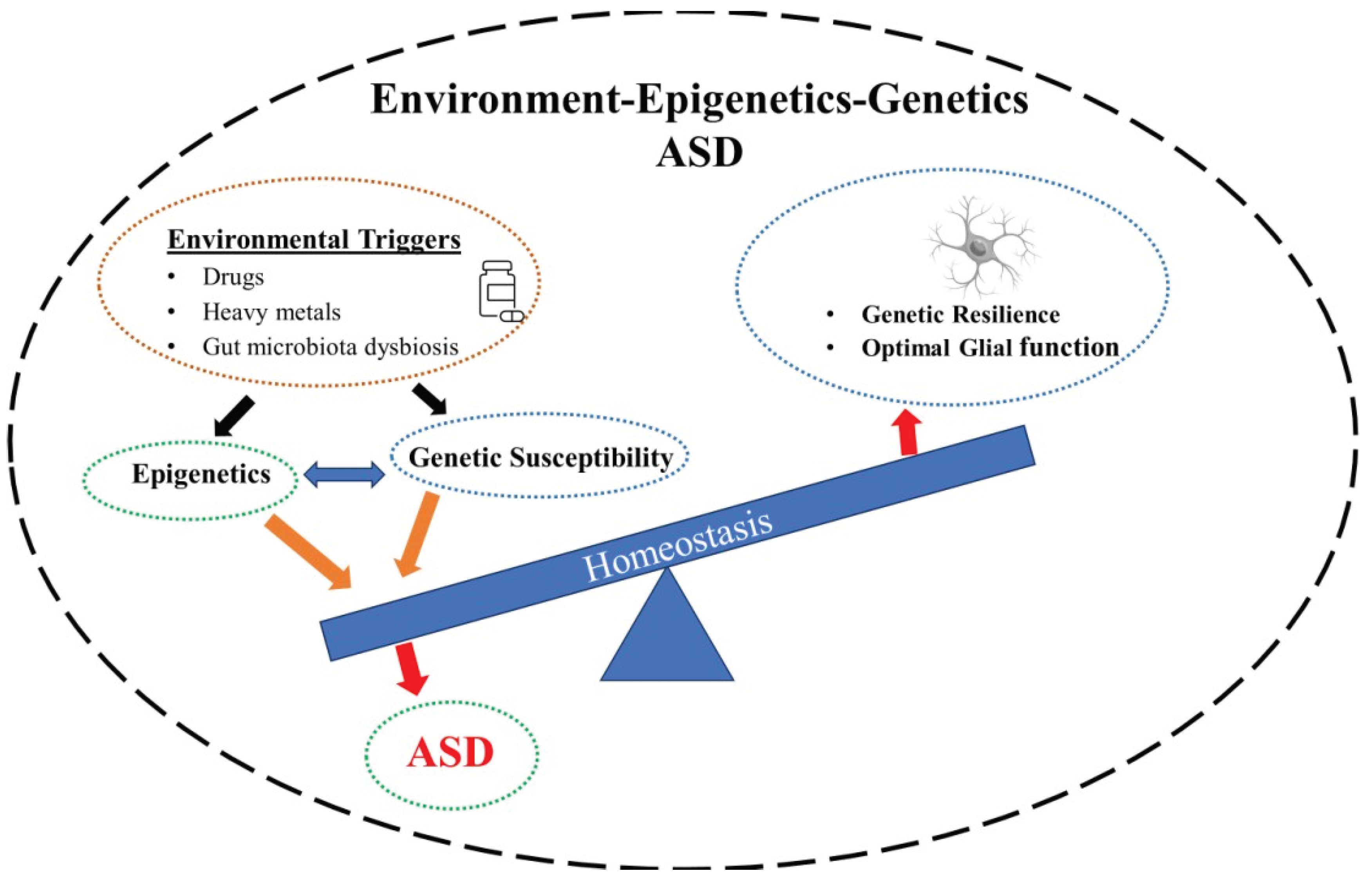
6. Implications for Novel Interventions in ASD
7. Conclusion
Acknowledgements
References
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef]
- Bailey, A.; Le Couteur, A.; Gottesman, I.; Bolton, P.; Simonoff, E.; Yuzda, E.; Rutter, M. Autism as a Strongly Genetic Disorder: Evidence from a British Twin Study. Psychol. Med. 1995, 25, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Masini, E.; Loi, E.; Vega-Benedetti, A.F.; Carta, M.; Doneddu, G.; Fadda, R.; Zavattari, P. An Overview of the Main Genetic, Epigenetic and Environmental Factors Involved in Autism Spectrum Disorder Focusing on Synaptic Activity. Int. J. Mol. Sci. 2020, 21, 8290. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.Y.; Kabiraj, G.; Ahmed, M.A.; Adam, M.; Mannuru, S.P.; Ramesh, V.; Shahzad, A.; Chaduvula, P.; Khan, S. A Systematic Review of the Link Between Autism Spectrum Disorder and Acetaminophen: A Mystery to Resolve. Cureus 2022, 14, e26995. [Google Scholar] [CrossRef] [PubMed]
- Benachenhou, S.; Laroui, A.; Dionne, O.; Rojas, D.; Toupin, A.; Çaku, A. Cholesterol Alterations in Fragile X Syndrome, Autism Spectrum Disorders and Other Neurodevelopmental Disorders. In International Review of Neurobiology; Elsevier, 2023; Vol. 173, pp. 115–139. ISBN 978-0-443-13729-7. [Google Scholar] [CrossRef]
- Tizabi, Y.; Bennani, S.; El Kouhen, N.; Getachew, B.; Aschner, M. Interaction of Heavy Metal Lead with Gut Microbiota: Implications for Autism Spectrum Disorder. Biomolecules 2023, 13, 1549. [Google Scholar] [CrossRef] [PubMed]
- Andalib, S.; Emamhadi, M.R.; Yousefzadeh-Chabok, S.; Shakouri, S.K.; Høilund-Carlsen, P.F.; Vafaee, M.S.; Michel, T.M. Maternal SSRI Exposure Increases the Risk of Autistic Offspring: A Meta-Analysis and Systematic Review. Eur. Psychiatry 2017, 45, 161–166. [Google Scholar] [CrossRef]
- Ji, Y.; Azuine, R.E.; Zhang, Y.; Hou, W.; Hong, X.; Wang, G.; Riley, A.; Pearson, C.; Zuckerman, B.; Wang, X. Association of Cord Plasma Biomarkers of In Utero Acetaminophen Exposure With Risk of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in Childhood. JAMA Psychiatry 2020, 77, 180–189. [Google Scholar] [CrossRef]
- Gidaya, N.B.; Lee, B.K.; Burstyn, I.; Michael, Y.; Newschaffer, C.J.; Mortensen, E.L. In Utero Exposure to β-2-Adrenergic Receptor Agonist Drugs and Risk for Autism Spectrum Disorders. Pediatrics 2016, 137, e20151316. [Google Scholar] [CrossRef]
- Christensen, J.; Grønborg, T.K.; Sørensen, M.J.; Schendel, D.; Parner, E.T.; Pedersen, L.H.; Vestergaard, M. Prenatal Valproate Exposure and Risk of Autism Spectrum Disorders and Childhood Autism. JAMA 2013, 309, 1696. [Google Scholar] [CrossRef]
- Rubenstein, E.; Young, J.C.; Croen, L.A.; DiGuiseppi, C.; Dowling, N.F.; Lee, L.-C.; Schieve, L.; Wiggins, L.D.; Daniels, J. Brief Report: Maternal Opioid Prescription from Preconception Through Pregnancy and the Odds of Autism Spectrum Disorder and Autism Features in Children. J. Autism Dev. Disord. 2019, 49, 376–382. [Google Scholar] [CrossRef]
- Hansen, J.B.; Bilenberg, N.; Timmermann, C.A.G.; Jensen, R.C.; Frederiksen, H.; Andersson, A.-M.; Kyhl, H.B.; Jensen, T.K. Prenatal Exposure to Bisphenol A and Autistic- and ADHD-Related Symptoms in Children Aged 2 And5 Years from the Odense Child Cohort. Environ. Health 2021, 20, 24. [Google Scholar] [CrossRef]
- Kim, J.I.; Lee, J.; Lee, K.-S.; Lee, Y.A.; Shin, C.H.; Hong, Y.-C.; Kim, B.-N.; Lim, Y.-H. Association of Phthalate Exposure with Autistic Traits in Children. Environ. Int. 2021, 157, 106775. [Google Scholar] [CrossRef]
- El-Ansary, A.K.; Bacha, A.B.; Kotb, M. Etiology of Autistic Features: The Persisting Neurotoxic Effects of Propionic Acid. J. Neuroinflammation 2012, 9, 661. [Google Scholar] [CrossRef]
- Williams, L.A.; LaSalle, J.M. Future Prospects for Epigenetics in Autism Spectrum Disorder. Mol. Diagn. Ther. 2022, 26, 569–579. [Google Scholar] [CrossRef]
- LaSalle, J.M. Epigenomic Signatures Reveal Mechanistic Clues and Predictive Markers for Autism Spectrum Disorder. Mol. Psychiatry 2023, 28, 1890–1901. [Google Scholar] [CrossRef]
- Sandin, S.; Lichtenstein, P.; Kuja-Halkola, R.; Hultman, C.; Larsson, H.; Reichenberg, A. The Heritability of Autism Spectrum Disorder. JAMA 2017, 318, 1182. [Google Scholar] [CrossRef] [PubMed]
- Taleb, A.; Lin, W.; Xu, X.; Zhang, G.; Zhou, Q.-G.; Naveed, M.; Meng, F.; Fukunaga, K.; Han, F. Emerging Mechanisms of Valproic Acid-Induced Neurotoxic Events in Autism and Its Implications for Pharmacological Treatment. Biomed. Pharmacother. 2021, 137, 111322. [Google Scholar] [CrossRef] [PubMed]
- Ghirardi, L.; Kuja-Halkola, R.; Butwicka, A.; Martin, J.; Larsson, H.; D’Onofrio, B.M.; Lichtenstein, P.; Taylor, M.J. Familial and Genetic Associations between Autism Spectrum Disorder and Other Neurodevelopmental and Psychiatric Disorders. J. Child Psychol. Psychiatry 2021, 62, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Yip, B.H.K.; Windham, G.C.; Sourander, A.; Francis, R.; Yoffe, R.; Glasson, E.; Mahjani, B.; Suominen, A.; Leonard, H.; et al. Association of Genetic and Environmental Factors With Autism in a 5-Country Cohort. JAMA Psychiatry 2019, 76, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Butler, M.G. The Autism Spectrum: Behavioral, Psychiatric and Genetic Associations. Genes 2023, 14, 677. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.S.; Wassman, E.R.; Baxter, A.L.; Hensel, C.H.; Martin, M.M.; Prasad, A.; Twede, H.; Vanzo, R.J.; Butler, M.G. Chromosomal Microarray Analysis of Consecutive Individuals with Autism Spectrum Disorders Using an Ultra-High Resolution Chromosomal Microarray Optimized for Neurodevelopmental Disorders. Int. J. Mol. Sci. 2016, 17, 2070. [Google Scholar] [CrossRef]
- Dhaliwal, N.; Choi, W.W.Y.; Muffat, J.; Li, Y. Modeling PTEN Overexpression-Induced Microcephaly in Human Brain Organoids. Mol. Brain 2021, 14, 131. [Google Scholar] [CrossRef]
- Al Aboud, N.M.; Tupper, C.; Jialal, I. Genetics, Epigenetic Mechanism. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Kanherkar, R.R.; Bhatia-Dey, N.; Csoka, A.B. Epigenetics across the Human Lifespan. Front. Cell Dev. Biol. 2014, 2. [Google Scholar] [CrossRef]
- Csoka, A.B.; Szyf, M. Epigenetic Side-Effects of Common Pharmaceuticals: A Potential New Field in Medicine and Pharmacology. Med. Hypotheses 2009, 73, 770–780. [Google Scholar] [CrossRef]
- Kanherkar, R.R.; Getachew, B.; Ben-Sheetrit, J.; Varma, S.; Heinbockel, T.; Tizabi, Y.; Csoka, A.B. The Effect of Citalopram on Genome-Wide DNA Methylation of Human Cells. Int. J. Genomics 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Kanherkar, R.R.; Stair, S.E.; Bhatia-Dey, N.; Mills, P.J.; Chopra, D.; Csoka, A.B. Epigenetic Mechanisms of Integrative Medicine. Evid. Based Complement. Alternat. Med. 2017, 2017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- A. Alhazzaa, R.; Heinbockel, T.; B. Csoka, A. Diabetes and Epigenetics. In Biochemistry; Anwar, M., Farooq, Z., Ahmad Rather, R., Tauseef, M., Heinbockel, T., Eds.; IntechOpen, 2022; Vol. 35, ISBN 978-1-83880-993-5. [Google Scholar]
- Alhazzaa, R.A.; McKinley, R.E.; Getachew, B.; Tizabi, Y.; Heinbockel, T.; Csoka, A.B. Epigenetic Changes Induced by High Glucose in Human Pancreatic Beta Cells. J. Diabetes Res. 2023, 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Minchin, S.; Lodge, J. Understanding Biochemistry: Structure and Function of Nucleic Acids. Essays Biochem. 2019, 63, 433–456. [Google Scholar] [CrossRef] [PubMed]
- Paro, R.; Grossniklaus, U.; Santoro, R.; Wutz, A. Biology of Chromatin. In Introduction to Epigenetics; Learning Materials in Biosciences; Springer International Publishing: Cham, 2021; pp. 1–28. ISBN 978-3-030-68669-7. [Google Scholar]
- Ghannam, J.Y.; Wang, J.; Jan, A. Biochemistry, DNA Structure. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Dombrowski, M.; Engeholm, M.; Dienemann, C.; Dodonova, S.; Cramer, P. Histone H1 Binding to Nucleosome Arrays Depends on Linker DNA Length and Trajectory. Nat. Struct. Mol. Biol. 2022, 29, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Grant, P.A. The Role of DNA Methylation and Histone Modifications in Transcriptional Regulation in Humans. Subcell. Biochem. 2013, 61, 289–317. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, Y. DNA Methylation in Human Diseases. Genes Dis. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Liu, R.; Wu, J.; Guo, H.; Yao, W.; Li, S.; Lu, Y.; Jia, Y.; Liang, X.; Tang, J.; Zhang, H. Post-Translational Modifications of Histones: Mechanisms, Biological Functions, and Therapeutic Targets. MedComm 2023, 4, e292. [Google Scholar] [CrossRef]
- Rasmi, Y.; Shokati, A.; Hassan, A.; Aziz, S.G.-G.; Bastani, S.; Jalali, L.; Moradi, F.; Alipour, S. The Role of DNA Methylation in Progression of Neurological Disorders and Neurodegenerative Diseases as Well as the Prospect of Using DNA Methylation Inhibitors as Therapeutic Agents for Such Disorders. IBRO Neurosci. Rep. 2023, 14, 28–37. [Google Scholar] [CrossRef]
- Martínez-Iglesias, O.; Carrera, I.; Carril, J.C.; Fernández-Novoa, L.; Cacabelos, N.; Cacabelos, R. DNA Methylation in Neurodegenerative and Cerebrovascular Disorders. Int. J. Mol. Sci. 2020, 21, 2220. [Google Scholar] [CrossRef]
- Kimura, R.; Nakata, M.; Funabiki, Y.; Suzuki, S.; Awaya, T.; Murai, T.; Hagiwara, M. An Epigenetic Biomarker for Adult High-Functioning Autism Spectrum Disorder. Sci. Rep. 2019, 9, 13662. [Google Scholar] [CrossRef] [PubMed]
- Boivin, V.; Deschamps-Francoeur, G.; Couture, S.; Nottingham, R.M.; Bouchard-Bourelle, P.; Lambowitz, A.M.; Scott, M.S.; Abou-Elela, S. Simultaneous Sequencing of Coding and Noncoding RNA Reveals a Human Transcriptome Dominated by a Small Number of Highly Expressed Noncoding Genes. RNA N. Y. N 2018, 24, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, Y.-X.; Gu, L.-J.; Cheng, Y. Understanding Autism Spectrum Disorders with Animal Models: Applications, Insights, and Perspectives. Zool. Res. 2021, 42, 800–824. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.U.; Beaudet, A.L.; Madduri, N.; Bacino, C.A. Autism in Angelman Syndrome: Implications for Autism Research. Clin. Genet. 2004, 66, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Beygo, J.; Grosser, C.; Kaya, S.; Mertel, C.; Buiting, K.; Horsthemke, B. Common Genetic Variation in the Angelman Syndrome Imprinting Centre Affects the Imprinting of Chromosome 15. Eur. J. Hum. Genet. EJHG 2020, 28, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Madaan, M.; Mendez, M.D. Angelman Syndrome. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Ryan, N.M.; Heron, E.A. Evidence for Parent-of-Origin Effects in Autism Spectrum Disorder: A Narrative Review. J. Appl. Genet. 2023, 64, 303–317. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. The Human Brain in Numbers: A Linearly Scaled-up Primate Brain. Front. Hum. Neurosci. 2009, 3, 31. [Google Scholar] [CrossRef]
- Shi, J.; Huang, S. Comparative Insight into Microglia/Macrophages-Associated Pathways in Glioblastoma and Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 25, 16. [Google Scholar] [CrossRef]
- Kim, J.D.; Copperi, F.; Diano, S. Microglia in Central Control of Metabolism. Physiology 2024, 39, 5–17. [Google Scholar] [CrossRef]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite Synapses: Astrocytes Process and Control Synaptic Information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef]
- Allen, N.J.; Eroglu, C. Cell Biology of Astrocyte-Synapse Interactions. Neuron 2017, 96, 697–708. [Google Scholar] [CrossRef]
- Novikov, N.I.; Brazhnik, E.S.; Kitchigina, V.F. Pathological Correlates of Cognitive Decline in Parkinson’s Disease: From Molecules to Neural Networks. Biochem. Mosc. 2023, 88, 1890–1904. [Google Scholar] [CrossRef]
- Manu, D.R.; Slevin, M.; Barcutean, L.; Forro, T.; Boghitoiu, T.; Balasa, R. Astrocyte Involvement in Blood–Brain Barrier Function: A Critical Update Highlighting Novel, Complex, Neurovascular Interactions. Int. J. Mol. Sci. 2023, 24, 17146. [Google Scholar] [CrossRef]
- Fernandes, V.M.; Auld, V.; Klämbt, C. Glia as Functional Barriers and Signaling Intermediaries. Cold Spring Harb. Perspect. Biol. 2024, 16, a041423. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R.; Brandmann, M.; Hohnholt, M.C.; Blumrich, E.-M. Glutathione-Dependent Detoxification Processes in Astrocytes. Neurochem. Res. 2015, 40, 2570–2582. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, M.S.; Jackson, J.; Sheu, S.-H.; Chang, C.-L.; Weigel, A.V.; Liu, H.; Pasolli, H.A.; Xu, C.S.; Pang, S.; Matthies, D.; et al. Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 2019, 177, 1522–1535.e14. [Google Scholar] [CrossRef]
- Fiacco, T.A.; McCarthy, K.D. Multiple Lines of Evidence Indicate That Gliotransmission Does Not Occur under Physiological Conditions. J. Neurosci. 2018, 38, 3–13. [Google Scholar] [CrossRef]
- Lalo, U.; Koh, W.; Lee, C.J.; Pankratov, Y. The Tripartite Glutamatergic Synapse. Neuropharmacology 2021, 199, 108758. [Google Scholar] [CrossRef]
- Rasia-Filho, A.A.; Calcagnotto, M.E.; Von Bohlen Und Halbach, O. Glial Cell Modulation of Dendritic Spine Structure and Synaptic Function. In Dendritic Spines; Rasia-Filho, A.A., Calcagnotto, M.E., Von Bohlen Und Halbach, O., Eds.; Advances in Neurobiology; Springer International Publishing: Cham, 2023; Vol. 34, pp. 255–310. ISBN 978-3-031-36158-6. [Google Scholar]
- Channels and Transporters in Astrocyte Volume Regulation in Health and Disease. Cell. Physiol. Biochem. 2022, 56, 12–30. [CrossRef] [PubMed]
- Clayton, R.W.; Lovell-Badge, R.; Galichet, C. The Properties and Functions of Glial Cell Types of the Hypothalamic Median Eminence. Front. Endocrinol. 2022, 13, 953995. [Google Scholar] [CrossRef]
- Chen, X.; Holtzman, D.M. Emerging Roles of Innate and Adaptive Immunity in Alzheimer’s Disease. Immunity 2022, 55, 2236–2254. [Google Scholar] [CrossRef] [PubMed]
- Kofler, J.; Wiley, C.A. Microglia: Key Innate Immune Cells of the Brain. Toxicol. Pathol. 2011, 39, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Ebling, F.J.P.; Lewis, J.E. Tanycytes and Hypothalamic Control of Energy Metabolism. Glia 2018, 66, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, K.A.; Huang, N.; Xie, Y.; LiCausi, F.; Li, S.; Li, Y.; Sheng, Z.-H. Oligodendrocytes Enhance Axonal Energy Metabolism by Deacetylation of Mitochondrial Proteins through Transcellular Delivery of SIRT2. Neuron 2021, 109, 3456–3472.e8. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Petidier, M.; Guerri, C.; Moreno-Manzano, V. Toll-like Receptors 2 and 4 Differentially Regulate the Self-Renewal and Differentiation of Spinal Cord Neural Precursor Cells. Stem Cell Res. Ther. 2022, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Wies Mancini, V.S.B.; Mattera, V.S.; Pasquini, J.M.; Pasquini, L.A.; Correale, J.D. Microglia-derived Extracellular Vesicles in Homeostasis and Demyelination/Remyelination Processes. J. Neurochem. 2024, 168, 3–25. [Google Scholar] [CrossRef]
- Rahman, S.; Alzarea, S. Glial Mechanisms Underlying Major Depressive Disorder: Potential Therapeutic Opportunities. In Progress in Molecular Biology and Translational Science; Elsevier, 2019; Vol. 167, pp. 159–178. ISBN 978-0-12-818855-2. [Google Scholar]
- Scuderi, C.; Verkhratsky, A.; Parpura, V.; Li, B. Neuroglia in Psychiatric Disorders. In Astrocytes in Psychiatric Disorders; Li, B., Parpura, V., Verkhratsky, A., Scuderi, C., Eds.; Advances in Neurobiology; Springer International Publishing: Cham, 2021; Vol. 26, pp. 3–19. ISBN 978-3-030-77374-8. [Google Scholar]
- Hanslik, K.L.; Marino, K.M.; Ulland, T.K. Modulation of Glial Function in Health, Aging, and Neurodegenerative Disease. Front. Cell. Neurosci. 2021, 15, 718324. [Google Scholar] [CrossRef]
- Zhu, H.; Guan, A.; Liu, J.; Peng, L.; Zhang, Z.; Wang, S. Noteworthy Perspectives on Microglia in Neuropsychiatric Disorders. J. Neuroinflammation 2023, 20, 223. [Google Scholar] [CrossRef] [PubMed]
- Shared and Disease-Specific Glial Gene Expression Changes in Neurodegenerative Diseases. Nat. Aging 2023, 3, 246–247. [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, Z.; Lu, G.; Chan, W.; Zhang, Y.; Wong, G.C. Microglia Activation, Classification and Microglia-Mediated Neuroinflammatory Modulators in Subarachnoid Hemorrhage. Neural Regen. Res. 2022, 17, 1404. [Google Scholar] [CrossRef]
- De Marchi, F.; Munitic, I.; Vidatic, L.; Papić, E.; Rački, V.; Nimac, J.; Jurak, I.; Novotni, G.; Rogelj, B.; Vuletic, V.; et al. Overlapping Neuroimmune Mechanisms and Therapeutic Targets in Neurodegenerative Disorders. Biomedicines 2023, 11, 2793. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Martins, S.; Ferreira, P.A.; Cardoso, A.M.S.; Guedes, J.R.; Peça, J.; Cardoso, A.L. The Old Guard: Age-Related Changes in Microglia and Their Consequences. Mech. Ageing Dev. 2021, 197, 111512. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, Z. The Impact of Microglia on Neurodevelopment and Brain Function in Autism. Biomedicines 2024, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-X.; Kim, G.H.; Tan, J.-W.; Riso, A.E.; Sun, Y.; Xu, E.Y.; Liao, G.-Y.; Xu, H.; Lee, S.-H.; Do, N.-Y.; et al. Elevated Protein Synthesis in Microglia Causes Autism-like Synaptic and Behavioral Aberrations. Nat. Commun. 2020, 11, 1797. [Google Scholar] [CrossRef]
- Pathak, D.; Sriram, K. Neuron-Astrocyte Omnidirectional Signaling in Neurological Health and Disease. Front. Mol. Neurosci. 2023, 16, 1169320. [Google Scholar] [CrossRef]
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front. Neurosci. 2022, 16, 824888. [Google Scholar] [CrossRef]
- Peltier, M.R.; Behbodikhah, J.; Renna, H.A.; Ahmed, S.; Srivastava, A.; Arita, Y.; Kasselman, L.J.; Pinkhasov, A.; Wisniewski, T.; De Leon, J.; et al. Cholesterol Deficiency as a Mechanism for Autism: A Valproic Acid Model. J. Investig. Med. 2024, 72, 80–87. [Google Scholar] [CrossRef]
- Gzielo, K.; Nikiforuk, A. Astroglia in Autism Spectrum Disorder. Int. J. Mol. Sci. 2021, 22, 11544. [Google Scholar] [CrossRef]
- Allen, M.; Huang, B.S.; Notaras, M.J.; Lodhi, A.; Barrio-Alonso, E.; Lituma, P.J.; Wolujewicz, P.; Witztum, J.; Longo, F.; Chen, M.; et al. Astrocytes Derived from ASD Individuals Alter Behavior and Destabilize Neuronal Activity through Aberrant Ca2+ Signaling. Mol. Psychiatry 2022, 27, 2470–2484. [Google Scholar] [CrossRef] [PubMed]
- Vakilzadeh, G.; Martinez-Cerdeño, V. Pathology and Astrocytes in Autism. Neuropsychiatr. Dis. Treat. 2023, Volume 19, 841–850. [Google Scholar] [CrossRef]
- Michalski, J.-P.; Kothary, R. Oligodendrocytes in a Nutshell. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, J. Neuronal Activity and Remyelination: New Insights into the Molecular Mechanisms and Therapeutic Advancements. Front. Cell Dev. Biol. 2023, 11, 1221890. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.-J.; Pérez-Acuña, D.; Rhee, K.H.; Lee, S.-J. Changes in Oligodendroglial Subpopulations in Parkinson’s Disease. Mol. Brain 2023, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.; Tadi, P. Histology, Schwann Cells. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2024. [Google Scholar]
- Usui, N. Possible Roles of Deep Cortical Neurons and Oligodendrocytes in the Neural Basis of Human Sociality. Anat. Sci. Int. 2024, 99, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Graciarena, M.; Seiffe, A.; Nait-Oumesmar, B.; Depino, A.M. Hypomyelination and Oligodendroglial Alterations in a Mouse Model of Autism Spectrum Disorder. Front. Cell. Neurosci. 2019, 12, 517. [Google Scholar] [CrossRef]
- Bsibsi, M.; Nomden, A.; Van Noort, J.M.; Baron, W. Toll-like Receptors 2 and 3 Agonists Differentially Affect Oligodendrocyte Survival, Differentiation, and Myelin Membrane Formation. J. Neurosci. Res. 2012, 90, 388–398. [Google Scholar] [CrossRef]
- Kumar, V. Toll-like Receptors in the Pathogenesis of Neuroinflammation. J. Neuroimmunol. 2019, 332, 16–30. [Google Scholar] [CrossRef]
- Hill, R.A.; Nishiyama, A. NG2 Cells (Polydendrocytes): Listeners to the Neural Network with Diverse Properties. Glia 2014, 62, 1195–1210. [Google Scholar] [CrossRef]
- Kirdajova, D.; Anderova, M. NG2 Cells and Their Neurogenic Potential. Curr. Opin. Pharmacol. 2020, 50, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-P.; Zhao, J.; Li, S. Roles of NG2 Glial Cells in Diseases of the Central Nervous System. Neurosci. Bull. 2011, 27, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Dimou, L.; Gallo, V. NG 2-glia and Their Functions in the Central Nervous System. Glia 2015, 63, 1429–1451. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Errede, M.; Girolamo, F.; Morando, S.; Ivaldi, F.; Panini, N.; Bendotti, C.; Perris, R.; Furlan, R.; Virgintino, D.; et al. NG2, a Common Denominator for Neuroinflammation, Blood–Brain Barrier Alteration, and Oligodendrocyte Precursor Response in EAE, Plays a Role in Dendritic Cell Activation. Acta Neuropathol. (Berl.) 2016, 132, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Q.; Yang, Q.; Gu, H.; Yin, Y.; Li, Y.; Hou, J.; Chen, R.; Sun, Q.; Sun, Y.; et al. NG2 Glia Regulate Brain Innate Immunity via TGF-Β2/TGFBR2 Axis. BMC Med. 2019, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Geng, P.; Zhao, X.; Wang, Q.; Liu, C.; Guo, C.; Dong, W.; Jin, X. The NG2-Glia Is a Potential Target to Maintain the Integrity of Neurovascular Unit after Acute Ischemic Stroke. Neurobiol. Dis. 2023, 180, 106076. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, A.; Tascio, D.; Jabs, R.; Boehlen, A.; Domingos, C.; Skubal, M.; Huang, W.; Kirchhoff, F.; Henneberger, C.; Bilkei-Gorzo, A.; et al. Dysfunction of NG2 Glial Cells Affects Neuronal Plasticity and Behavior. Glia 2023, 71, 1481–1501. [Google Scholar] [CrossRef]
- Carter, M.; Scherer, S. Autism Spectrum Disorder in the Genetics Clinic: A Review. Clin. Genet. 2013, 83, 399–407. [Google Scholar] [CrossRef]
- Tilot, A.K.; Gaugler, M.K.; Yu, Q.; Romigh, T.; Yu, W.; Miller, R.H.; Frazier, T.W.; Eng, C. Germline Disruption of Pten Localization Causes Enhanced Sex-Dependent Social Motivation and Increased Glial Production. Hum. Mol. Genet. 2014, 23, 3212–3227. [Google Scholar] [CrossRef]
- Lee, H.; Thacker, S.; Sarn, N.; Dutta, R.; Eng, C. Constitutional Mislocalization of Pten Drives Precocious Maturation in Oligodendrocytes and Aberrant Myelination in Model of Autism Spectrum Disorder. Transl. Psychiatry 2019, 9, 13. [Google Scholar] [CrossRef]
- Sarn, N.; Thacker, S.; Lee, H.; Eng, C. Germline Nuclear-Predominant Pten Murine Model Exhibits Impaired Social and Perseverative Behavior, Microglial Activation, and Increased Oxytocinergic Activity. Mol. Autism 2021, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, G.; Sula, A.; Miles, A.J.; Ng, L.C.T.; Torella, R.; Pryde, D.C.; DeCaen, P.G.; Wallace, B.A. Valproic Acid Interactions with the NavMs Voltage-Gated Sodium Channel. Proc. Natl. Acad. Sci. 2019, 116, 26549–26554. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Ismail, F. A Comprehensive Review on Pharmacological Applications and Drug-Induced Toxicity of Valproic Acid. Saudi Pharm. J. 2023, 31, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.K.; Kukal, S.; Paul, P.R.; Bora, S.; Singh, A.; Kukreti, S.; Saso, L.; Muthusamy, K.; Hasija, Y.; Kukreti, R. Insights into Structural Modifications of Valproic Acid and Their Pharmacological Profile. Molecules 2021, 27, 104. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, M.; Ricci, E.; Ceraldi, R.; Nigro, A.; Bonofiglio, D.; Lanzino, M.; Morelli, C. From HDAC to Voltage-Gated Ion Channels: What’s Next? The Long Road of Antiepileptic Drugs Repositioning in Cancer. Cancers 2022, 14, 4401. [Google Scholar] [CrossRef]
- Gibbons, H.M.; Smith, A.M.; Teoh, H.H.; Bergin, P.M.; Mee, E.W.; Faull, R.L.M.; Dragunow, M. Valproic Acid Induces Microglial Dysfunction, Not Apoptosis, in Human Glial Cultures. Neurobiol. Dis. 2011, 41, 96–103. [Google Scholar] [CrossRef]
- Mony, T.J.; Lee, J.W.; Kim, S.S.; Chun, W.; Lee, H.J. Early Postnatal Valproic Acid Exposure Increase the Protein Level of Astrocyte Markers in Frontal Cortex of Rat. Clin. Psychopharmacol. Neurosci. 2018, 16, 214–217. [Google Scholar] [CrossRef]
- Lee, H.J.; Dreyfus, C.; DiCicco-Bloom, E. Valproic Acid Stimulates Proliferation of Glial Precursors during Cortical Gliogenesis in Developing Rat. Dev. Neurobiol. 2016, 76, 780–798. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, Y.; Li, Z.; Ye, L.; Lu, Q.; Zhou, Y.; Yuan, Y.; Jiang, T.; Xie, L.; Liu, Y.; et al. Valproic Acid Affects Neuronal Fate and Microglial Function via Enhancing Autophagic Flux in Mice after Traumatic Brain Injury. J. Neurochem. 2020, 154, 284–300. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z.; Yin, H.; Du, Z.; Xie, R.; Yang, L.; Cai, Y.; Wang, L.; Zhang, D.; Li, X.; Liu, T.; et al. Valproic Acid Exposure Decreases Neurogenic Potential of Outer Radial Glia in Human Brain Organoids. Front. Mol. Neurosci. 2022, 15, 1023765. [Google Scholar] [CrossRef] [PubMed]
- Traetta, M.E.; Uccelli, N.A.; Zárate, S.C.; Gómez Cuautle, D.; Ramos, A.J.; Reinés, A. Long-Lasting Changes in Glial Cells Isolated From Rats Subjected to the Valproic Acid Model of Autism Spectrum Disorder. Front. Pharmacol. 2021, 12, 707859. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, A.; Greenhalgh, T. Sodium Valproate in Pregnancy: What Are the Risks and Should We Use a Shared Decision-Making Approach? BMC Pregnancy Childbirth 2018, 18, 200. [Google Scholar] [CrossRef]
- Ornoy, A. Valproic Acid in Pregnancy: How Much Are We Endangering the Embryo and Fetus? Reprod. Toxicol. 2009, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Q.; Yan, T.; Zhang, Y.; Xu, H.; Yu, H.; Tu, Z.; Guo, X.; Jiang, Y.; Li, X.; et al. Maternal Valproic Acid Exposure Leads to Neurogenesis Defects and Autism-like Behaviors in Non-Human Primates. Transl. Psychiatry 2019, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tan, M.; Cheng, B.; Wang, S.; Xiao, L.; Zhu, J.; Wu, Q.; Lai, X.; Zhang, Q.; Chen, J.; et al. Valproic Acid Induces Autism-Like Synaptic and Behavioral Deficits by Disrupting Histone Acetylation of Prefrontal Cortex ALDH1A1 in Rats. Front. Neurosci. 2021, 15, 641284. [Google Scholar] [CrossRef]
- Zarate-Lopez, D.; Torres-Chávez, A.L.; Gálvez-Contreras, A.Y.; Gonzalez-Perez, O. Three Decades of Valproate: A Current Model for Studying AutismSpectrum Disorder. Curr. Neuropharmacol. 2024, 22, 260–289. [Google Scholar] [CrossRef]
- RESERVED, I.U.-A.R. Orphanet: Fetal Valproate Spectrum Disorder. Available online: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=en&Expert=1906 (accessed on 15 December 2023).
- Sharma, A.R.; Batra, G.; Saini, L.; Sharma, S.; Mishra, A.; Singla, R.; Singh, A.; Singh, R.S.; Jain, A.; Bansal, S.; et al. Valproic Acid and Propionic Acid Modulated Mechanical Pathways Associatedwith Autism Spectrum Disorder at Prenatal and Neonatal Exposure. CNS Neurol. Disord. - Drug Targets 2022, 21, 399–408. [Google Scholar] [CrossRef]
- Mony, T.J.; Lee, J.W.; Dreyfus, C.; DiCicco-Bloom, E.; Lee, H.J. Valproic Acid Exposure during Early Postnatal Gliogenesis Leads to Autistic-like Behaviors in Rats. Clin. Psychopharmacol. Neurosci. 2016, 14, 338–344. [Google Scholar] [CrossRef]
- Matsushima, T.; Izumi, T.; Vallortigara, G. The Domestic Chick as an Animal Model of Autism Spectrum Disorder: Building Adaptive Social Perceptions through Prenatally Formed Predispositions. Front. Neurosci. 2024, 18, 1279947. [Google Scholar] [CrossRef]
- Zahedi, E.; Sadr, S.S.; Sanaeierad, A.; Roghani, M. Valproate-Induced Murine Autism Spectrum Disorder Is Associated with Dysfunction of Amygdala Parvalbumin Interneurons and Downregulation of AMPK/SIRT1/PGC1α Signaling. Metab. Brain Dis. 2023, 38, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, T.; Hattori, S.; Fujimura, K.; Shibata, S.; Miyakawa, T.; Takahashi, T. In Utero Exposure to Valproic Acid throughout Pregnancy Causes Phenotypes of Autism in Offspring Mice. Dev. Neurosci. 2023, 45, 223–233. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, Y.-J.; Kim, S.J.; Lee, J.D.; Kim, S.; Ko, M.J.; Kim, J.-W.; Shin, C.Y.; Kim, K.-B. Metabolomics Profiling of Valproic Acid-Induced Symptoms Resembling Autism Spectrum Disorders Using 1H NMR Spectral Analysis in Rat Model. J. Toxicol. Environ. Health A 2022, 85, 1–13. [Google Scholar] [CrossRef]
- Guerra, M.; Medici, V.; Weatheritt, R.; Corvino, V.; Palacios, D.; Geloso, M.C.; Farini, D.; Sette, C. Fetal Exposure to Valproic Acid Dysregulates the Expression of Autism-Linked Genes in the Developing Cerebellum. Transl. Psychiatry 2023, 13, 114. [Google Scholar] [CrossRef]
- Tripathy, D.; Grammas, P. Acetaminophen Inhibits Neuronal Inflammation and Protects Neurons from Oxidative Stress. J. Neuroinflammation 2009, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.Z.; Swan, S.H.; Kriebel, D.; Liew, Z.; Taylor, H.S.; Bornehag, C.-G.; Andrade, A.M.; Olsen, J.; Jensen, R.H.; Mitchell, R.T.; et al. Paracetamol Use during Pregnancy — a Call for Precautionary Action. Nat. Rev. Endocrinol. 2021, 17, 757–766. [Google Scholar] [CrossRef]
- Wu, K.; Lu, W.; Yan, X. Potential Adverse Actions of Prenatal Exposure of Acetaminophen to Offspring. Front. Pharmacol. 2023, 14, 1094435. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.; Morelli, G.; Rastogi, M.; Savardi, A.; Fumagalli, A.; Petretto, A.; Bartolucci, M.; Varea, E.; Catelani, T.; Contestabile, A.; et al. Rescuing Over-Activated Microglia Restores Cognitive Performance in Juvenile Animals of the Dp(16) Mouse Model of Down Syndrome. Neuron 2020, 108, 887–904.e12. [Google Scholar] [CrossRef]
- Park, M.H.; Lee, M.; Nam, G.; Kim, M.; Kang, J.; Choi, B.J.; Jeong, M.S.; Park, K.H.; Han, W.H.; Tak, E.; et al. N, N ′-Diacetyl- p -Phenylenediamine Restores Microglial Phagocytosis and Improves Cognitive Defects in Alzheimer’s Disease Transgenic Mice. Proc. Natl. Acad. Sci. 2019, 116, 23426–23436. [Google Scholar] [CrossRef]
- Zhao, W.-X.; Zhang, J.-H.; Cao, J.-B.; Wang, W.; Wang, D.-X.; Zhang, X.-Y.; Yu, J.; Zhang, Y.-Y.; Zhang, Y.-Z.; Mi, W.-D. Acetaminophen Attenuates Lipopolysaccharide-Induced Cognitive Impairment through Antioxidant Activity. J. Neuroinflammation 2017, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Kwak, S.G.; Park, J.-S.; Park, D. The Effectiveness of Nonsteroidal Anti-Inflammatory Drugs and Acetaminophen in Reduce the Risk of Amyotrophic Lateral Sclerosis? A Meta-Analysis. Sci. Rep. 2020, 10, 14759. [Google Scholar] [CrossRef] [PubMed]
- Lalert, L.; Tantarungsee, N.; Chotipinit, T.; Ji-au, W.; Srikiatkhachorn, A.; Maneesri-le Grand, S. Long-Term Paracetamol Treatment Impairs Cognitive Function and Brain-Derived Neurotrophic Factor in Adult Rat Brain. Sci. Pharm. 2023, 91, 11. [Google Scholar] [CrossRef]
- Bernal, J. Thyroid Hormones in Brain Development and Function. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., Kalra, S., Kaltsas, G., Kapoor, N., Koch, C., Kopp, P., Korbonits, M., Kovacs, C.S., Kuohung, W., Laferrère, B., Levy, M., McGee, E.A., McLachlan, R., New, M., Purnell, J., Sahay, R., Shah, A.S., Singer, F., Sperling, M.A., Stratakis, C.A., Trence, D.L., Wilson, D.P., Eds.; MDText.com, Inc.: South Dartmouth (MA), 2000. [Google Scholar]
- Klein, R.M.; Motomura, V.N.; Debiasi, J.D.; Moreira, E.G. Gestational Paracetamol Exposure Induces Core Behaviors of Neurodevelopmental Disorders in Infant Rats and Modifies Response to a Cannabinoid Agonist in Females. Neurotoxicol. Teratol. 2023, 99, 107279. [Google Scholar] [CrossRef]
- Bührer, C.; Endesfelder, S.; Scheuer, T.; Schmitz, T. Paracetamol (Acetaminophen) and the Developing Brain. Int. J. Mol. Sci. 2021, 22, 11156. [Google Scholar] [CrossRef]
- Kwok, J.; Luedecke, E.; Hall, H.A.; Murray, A.L.; Auyeung, B. Analgesic Drug Use in Pregnancy and Neurodevelopment Outcomes: An Umbrella Review. Neurosci. Biobehav. Rev. 2022, 136, 104607. [Google Scholar] [CrossRef] [PubMed]
- Blecharz-Klin, K.; Wawer, A.; Jawna-Zboińska, K.; Pyrzanowska, J.; Piechal, A.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Early Paracetamol Exposure Decreases Brain-Derived Neurotrophic Factor (BDNF) in Striatum and Affects Social Behaviour and Exploration in Rats. Pharmacol. Biochem. Behav. 2018, 168, 25–32. [Google Scholar] [CrossRef]
- Andrade, C. Use of Acetaminophen (Paracetamol) during Pregnancy and the Risk of Autism Spectrum Disorder in the Offspring. J. Clin. Psychiatry 2016, 77, e152-154. [Google Scholar] [CrossRef]
- Alemany, S.; Avella-García, C.; Liew, Z.; García-Esteban, R.; Inoue, K.; Cadman, T.; López-Vicente, M.; González, L.; Riaño Galán, I.; Andiarena, A.; et al. Prenatal and Postnatal Exposure to Acetaminophen in Relation to Autism Spectrum and Attention-Deficit and Hyperactivity Symptoms in Childhood: Meta-Analysis in Six European Population-Based Cohorts. Eur. J. Epidemiol. 2021, 36, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.Z.; Kriebel, D.; Herbert, M.R.; Bornehag, C.-G.; Swan, S.H. Prenatal Paracetamol Exposure and Child Neurodevelopment: A Review. Horm. Behav. 2018, 101, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Avella-Garcia, C.B.; Julvez, J.; Fortuny, J.; Rebordosa, C.; García-Esteban, R.; Galán, I.R.; Tardón, A.; Rodríguez-Bernal, C.L.; Iñiguez, C.; Andiarena, A.; et al. Acetaminophen Use in Pregnancy and Neurodevelopment: Attention Function and Autism Spectrum Symptoms. Int. J. Epidemiol. 2016, 45, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.H.; Rafikian, E.E.; Hamblin, P.B.; Strait, M.D.; Yang, M.; Pearson, B.L. Sex-Specific Neurobehavioral and Prefrontal Cortex Gene Expression Alterations Following Developmental Acetaminophen Exposure in Mice. Neurobiol. Dis. 2023, 177, 105970. [Google Scholar] [CrossRef]
- Bittker, S.S.; Bell, K.R. Postnatal Acetaminophen and Potential Risk of Autism Spectrum Disorder among Males. Behav. Sci. 2020, 10, 26. [Google Scholar] [CrossRef]
- Glasgow, A.M.; Chase, P.H. Effect of Propionic Acid on Fatty Acid Oxidation and Ureagenesis. Pediatr. Res. 1976, 10, 683–686. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.-P.; Zhang, Y.-N.; Li, Y.; Gu, L.-T.; Sun, H.-H.; Liu, M.-D.; Zhou, H.-L.; Wang, Y.-S.; Xu, Z.-X. Short-Chain Fatty Acids in Diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef]
- Hekim Medical Company, Istanbul, Turkey; Dogan, M.; Albayrak, Y.; Department of Psychiatry, Tekirdag Namik Kemal University Faculty of Medicine, Tekirdag, Turkey; Erbas, O.; Department of Physiology, Demiroglu Bilim University Faculty of Medicine, Istanbul, Turkey. Torasemide Improves the Propionic Acid-Induced Autism in Rats: A Histopathological and Imaging Study. ALPHA PSYCHIATRY 2023, 24, 22–31. [Google Scholar] [CrossRef]
- Abdelli, L.S.; Samsam, A.; Naser, S.A. Propionic Acid Induces Gliosis and Neuro-Inflammation through Modulation of PTEN/AKT Pathway in Autism Spectrum Disorder. Sci. Rep. 2019, 9, 8824. [Google Scholar] [CrossRef]
- Frye, R.E.; Melnyk, S.; MacFabe, D.F. Unique Acyl-Carnitine Profiles Are Potential Biomarkers for Acquired Mitochondrial Disease in Autism Spectrum Disorder. Transl. Psychiatry 2013, 3, e220–e220. [Google Scholar] [CrossRef]
- Macfabe, D. Autism: Metabolism, Mitochondria, and the Microbiome. Glob. Adv. Health Med. 2013, 2, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Lobzhanidze, G.; Lordkipanidze, T.; Zhvania, M.; Japaridze, N.; MacFabe, D.F.; Pochkidze, N.; Gasimov, E.; Rzaev, F. Effect of Propionic Acid on the Morphology of the Amygdala in Adolescent Male Rats and Their Behavior. Micron Oxf. Engl. 1993 2019, 125, 102732. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-E.J.; McDougle, C.J.; Hooker, J.M.; Zürcher, N.R. Epigenetics of Autism Spectrum Disorder: Histone Deacetylases. Biol. Psychiatry 2022, 91, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Pobbe, R.L.H.; Pearson, B.L.; Blanchard, D.C.; Blanchard, R.J. Oxytocin Receptor and Mecp2308/Y Knockout Mice Exhibit Altered Expression of Autism-Related Social Behaviors. Physiol. Behav. 2012, 107, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Zhubi, A.; Chen, Y.; Guidotti, A.; Grayson, D.R. Epigenetic Regulation of RELN and GAD1 in the Frontal Cortex (FC) of Autism Spectrum Disorder (ASD) Subjects. Int. J. Dev. Neurosci. 2017, 62, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.; Stoccoro, A.; Cagiano, R.; Nicolì, V.; Ricciardi, R.; Tancredi, R.; Trovato, R.; Santorelli, F.M.; Calderoni, S.; Muratori, F.; et al. Correlation among Maternal Risk Factors, Gene Methylation and Disease Severity in Females with Autism Spectrum Disorder. Epigenomics 2022, 14, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Karimi, P.; Ghahfarroki, M.S.; Lorigooini, Z.; Shahrani, M.; Amini-Khoei, H. Umbelliprenin via Increase in the MECP2 and Attenuation of Oxidative Stress Mitigates the Autistic-like Behaviors in Mouse Model of Maternal Separation Stress. Front. Pharmacol. 2024, 14, 1300310. [Google Scholar] [CrossRef] [PubMed]
- Rylaarsdam, L.; Guemez-Gamboa, A. Genetic Causes and Modifiers of Autism Spectrum Disorder. Front. Cell. Neurosci. 2019, 13, 385. [Google Scholar] [CrossRef]
- Banker, S.M.; Gu, X.; Schiller, D.; Foss-Feig, J.H. Hippocampal Contributions to Social and Cognitive Deficits in Autism Spectrum Disorder. Trends Neurosci. 2021, 44, 793–807. [Google Scholar] [CrossRef]
- Maud, C.; Ryan, J.; McIntosh, J.E.; Olsson, C.A. The Role of Oxytocin Receptor Gene (OXTR) DNA Methylation (DNAm) in Human Social and Emotional Functioning: A Systematic Narrative Review. BMC Psychiatry 2018, 18, 154. [Google Scholar] [CrossRef]
- Chen, X.; Nishitani, S.; Haroon, E.; Smith, A.K.; Rilling, J.K. OXTR Methylation Modulates Exogenous Oxytocin Effects on Human Brain Activity during Social Interaction. Genes Brain Behav. 2020, 19, e12555. [Google Scholar] [CrossRef]
- Scala, M.; Grasso, E.A.; Di Cara, G.; Riva, A.; Striano, P.; Verrotti, A. The Pathophysiological Link Between Reelin and Autism: Overview and New Insights. Front. Genet. 2022, 13, 869002. [Google Scholar] [CrossRef]
- Kraan, C.M.; Godler, D.E.; Amor, D.J. Epigenetics of Fragile X Syndrome and Fragile X-related Disorders. Dev. Med. Child Neurol. 2019, 61, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Dolskiy, A.A.; Pustylnyak, V.O.; Yarushkin, A.A.; Lemskaya, N.A.; Yudkin, D.V. Inhibitors of Histone Deacetylases Are Weak Activators of the FMR1 Gene in Fragile X Syndrome Cell Lines. BioMed Res. Int. 2017, 2017, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Frasch, M.G.; Yoon, B.-J.; Helbing, D.L.; Snir, G.; Antonelli, M.C.; Bauer, R. Autism Spectrum Disorder: A Neuro-Immunometabolic Hypothesis of the Developmental Origins. Biology 2023, 12, 914. [Google Scholar] [CrossRef] [PubMed]
- Lampiasi, N.; Bonaventura, R.; Deidda, I.; Zito, F.; Russo, R. Inflammation and the Potential Implication of Macrophage-Microglia Polarization in Human ASD: An Overview. Int. J. Mol. Sci. 2023, 24, 2703. [Google Scholar] [CrossRef] [PubMed]
- Si, T.-E.; Li, Z.; Zhang, J.; Su, S.; Liu, Y.; Chen, S.; Peng, G.-H.; Cao, J.; Zang, W. Epigenetic Mechanisms of Müller Glial Reprogramming Mediating Retinal Regeneration. Front. Cell Dev. Biol. 2023, 11, 1157893. [Google Scholar] [CrossRef]
- Kular, L.; Klose, D.; Urdánoz-Casado, A.; Ewing, E.; Planell, N.; Gomez-Cabrero, D.; Needhamsen, M.; Jagodic, M. Epigenetic Clock Indicates Accelerated Aging in Glial Cells of Progressive Multiple Sclerosis Patients. Front. Aging Neurosci. 2022, 14, 926468. [Google Scholar] [CrossRef]
- Abdolmaleky, H.M.; Martin, M.; Zhou, J.-R.; Thiagalingam, S. Epigenetic Alterations of Brain Non-Neuronal Cells in Major Mental Diseases. Genes 2023, 14, 896. [Google Scholar] [CrossRef]
- Imado, E.; Sun, S.; Abawa, A.R.; Tahara, T.; Kochi, T.; Huynh, T.N.B.; Asano, S.; Hasebe, S.; Nakamura, Y.; Hisaoka-Nakashima, K.; et al. Prenatal Exposure to Valproic Acid Causes Allodynia Associated with Spinal Microglial Activation. Neurochem. Int. 2022, 160, 105415. [Google Scholar] [CrossRef]
- Thiele, K.; Solano, M.E.; Huber, S.; Flavell, R.A.; Kessler, T.; Barikbin, R.; Jung, R.; Karimi, K.; Tiegs, G.; Arck, P.C. Prenatal Acetaminophen Affects Maternal Immune and Endocrine Adaptation to Pregnancy, Induces Placental Damage, and Impairs Fetal Development in Mice. Am. J. Pathol. 2015, 185, 2805–2818. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, M.L.; Geiger, S.D.; Schantz, S.L. The Relationship of Prenatal Acetaminophen Exposure and Attention-Related Behavior in Early Childhood. Neurotoxicol. Teratol. 2024, 101, 107319. [Google Scholar] [CrossRef] [PubMed]
- De Campos Vidal, B.; Mello, M.L.S. Sodium Valproate (VPA) Interactions with DNA and Histones. Int. J. Biol. Macromol. 2020, 163, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Gervin, K.; Nordeng, H.; Ystrom, E.; Reichborn-Kjennerud, T.; Lyle, R. Long-Term Prenatal Exposure to Paracetamol Is Associated with DNA Methylation Differences in Children Diagnosed with ADHD. Clin. Epigenetics 2017, 9, 77. [Google Scholar] [CrossRef]
- Rozenkrantz, L.; Zachor, D.; Heller, I.; Plotkin, A.; Weissbrod, A.; Snitz, K.; Secundo, L.; Sobel, N. A Mechanistic Link between Olfaction and Autism Spectrum Disorder. Curr. Biol. 2015, 25, 1904–1910. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, G.; Shen, Y.; Ou, J.; Liu, Y.; Huang, L.; Zeng, Y.; Lin, J.; Liu, R.; Wu, R.; et al. Odor Identification Impairment in Autism Spectrum Disorder Might Be Associated with Mitochondrial Dysfunction. Asian J. Psychiatry 2022, 72, 103072. [Google Scholar] [CrossRef]
- Frye, R.E. Mitochondrial Dysfunction in Autism Spectrum Disorder: Unique Abnormalities and Targeted Treatments. Semin. Pediatr. Neurol. 2020, 35, 100829. [Google Scholar] [CrossRef]
- Wang, J.; Blaze, J.; Haghighi, F.; Kim-Schulze, S.; Raval, U.; Trageser, K.J.; Pasinetti, G.M. Characterization of 3(3,4-Dihydroxy-Phenyl) Propionic Acid as a Novel Microbiome-Derived Epigenetic Modifier in Attenuation of Immune Inflammatory Response in Human Monocytes. Mol. Immunol. 2020, 125, 172–177. [Google Scholar] [CrossRef]
- Buchanan, E.; Mahony, C.; Bam, S.; Jaffer, M.; Macleod, S.; Mangali, A.; Van Der Watt, M.; De Wet, S.; Theart, R.; Jacobs, C.; et al. Propionic Acid Induces Alterations in Mitochondrial Morphology and Dynamics in SH-SY5Y Cells. Sci. Rep. 2023, 13, 13248. [Google Scholar] [CrossRef]
- Stein, R.A.; Riber, L. Epigenetic Effects of Short-Chain Fatty Acids from the Large Intestine on Host Cells. microLife 2023, 4, uqad032. [Google Scholar] [CrossRef]
- Cristiano, C.; Hoxha, E.; Lippiello, P.; Balbo, I.; Russo, R.; Tempia, F.; Miniaci, M.C. Maternal Treatment with Sodium Butyrate Reduces the Development of Autism-like Traits in Mice Offspring. Biomed. Pharmacother. 2022, 156, 113870. [Google Scholar] [CrossRef]
- Li, Y.; Liu, A.; Chen, K.; Li, L.; Zhang, X.; Zou, F.; Zhang, X.; Meng, X. Sodium Butyrate Alleviates Lead-Induced Neuroinflammation and Improves Cognitive and Memory Impairment through the ACSS2/H3K9ac/BDNF Pathway. Environ. Int. 2024, 184, 108479. [Google Scholar] [CrossRef] [PubMed]
- Tizabi, Y.; Bennani, S.; El Kouhen, N.; Getachew, B.; Aschner, M. Interaction of Heavy Metal Lead with Gut Microbiota: Implications for Autism Spectrum Disorder. Biomolecules 2023, 13, 1549. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Aljazi, M.B.; Wu, Y.; He, J. Vorinostat, a Histone Deacetylase Inhibitor, Ameliorates the Sociability and Cognitive Memory in an Ash1L-Deletion-Induced ASD/ID Mouse Model. Neurosci. Lett. 2021, 764, 136241. [Google Scholar] [CrossRef] [PubMed]
- Alonazi, M.; Ben Bacha, A.; Al Suhaibani, A.; Almnaizel, A.T.; Aloudah, H.S.; El-Ansary, A. Psychobiotics Improve Propionic Acid-Induced Neuroinflammation in Juvenile Rats, Rodent Model of Autism. Transl. Neurosci. 2022, 13, 292–300. [Google Scholar] [CrossRef]
- Alsubaiei, S.R.M.; Alfawaz, H.A.; Bhat, R.S.; El-Ansary, A. Nutritional Intervention as a Complementary Neuroprotective Approach against Propionic Acid-Induced Neurotoxicity and Associated Biochemical Autistic Features in Rat Pups. Metabolites 2023, 13, 738. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.; Kaliszewska, A.; Uceda, S.; Reiriz, M.; Arias, N. Targeting DNA Methylation in the Adult Brain through Diet. Nutrients 2021, 13, 3979. [Google Scholar] [CrossRef]
- Swarnkar, G.; Semenkovich, N.P.; Arra, M.; Mims, D.K.; Naqvi, S.K.; Peterson, T.; Mbalaviele, G.; Wu, C.-L.; Abu-Amer, Y. DNA Hypomethylation Ameliorates Erosive Inflammatory Arthritis by Modulating Interferon Regulatory Factor-8. Proc. Natl. Acad. Sci. 2024, 121, e2310264121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




