Submitted:
01 March 2024
Posted:
04 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
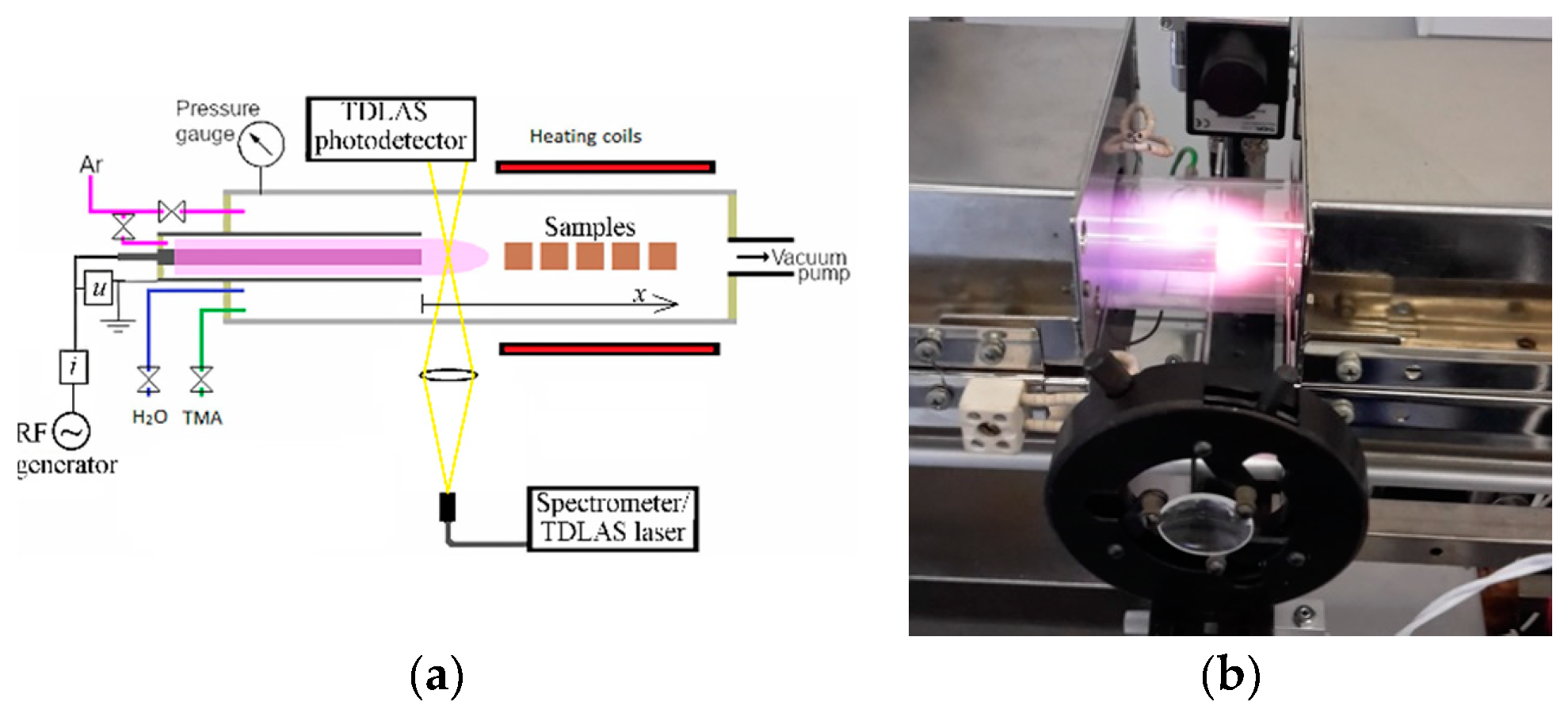
2. Materials and Methods
3. Results and Discussion
3.1. Plasma Characteristics
3.1.1. Discharge Appearance and the Choice of Generator Power
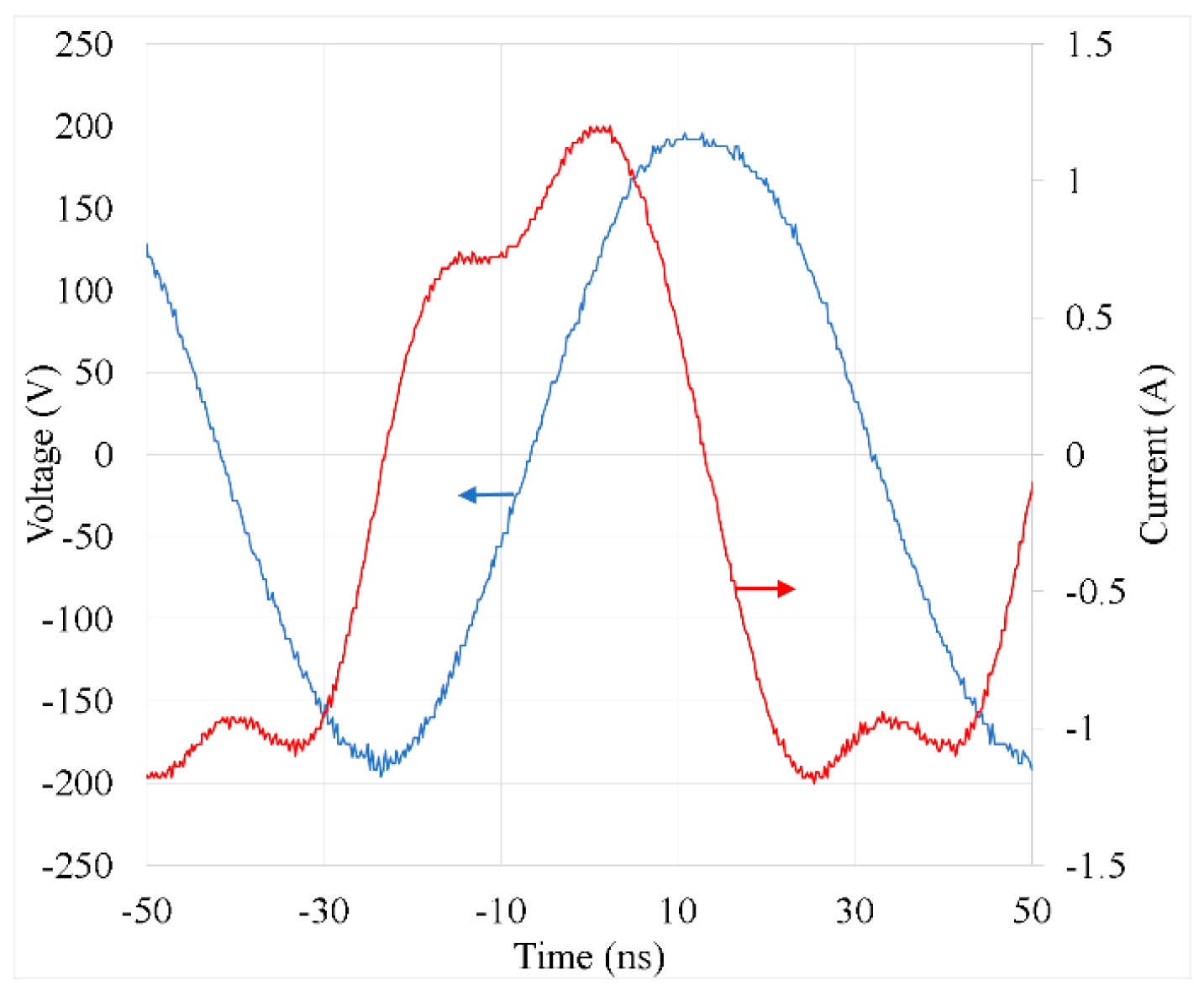
3.1.2. Electrical Characteristics
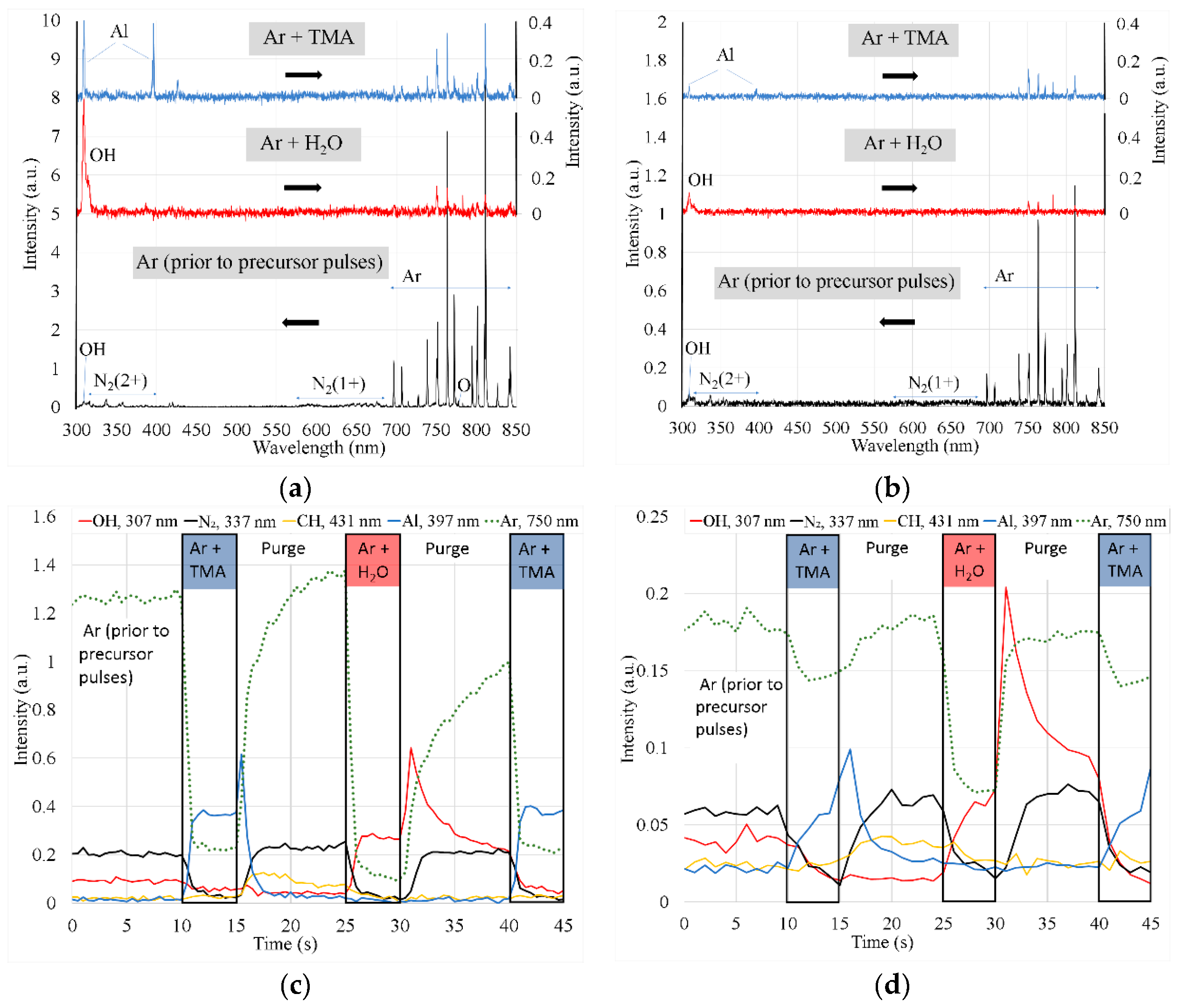
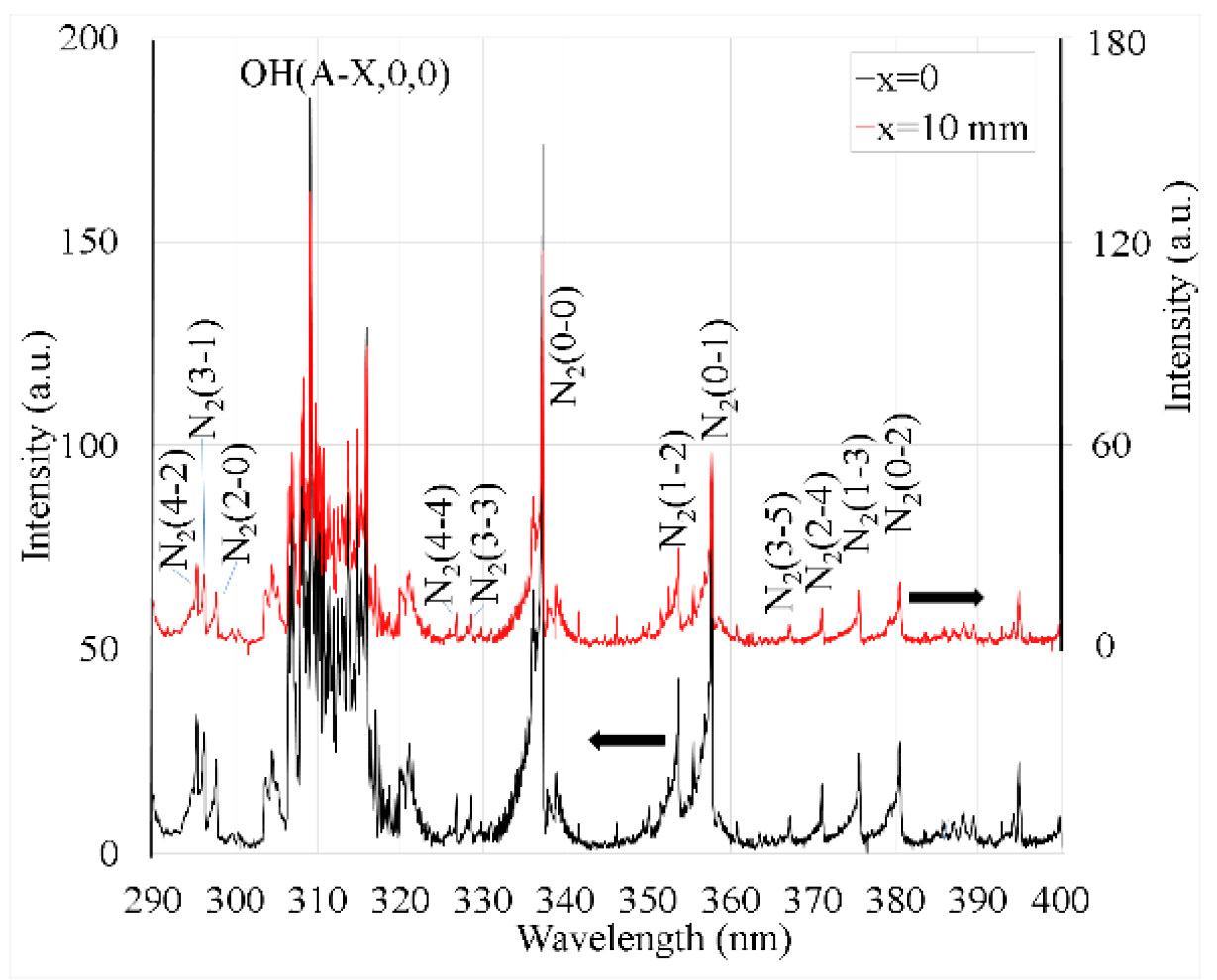
3.1.3. Plasma spectrum
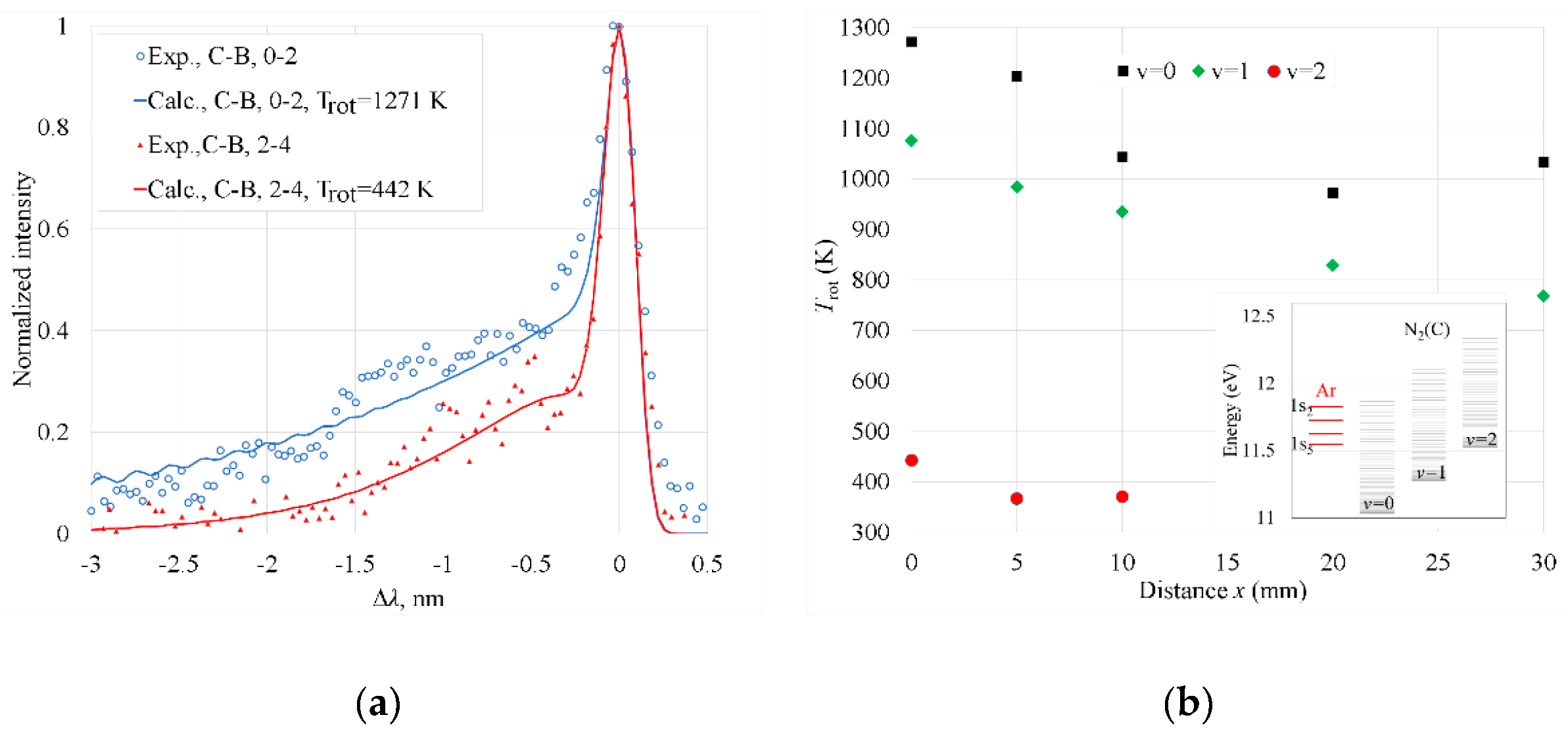
3.1.4. Gas Temperature
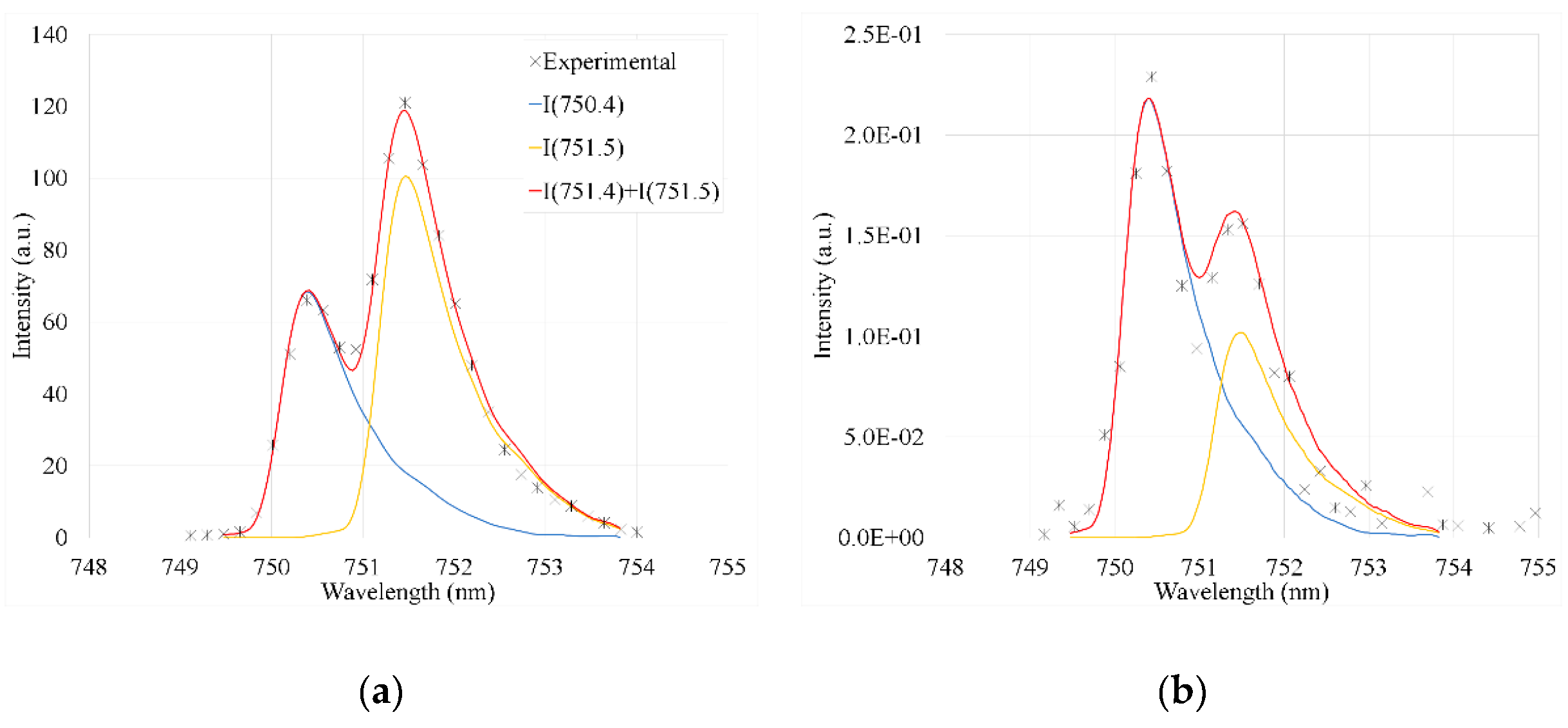
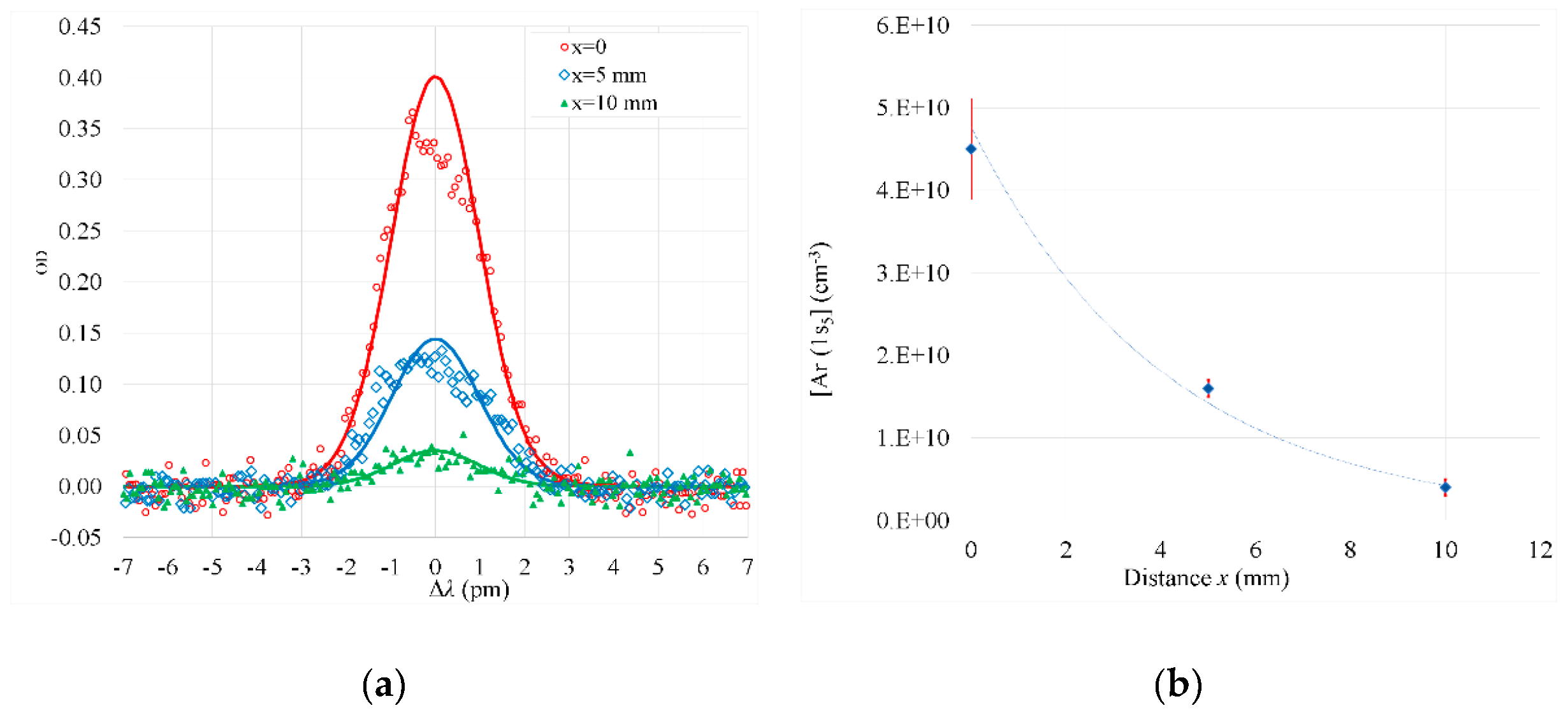
3.1.5. Line-Integrated Concentration of Ar Metastable, 1s5 State Atoms
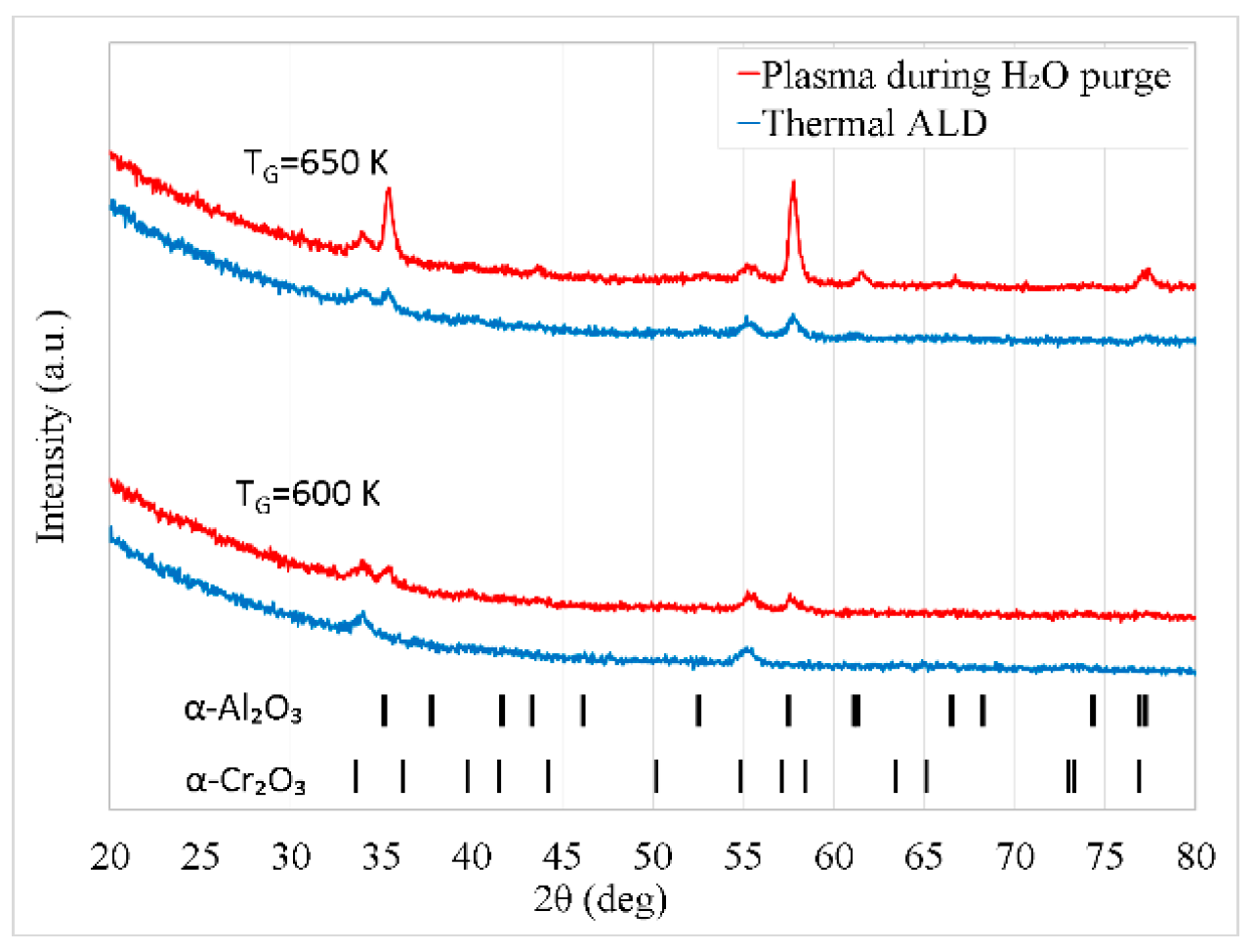
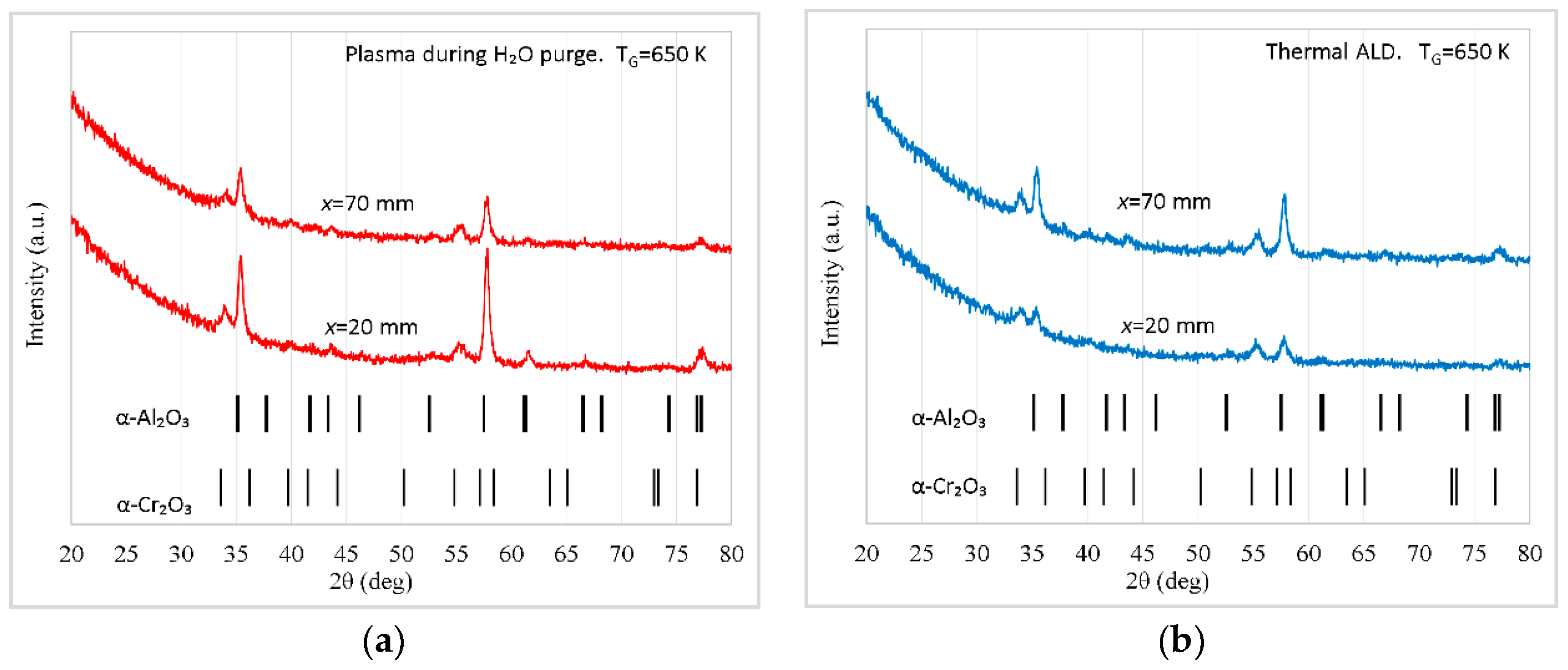
3.2. Effect of Plasma on Film Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boris, D.R.; Wheeler, V.D.; Nepal, N.; Qadri, S.B.; Walton, S.G.; Eddy, C. (Chip) R. The Role of Plasma in Plasma-Enhanced Atomic Layer Deposition of Crystalline Films. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 2020, 38. [Google Scholar] [CrossRef]
- Chang, J.; Chang, J.P. Achieving Atomistic Control in Materials Processing by Plasma-Surface Interactions. J. Phys. D. Appl. Phys. 2017, 50. [Google Scholar] [CrossRef]
- Faraz, T.; Arts, K.; Karwal, S.; Knoops, H.C.M.; Kessels, W.M.M. Energetic Ions during Plasma-Enhanced Atomic Layer Deposition and Their Role in Tailoring Material Properties. Plasma Sources Sci. Technol. 2019, 28. [Google Scholar] [CrossRef]
- Faraz, T.; Knoops, H.C.M.; Verheijen, M.A.; Van Helvoirt, C.A.A.; Karwal, S.; Sharma, A.; Beladiya, V.; Szeghalmi, A.; Hausmann, D.M.; Henri, J.; et al. Tuning Material Properties of Oxides and Nitrides by Substrate Biasing during Plasma-Enhanced Atomic Layer Deposition on Planar and 3D Substrate Topographies. ACS Appl. Mater. Interfaces 2018, 10, 13158–13180. [Google Scholar] [CrossRef]
- Profijt, H.B.; Kudlacek, P.; van de Sanden, M.C.M.; Kessels, W.M.M. Ion and Photon Surface Interaction during Remote Plasma ALD of Metal Oxides. J. Electrochem. Soc. 2011, 158, G88. [Google Scholar] [CrossRef]
- Knoops, H.C.M.; Faraz, T.; Arts, K.; Kessels, W.M.M. (Erwin) Status and Prospects of Plasma-Assisted Atomic Layer Deposition. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 2019, 37. [Google Scholar] [CrossRef]
- Profijt, H.B.; Potts, S.E.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-Assisted Atomic Layer Deposition: Basics, Opportunities, and Challenges. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 2011, 29. [Google Scholar] [CrossRef]
- Wang, Z. Bin; Li, J.T.; Zhu, X.M.; Pu, Y.K. Determination of the de-Excitation Probability of Argon Metastable (1s5 and 1s3) Atoms on Aluminum, Stainless Steel, Silicon, Quartz and Pyrex Surfaces. J. Phys. D. Appl. Phys. 2015, 48. [Google Scholar] [CrossRef]
- Wheeler, V.D.; Nepal, N.; Boris, D.R.; Qadri, S.B.; Nyakiti, L.O.; Lang, A.; Koehler, A.; Foster, G.; Walton, S.G.; Eddy, C.R.; et al. Phase Control of Crystalline Ga2O3 Films by Plasma-Enhanced Atomic Layer Deposition. Chem. Mater. 2020, 32, 1140–1152. [Google Scholar] [CrossRef]
- Nepal, N.; Mahadik, N.A.; Nyakiti, L.O.; Qadri, S.B.; Mehl, M.J.; Hite, J.K.; Eddy, C.R. Epitaxial Growth of Cubic and Hexagonal InN Thin Films via Plasma-Assisted Atomic Layer Epitaxy. Cryst. Growth Des. 2013, 13, 1485–1490. [Google Scholar] [CrossRef]
- Fang, Z.; Williams, P.A.; Odedra, R.; Jeon, H.; Potter, R.J. Gadolinium Nitride Films Deposited Using a PEALD Based Process. J. Cryst. Growth 2012, 338, 111–117. [Google Scholar] [CrossRef]
- Fan, Q.; Sang, L.; Jiang, D.; Yang, L.; Zhang, H.; Chen, Q.; Liu, Z. Plasma Enhanced Atomic Layer Deposition of Cobalt Nitride with Cobalt Amidinate. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 2019, 37. [Google Scholar] [CrossRef]
- Boris, D.R.; Wheeler, V.D.; Avila, J.R.; Qadri, S.B.; Eddy, C.R.; Walton, S.G. Role of Plasma Properties in Controlling Crystallinity and Phase in Oxide Films Grown by Plasma-Enhanced Atomic Layer Epitaxy. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 2019, 37. [Google Scholar] [CrossRef]
- Raud, S.; Raud, J.; Aarik, L.; Jõgi, I.; Kivimäe, M.; Piller, C.T.; Plaas, M.; Plank, T.; Talviste, R.; Teesalu, T.; et al. The Production of Reactive Chlorine Species (RCS) by Argon and Helium Plasma Jets and the Sensitivity of Liver Cancer Cell HepG2 to RCS and H2O2 Treatment. Plasma Process. Polym. 2023, 1–11. [Google Scholar] [CrossRef]
- Napari, M.; Tarvainen, O.; Kinnunen, S.; Arstila, K.; Julin, J.; Fjellvåg, S.; Weibye, K.; Nilsen, O.; Sajavaara, T. The α and γ Plasma Modes in Plasma-Enhanced Atomic Layer Deposition with O2-N2 Capacitive Discharges. J. Phys. D. Appl. Phys. 2017, 50. [Google Scholar] [CrossRef]
- Catalin, C.; Dumitrache, C.; Bazavan, M.; Mitu, B.; Marascu, V.; Stancu, S.; Vizireanu, S.; Dinescu, G. Maximization of RONS Generation by Selection of Gas Nature and Injection Type in One-Electrode Tubular RF Cold Plasma Source. In Proceedings of the 2nd Annual Meeting of COST Action PlasTHER “Therapeutical applications of cold plasmas”; Bologna; 2023. [Google Scholar]
- Aarik, L.; Piller, C.T.; Raud, J.; Talviste, R.; Jõgi, I.; Aarik, J. Atomic Layer Deposition of α-Al2O3 from Trimethylaluminum and H2O: Effect of Process Parameters and Plasma Excitation on Structure Development. J. Cryst. Growth 2023, 609, 6–11. [Google Scholar] [CrossRef]
- Aarik, L.; Mändar, H.; Tarre, A.; Piirsoo, H.M.; Aarik, J. Mechanical Properties of Crystalline and Amorphous Aluminum Oxide Thin Films Grown by Atomic Layer Deposition. Surf. Coatings Technol. 2022, 438. [Google Scholar] [CrossRef]
- Raud, J.; Jõgi, I.; Matisen, L.; Navratil, Z.; Talviste, R.; Trunec, D.; Aarik, J. Characterization of Ar / N 2 / H 2 Middle-Pressure RF Discharge and Application of the Afterglow Region for Nitridation of GaAs. J. Phys. D. Appl. Phys. 2017, 50, 505201. [Google Scholar] [CrossRef]
- Jõgi, I.; Raud, J.; Hein, K.; Laan, M. Spectral Characterization of Medium-Pressure RF Discharge in Argon-Oxygen Mixture. J. Phys. D. Appl. Phys. 2014, 47. [Google Scholar] [CrossRef]
- Rezaei, F.; Gorbanev, Y.; Chys, M.; Nikiforov, A.; Van Hulle, S.W.H.; Cos, P.; Bogaerts, A.; De Geyter, N. Investigation of Plasma-Induced Chemistry in Organic Solutions for Enhanced Electrospun PLA Nanofibers. Plasma Process. Polym. 2018, 15, 1–18. [Google Scholar] [CrossRef]
- Thimsen, E.; Kortshagen, U.R.; Aydil, E.S. Nonthermal Plasma Synthesis of Metal Sulfide Nanocrystals from Metalorganic Vapor and Elemental Sulfur. J. Phys. D. Appl. Phys. 2015, 48. [Google Scholar] [CrossRef]
- Kokai, F.; Yamamoto, K.; Koga, Y.; Fujiwara, S.; Heimann, R.B. Characterization of Ablation Plumes and Carbon Nitride Films Produced by Reactive Pulsed Laser Deposition in the Presence of a Magnetic Field. Appl. Phys. A Mater. Sci. Process. 1998, 66, 403–406. [Google Scholar] [CrossRef]
- Pearse, R.W.B.; Gaydon, A.G. The Identification of Molecular Spectra; second edi.; Chapman & Hall LTD.: London, 1950. [Google Scholar]
- Morozov, A.; Krücken, R.; Ottenthal, T.; Ulrich, A.; Wieser, J. Ultraviolet Emission from Argon Water-Vapor Mixtures Excited with Low-Energy Electron Beams. Appl. Phys. Lett. 2005, 86, 3–5. [Google Scholar] [CrossRef]
- Tabayashi, K.; Shobatake, K. Dissociative Excitation of Water by Metastable Rare Gas Atoms: Rg(3P0,2) +H2O→Rg+OH(A 2Σ+) +H (Rg=Ar,Kr). J. Chem. Phys. 1988, 88, 835–844. [Google Scholar] [CrossRef]
- Mikhailov, D. V.; Pastor, A.A.; Serdobintsev, P.Y.; Timofeev, N.A.; Khodorkovskii, M.A. Discharge Plasma in an Argon–water Vapor Mixture as a Gas Laser Medium. Russ. J. Phys. Chem. B 2015, 9, 593–595. [Google Scholar] [CrossRef]
- Raud, J.; Laan, M.; Jgi, I. Rotational Temperatures of N<inf>2</inf>(C,0) and OH(A,0) as Gas Temperature Estimates in the Middle Pressure Ar/O<inf>2</inf> Discharge. J. Phys. D. Appl. Phys. 2011, 44. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Sadeghi, N. Rotational and Vibrational Distributions of N2(C) Excited by State-Selected Ar(3P2) and Ar(3P0) Metastable Atoms. Chem. Phys. 1983, 79, 41–55. [Google Scholar] [CrossRef]
- Raud, S.; Raud, J.; Jõgi, I.; Piller, C.T.; Plank, T.; Talviste, R.; Teesalu, T.; Vasar, E. The Production of Plasma Activated Water in Controlled Ambient Gases and Its Impact on Cancer Cell Viability. Plasma Chem. Plasma Process. 2021, 41, 1381–1395. [Google Scholar] [CrossRef]
- Xiong, Q.; Nikiforov, A.Y.; Lu, X.P.; Leys, C. High-Speed Dispersed Photographing of an Open-Air Argon Plasma Plume by a Grating-ICCD Camera System. J. Phys. D. Appl. Phys. 2010, 43. [Google Scholar] [CrossRef]
- Luque, J.; Crosley, D.R. LIFBASE: Database and Spectral Simulation Program (Version 1.5). SRI Int. Rep. 1999, 9. [Google Scholar]
- Miura, N.; Hopwood, J. Spatially Resolved Argon Microplasma Diagnostics by Diode Laser Absorption. J. Appl. Phys. 2011, 109, 2458–2464. [Google Scholar] [CrossRef]
- Muñoz, J.; Dimitrijević, M.S.; Yubero, C.; Calzada, M.D. Using the van Der Waals Broadening of Spectral Atomic Lines to Measure the Gas Temperature of an Argon-Helium Microwave Plasma at Atmospheric Pressure. Spectrochim. Acta - Part B At. Spectrosc. 2009, 64, 167–172. [Google Scholar] [CrossRef]
- 35. Raizer, Yuri, P. Gas Discharge Physics, Allen, J.E., Ed.; Springer-Verlag Berlin Heidelberg 1991, 1991; ISBN 131 3-540-19462-2Springer-Verlag Berlin Heidelberg New York.
- Kramida, A., Ralchenko, Yu., Reader, J., and N.A.T. NIST Atomic Spectra Database (Ver. 5.10), [Online].
- Zhu, X.M.; Pu, Y.K. A Simple Collisional-Radiative Model for Low-Temperature Argon Discharges with Pressure Ranging from 1 Pa to Atmospheric Pressure: Kinetics of Paschen 1s and 2p Levels. J. Phys. D. Appl. Phys. 2010, 43. [Google Scholar] [CrossRef]
- Puurunen, R.L. Surface Chemistry of Atomic Layer Deposition: A Case Study for the Trimethylaluminum/water Process. J. Appl. Phys. 2005, 97. [Google Scholar] [CrossRef]
- Gosset, L.G.; Damlencourt, J.F.; Renault, O.; Rouchon, D.; Holliger, P.; Ermolieff, A.; Trimaille, I.; Ganem, J.J.; Martin, F.; Séméria, M.N. Interface and Material Characterization of Thin Al2O3 Layers Deposited by ALD Using TMA/H2O. J. Non. Cryst. Solids 2002, 303, 17–23. [Google Scholar] [CrossRef]
- Talviste, R.; Raud, S.; Jogi, I.; Plank, T.; Raud, J.; Teesalu, T. Investigation of a He Micro Plasma-Jet Utilized for Treatment of Prostate Cancer Cells. Plasma Res. Express 2019, 1. [Google Scholar] [CrossRef]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Hampson, R.F.; Kerr (Chairman), J.A.; Troe, J. Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry: Supplement III. IUPAC Subcommittee on Gas Kinetic Data Evaluation for Atmospheric Chemistry. J. Phys. Chem. Ref. Data 1989, 18. [Google Scholar] [CrossRef]
- Bruggeman, P.; Schram, D.; González, M.Á.; Rego, R.; Kong, M.G.; Leys, C. Characterization of a Direct Dc-Excited Discharge in Water by Optical Emission Spectroscopy. Plasma Sources Sci. Technol. 2009, 18. [Google Scholar] [CrossRef]
- Del Greco, F.P.; Kaufman, F. Lifetime and Reactions of OH Radicals in Discharge-Flow Systems. Discuss. Faraday Soc. 1962, 33, 128–138. [Google Scholar] [CrossRef]
- Tochikubo, F.; Uchida, S.; Watanabe, T. Study on Decay Characteristics of OH Radical Density in Pulsed Discharge in Ar/H2O. Japanese J. Appl. Physics, Part 1 Regul. Pap. Short Notes Rev. Pap. 2004, 43, 315–320. [Google Scholar] [CrossRef]
| ALD Process | x, mm | GPC, nm | , g/cm3 | MGPC, ng/cm2 | Roughness, nm |
|---|---|---|---|---|---|
| Thermal ALD | 20 | 0.104 ± 0.002 | 3.21 ± 0.06 | 33.4 ± 0.9 | 2.4 ± 0.1 |
| PEALD | 20 | 0.094 ± 0.002 | 3.43 ± 0.07 | 32 ± 1 | 3.3 ± 0.1 |
| Thermal ALD | 70 | 0.105 ± 0.002 | 3.27 ± 0.06 | 34.3 ± 0.9 | 1.5 ± 0.1 |
| PEALD | 70 | 0.092 ± 0.002 | 3.25 ± 0.06 | 29.9 ± 0.9 | 1.5 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





