1. Introduction
Conservation and sustainable management of forest trees are vital not only for the goods and services they provide but also for the mitigation of climate change effects. This is especially important for multipurpose forest trees because, besides timber, they provide essential non-timber forest products, such as food, fodder, fuel wood, medicines, biofuels, shade, and support soil conservation. Sustainability of forest trees could be achieved by conserving their natural forest genetic resources
in-situ and planting genetically improved stock to meet the demands. Both practices require the existence of diversity, especially genetic diversity, which provides the basis for conservation and sustainable management of forest trees [1-4] and is essential for genetic improvement and adaptive potential under climate change [
4]. Morphometric diversity can often reflect underlying genetic diversity. Therefore, understanding morphological and genetic diversity is crucial in forest trees. Among the morphometric traits, seeds and fruits are extremely important reproductive traits because survival and reproduction are essential for the existence of a species. Furthermore, seed size is a functional trait under selection in plants and affects quality and fitness of the offspring [
5,
6] and local adaptation of the species [
7]. Seeds and fruits are also of economic importance in many forest trees. Therefore, knowledge of morphometric variation in seed and fruit characteristics is of basic and applied importance. The first step in tree breeding is usually collecting seeds and fruits for conducting provenance tests and examining provenance variation in seed, fruit, and offspring growth traits.
Albizia lebbek (L.) Benth, commonly known as Indian siris, black siris or siris, is a fast-growing multipurpose tree belonging to the family Fabaceae and subfamily Mimosoideae. It is native to the tropical and subtropical regions of Asia and Africa and extensively distributed in the Indian subcontinent [
8]. Due to its adaptability to various climatic conditions,
A. lebbeck has a wide natural distribution range in India. It is also commonly planted along roadsides and gardens as a shade and avenue tree.
A. lebbek has high economic, ecological and environmental importance. It is valued for various end uses including timber, fuel wood, shade tree in agroforestry systems [
9], fodder for livestock [
10] and a wide range of medicines [
11]. The fruit pods of
A. lebbeck are a source to produce microporous activated carbon used for water and sewage treatments and air purification [
12], potential source for bioenergy and bioethanol production [
13,
14] and a low-cost biosorbent for water softening [
15] and removal of phenol from aqueous solutions [
12,
16].
A. lebbek seeds have extensive medicinal applications for the treatment of diverse ailments [
17,
11]. Furthermore, this species has applications for soil conservation and erosion control owing to its shallow root system and ability to improve soil fertility due to its symbiotic association with
Rhizobia [
18]. Therefore,
A. lebbek could be called a “wonder tree”. Hence, conservation and sustainable management and genetic improvement of
A. lebbek are of crucial importance.
Despite the huge ecological, economic, and environmental importance of
A. lebbek, almost nothing is known about geographical, morphological and genetic variation in this species. From the adaptability of
A. lebbeck to a wide range of edaphic and ecological conditions, high morphological and genetic diversity is expected in this species. Also, there is no information on relationships of morphometric or genetic traits with bioclimatic factors, which is necessary to understand genetic basis of local adaptation to climate in this species as well as its responses and adaptation to climate change. We are also not aware of any active tree improvement and genetic resource conservation program for this species. To our knowledge, only one study has been published on morphological variation in
A. lebbek provenances [
19], which was conducted to examine variation in three seed traits from a very small part of the species range in Himachal Pradesh State of India. Also, Bagchi and Emannuel [
20] reported variation in pod length and number of seeds per pod for six trees of
A. lebbek. No study has been published reporting range-wide or limited range (beyond one state) morphological variation in this species. Indeed, most of the studies on morphological variation within the
Albizzia genus are limited to examining variation in seed, pod and/or seedling traits in two species:
A. procera [
21,
22,
23] and
A. chinensis [
24]. Therefore, there is an urgent need to examine morphological and genetic diversity in
A. lebbek that can support its genetic improvement and genetic resource conservation and sustainable management programs.
In order to meet the demands for various products and services from A. lebbek as described above, there is great need to genetically improve the traits of interest. Besides growth and wood traits, seed size and pod size are of significant importance because seeds and pods are of economic and environmental importance in this species as discussed above. One of us (Om P. Rajora) started a tree improvement program of this species in 1981 under the Indo-Danish Project on Tree Improvement, at Forest Research Institute, Dehradun, India. As usual, the first step for initiating a tree improvement program is to collect seeds from various seed sources, conduct provenance tests and examine provenance variation in traits of interest. This paper reports those first efforts. No other genetic improvement has been reported for A. lebbek to our knowledge despite four decades have passed since the first efforts were made, and Om Rajora came to Canada.
The objective of this study was to examine morphological variation in seed and fruit pod traits and seed germination of A. lebbek from its natural range in northern India and correlations among the studied seed and pod traits and seed germination, and to understand the influence of bioclimatic factors of seed sources on the pod and seed traits variation. We expected high morphological variation in seed size and pod size over the sampled range because of wide differences in ecological, edaphic and climate conditions.
2. Materials and Methods
2.1. Provenances and Pod Collection
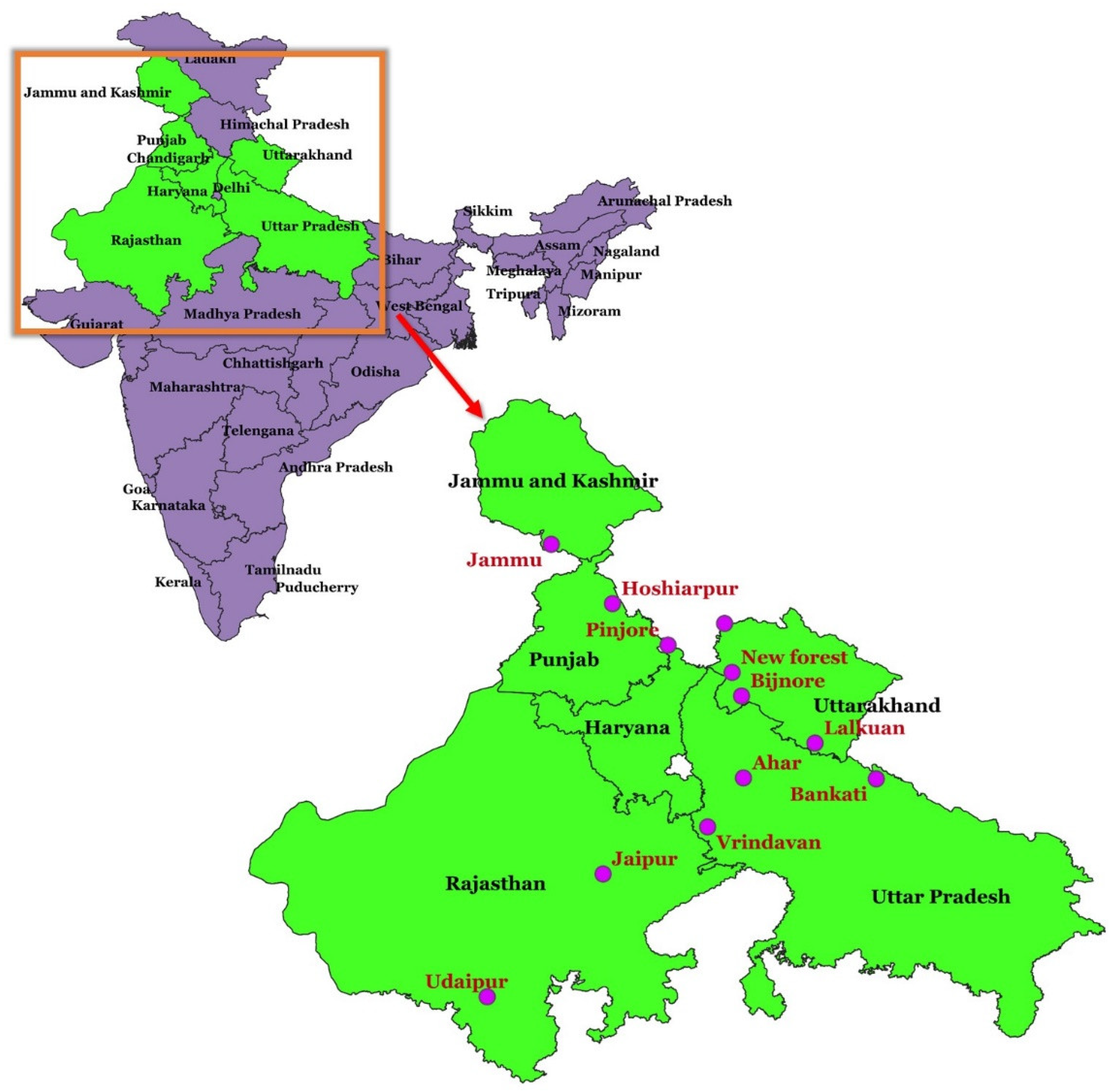
Twelve provenances (seed sources) of
A. lebbek located in six states (Jammu and Kashmir, Punjab, Haryana, Uttar Pradesh, Uttarakhand, and Rajasthan) in northern India were sampled. These locations differed in climatic and ecological conditions, spanning over various latitudes, longitudes, altitudes and rainfall regimes (
Table 1;
Figure 1). Clean matured unopened brown pods with seeds were collected from at least 10 different siris trees per provenance in 1981 and placed in bulk in one gunny bag.
The collected pods were brought to Forest Research Institute and Colleges, Dehradun, India for further processing and measurements. The pods were subsequently sun dried
2.2. Pod Measurement
Four replicates of 25 pods each were randomly drawn from each provenance for the measurement of pod morphological traits. The length and width of each of the 100 pods per provenance were measured using a ruler to a mm resolution. The pod width to pod length ratio was calculated for each measured pod. The number of seeds per pod was counted and the ratio of the number of seeds per pod to the pod length was calculated.
2.3. Seed Extraction and Seed Traits Measurement
Seeds were manually extracted from dried pods separately for each provenance. The number of insect infected seeds was counted in four replicates each of randomly selected 100 seeds. Discolored, stained, and damaged seeds were discarded.
Seed length and seed width were determined for 100 seeds in four replicates of 25 seeds each drawn randomly from each seed lot per provenance. Individual seeds were measured for their maximum length and width to a mm resolution using a ruler. Seed width/seed length and seed width × seed length of each measured seed were calculated. Fresh seed weight was measured in four replicates of 100 seeds each per provenance, and 1000 seed weight was calculated from these measurements. The moisture percent of the seeds was calculated as [(fresh weight – dry weight)/fresh weight) × 100] in four replicates of 100 seeds each per provenance. The seed dry weight was determined by drying the seeds at 105 + 1oC in an oven according to the International Seed Testing Association Rules (International Seed Testing Association 1966).
2.3. Seed Germination
Seed germination was conducted using four replicates of 100 seeds each randomly drawn from each seed lot from 12 May 1981 to 3 June 1981. The seeds were sown in soil contained in pots, which were placed in a glasshouse at the Forest Research Institute and Colleges, Dehradun, India under natural light, temperature and humidity conditions. Seed germination was monitored, and the germinated seeds were counted daily when the radicle reached about 1 cm. Maximum seed germination was considered when no further seed germination was observed, and the percentage was calculated from maximum seed germination counts at the end of the experiment. The seed germination value (SGV) was calculated for each replicate following the procedure described by [
25]
where PV is the peak value of seed germination, which is the highest seed germination percentage divided by the number of days since the start of the experiment, and MDG is the mean daily germination value.
2.4. Data Analysis
The mean, standard deviation (SD) and coefficient of variation (CV) for each measured and derived pod and seed morphological traits and seed germination were calculated for each provenance using SPSS 19 (SPSS, Inc., Chicago, IL, USA). Tukey’s multiple comparison test was conducted to test the significance of differences in the means of pod and seed traits among the 12 provenances. Analysis of variance (ANOVA) was performed to test the significance of differences in the measured and derived traits due to provenances and replicates using the Univariate General Linear Model in SPSS 19 (SPSS, Inc., Chicago, IL, USA). Arcsin transformed data was used for ANOVA for pod width/pod length and seed width/seed length ratios, insect infected seeds percentage, seed moisture percentage, and seed germination percentage.
The Generalized Linear Model was used for variance component analysis and estimation of provenance (σ2prov) and error (σ2e) was conducted using restricted maximum likelihood method. The contribution of provenance variation to the total variance was determined as the proportion of the total phenotypic variance due to provenance as follows:
Correlation between the pod and seed parameters and its statistical significance was determined using Pearson correlation coefficient executed in SPSS 19 (SPSS, Inc., Chicago, IL, USA). Further, to determine whether the variation in seed and pod traits is clinal or not related to provenances’ geographical locations, correlation of the pod and seed traits with geographical coordinates (longitude and latitude), altitude and rainfall was determined. Additionally, 19 climatic variables were downloaded from the Worldclim data portal (
https://www.worldclim.org/data/bioclim.html) and correlation of each of these climate factors with each of the pod and seed traits was determined. Principal component analysis (PCA) was conducted using FactoMineR [
26] to condense the studied traits into principal components and explore the continuum of trait variation. Hierarchical clustering analysis was conducted using factoextra [
27] to generate a UPGMA dendrogram and group the provenances into clusters based on the Euclidean distances calculated from the studied traits. All the graphical representations were performed using the R statistical environment software [
28].
3. Results
3.1. Pod Traits
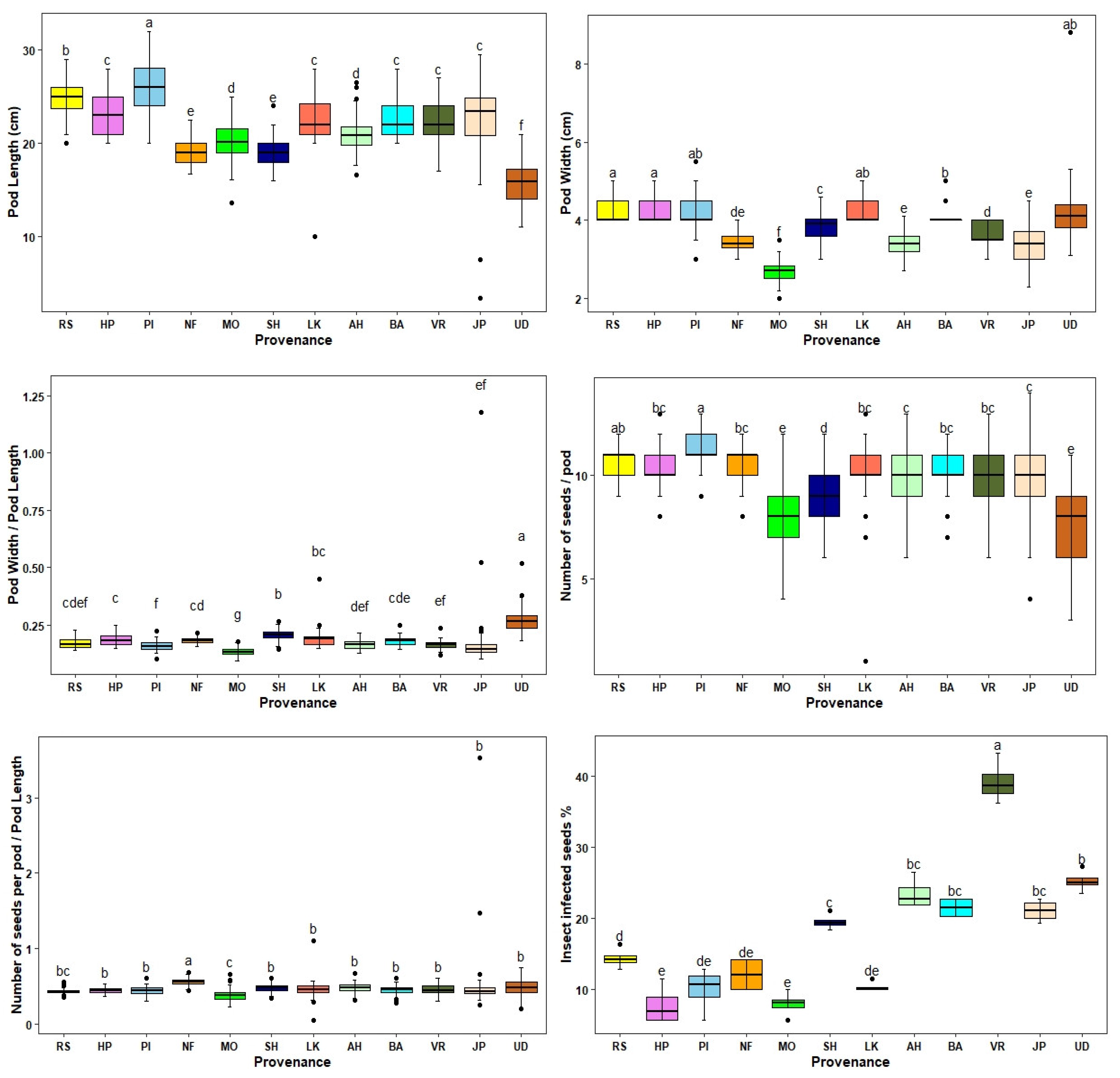
The pod characteristics of
A. lebbek showed high inter-provenance variation (
Table 2). The mean pod length over the provenances varied from 15.7 cm to 26.2 cm, with an overall mean of 21.6 cm. The mean number of seeds per pod varied from 7.5 to 11.3, with an overall mean of 3.8 (
Table 2;
Figure 2). The highest pod length and maximum number of seeds per pod were recorded for the Pinjore provenance followed by the Jammu provenance, and the lowest were documented for the Udaipur provenance. The mean pod width varied from 2.7 cm to 4.3 cm, with Hoshiarpur provenance documenting the highest pod width followed by Jammu, whereas lowest pod width was observed for the Mohand provenance (
Table 2;
Figure 2). The mean pod width/pod length varied from 0.13 to 0.27 over the provenances with an overall mean of 0.18, whereas the number of seeds per pod length varied from 0.38 to 0.55, with an overall mean of 0.46 (
Table 2). The variation in all measured and derived pod traits was highly significant among the provenances (
Table 3), whereas the variation between replicates was not significant (
Table 3).
A significant proportion of the total variation in pod length (59.2) and pod width (65.2%) was due to the provenance effect (
Table 3). In contrast, the provenance effect on the number of seeds per pod and the number of seeds per pod / pod length was lower than the error (environment) effect (
Table 3)
3.2. Seed Traits
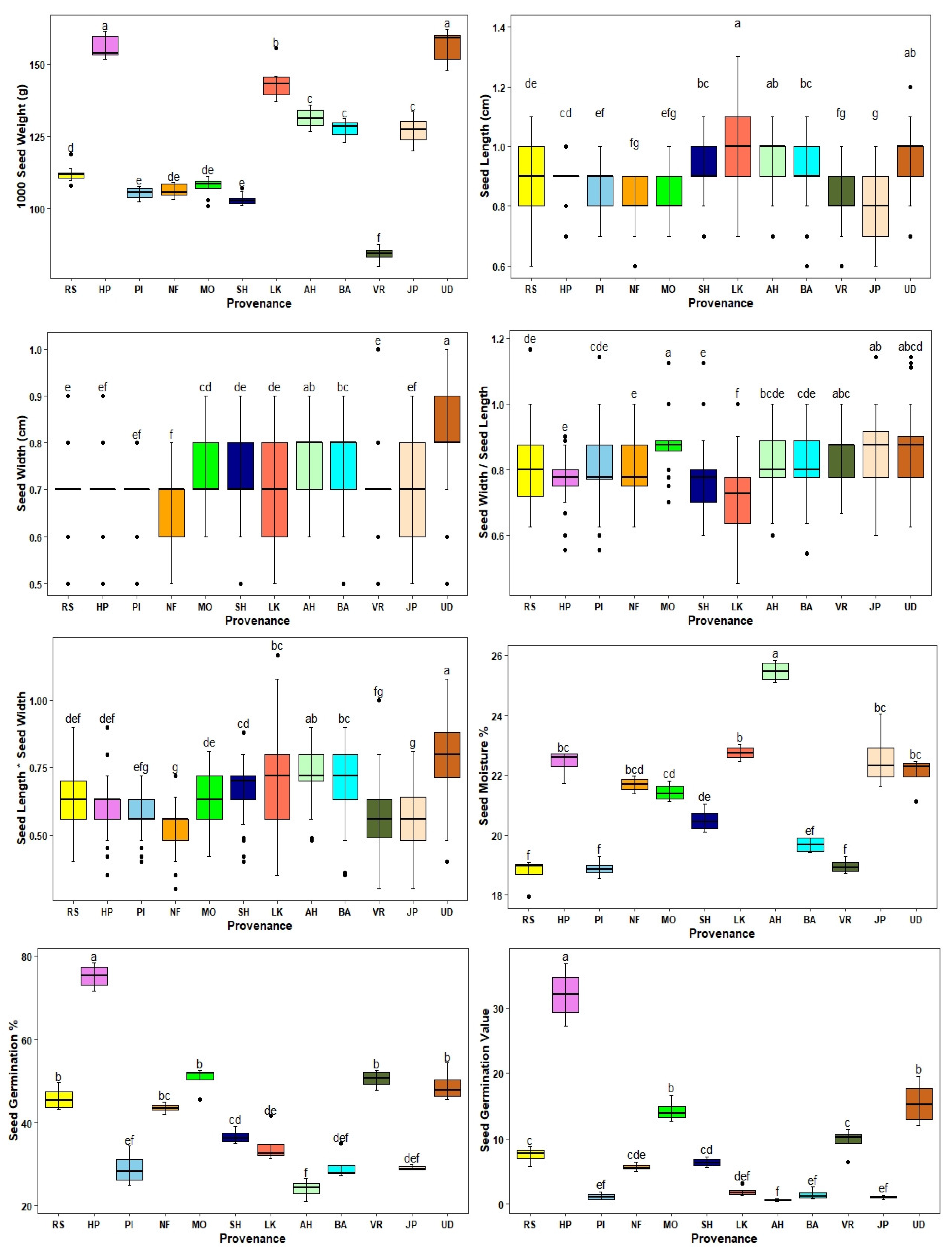
Highly significant variation for the measured and derived seed traits and seed germination was observed among the 12
A. lebbek provenances (
Table 4;
Figure 3). The mean 1000 seed weight varied from 84.19 g to 156.35 g over the provenances, with an overall mean of 121.66 g. Seeds from the Udaipur provenance had the highest 1000 seed weight followed by the Hoshiarpur, Lalkuan and Ahar provenances, whereas the lowest 1000 seed weight was observed for seeds from Vrindavan. The mean seed length and seed width varied over the provenances from 0.81cm, and 0.66 cm to 0.99 cm, and 0.81 cm, respectively, with an overall mean of 0.89 cm and 0.72 cm (
Table 4). The highest seed length was observed for the Lalkuan provenance followed by Udaipur and Ahar provenances, whereas the highest seed width was recorded for the Udaipur provenance followed by Ahar. The lowest seed length and seed width were documented for the Jaipur, and New Forest provenances, respectively (
Table 4;
Figure 3). The highest insect infected seeds were observed for the Vrindavan and the lowest for the Hoshiarpur provenance (
Table 4). The mean seed germination % varied from 24.09 to 75.17 (overall mean 41.46%) and the mean seed germination value varied from 0.59 to 31.97 (overall mean 8.08), with seeds collected from Hoshiarpur documenting the highest values and seeds from Ahar the lowest values (
Table 4;
Figure 3). The moisture percentage of seeds varied from 18.74 (Jammu) to 25.47 (Ahar) with an overall mean of 21.26 (
Table 4). No single provenance had the highest or lowest values for all seed traits.
ANOVA revealed highly significant differences (
p < 0.001) for all seed morphological traits and seed germination among the provenances, and no significant differences among the replicates except for seed germination value (
Table 3).The provenance effect was predominant for most of the seed traits including 1000 seed weight, seed germination, seed germination value, seed moisture, and the insect-infected seeds accounting for over 90% of the total variance (
Table 3). However, the provenance effect was lower than the error effect for seed length and seed width, and their derived traits (
Table 3).
3.2. Correlation Between Pod and Seed Traits
The correlation coefficients among the pod and seed traits ranged from highly negative (r=-0.65) to highly positive (r=0.96) (
Table 5). However, only a small number of inter-trait correlations were statistically significant. And the ones that were significant were expected. The results revealed a significant positive correlation of seed length or seed width with 1000 seed weight, as expected (
Table 5). Similarly, a highly significant (
p<0.01) positive correlation was observed between the number of seeds per pod and pod length (r = 0.789), and between seed germination % and seed germination value (r=0.96). Furthermore, 1000 seed weight exhibited a significant positive correlation with seed moisture percent (
Table 5). A significant negative correlation was observed between pod width and SW/SL, and between the numbers of seeds per pod with seed width (
Table 5).
3.3. Correlation of Pod and Seed Traits with Geographic and Climatic Factors
Except for the percentage insect infected seeds, none of the measured or calculated pod or seed morphological traits and seed germination traits showed significant correlation with longitude, latitude, altitude, or rainfall (
Table 6). The insect infected seeds percentage showed a significant negative correlation with latitude and four precipitation related bioclimatic factors but a significant positive correlation with precipitation seasonality (
Table 6).
The majority of correlations between seed or pod traits with temperature bioclimatic factors were positive; however, correlations of only some pod traits were statistically significant (
Table 6). On the other hand, majority of the correlations of seed and pod traits with the precipitation bioclimatic factors were negative, only correlations of seed width and seed width/seed length with a few precipitation factors were statistically significant (
Table 6). A significant negative correlation was observed between seed width and precipitation of the warmest quarter, between seed width/seed length with each of precipitation of the wettest month, precipitation of the wettest quarter, and precipitation of the warmest quarter (
Table 6). On the contrary, NSPP/PL showed a highly significant positive correlation with precipitation of the wettest month as well as precipitation seasonality (
Table 6). Among the pod traits, pod width showed significant positive correlations with several temperature related climatic factors, i.e., annual mean temperature, mean diurnal range, mean temperature of wettest, warmest and coldest quarter, minimum and maximum temperatures of the coldest and warmest month (
Table 6). Also, a significant positive correlation of pod length with temperature seasonality was observed, and pod width/pod length was found to be significantly negatively correlated with isothermality (
Table 6).
3.4. Clustering of the Provenances for Pod and Seed Traits and Climatic Factors
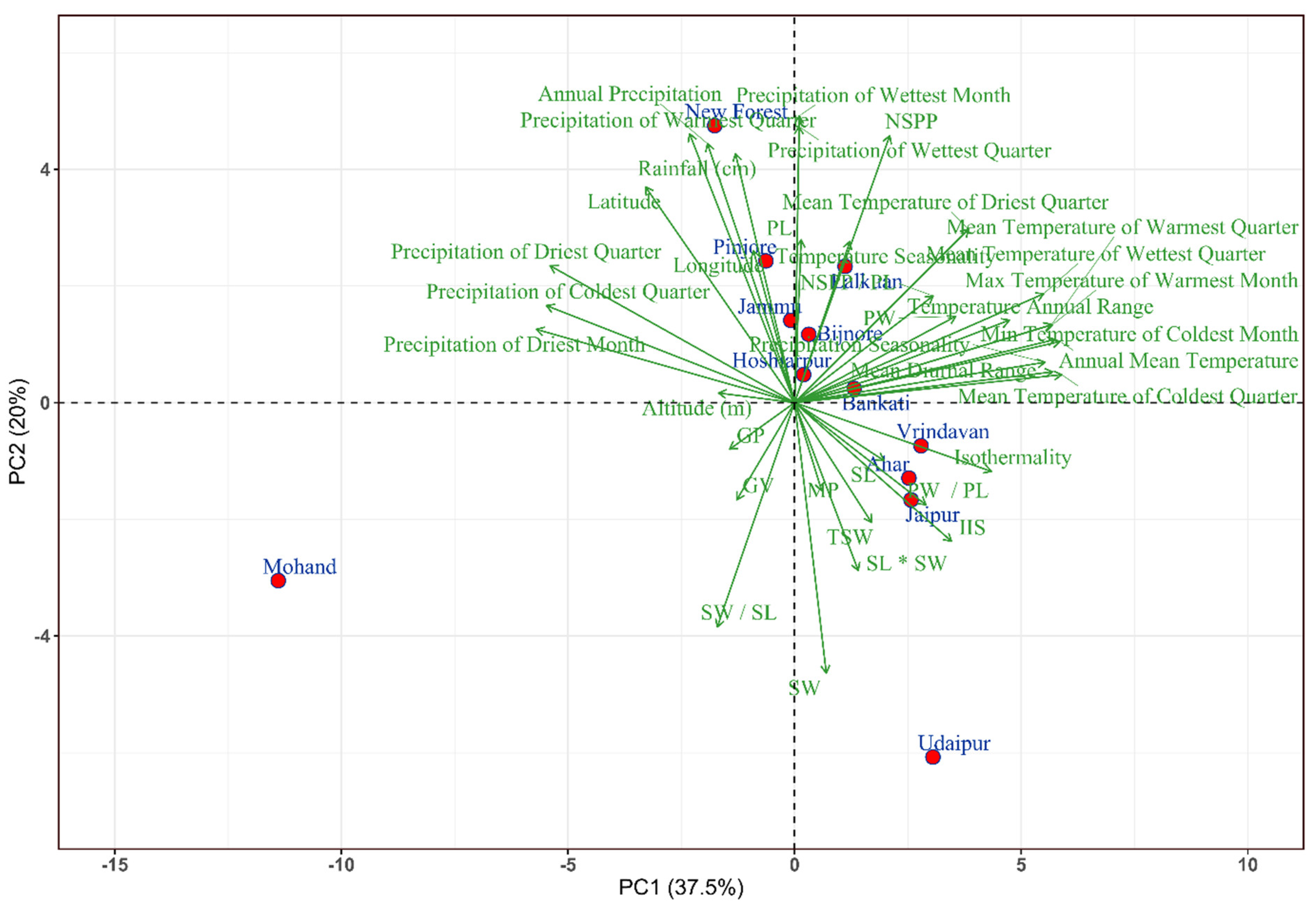
The first two principal components from the principal component analysis explained 37.5%, and 20% of the total variation, respectively (
Table S1;
Figure 4). The first principal component (PC1) was highly positively correlated with pod width (0.59,
p < 0.05) and % insect infected seeds (0.58,
p < 0.05) (
Table S2). The second principal component (PC2) showed a significant positive correlation with the number of seeds/pod (0.76,
p < 0.01) and a significant high negative correlation with seed width (-0.64,
p < 0.01) and seed width/seed length (-0.77,
p < 0.05) (
Table S2). PC1 representing pod width and IIS, was significantly and positively correlated with the mean temperature of the coldest quarter, annual mean temperature, minimum and maximum temperatures of the coldest and warmest month from the climatic factors whereas PC2 representing number of seeds per pod, seed width, and seed width/seed length was significantly correlated with annual precipitation, precipitation of wettest month, wettest and warmest quarter variables (
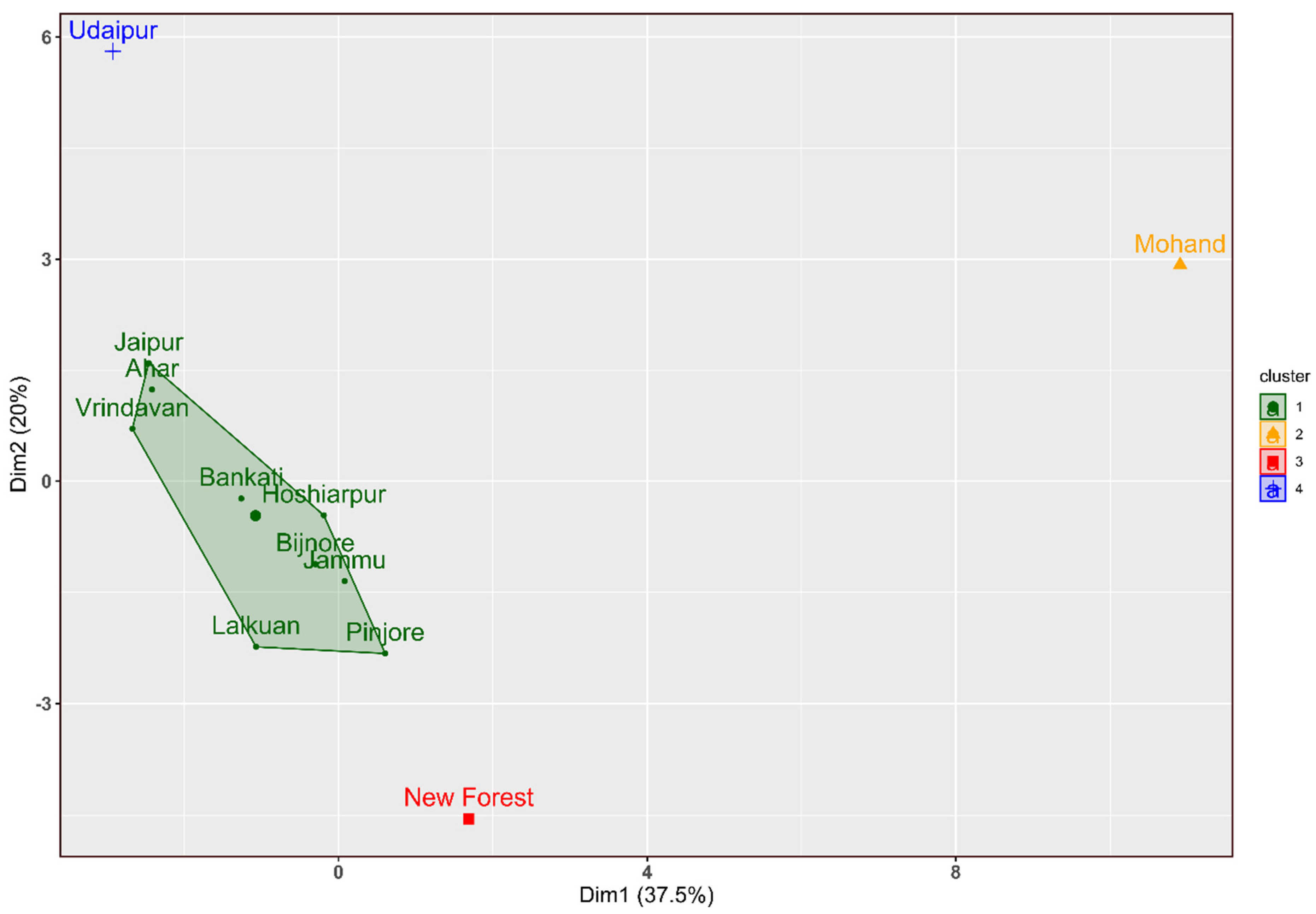
Table S2). The PC1 and PC2 separated New Forest, Mohand and Udaipur provenances from the rest, which were clustered together (
Figure 4). Furthermore, the provenances from Udaipur, Ahar, Jaipur, and Vrindavan clustered in the right lower quarter of the plot had larger seed size (seed length and seed width), seed weight, and higher seed moisture percent (
Figure 4;
Table 3).
Hierarchical clustering analysis grouped provenances into clusters according to their similarity based on the Euclidean distances calculated from the studied pod and seed traits. The UPGMA clustering classified the provenances into four major groups (
Figure 5), which was consistent with that observed from PCA (
Figure 4). The largest group (Group 1) consisted of nine provenances (JP, VR, AH, BA, LK, SH, PI, HP, and RS), while groups 2, 3, and 4 were represented by one provenance each: Mohand, New Forest, and Udaipur respectively (
Figure 5).
4. Discussion
We have demonstrated that seed and pod size and seed germination traits have highly significant inter-provenance variation in A. lebbek in its range in northern India. We have also shown that the variation in all traits except percent insect infected seeds (IIS) is not clinal related to latitude, longitude, altitude, or rainfall. The variation in IIS showed clinal variation related to latitude. Both the seed size and pod size appear to be positively affected by temperature bioclimatic factors and negatively by precipitation bioclimatic factors.
4.1. Provenance Variation in Seed and Pod Traits
Our study shows that morphometric traits related to seed size (seed length, seed width, seed width/seed length, seed length × seed width) and pod size (pod length, pod width, pod width/pod length), number of seed per pod, seed mass (1000 seed weight), seed germination (seed germination %, seed germination value), and percent insect infected seeds have highly significant variation among the studied
A. lebbek provenances from the species’ range in northern India. Even the geographically closer provenances, such as Mohand and New Forest, showed significant inter-provenance variation in most traits (
Table 2 and
Table 4;
Figure 2 and
Figure 3). For several traits, including seed weight, seed germination, seed germination value, seed moisture, and insect-infected seeds, the provenance effect contributed
>95% to the variability observed (
Table 3). Our results also demonstrate that none of the provenance has the highest or lowest values of all traits. The morphometric variation in the traits studied here could be conditioned by genetic, environment and genotype × environment factors. A well-designed provenance test is required to determine the extent to which each of these factors contributed to the observed variation in seed and pod traits of
A. lebbek. Among the traits studied, seed germination test could be considered as a replicated provenance test under the same conditions. The provenance effect contributed to 95.5% of the total variation for seed germination, which suggests that genetic factors likely contributed highly to the total variation in seed germination. Our results of significant provenance variation in seed and pod traits of
A. lebbek are consistent with similar observations for seed length, seed weight and 1000 seed weight reported for
A. lebbek provenances from the Indian state of Himachal Pradesh [
19], as well as for seed and/or pod traits of its sister species
Albizia procera [
21,
22,
23], and
Albizia chinensis [
24,
29]. Our results are also consistent with those reported for other tropical angiosperm forest trees and annual plants, including
Dalbergia sissoo [30-35],
Pongamia pinnata [
36,
37,
38,
39],
Ambrosia artemisiifolia [
40],
Carpobrotus edulis [
41],
Faidherbia albida [
42],
Magnolia officinalis [
43],
Parkia timoriana [
44], and
Cordia africana [
45]. Consistent with our observations, high provenance effect (>90%) was reported for seed weight in
Albizia chinensis,
Albizia procera,
Dalbergia sissoo,
Cordia africana and
Pongamia pinnata [
29,
23,
33,
34,
45,
46]. However, the extent of provenance effect contributing to the total variation was found to be lower for seed germination in
Albizia chinensis (66%) [
29] and
Albizia procera (6%) [
21].
The range and average seed length and seed width observed in our study (seed length 0.81-0.99 cm, mean 0.89 cm; seed width 0.66-0.81 cm, mean 0.72 cm) were similar to those reported for
A. lebbek provenances from Himachal Pradesh (seed length 0.84-0.99 cm, mean 0.90 cm; seed width 0.68-0.84 cm, mean 0.74 cm [
19]). However, both the pod size (pod length and pod width) and seed size (seed length and seed width) of
A. lebbek we recorded are larger than those reported for its sister species
Albizzia procera [
21,
22], indicating that fruit pods and seeds of
A. lebbek are larger than that of
A. procera.
4.2. Interrelationships between Seed and Pod Traits
Significant positive or negative correlations observed between seed and pod traits (
Table 5) are expected. For example, significant positive correlation of seed length with seed width, seed length and seed length × seed width with 1000 seed weight, number of seeds per pod with pod length, and percent seed germination with seed germination value, all are expected and make sense. This suggests that selection for seed size or seed mass could be made from seed length and seed width. Significant negative correlations were mainly observed between the original measured and their derived traits (
Table 5), which are also all expected. Similar correlation results that we observed were also reported in sister
Albizzia and other tropical forest tree species:
Albizzia chinensis [
29],
Albizia procera [
22],
Dalbergia sissoo [
30,
32,
34],
Pongamia pinnata [
36,
39],
Magnolia officinalis [
43], and
Faidherbia albida [
42]. Although positive correlation was observed between seed mass or seed size traits with seed germination (
Table 5), the correlation was not statistically significant. This suggests that seed mass or seed size does not significantly influence seed germination in the studied
A. lebbek provenances. We expected a significant positive correlation because a larger seed mass can provide more stored resources and energy for seed germination. Previous studies in
Pongamia pinnata,
Aquilaria malaccensis,
Dalbergia sissoo,
Albizia procera, and
A. chinensis reported a direct association of seed weight and seed size with germination percentage [
23,
24,
47,
48,
49]. There may be some intrinsic and extrinsic factors, such as local adaptation of the provenances, temperature, seed germination medium that may have contributed to non-significant correlations of seed size and seed mass with seed germination percentage in our study. Additional more in-depth work is needed to determine those factors.
4.3. Relationships of Seed and Pod Traits with Geocoordinates, and Pattern of Variation
The results of our study show that the variation in all seed and pod traits studied, except insect infected seeds, is not clinal related to longitude, latitude, altitude, or rainfall in
A. lebbek over its range in northern India. Differences in latitude and longitude are normally associated with differences in temperature and precipitation. The 12
A. lebbek provenances sampled in this study spanned over about 4 degrees in latitude and 8 degrees in longitude. It appears that the differences in latitude, longitude, altitude, and rainfall amount over the study area do not create gradients strong enough that can exert selection pressures to create clinal variation in the studied seed and pod traits in
A. lebbek. Even some geographically closest provenances, such as Mohand and New Forest which are about 30 km apart, were highly differentiated from each other from both the principal component and cluster analyses (
Figure 4 and
Figure 5). Non-clinal pattern of variation in seed length, seed width, and 1000 seed weight was also previously reported for 15
A. lebbek provenances from Himachal Pradesh [
19]. It seems that the local soil, climate and other conditions likely affect the variation in seed and pod traits in
A. lebbek and the geographical diversity does not apparently reflect the morphological diversity in seed and pod traits. On the contrary, clinal pattern of variation was reported for seed traits in
Dalbergia sissoo [
30,
32,
33],
Pongamia pinnata [
46],
Cordia africana [
45],
Faidherbia albida [
42],
Magnolia officinalis [
43], and
Ambrosia artemisiifolia [
40]. Our results suggest clinal variation in insect infected seeds related to latitude with increased insect infected seed with decreased latitude. This is consistent with the well-known fact that species diversity, including that of insects, and their niche increase from north toward the equator, i.e., from higher latitudes to lower latitudes. This may be the case with more insects infecting
A. lebbek seeds with decreasing latitude.
Nine of the 12 provenances formed one group based on both the principal component and cluster analyses (
Figure 4 and
Figure 5) This suggests their common ancestral origin. Three provenances, New Forest, Mohand and Udaipur that clustered individually separate from the large group of nine provenances, may represent ecotypic variation in the studied seed and pod traits. For example, Mohand has distinct climatic conditions including the lowest mean annual temperature (10.1
oC), isothermality, and precipitation seasonality and Udaipur provenance is located in the Rajasthan’s desert lands, and the area has hot climate (mean annual temperature 24.1 °C). The New Forest provenance is located in the Himalayan Doon valley and its climate and soil conditions are different from that of other provenances. Ecotypic variation in seed size has also been reported for a perennial grass
Panicum hallii [
7]. Differentiation of New Forest and Mohand provenances may also be due to their location close to the species range margin ([
8];
https://indiabiodiversity.org/species/show/31039) because marginal populations are expected to be genetically differentiated from the central populations [
50,
51,
52]. Jammu provenance location is also marginal ([
8];
https://indiabiodiversity.org/species/show/31039) but it was not differentiated from other provenances within the major group. Population genetics and genomics studies are needed to address the central-marginal population differentiation issues in
A. lebbek.
4.4. Relationships of Seed and Pod Traits with Bioclimatic Factors
The local bioclimatic factors/conditions can influence inter-provenance variation in seed and pod traits and has been reported for seed traits in
Faidherbia albida [
42],
Magnolia officinalis [
43],
Parkia timoriana [
44],
Carpobrotus edulis [
41], and
Cordia africana [
45]. We observed negative correlations of seed traits, including 1000 seed weight, with most of the precipitation bioclimatic factors, although the correlations were statistically significant only for seed width and seed width/seed length ratio (
Table 6). This suggests that higher precipitation may negatively affect seed size and seed mass in
A. lebbek. Vakshasya
et al. [
30] also reported negative correlation of 1000 seed weight with rainfall in
Dalbergia sissoo. A mixed pattern of correlations was observed between seed size or seed mass with temperature factors, with seed mass and seed length showing positive but insignificant correlations (
Table 6). This suggests that temperature may have positive effects on these seed traits. Similar results were reported in other tree species wherein post monsoon temperature positively correlated with pod length in
Parkia timoriana [
44], annual mean temperature positively correlated with seed length, seed width and seed weight in
Magnolia officinalis [
43]. However, seed length, seed width and seed weight were found to be negatively correlated with temperature in
Faidherbia albida [
42].
The positive correlations of pod length and pod width with most the temperature factors, especially significant correlations of pod width with several temperature bioclimatic factors (
Table 6), suggest that temperature has positive effect on pod size. As seen for seed traits, negative correlations of pod traits with several precipitation bioclimatic factors indicate that higher precipitation likely has negative effects on pod size and number of seeds per pod. These correlation results are also supported by the PCA results where PC1 showed significant positive correlations with several temperature bioclimatic factors and pod width, and PC2 showed significant positive correlations with several precipitation bioclimatic factors and significant negative correlations with seed width and seed width/seed length (
Table S2). Meenakshi
et al. [
22] also reported a significant negative correlation of rainfall with pod length, pod width, and number of seeds per pod in
A. procera. And faster fruit growth with increased temperature was reported for
Theobroma cacao [
53] and lower temperature regimes were found to strongly delay pod and seed development in
Vicia faba [
54]. Overall, our results suggest that temperature factors have positive effect on seed size, seed mass, pod size and number of seeds per pod whereas precipitation factors have negative effect on these traits as well on the insect infected seeds in the studied
A. lebbek provenances. It has been shown that seed size contributes to local adaptation in plants (e.g., [
7] and references there in). Temperature and precipitation are the two most important bioclimatic factors of local adaptation significance [
55]. Because seed size and pod size have shown significant correlations with these bioclimatic factors in
A. lebbek in this study, it can be inferred that variation in seed and pod size, and seed mass may impact local adaptation in
A. lebbek like in many other plants ([
7] and references there in).
5. Significance and Implications
The results provide the basic information on provenance variation in
A. lebbek and have significance for provenance and progeny tests, genetic improvement, population genetics and genomics studies and conservation and sustainable management of
A. lebbek genetic resources. Highly significant variation observed for seed and pod traits suggests that genetic improvement of these traits is feasible through selection and breeding. Seed size is an ecologically functional trait in plants which influences the fitness of offsprings in various ways at different stages [
5,
6]. In
A. procera, seedlings originated from large seeds were found to be taller and heavier and had a greater leaf area and tolerance to extreme water stress than seedlings from small seeds [
56]. This may be the case in
A. lebbek. Also, in
A. lebbek both seeds and pods are economically important as seeds are used for medicinal purposes [
11] and pods for bioenergy production [
14]. Our study suggests that a range-wide provenance test of
A. lebbek should be conducted at several locations to understand range-wide variation not only in seed and pod traits but also in other economically and ecologically important traits, such as growth and adaptation. Our results provide the foundation for such studies. Furthermore, well designed progeny tests should be conducted to determine heritability and genetic correlations of seed, pod and other traits.
A. lebbek has shown high morphometric variation in seed and pod traits. Whether such diversity exists at the genetic level, population genetics and genomics studies [
57] should be undertaken to examine both neutral and adaptive genetic variation [
57,
58]. Our results in combination with future population genetics and genomics studies can assist in conservation and sustainable management of
A. lebbek genetic resources. Our results indicate that seed and pod traits are likely involved in
A. lebbek local adaptation to climate; thus, this species could provide an excellent model to examine the genetic architecture of local adaptation to climate in widely distributed tropical forest trees.
6. Conclusions
We have for the first-time reported provenance variation in seed and pod traits and seed germination of A. lebbek and their relationships with geocoordinates and bioclimatic factors from the species’ range in northern India. A. lebbek has high variation in seed and pod size, seed mass, number of sees per pod, and seed germination, and the variation in these traits is not clinal in relation to latitude, longitude, altitude, or rainfall but there is an indication of the existence of ecotypic variation. Because seed size has been established to be under selection, a high genetic diversity is expected in the studied A. lebbek provenances. Based on huge morphometric variation in seed and pod traits, we can conclude that genetic improvement in these traits is feasible by selection and breeding. However, well designed progeny tests should be conducted to determine the extent of genetic control of these traits. Both seed and pod traits are likely influenced by bioclimatic factors, positively by temperature and negatively by precipitation factors. Our study can serve as the foundation or starting point for a range-wide provenance test, and population, conservation, and landscape genomics studies in A. lebbek and their application in conservation and sustainable management of A. lebbek genetic resources especially under changing climate conditions. A. lebbek is widely planted in India. Seeds from many diverse seed trees should be used to maintain morphometric and genetic diversity in the plantations.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S2: Eigen values and percentage of variance from the principal component analysis. Table S1: Pearson’s correlation coefficients and scores of the first two principal components
Author Contributions
Conception, experimental design, field collection, data collection: OPR; data analysis: ABMP; Manuscript writing and revision: OPR, ABMP; Overall direction and principal investigator: OPR; Supervision of ABMP and MS editing: MGD.
Funding
The research work was done as a part of the Indo-Danish Project on Tree Improvement when Om P. Rajora was working as a Research Officer at the Forest Research Institute and Colleges, Dehradun, India. The project was funded by the Government of India and Indo-Danish Project on Tree Improvement.
Data availability statement
The data is available from the corresponding author upon request.
Acknowledgments
We thank the Forest Research Institute for providing transportation for the field work, State Forest Departments for providing the access and locating the sites, and B.S. Nainwal for his help with field collection.
References
- Rajora, O.P. Genetic biodiversity impacts of silvicultural practices and phenotypic selection in white spruce. Theor. Appl. Genet. 1999, 99, 954–961. [Google Scholar] [CrossRef]
- Rajora, O.P.; Mosseler, A. Challenges and opportunities for conservation of forest genetic resources. Euphytica. 2001, 118, 197–212. [Google Scholar] [CrossRef]
- Hoban, S.; Bruford, M.; Jackson, J.D.U.; Lopes-Fernandes, M.; Heuertz, M.; Hohenlohe, P.A.; Paz-Vinas, I.; Sjögren-Gulve, P.; Segelbacher, G.; Vernesi, C.; Aitken, S. Genetic diversity targets and indicators in the CBD post-2020 Global Biodiversity Framework must be improved. Biol. Conserv. 2020, 248, 108654. [Google Scholar] [CrossRef]
- Rajora, O.P.; Zinck, J.W.R. Genetic diversity, structure and effective population size of old-growth versus second-growth populations of keystone and long-lived conifer, eastern white pine (Pinus strobus): Conservation value and climate adaptation potential. Front. Genet. 2021, 12, 650299. [Google Scholar] [CrossRef] [PubMed]
- Moles, A. T.; Westoby, M. Seedling survival and seed size: A synthesis of the literature. J. Ecol. 2004, 92, 372–383. [Google Scholar] [CrossRef]
- Larios, E.; Venable, D.L. Selection for seed size: The unexpected effects of water availability and density. Funct. Ecol. 2018, 32, 2216–2224. [Google Scholar] [CrossRef]
- Razzaque, S.; Heckman, R.W.; Juenger, T.E. Seed size variation impacts local adaptation and life-history strategies in a perennial grass. Proc. R. Soc. B. 2023, 290, 20222460. [Google Scholar] [CrossRef]
- Parrotta, J. A. Albizia Lebbek (L.) Benth. In Tropical tree seed manual; Vozzo, J.A., Ed.; US Department of Agriculture, Forest Service: Washington, DC, USA, 2002. [Google Scholar]
- Singh, S. P. Favourite agroforestry trees; Agrotech Publishing Academy: Udaipur, India, 1995. [Google Scholar]
- Singh, R. V. Fodder trees of India; Oxford and IBH Publishing Company: New Delhi, India, 1982. [Google Scholar]
- Balkrishna, A.; Sakshi; Chauhan, M.; Dabas, A.; Arya, V. A comprehensive insight into the phytochemical, pharmacological potential, and traditional medicinal uses of Albizia lebbeck (L.) Benth. Evid. Based Complement. Alternat. Med. 2022, 2022, 5359669. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M. J.; Theydan, S. K. Adsorption of p-chlorophenol onto microporous activated carbon from Albizia lebbeck seed pods by one-step microwave assisted activation. J. Anal. Appl. Pyrolysis. 2013, 100, 253–260. [Google Scholar] [CrossRef]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Simons, A. Agroforestree Database: a tree reference and selection guide version 4.0, World Agroforestry Centre, Kenya, 2009. https://www.worldagroforestry.org/output/agroforestree-database accessed on 15-02-2024.
- Rajamohan, S.; Chidambaresh, S.; Sundarrajan, H.; Balakrishnan, S.; Sirohi, R.; Cao, D.N.; Hoang, A.T. Investigation of thermodynamic and kinetic parameters of Albizia lebbeck seed pods using thermogravimetric analysis. Bioresour. Technol. 2023, 384, 129333. [Google Scholar] [CrossRef]
- Fernandez, N.; Chacin, E.; Garcia, C.; Alastre, N.; Leal, F.; Forster, C. F. (1996) Pods from Albizia Lebbek as a Novel Water Softening Biosorbent. Environ. Technol. 1996, 17, 541–546. [Google Scholar] [CrossRef]
- Alabi, A.H., Buhari-Alade, A.I., Sholaru, F.O.; Awoyemi, R.F. Biosorption of phenols and dyes on Albizia lebbeck (Rattle seed) pod: equilibrium and kinetic studies. Int. J. Environ. Sci. 2016, 5, 154–165. [Google Scholar]
- Tomar, S.; Jawanjal, P. Critical review of Albizia lebbeck-A multi potent drug. J. Ayu. Her. Med. 2019, 5, 76–81. [Google Scholar] [CrossRef]
- Khera, N.; Singh, R. P. Germination of some multipurpose tree species in five provenances in response to variation in light, temperature, substrate and water stress. Trop. Ecol. 2005, 46, 203–217. [Google Scholar]
- Thakur, I. K.; Dhuppe, S.; Sharma, J. P. Phenotypic variation and seed characters evaluation in different provenances of Albizia lebbeck (L.) Benth. Ind. J. For. 2014, 37, 35–40. [Google Scholar]
- Bagchi, S.; Emmanuel, C. J. S. K. Analysis of variability for pod length and seed count in Albizia lebbek, benth. Myforest 1984, 20. [Google Scholar]
- Tiwari S., K.; Dhuria, S. S. Variability studies of pod and seed characteristics of Albizia procera in Chhattisgarh. Int. J. Sci. Res. Biol. Sci. 2018, 5, 27–31. [Google Scholar]
- Meenakshi; Rana, N.; Ghabru, A. Influence of geographic variation on morphometric traits of seeds, pods and germination behavior of Albizia procera. Int. J. Chem. Stud. 2019, 7, 1626–1631. [Google Scholar]
- Meenakshi,; Rana, N.; Bharti, Chauhan, A.; Sankhyan, N.; Ghabru, A. Exploring intraspecific provenance variation in seed morphological traits of Albizia procera in mid-Himalayan region of India. J. Trop. For. Sci. 2023, 35, 168–178. [Google Scholar]
- Dhanai, C.S.; Uniyal, A.K.; Todaria, N.P. Source variation in Albizia chinensis (Osbeck) Mer.: seed and seedling characteristics. Silvae Genet. 2003, 52, 259–266. [Google Scholar]
- Czabator, F. J. Germination value: an index combining speed and completeness of pine seed germination. For. Sci. 1962, 8, 386–96. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: an R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. factoextra: Extract and visualize the results of multivariate data analyses Package; R Foundation for Statistical Computing: Vienna, Austria, 2017.
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2007.
- Sachan, M.S.; Tomar, J. M. S.; Bhatt, B. P. Genetic parameters for seed traits in Albizia chinensis (Osbeck) MERR. J. interacad. 2006, 10, 293–297. [Google Scholar]
- Vakshasya, R. K.; Rajora, O. P.; Rawat, M. S. Seed and seedling traits of Dalbergia sissoo Roxb.: Seed source variation studies among ten sources in India. For. Ecol. Manag. 1992, 48, 265–275. [Google Scholar] [CrossRef]
- Gera, M.; Gera, N.; Ginwal, H.S. Seed trait variation in Dalbergia sissoo Roxb. Seed Sci. Technol. 2000, 28, 467–475. [Google Scholar]
- Singh, N.; Pokhriyal, T. C. Variations in pod and seed traits in six different Dalbergia sissoo seed sources. J. Trop. For. Sci. 2001, 4, 162–176. [Google Scholar]
- Singh, B.; Bhatt, B.P. Provenance variation in pod, seed and seedling traits of Dalbergia sissoo (Roxb. ex dc), Central Himalaya, India. Trop. Agric. Res. Ext. 2008, 11, 39–44. [Google Scholar] [CrossRef]
- Singh, O.; Sofi, A.H. Variability in seed traits and genetic divergence in a clonal seed orchard of Dalbergia sissoo Roxb. J. For. Res. 2012, 23, 109–114. [Google Scholar] [CrossRef]
- Dubey, S.; Tripathi, S. Studies on Fifteen Seed Source Variation in seed Traits of Dalbergia Sissoo (Roxb). Int. J. Sci. Res. 2018, 7. [Google Scholar]
- Kaushik, N.; Kumar, S.; Kumar, K.; Beniwal, R.; Kaushik, N.; Roy, S. Genetic variability and association studies in pod and seed traits of Pongamia pinnata (L.) Pierre in Haryana. India. Genet. Resour. Crop Evol. 2007, 54, 1827–1832. [Google Scholar] [CrossRef]
- Divakara, B. N.; Alur, A. S.; Tripati, S. Genetic variability and relationship of pod and seed traits in Pongamia Pinnata (L.) Pierre., a potential agroforestry tree. Int. J. Plant Prod. 2010, 4, 129–142. [Google Scholar]
- Sahoo, D. P.; Rout, G. R.; Das, S.; Aparajita, S.; Mahapatra, A. K. Genotypic variability and correlation studies in pod and seed characteristics of Pongamia pinata (L) Pierre in Orissa, India. Int. J. For. Res. 2011, 728985. [Google Scholar]
- Gawali, A.; Wagh, R.; Sonawane, C. Evaluation of genetic variability and correlation in pod and seed traits of Pongamia pinnata (L.) Pierre. germplasm for genetic tree improvement. For. Res. 2015, 4, 1–6. [Google Scholar]
- Zhou, L.; Yu, H.; Yang, K.; Chen, L.; Yin, W.; Ding, J. Latitudinal and longitudinal trends of seed traits indicate adaptive strategies of an invasive plant. Front. Plant Sci. 2021, 12, 657813. [Google Scholar] [CrossRef]
- Fenollosa, E.; Jené, L.; Munné-Bosch, S. Geographic patterns of seed trait variation in an invasive species: how much can close populations differ? Oecologia. 2021, 196, 747–761. [Google Scholar] [CrossRef]
- Koech, G.; Ofori, D.; Muigai, A.W.; Makobe, M.; Muruiki, J.; Mowo, G. J.; Jamnadas, R. Genetic variability and divergence of seed traits and seed germination of five provenances of Faidherbia albida (Delile) A. Chev. Afr. J. Plant Sci. 2014, 8, 482–491. [Google Scholar]
- Shu, X.; Yang, X.; Yang, Z. Variation in seed and seedling traits among fifteen Chinese provenances of Magnolia officinalis. Not. Bot. Hortic. Agrobot. 2012, 40, 274–283. [Google Scholar] [CrossRef]
- Thangjam, U.; Sahoo, U.K.; Thong, P. Characterization of morphometric, reproductive and seedling traits of Parkia timoriana in Northeast India. Silva Fenn. 2020, 54, 1–16. [Google Scholar] [CrossRef]
- Loha, A.; Tigabu, M.; Teketay, D.; Lundkvist, K.; Fries, A. Provenance variation in seed morphometric traits, germination, and seedling growth of Cordia africana Lam. New For. 2006, 32, 71–86. [Google Scholar] [CrossRef]
- Pavithra, H. R., Gowda, B., Prasann, K. T., Shivanna, M. V. Pod and seed traits in candidate plus trees of Pongamia pinnata (L.) Pierre from southern peninsular India in relation to provenance variation and genetic variability. J. Crop Sci. Biotechnol. 2013, 16, 131–142. [Google Scholar] [CrossRef]
- Devagiri, G.M.; Dhiman, R.C.; Thapliyal, R.C.; Patil, C.S.P.; Kumar, N. Genetic analysis of traits related to seed germination and vigour among provenances of Shisham (Dalbergia sissoo Roxb.). Ann. For. 2004, 12, 161–174. [Google Scholar]
- Palanikumaran, B.; Parthiban, K. T.; Sekar, I.; Umarani, R.; Amirtham, D. Variability studies for seed and seedling traits in Pongamia pinnata (L.) Pierre at Tamil Nadu. Electron. J. Plant Breed. 2016, 7, 657–665. [Google Scholar] [CrossRef]
- Dubey, S.; Dey, A. N.; Roy, S.; Saha, A. Studies on seed source variation in seed traits of Aquilaria malaccensis (Agar). Int. j. curr. microbiol. appl. sci. 2020, 9, 1355–1369. [Google Scholar] [CrossRef]
- Chhatre, V.E.; Rajora, O.P. Genetic divergence and signatures of natural selection in marginal populations of a keystone, long-lived conifer, eastern white pine (Pinus strobus) from Northern Ontario. PLoS ONE. 2014, 9, e97291. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Rajora, O.P. Genetic diversity and differentiation of core versus peripheral populations of eastern white cedar, Thuja occidentalis L. (Cupressaceae). Am. J. Bot. 2012, 99, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Götz, J.; Rajora, O.P.; Gailing, O. Genetic structure of natural northern range-margin mainland, peninsular, and island populations of northern red oak (Quercus rubra L.). Front. Ecol. Evol. 2022, 10, 907414. [Google Scholar] [CrossRef]
- Daymond, A. J.; Hadley, P. Differential effects of temperature on fruit development and bean quality of contrasting genotypes of cacao (Theobroma cacao). Ann Appl Biol. 2008, 153, 175–185. [Google Scholar] [CrossRef]
- Lundby, A.M.; Waalen, W.; Uhlen, A.K.; Knutsen, S.H. ; Wold, A-B. Effect of temperature during flowering, pod set, and seed development on yield components and accumulation of protein, starch, and low molecular weight carbohydrates in two faba bean (Vicia faba L.) cultivars. Legum. sci. 2023, e212. [Google Scholar] [CrossRef]
- Rajora, O.P.; Eckert, A.J.; Zinck, J.W.R. Single-locus versus multilocus patterns of local adaptation to climate in eastern white pine (Pinus strobus, Pinaceae). PLoS ONE. 2016, 11, e0158691. [Google Scholar] [CrossRef]
- Khurana, E.; Singh, J.S. Influence of seed size on seedling growth of Albizia procera under different soil water levels. Ann. Bot. 2000, 86, 1185–1192. [Google Scholar] [CrossRef]
- Rajora, O.P. Population Genomics: Concepts, Approaches and Application, Springer Nature: Switzerland AG, 2019; 823pp.
- Luikart, G.; Kardos, M.; Hand, B.; Rajora, O.P.; Aitkin, S.; Hohenlohe, P.A. Population genomics: Advancing understanding of nature. In Population Genomics: Concepts, Approaches and Applications, Rajora O.P., Eds.; Springer Nature: Switzerland AG, 2019; pp. 3–79. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).