Submitted:
01 March 2024
Posted:
04 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Maternal Oral Health during Pregnancy
3. DSCs
3.1. Types and Sources of DSCs
3.2. Clinical Significance of DSCs in Regenerative Dentistry
3.3. Standardization of Protocols
3.3.1. Isolation of DSCs
3.3.2. Enhancing Research Efficiency: Protocols, Quality Control, Collaboration, and Accessibility
3.4. Translating research findings into clinically relevant therapies
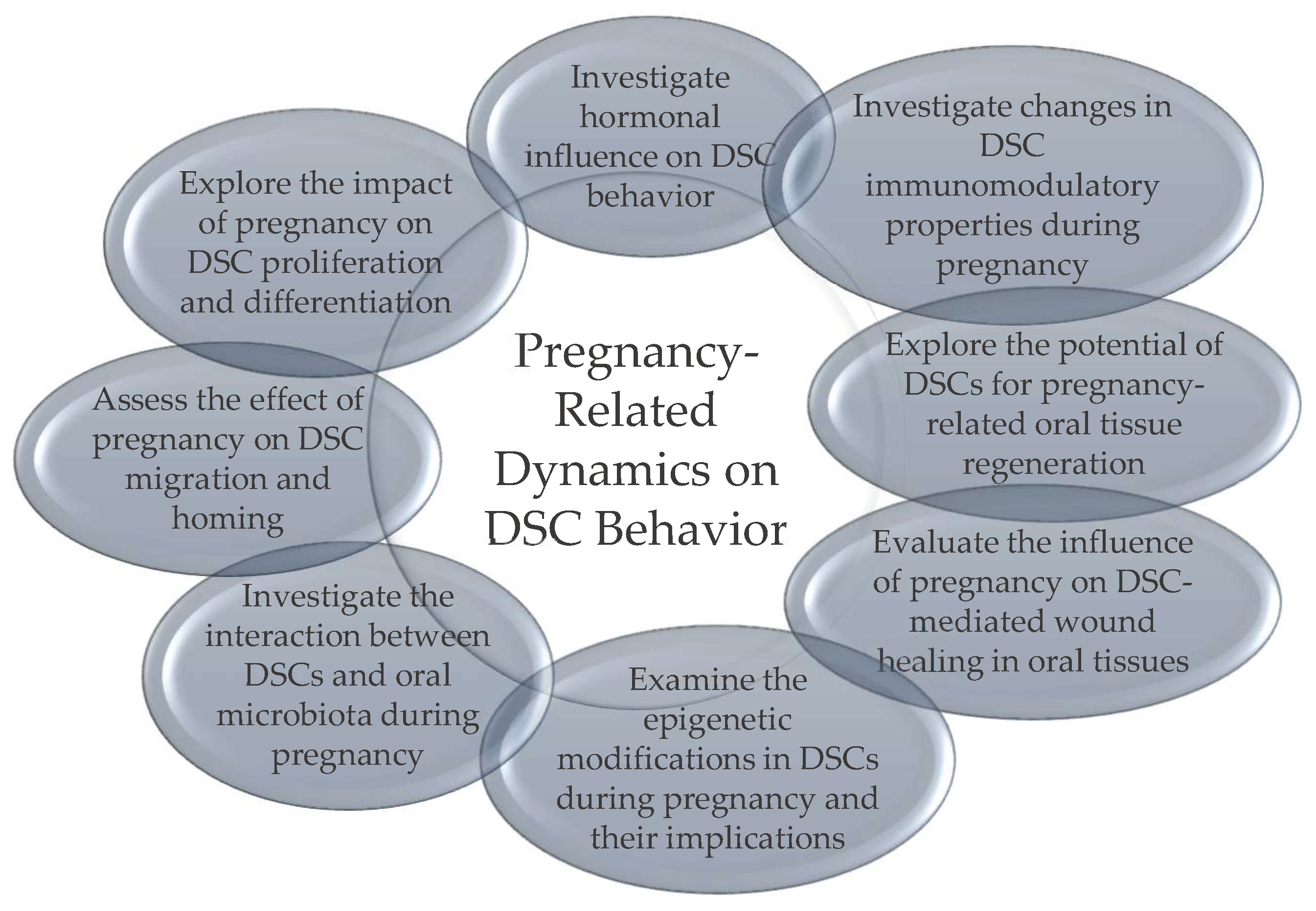
3.5. DSCs Behavior during Pregnancy
3.5.1. Understanding the Impact of Pregnancy on DSC Behavior: Current Knowledge and Research Gaps
4. Future Directions and Research Recommendations
4.1. Novel Therapeutic Strategies Leveraging DSCs for Maternal Oral Health Enhancement
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinberg, B.J. Women's oral health issues. J Calif Dent Assoc. 2000, 28, 663–7. [Google Scholar] [CrossRef]
- Hartnett, E.; Haber, J.; Krainovich-Miller, B.; Bella, A.; Vasilyeva, A.; Lange Kessler, J. Oral Health in Pregnancy. J Obstet Gynecol Neonatal Nurs. 2016, 45, 565–73. [Google Scholar] [CrossRef]
- Saadaoui, M.; Singh, P.; Al Khodor, S. Oral microbiome and pregnancy: A bidirectional relationship. J Reprod Immunol. 2021, 145, 103293. [Google Scholar] [CrossRef]
- Boggess, K.A. Choosing the left fork: Steven Offenbacher and understanding maternal periodontal disease and adverse pregnancy outcomes. J Periodontol. 2020, 91, S40–S44. [Google Scholar] [CrossRef]
- Daskalakis, G.; Psarris, A.; Koutras, A, et al. Maternal Infection and Preterm Birth: From Molecular Basis to Clinical Implications. Children (Basel) 2023, 10, 907. [CrossRef]
- Bushehab, N.M.E.; Sreedharan, J.; Reddy, S.; D'souza, J.; Abdelmagyd, H. Oral Hygiene Practices and Awareness of Pregnant Women about the Effects of Periodontal Disease on Pregnancy Outcomes. Int J Dent. 2022, 2022, 5195278. [Google Scholar] [CrossRef]
- Han, Y.W.; Redline, R.W.; Li, M.; Yin, L.; Hill, G.B.; McCormick, T.S. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. 2004, 72, 2272–9. [Google Scholar] [CrossRef]
- Vidmar Šimic, M.; Maver, A.; Zimani, A.N.; Hočevar, K.; Peterlin, B.; Kovanda, A.; Premru-Sršen, T. Oral microbiome and preterm birth. Front Med (Lausanne). 2023, 10, 1177990. [Google Scholar] [CrossRef]
- Lagadec, N.; Steinecker, M.; Kapassi, A.; Magnier, A.M.; Chastang, J.; Robert, S.; et al. Factors influencing the quality of life of pregnant women: a systematic review. BMC Pregnancy Childbirth 2018, 18, 455. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000, 97, 13625–30. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003, 100, 5807–12. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–55. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–65. [Google Scholar] [CrossRef]
- Botelho, J.; Cavacas, M.A.; Machado, V.; Mendes, J.J. Dental stem cells: recent progresses in tissue engineering and regenerative medicine. Ann Med. 2017, 49, 644–651. [Google Scholar] [CrossRef]
- Zhai, Q.; Dong, Z.; Wang, W.; Li, B.; Jin, Y. Dental stem cell and dental tissue regeneration. Front Med. 2019, 13, 152–159. [Google Scholar] [CrossRef]
- Miran, S.; Mitsiadis, T.A.; Pagella, P. Innovative Dental Stem Cell-Based Research Approaches: The Future of Dentistry. Stem Cells Int. 2016, 2016, 7231038. [Google Scholar] [CrossRef]
- Vaswani, B.K.; Mundada, B.P.; Bhola, N.; Paul, P.; Reche, A.; Ahuja, K.P. Stem-Cell Therapy: Filling Gaps in Oro-Maxillofacial Region. Cureus 2023, 15, e47171. [Google Scholar] [CrossRef]
- Bansal, R.; Jain, A. Current overview on dental stem cells applications in regenerative dentistry. J Nat Sci Biol Med. 2015, 6, 29–34. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Chen, C.; Xu, X.; et al. Regulation of the Stem Cell-Host Immune System Interplay Using Hydrogel Coencapsulation System with an Anti-Inflammatory Drug. Adv Funct Mater. 2015, 25, 2296–2307. [Google Scholar] [CrossRef]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Volponi, A.A.; Pang, Y.; Sharpe, P.T. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010, 20, 715–22. [Google Scholar] [CrossRef]
- Offenbacher, S.; Lieff, S.; Boggess, K.A.; et al. Maternal periodontitis and prematurity. Part I: Obstetric outcome of prematurity and growth restriction. Ann Periodontol. 2001, 6, 164–74. [Google Scholar] [CrossRef]
- Beckers, K.F.; Sones, J.L. Maternal microbiome and the hypertensive disorder of pregnancy, preeclampsia. Am J Physiol Heart Circ Physiol. 2020, 318, H1–H10. [Google Scholar] [CrossRef]
- Ye, C.; Kapila, Y. Oral microbiome shifts during pregnancy and adverse pregnancy outcomes: Hormonal and Immunologic changes at play. Periodontol 2000. 2021, 87, 276–281. [Google Scholar] [CrossRef]
- Balan, P.; Chong, Y.S.; Umashankar, S.; et al. Keystone Species in Pregnancy Gingivitis: A Snapshot of Oral Microbiome During Pregnancy and Postpartum Period. Front Microbiol. 2018, 9, 2360. [Google Scholar] [CrossRef]
- Iida, H. Oral Health Interventions During Pregnancy. Dent Clin North Am. 2017, 61, 467–481. [Google Scholar] [CrossRef]
- Steinberg, B.J.; Hilton, I.V.; Iida, H.; Samelson, R. Oral health and dental care during pregnancy. Dent Clin North Am. 2013, 57, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, E.; Haber, J.; Krainovich-Miller, B.; Bella, A.; Vasilyeva, A.; Lange Kessler, J. Oral Health in Pregnancy. J Obstet Gynecol Neonatal Nurs. 2016, 45, 565–73. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, R.A.; Jacobson, L.T.; John, J.; et al. Correlates of Early Prenatal Care Access among U. S. Women: Data from the Pregnancy Risk Assessment Monitoring System (PRAMS). Matern Child Health J. 2022, 26, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Przeklasa-Bierowiec, A.; Jakubik, A.; Szczeklik, K.; Majewska, I.; Marcinek, A.; Pytko-Polończyk, J. Awareness of oral health prophylaxis in pregnant women. Folia Med Cracov. 2020, 60, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Sui, B.; Wu, D.; Xiang, L.; Fu, Y.; Kou, X.; Shi, S. Dental Pulp Stem Cells: From Discovery to Clinical Application. J Endod. 2020, 46, S46–S55. [Google Scholar] [CrossRef] [PubMed]
- Bar, J.K.; Lis-Nawara, A.; Grelewski, P.G. Dental Pulp Stem Cell-Derived Secretome and Its Regenerative Potential. Int J Mol Sci. 2021, 22, 12018. [Google Scholar] [CrossRef] [PubMed]
- Tomokiyo, A.; Wada, N.; Maeda, H. Periodontal Ligament Stem Cells: Regenerative Potency in Periodontium. Stem Cells Dev. 2019, 28, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Lyu, P.; Song, Y.; Li, P.; Song, D.; Cui, C.; Fan, Y. Function of Dental Follicle Progenitor/Stem Cells and Their Potential in Regenerative Medicine: From Mechanisms to Applications. Biomolecules. 2021, 11, 997. [Google Scholar] [CrossRef]
- Song, W.P.; Jin, L.Y.; Zhu, M.D.; Wang, H.; Xia, D.S. Clinical trials using dental stem cells: 2022 update. World J Stem Cells. 2023, 15, 31–51. [Google Scholar] [CrossRef]
- Rai, S.; Kaur, M.; Kaur, S. Applications of stem cells in interdisciplinary dentistry and beyond: an overview. Ann Med Health Sci Res. 2013, 3, 245–54. [Google Scholar] [CrossRef]
- Chalisserry, E.P.; Nam, S.Y.; Park, S.H.; Anil, S. Therapeutic potential of dental stem cells. J Tissue Eng. 2017, 8, 2041731417702531. [Google Scholar] [CrossRef]
- Hilkens, P.; Driesen, R.B.; Wolfs, E.; et al. Cryopreservation and Banking of Dental Stem Cells. Adv Exp Med Biol. 2016, 951, 199–235. [Google Scholar] [CrossRef]
- Gronthos, S.; Arthur, A.; Bartold, P.M.; Shi, S. A method to isolate and culture expand human dental pulp stem cells. Methods Mol Biol. 2011, 698, 107–21. [Google Scholar] [CrossRef]
- Smith, J.G.; Smith, A.; Shelton, R.M.; Cooper, P.R. Dental Pulp Cell Behavior in Biomimetic Environments. J Dent Res. 2015, 94, 1552–9. [Google Scholar] [CrossRef]
- Pisciotta, A.; Carnevale, G.; Meloni, S.; et al. Human dental pulp stem cells (hDPSCs): isolation, enrichment and comparative differentiation of two sub-populations. BMC Dev Biol. 2015, 15, 14. [Google Scholar] [CrossRef]
- Bacon, K.; Lavoie, A.; Rao, B.M.; Daniele, M.; Menegatti, S. Past, Present, and Future of Affinity-based Cell Separation Technologies. Acta Biomater. 2020, 112, 29–51. [Google Scholar] [CrossRef]
- Dieterle, M.P.; Gross, T.; Steinberg, T.; et al. Characterization of a Stemness-Optimized Purification Method for Human Dental-Pulp Stem Cells: An Approach to Standardization. Cells 2022, 11, 3204. [Google Scholar] [CrossRef]
- Wang, X.; Sha, X.J.; Li, G.H.; et al. Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch Oral Biol. 2012, 57, 1231–40. [Google Scholar] [CrossRef]
- Jeon, M.; Song, J.S.; Choi, B.J.; Choi, H.J.; Shin, D.M.; Jung, H.S.; Kim, S.O. In vitro and in vivo characteristics of stem cells from human exfoliated deciduous teeth obtained by enzymatic disaggregation and outgrowth. Arch Oral Biol. 2014, 59, 1013–23. [Google Scholar] [CrossRef]
- Behfarnia, P.; Fazlalizadeh, S.; Nasr-Esfahani, M.H.; Ejeian, F.; Mogharehabed, A. Isolation and characterization of human periodontal ligament stem cells under the terms of use in clinical application: A pilot study. Dent Res J (Isfahan). 2023, 20, 105. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Xu, X.; Lin, J.; Fan, L.; Zheng, Y.; Kuang, W. Dental Stem Cell in Tooth Development and Advances of Adult Dental Stem Cell in Regenerative Therapies. Curr Stem Cell Res Ther. 2015, 10, 375–83. [Google Scholar] [CrossRef]
- Aydin, S.; Şahin, F. Stem Cells Derived from Dental Tissues. Adv Exp Med Biol. 2019, 1144, 123–132. [Google Scholar] [CrossRef]
- Karaöz, E.; Doğan, B.N.; Aksoy, A.; et al. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem Cell Biol. 2010, 133, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Chouaib, B.; Cuisinier, F.; Collart-Dutilleul, P.Y. Dental stem cell-conditioned medium for tissue regeneration: Optimization of production and storage. World J Stem Cells. 2022, 14, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–7. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008, 34, 166–71. [Google Scholar] [CrossRef]
- Huang, G.T.; Sonoyama, W.; Liu, Y.; Liu, H.; Wang, S.; Shi, S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008, 34, 645–51. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Shakesheff, K.M.; White, L.J. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Eng Regen Med. 2015, 9, 1205–16. [Google Scholar] [CrossRef]
- Lombaert, I.M.; Brunsting, J.F.; Wierenga, P.K.; et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One 2008, 3, e2063. [Google Scholar] [CrossRef]
| Oral Health Indicator | Description | Correlation with Pregnancy-Related Complications |
|---|---|---|
| Gingival Inflammation | Swelling, redness, and tenderness of the gingiva | Increased risk of preterm birth, low birth weight |
| Periodontal Disease | Inflammation and infection of the gums and surrounding tissues | Linked to preterm birth, preeclampsia, and gestational diabetes |
| Dental Caries | Tooth decay resulting from bacterial acid demineralization | May exacerbate the risk of preterm birth and low birth weight |
| Pregnancy Gingivitis | Gingival inflammation exacerbated by hormonal changes | Associated with adverse pregnancy outcomes, including preterm birth |
| Oral Candidiasis | Fungal infection characterized by white patches in the mouth | May indicate immunosuppression, potential risk factor for preterm birth |
| Oral Lesions | Abnormalities in the oral mucosa, including ulcers, red or white patches | Linked to nutritional deficiencies, may affect maternal health and fetal development |
| Pregnancy Tumors | Also known as pyogenic granulomas or granuloma gravidarum | Presence may indicate hormonal imbalances; typically resolve postpartum |
| Salivary Changes | Altered salivary composition, dry mouth | Changes in salivary flow and composition may impact oral health and contribute to the development of gestational diabetes |
| DSC Type | Source | Characteristics |
|---|---|---|
| Dental Pulp Stem Cells (DPSCs) | Dental pulp of teeth | - Multipotent mesenchymal stem cells########- Self-renewal capabilities########- Differentiation into odontoblasts, osteoblasts, adipocytes, and neural cells########- High proliferative capacity########- Expression of mesenchymal stem cell markers (CD73, CD90, CD105) |
| Stem Cells from Human Exfoliated Deciduous Teeth (SHEDs) | Dental pulp of deciduous teeth | - High proliferation rates########- Multipotent differentiation capabilities########- Differentiation into odontoblasts, adipocytes, and neural cells########- Accessibility and ease of isolation########- Robust regenerative potential |
| Periodontal Ligament Stem Cells (PDLSCs) | Periodontal ligament | - Self-renewal capacity########- Multilineage differentiation potential (osteoblasts, chondrocytes, adipocytes)########- Crucial role in maintaining periodontal tissue homeostasis########- Potential in regenerative therapies for periodontal tissue repair |
| Dental Follicle Progenitor Cells (DFPCs) | Dental follicle surrounding tooth germ | - Self-renewal ability########- Multilineage differentiation potential (osteoblasts, adipocytes, cementoblasts)########- Vital role in tooth eruption and periodontal tissue formation########- Potential for tooth and periodontal tissue regeneration |
| Specific Aspect | Description |
|---|---|
| Standardized Differentiation Protocols | Protocols established for obtaining differentiated cells from various lineages |
| - Odontoblastic Differentiation | Induce differentiation with specific odontogenic medium; monitor gene expression and mineralization |
| - Osteogenic Differentiation | Culture cells in osteogenic medium containing dexamethasone and β-glycerophosphate; assess osteogenic markers and mineralized matrix |
| - Adipogenic Differentiation | Induce adipogenesis using adipogenic medium; confirm differentiation by lipid staining and adipogenic marker expression |
| - Neurogenic Differentiation | Expose cells to neurogenic differentiation medium; evaluate neural marker expression and neurite formation |
| Quality Control Measures | Measures ensuring the correctness of produced data |
| - Viability Assessment | Employ assays (e.g., Trypan Blue exclusion) to determine cell viability |
| - Phenotypic Characterization | Confirm stem cell markers (e.g., CD146, STRO-1) via flow cytometry |
| - Sterility Testing | Regularly perform microbial testing to ensure aseptic conditions |
| - Functional Assays | Validate stem cell functionality through differentiation assays |
| Interdisciplinary Collaboration | Enhanced potential of studies through multidisciplinary collaboration |
| - Regular Consultation | Engage with experts in stem cell biology, dentistry, and regenerative medicine |
| - Open Communication | Establish platforms for sharing protocols and research findings. |
| - Collaborative Research Projects | Foster joint initiatives to refine and optimize protocols collaboratively |
| Access to Protocols | Methods to access and share protocols effectively |
| - Online Repositories | Contribute to and utilize online platforms for protocol sharing. |
| - Scientific Journals | Encourage publication of detailed protocols alongside research articles |
| - Consortium Initiatives | Support consortiums promoting standardized protocols in dental stem cell research |
| Steps | Description |
|---|---|
| DSC ########Isolation | - Following the International Society for Cellular Therapy guidelines for minimal criteria [51] ########- Source cells from well-established locations such as dental pulp, deciduous teeth, periodontal ligament, and dental follicle [10,11,12,13] ########- Ensure ethical considerations and regulatory approvals ########- Use aseptic techniques during tissue collection and cell isolation |
| DSC Differentiation | - Choose appropriate differentiation protocols based on desired cell type (e.g., odontoblasts, osteoblasts, adipocytes, neural cells) ########- Optimize culture conditions for specific lineage differentiation########- Validate differentiation outcomes through morphological, molecular, and functional assays ########- Adjust protocols based on assessment results |
| Quality Control | - Implement rigorous quality control measures at each step of isolation and differentiation ########- Monitor cell viability, morphology, and phenotype throughout the process########- Use standardized assays to confirm multipotency and differentiation potential |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





