Submitted:
28 February 2024
Posted:
06 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
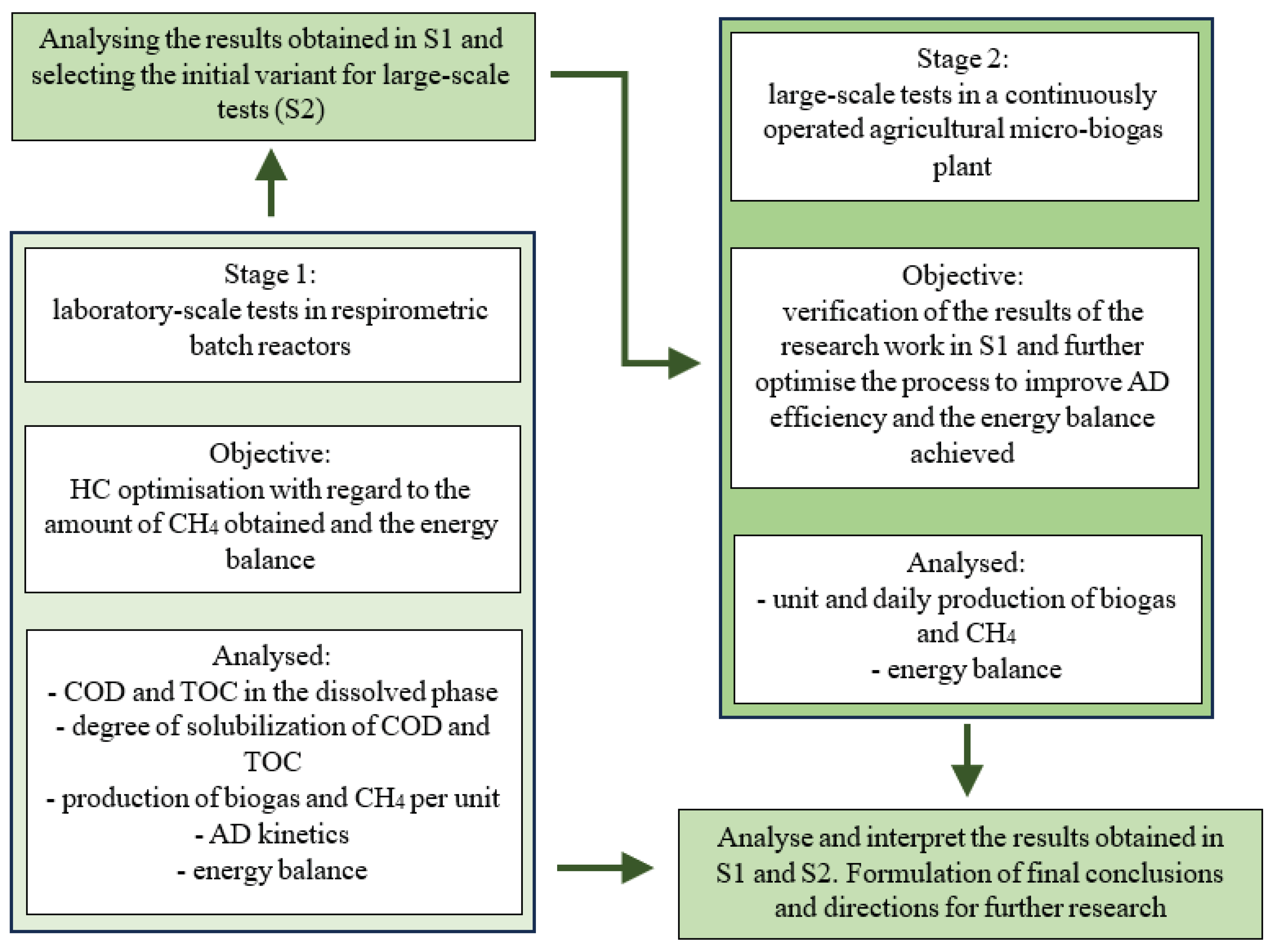
2.1. Organisation of the Experiment
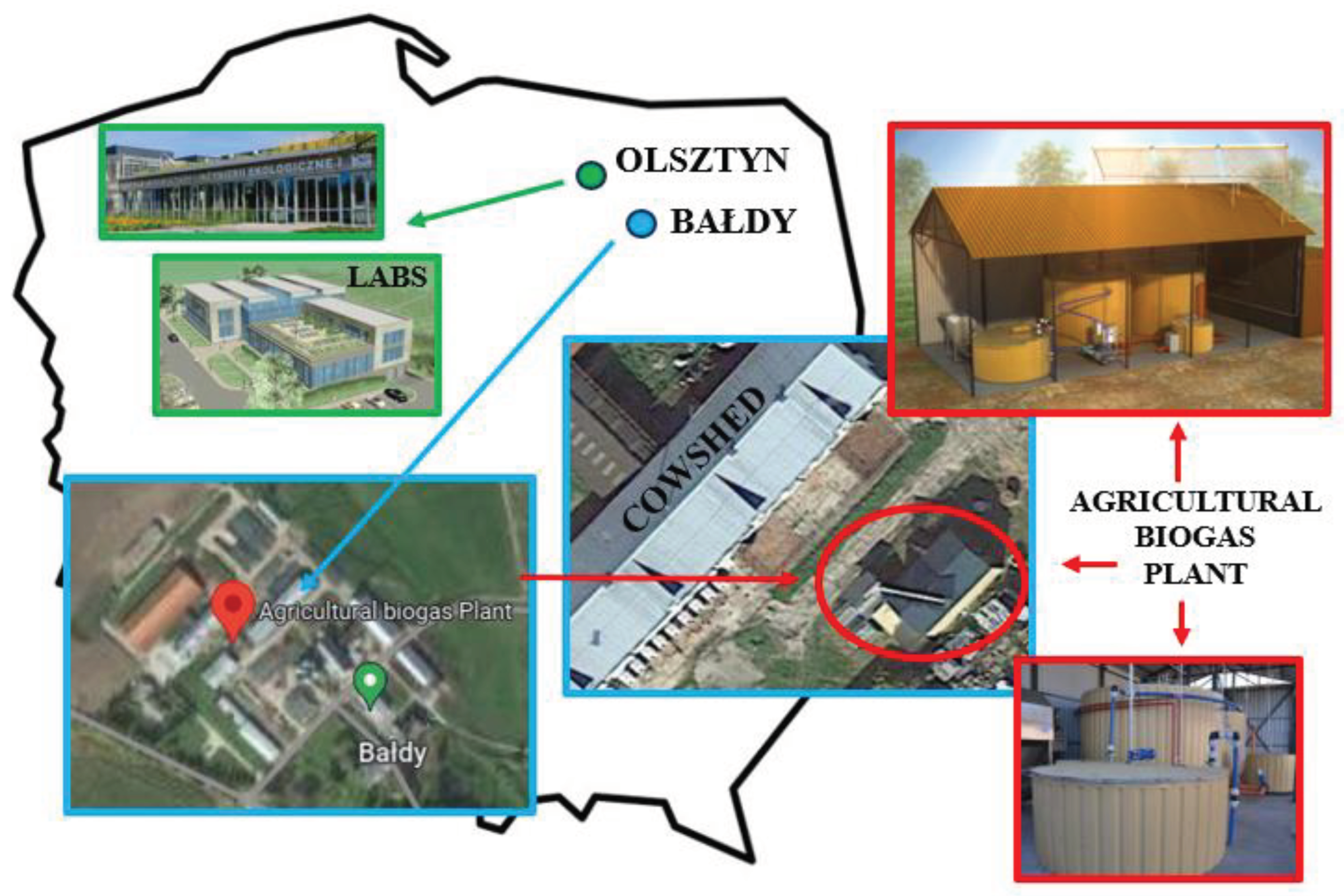
2.2. Location of Research
2.3. Materials
2.4. Hydrodynamic Cavitators
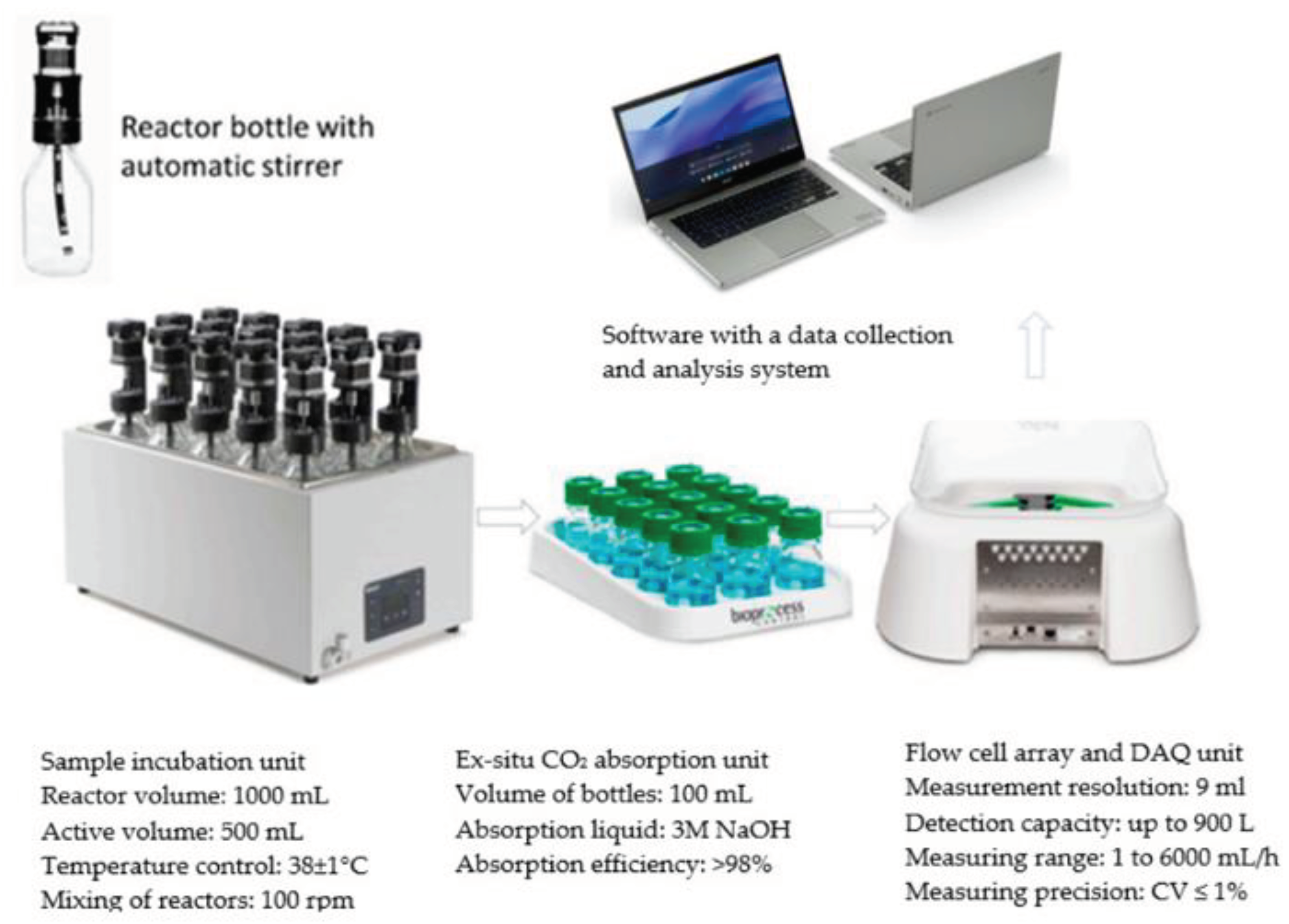
2.5. Stations for Anaerobic Digestion (AD)
2.6. Analytical Methods
2.7. Calculations and Statistical Methods
[(sCODS1 or sTOCS1 − sCODS0 or sTOCS0)/(CODT0 or TOCT0 − sCODS0 or sTOCS0)]×100
3. Results and Discussion
3.1. Stage 1—Laboratory-Scale Research
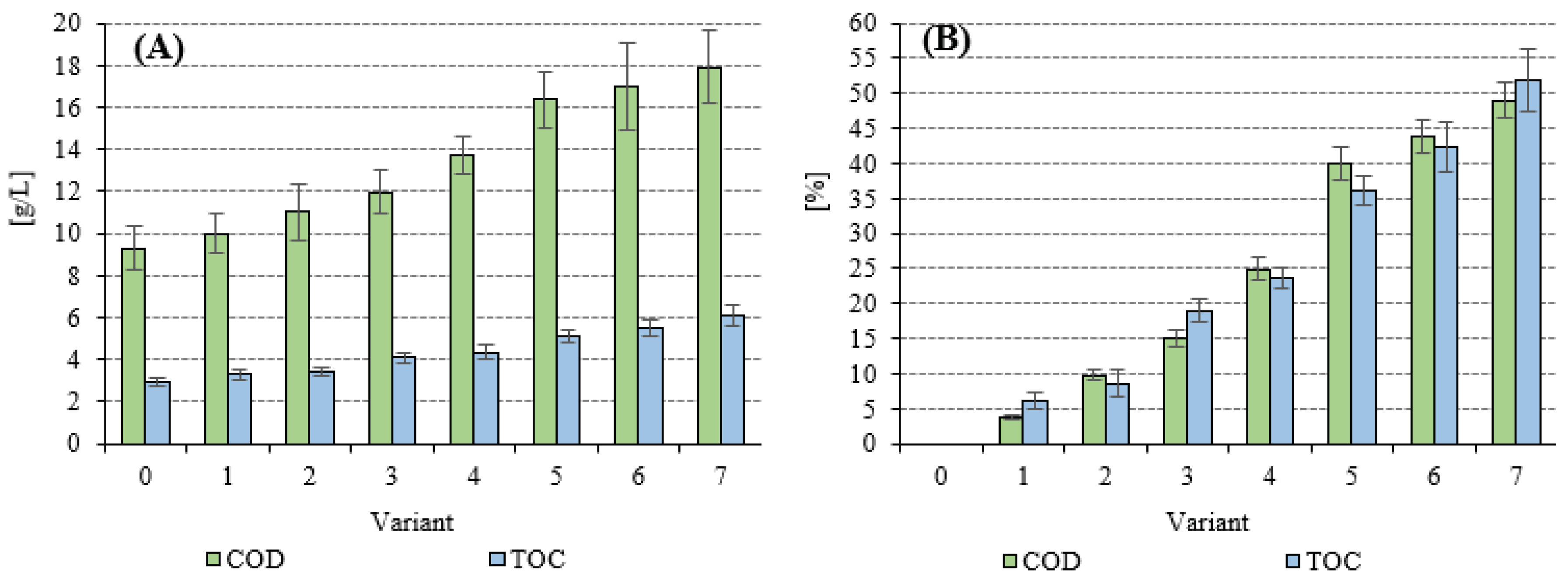
3.2. Stage 2—Full-Scale Research
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Teleszewski, T.; Żukowski, M. The Influence of Sludge on Thermal Performance of Heat Exchanger Tubes Inside in an Anaerobic Digester. Annu. Set Environ. Prot. 2018, 20, 763–779. [Google Scholar]
- Karolinczak, B.; Walczak, J.; Bogacka, M.; Zubrowska-Sudol, M. Life Cycle Assessment of Sewage Sludge Mono-Digestion and Co-Digestion with the Organic Fraction of Municipal Solid Waste at a Wastewater Treatment Plant. Sci. Total Environ. 2024, 907, 167801. [Google Scholar] [CrossRef] [PubMed]
- Eggemann, L.; Rau, F.; Stolten, D. The Ecological Potential of Manure Utilisation in Small-Scale Biogas Plants. Appl. Energy 2023, 331, 120445. [Google Scholar] [CrossRef]
- Gadirli, G.; Pilarska, A. A.; Dach, J.; Pilarski, K.; Kolasa-Więcek, A.; Borowiak, K. Fundamentals, Operation and Global Prospects for the Development of Biogas Plants—A Review. Energies 2024, Vol. 17, Page 568 2024, 17(3), 568. [Google Scholar] [CrossRef]
- Hafner, M.; Raimondi, P. P.; Bonometti, B. The Energy Sector and Energy Geopolitics in the MENA Region at a Crossroad; Perspectives on Development in the Middle East and North Africa (MENA) Region; Springer Nature: Cham, 2023. [Google Scholar] [CrossRef]
- Walczak, J.; Karolinczak, B.; Zubrowska-Sudol, M. Effect of Co-Digestion and Hydrodynamic Disintegration on the Methane Potential of Sewage Sludge and Organic Fraction of Municipal Solid Waste with Consideration of the Carbon Footprint. Energy 2023, 282, 128949. [Google Scholar] [CrossRef]
- Teleszewski, T. J.; Z˙ukowski, M. Analysis of Heat Loss of a Biogas Anaerobic Digester in Weather Conditions in Poland. J. Ecol. Eng. 2018, 19(4), 242–250. [Google Scholar] [CrossRef] [PubMed]
- Gajdzik, B.; Wolniak, R.; Nagaj, R.; Grebski, W. W.; Romanyshyn, T. Barriers to Renewable Energy Source (RES) Installations as Determinants of Energy Consumption in EU Countries. Energies 2023, Vol. 16, Page 7364 2023, 16(21), 7364. [Google Scholar] [CrossRef]
- Bumharter, C.; Bolonio, D.; Amez, I.; García Martínez, M. J.; Ortega, M. F. New Opportunities for the European Biogas Industry: A Review on Current Installation Development, Production Potentials and Yield Improvements for Manure and Agricultural Waste Mixtures. J. Clean. Prod. 2023, 388, 135867. [Google Scholar] [CrossRef]
- Banaszuk, P.; Kamocki, A. K.; Wysocka-Czubaszek, A.; Czubaszek, R.; Roj-Rojewski, S. Closing the Loop - Recovery of Nutrients and Energy from Wetland Biomass. Ecol. Eng. 2020, 143, 105643. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M. Technological Effectiveness of Sugar-Industry Effluent Methane Fermentation in a Fluidized Active Filling Reactor (FAF-R). Energies 2020, Vol. 13, Page 6626 2020, 13(24), 6626. [Google Scholar] [CrossRef]
- Lafratta, M.; Thorpe, R. B.; Ouki, S. K.; Shana, A.; Germain, E.; Willcocks, M.; Lee, J. Dynamic Biogas Production from Anaerobic Digestion of Sewage Sludge for On-Demand Electricity Generation. Bioresour. Technol. 2020, 310, 123415. [Google Scholar] [CrossRef] [PubMed]
- Markowski, M.; Białobrzewski, I.; Zieliński, M.; Debowski, M.; Krzemieniewski, M. Optimizing Low-Temperature Biogas Production from Biomass by Anaerobic Digestion. Renew. Energy 2014, 69, 219–225. [Google Scholar] [CrossRef]
- Li, Y.; Qi, C.; Zhang, Y.; Li, Y.; Wang, Y.; Li, G.; Luo, W. Anaerobic Digestion of Agricultural Wastes from Liquid to Solid State: Performance and Environ-Economic Comparison. Bioresour. Technol. 2021, 332, 125080. [Google Scholar] [CrossRef] [PubMed]
- Czubaszek, R.; Wysocka-Czubaszek, A.; Tyborowski, R. Methane Production Potential from Apple Pomace, Cabbage Leaves, Pumpkin Residue and Walnut Husks. Appl. Sci. 2022, Vol. 12, Page 6128 2022, 12(12), 6128. [Google Scholar] [CrossRef]
- Sarrion, A.; Ipiales, R. P.; de la Rubia, M. A.; Mohedano, A. F.; Diaz, E. Chicken Meat and Bone Meal Valorization by Hydrothermal Treatment and Anaerobic Digestion: Biofuel Production and Nutrient Recovery. Renew. Energy 2023, 204, 652–660. [Google Scholar] [CrossRef]
- Kasinski, S. Mesophilic and Thermophilic Anaerobic Digestion of Organic Fraction Separated during Mechanical Heat Treatment of Municipal Waste. Appl. Sci. 2020, Vol. 10, Page 2412 2020, 10(7), 2412. [Google Scholar] [CrossRef]
- Czubaszek, R.; Wysocka-Czubaszek, A.; Wichtmann, W.; Banaszuk, P. Specific Methane Yield of Wetland Biomass in Dry and Wet Fermentation Technologies. Energies 2021, Vol. 14, Page 8373 2021, 14(24), 8373. [Google Scholar] [CrossRef]
- Czubaszek, R.; Wysocka-Czubaszek, A.; Banaszuk, P. GHG Emissions and Efficiency of Energy Generation through Anaerobic Fermentation of Wetland Biomass. Energies 2020, Vol. 13, Page 6497 2020, 13(24), 6497. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dzienis, L. Giant Miscanthus as a Substrate for Biogas Production. J. Ecol. Eng. 2015, Vol. 16(nr 4). [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kisielewska, M.; Nowicka, A.; Rokicka, M.; Szwarc, K. Comparison of Ultrasonic and Hydrothermal Cavitation Pretreatments of Cattle Manure Mixed with Straw Wheat on Fermentative Biogas Production. Waste and Biomass Valorization 2019, 10(4), 747–754. [Google Scholar] [CrossRef]
- Bhatt, A. H.; Tao, L. Economic Perspectives of Biogas Production via Anaerobic Digestion. Bioeng. 2020, Vol. 7, Page 74 2020, 7(3), 74. [Google Scholar] [CrossRef]
- Park, J.; Cayetano, R. D. A.; Kim, G. B.; Jo, Y.; Kwon, Y.; Lei, Z.; Kim, S. H. Sludge Disintegration and Anaerobic Digestion Enhancement by Alkaline-Thermal Pretreatment: Economic Evaluation and Microbial Population Analysis. Bioresour. Technol. 2022, 346, 126594. [Google Scholar] [CrossRef] [PubMed]
- Coarita Fernandez, H.; Teixeira Franco, R.; Bayard, R.; Buffiere, P. Mechanical Pre-Treatments Evaluation of Cattle Manure Before Anaerobic Digestion. Waste and Biomass Valorization 2020, 11(10), 5175–5184. [Google Scholar] [CrossRef]
- Wang, S.; Yu, S.; Lu, Q.; Liao, Y.; Li, H.; Sun, L.; Wang, H.; Zhang, Y. Development of an Alkaline/Acid Pre-Treatment and Anaerobic Digestion (APAD) Process for Methane Generation from Waste Activated Sludge. Sci. Total Environ. 2020, 708, 134564. [Google Scholar] [CrossRef] [PubMed]
- Malik, S. N.; Madhu, K.; Mhaisalkar, V. A.; Vaidya, A. N.; Mudliar, S. N. Pretreatment of Yard Waste Using Advanced Oxidation Processes for Enhanced Biogas Production. Biomass and Bioenergy 2020, 142, 105780. [Google Scholar] [CrossRef]
- Preethi; Banu J, R.; Varjani, S.; P, S.; Tyagi, V. K.; Gunasekaran, M. Breakthrough in Hydrolysis of Waste Biomass by Physico-Chemical Pretreatment Processes for Efficient Anaerobic Digestion. Chemosphere 2022, 294, 133617. [CrossRef]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M. The Synergistic Effect of Simultaneous Ultrasound Heating and Disintegration on the Technological Efficiency and Energetic Balance of Anaerobic Digestion of High-Load Slaughter Poultry Sewage. Appl. Sci. 2023, Vol. 13, Page 2420 2023, 13(4), 2420. [Google Scholar] [CrossRef]
- Szaja, A.; Szulżyk-Cieplak, J.; Łagód, S.; Kuzioła, E. Recent Developments in the Application of Ultrasonication in Pre-Treatment of Municipal Sewage Sludge. J. Ecol. Eng. 2023, 24(12), 223–234. [Google Scholar] [CrossRef]
- Hidaka, T.; Nakamura, M.; Oritate, F.; Nishimura, F. Comparative Anaerobic Digestion of Sewage Sludge at Different Temperatures with and without Heat Pre-Treatment. Chemosphere 2022, 307, 135808. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Rusanowska, P.; Kazimierowicz, J. Influence of Microwave Radiation on Pollutant Removal and Biomethane Production Efficiency in Anaerobic Treatment of High-Load Poultry Wastewater. Appl. Sci. 2023, Vol. 13, Page 3553 2023, 13(6), 3553. [Google Scholar] [CrossRef]
- Zhang, Q.; Yao, Y.; Xi, X. Effects of Freezing–Thawing Pretreatment on Anaerobic Digestion of Wheat Straw and Its Kinetics Analysis. Clean Technol. Environ. Policy 2022, 24(1), 125–141. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M. Technological, Ecological, and Energy-Economic Aspects of Using Solidified Carbon Dioxide for Aerobic Granular Sludge Pre-Treatment Prior to Anaerobic Digestion. Int. J. Environ. Res. Public Heal. 2023, Vol. 20, Page 4234 2023, 20(5), 4234. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M. Long-Term Pre-Treatment of Municipal Sewage Sludge with Solidified Carbon Dioxide (SCO2)—Effect on Anaerobic Digestion Efficiency. Appl. Sci. 2023, Vol. 13, Page 3075 2023, 13(5), 3075. [Google Scholar] [CrossRef]
- Dauknys, R.; Mažeikienė, A.; Paliulis, D. Effect of Ultrasound and High Voltage Disintegration on Sludge Digestion Process. J. Environ. Manage. 2020, 270, 110833. [Google Scholar] [CrossRef]
- Kovačič, A.; Škufca, D.; Zupanc, M.; Gostiša, J.; Bizjan, B.; Krištofelc, N.; Dolenc, M. S.; Heath, E. The Removal of Bisphenols and Other Contaminants of Emerging Concern by Hydrodynamic Cavitation: From Lab-Scale to Pilot-Scale. Sci. Total Environ. 2020, 743, 140724. [Google Scholar] [CrossRef] [PubMed]
- Lanfranchi, A.; Tassinato, G.; Valentino, F.; Martinez, G. A.; Jones, E.; Gioia, C.; Bertin, L.; Cavinato, C. Hydrodynamic Cavitation Pre-Treatment of Urban Waste: Integration with Acidogenic Fermentation, PHAs Synthesis and Anaerobic Digestion Processes. Chemosphere 2022, 301, 134624. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zheng, Y.; Zhu, J. Recent Developments in Hydrodynamic Cavitation Reactors: Cavitation Mechanism, Reactor Design, and Applications. Engineering 2022, 19, 180–198. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, R.; Zhang, L.; Chen, J.; Li, M.; Wang, Y. Numerical Investigation of Effect of Geometric Parameters on Performance of Rotational Hydrodynamic Cavitation Reactor. Ultrason. Sonochem. 2024, 103, 106790. [Google Scholar] [CrossRef]
- Carpenter, J.; Badve, M.; Rajoriya, S.; George, S.; Saharan, V. K.; Pandit, A. B. Hydrodynamic Cavitation: An Emerging Technology for the Intensification of Various Chemical and Physical Processes in a Chemical Process Industry. Rev. Chem. Eng. 2017, 33(5), 433–468. [Google Scholar] [CrossRef]
- Tao, Y.; Cai, J.; Huai, X.; Liu, B.; Guo, Z. Application of Hydrodynamic Cavitation to Wastewater Treatment. Chem. Eng. Technol. 2016, 39(8), 1363–1376. [Google Scholar] [CrossRef]
- Montusiewicz, A.; Pasieczna-Patkowska, S.; Lebiocka, M.; Szaja, A.; Szymańska-Chargot, M. Hydrodynamic Cavitation of Brewery Spent Grain Diluted by Wastewater. Chem. Eng. J. 2017, 313, 946–956. [Google Scholar] [CrossRef]
- Mancuso, G.; Langone, M.; Di Maggio, R.; Toscano, A.; Andreottola, G. Effect of Hydrodynamic Cavitation on Flocs Structure in Sewage Sludge to Increase Stabilization for Efficient and Safe Reuse in Agriculture. Bioremediat. J. 2022, 26(1), 41–52. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J.; Nowicka, A.; Dudek, M. Application of Hydrodynamic Cavitation in the Disintegration of Aerobic Granular Sludge—Evaluation of Pretreatment Time on Biomass Properties, Anaerobic Digestion Efficiency and Energy Balance. Energies 2024, Vol. 17, Page 335 2024, 17(2), 335. [Google Scholar] [CrossRef]
- Garlicka, A.; Zubrowska-Sudol, M.; Umiejewska, K.; Roubinek, O.; Palige, J.; Chmielewski, A. Effects of Thickened Excess Sludge Pre-Treatment Using Hydrodynamic Cavitation for Anaerobic Digestion. Energies 2020, Vol. 13, Page 2483 2020, 13(10), 2483. [Google Scholar] [CrossRef]
- Seo, Y. H.; Yun, Y. M.; Lee, H.; Han, J. I. Pretreatment of Cheese Whey for Hydrogen Production Using a Simple Hydrodynamic Cavitation System under Alkaline Condition. Fuel 2015, 150, 202–207. [Google Scholar] [CrossRef]
- Prado, C. A.; Cunha, M. L. S.; Arruda, G. L.; Cruz-Santos, M. M.; Antunes, F. A. F.; Shibukawa, V. P.; Terán-Hilares, R.; da Silva, S. S.; Santos, J. C. Hydrodynamic Cavitation-Assisted Acid Pretreatment and Fed-Batch Simultaneous Saccharification and Co-Fermentation for Ethanol Production from Sugarcane Bagasse Using Immobilized Cells of Scheffersomyces Parashehatae. Bioresour. Technol. 2024, 394, 130234. [Google Scholar] [CrossRef] [PubMed]
- Samani, B. H.; Behruzian, M.; Najafi, G.; Fayyazi, E.; Ghobadian, B.; Behruzian, A.; Mofijur, M.; Mazlan, M.; Yue, J. The Rotor-Stator Type Hydrodynamic Cavitation Reactor Approach for Enhanced Biodiesel Fuel Production. Fuel 2021, 283, 118821. [Google Scholar] [CrossRef]
- Islam, S.; Ranade, V. V. Enhancement of Biomethane Potential of Brown Sludge by Pre-Treatment Using Vortex Based Hydrodynamic Cavitation. Heliyon 2023, 9, e18345. [Google Scholar] [CrossRef]
- Ranade, V. V.; Bhandari, V. M.; Nagarajan, S.; Sarvothaman, V. P.; Simpson, A. A. Hydrodynamic Cavitation: Devices, Design, and Applications; John Wiley & Sons, Ltd, 2022. [Google Scholar]
- Wang, B.; Su, H.; Zhang, B. Hydrodynamic Cavitation as a Promising Route for Wastewater Treatment – A Review. Chem. Eng. J. 2021, 412, 128685. [Google Scholar] [CrossRef]
- Zaresharif, M.; Ravelet, F.; Kinahan, D. J.; Delaure, Y. M. C. Cavitation Control Using Passive Flow Control Techniques. Phys. Fluids 2021, 33(12), 121301. [Google Scholar] [CrossRef]
- Zampeta, C.; Bertaki, K.; Triantaphyllidou, I. E.; Frontistis, Z.; Vayenas, D. V. Treatment of Real Industrial-Grade Dye Solutions and Printing Ink Wastewater Using a Novel Pilot-Scale Hydrodynamic Cavitation Reactor. J. Environ. Manage. 2021, 297, 113301. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A.; Pourfayaz, F.; Saifoddin, A. Techno-Economic Assessment and Sensitivity Analysis of Biodiesel Production Intensified through Hydrodynamic Cavitation. Energy Sci. Eng. 2021, 9(11), 1997–2018. [Google Scholar] [CrossRef]
- Skorb, E. V.; Möhwald, H.; Andreeva, D. V. Effect of Cavitation Bubble Collapse on the Modification of Solids: Crystallization Aspects. Langmuir 2016, 32(43), 11072–11085. [Google Scholar] [CrossRef] [PubMed]
- Blagojevič, M.; Rak, G.; Bizjan, B.; Kolbl Repinc, S. A Review on Rotary Generators of Hydrodynamic Cavitation for Wastewater Treatment and Enhancement of Anaerobic Digestion Process. Process. 2023, Vol. 11, Page 514 2023, 11(2), 514. [Google Scholar] [CrossRef]
- Zevnik, J.; Dular, M. Cavitation Bubble Interaction with a Rigid Spherical Particle on a Microscale. Ultrason. Sonochem. 2020, 69, 105252. [Google Scholar] [CrossRef] [PubMed]
- Gogate, P. R.; Patil, P. N. Combined Treatment Technology Based on Synergism between Hydrodynamic Cavitation and Advanced Oxidation Processes. Ultrason. Sonochem. 2015, 25(1), 60–69. [Google Scholar] [CrossRef]
- Save, S. S.; Pandit, A. B.; Joshi, J. B. Use of Hydrodynamic Cavitation for Large Scale Microbial Cell Disruption. Food Bioprod. Process. 1997, 75(1), 41–49. [Google Scholar] [CrossRef]
- Kim, H.; Sun, X.; Koo, B.; Yoon, J. Y. Experimental Investigation of Sludge Treatment Using a Rotor-Stator Type Hydrodynamic Cavitation Reactor and an Ultrasonic Bath. Process. 2019, Vol. 7, Page 790 2019, 7(11), 790. [Google Scholar] [CrossRef]
- Holkar, C. R.; Jadhav, A. J.; Pinjari, D. V.; Pandit, A. B. Cavitationally Driven Transformations: A Technique of Process Intensification. Ind. Eng. Chem. Res. 2019, 58(15), 5797–5819. [Google Scholar] [CrossRef]
- Bhat, A. P.; Gogate, P. R. Cavitation-Based Pre-Treatment of Wastewater and Waste Sludge for Improvement in the Performance of Biological Processes: A Review. J. Environ. Chem. Eng. 2021, 9(2), 104743. [Google Scholar] [CrossRef]
- Garuti, M.; Langone, M.; Fabbri, C.; Piccinini, S. Monitoring of Full-Scale Hydrodynamic Cavitation Pretreatment in Agricultural Biogas Plant. Bioresour. Technol. 2018, 247, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Langone, M.; Soldano, M.; Fabbri, C.; Pirozzi, F.; Andreottola, G. Anaerobic Digestion of Cattle Manure Influenced by Swirling Jet Induced Hydrodynamic Cavitation. Appl. Biochem. Biotechnol. 2018, 184(4), 1200–1218. [Google Scholar] [CrossRef] [PubMed]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; Delisi, R.; Albanese, L.; Zabini, F.; Gori, A.; Nascimento, L. B. dos S.; De Carlo, A.; Ferrini, F.; Ilharco, L. M.; Pagliaro, M. Real-Scale Integral Valorization of Waste Orange Peel via Hydrodynamic Cavitation. Process. 2019, Vol. 7, Page 581 2019, 7(9), 581. [Google Scholar] [CrossRef]
- Zupanc, M.; Humar, B. B.; Dular, M.; Gostiša, J.; Hočevar, M.; Repinc, S. K.; Krzyk, M.; Novak, L.; Ortar, J.; Pandur, Ž.; Stres, B.; Petkovšek, M. The Use of Hydrodynamic Cavitation for Waste-to-Energy Approach to Enhance Methane Production from Waste Activated Sludge. J. Environ. Manage. 2023, 347, 119074. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Yang, R.; Zhou, X.; Cao, G.; Zhu, R.; Ouyang, F. Effects of Mixed Alkali-Thermal Pretreatment on Anaerobic Digestion Performance of Waste Activated Sludge. J. Clean. Prod. 2020, 259, 120940. [Google Scholar] [CrossRef]
- Dębowski, M.; Kisielewska, M.; Zieliński, M.; Kazimierowicz, J. The Influence of the Ultrasound Disintegration of Microalgal–Bacterial Granular Sludge on Anaerobic Digestion Efficiency. Appl. Sci. 2023, Vol. 13, Page 7387 2023, 13(13), 7387. [Google Scholar] [CrossRef]
- Zawieja, I. Effect of Dry Ice Modification of Excess Sludge on the Methane Fermentation Process. Annu. Set Environ. Prot. 2018, 20(1), 558–573. [Google Scholar]
- Hu, K.; Jiang, J. Q.; Zhao, Q. L.; Lee, D. J.; Wang, K.; Qiu, W. Conditioning of Wastewater Sludge Using Freezing and Thawing: Role of Curing. Water Res. 2011, 45(18), 5969–5976. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M. Biohythane Production in Hydrogen-Oriented Dark Fermentation of Aerobic Granular Sludge (AGS) Pretreated with Solidified Carbon Dioxide (SCO2). Int. J. Mol. Sci. 2023, Vol. 24, Page 4442 2023, 24(5), 4442. [Google Scholar] [CrossRef]
- Li, X.; Zhu, T.; Zhang, K.; Lv, L.; Chai, T.; Shen, Y.; Wang, Y.; You, M.; Xie, Y. Effect of the Sequence Ultrasonic Operation on Anaerobic Degradation of Sewage Sludge. Int. Biodeterior. Biodegradation 2016, 112, 66–71. [Google Scholar] [CrossRef]
- Park, K. Y.; Kweon, J.; Chantrasakdakul, P.; Lee, K.; Cha, H. Y. Anaerobic Digestion of Microalgal Biomass with Ultrasonic Disintegration. Int. Biodeterior. Biodegradation 2013, 85, 598–602. [Google Scholar] [CrossRef]
- Lee, I.; Han, J. I. The Effects of Waste-Activated Sludge Pretreatment Using Hydrodynamic Cavitation for Methane Production. Ultrason. Sonochem. 2013, 20(6), 1450–1455. [Google Scholar] [CrossRef]
- Mancuso, G.; Langone, M.; Andreottola, G.; Bruni, L. Effects of Hydrodynamic Cavitation, Low-Level Thermal and Low-Level Alkaline Pre-Treatments on Sludge Solubilisation. Ultrason. Sonochem. 2019, 59, 104750. [Google Scholar] [CrossRef] [PubMed]
- Petkovšek, M.; Mlakar, M.; Levstek, M.; Stražar, M.; Širok, B.; Dular, M. A Novel Rotation Generator of Hydrodynamic Cavitation for Waste-Activated Sludge Disintegration. Ultrason. Sonochem. 2015, 26, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Luste, S.; Heinonen-Tanski, H.; Luostarinen, S. Co-Digestion of Dairy Cattle Slurry and Industrial Meat-Processing by-Products – Effect of Ultrasound and Hygienization Pre-Treatments. Bioresour. Technol. 2012, 104, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Ranade, V. V. Intensifying Extraction of Biomolecules from Macroalgae Using Vortex Based Hydrodynamic Cavitation Device. Ultrason. Sonochem. 2023, 94, 106347. [Google Scholar] [CrossRef]
- Terán Hilares, R.; dos Santos, J. C.; Ahmed, M. A.; Jeon, S. H.; da Silva, S. S.; Han, J. I. Hydrodynamic Cavitation-Assisted Alkaline Pretreatment as a New Approach for Sugarcane Bagasse Biorefineries. Bioresour. Technol. 2016, 214, 609–614. [Google Scholar] [CrossRef]
- Szaja, A.; Montusiewicz, A.; Lebiocka, M. Challenges of Hydrodynamic Cavitation of Organic Wastes. Appl. Sci. 2022, Vol. 12, Page 7936 2022, 12(15), 7936. [Google Scholar] [CrossRef]
- Ge, M.; Sun, C.; Zhang, G.; Coutier-Delgosha, O.; Fan, D. Combined Suppression Effects on Hydrodynamic Cavitation Performance in Venturi-Type Reactor for Process Intensification. Ultrason. Sonochem. 2022, 86, 106035. [Google Scholar] [CrossRef]
- Cimochowicz-Rybicka, M.; Rybicki, S. Application of Respirometric Tests for Assessment of Methanogenic Bacteria Activity in Wastewater Sludge Processing. Inżynieria Ekol. 2011, No. Nr 25, 30–42. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Bernat, K.; Zielińska, M.; Gusiatin, M. Z.; Wojnowska-Baryła, I.; Kulikowska, D. Valorization of Full-Scale Waste Aerobic Granular Sludge for Biogas Production and the Characteristics of the Digestate. Chemosphere 2022, 303, 135167. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, C.; Young Kim, J. Effects of Alkaline Thermal Hydrolysis on the Formation of Refractory Compounds and Energy Balance of Anaerobic Digestion of Cattle Manure. Appl. Energy 2023, 342, 121097. [Google Scholar] [CrossRef]
- Fardinpoor, M.; Perendeci, N. A.; Yılmaz, V.; Taştan, B. E.; Yılmaz, F. Effects of Hydrodynamic Cavitation-Assisted NaOH Pretreatment on Biofuel Production from Cyanobacteria: Promising Approach. Bioenergy Res. 2022, 15(1), 289–302. [Google Scholar] [CrossRef]
- Braeutigam, P.; Franke, M.; Ondruschka, B. Effect of Ultrasound Amplitude and Reaction Time on the Anaerobic Fermentation of Chicken Manure for Biogas Production. Biomass and Bioenergy 2014, 63, 109–113. [Google Scholar] [CrossRef]
- Hendriks, A. T. W. M.; Zeeman, G. Pretreatments to Enhance the Digestibility of Lignocellulosic Biomass. Bioresour. Technol. 2009, 100(1), 10–18. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. Pretreatment: The Key to Efficient Utilization of Lignocellulosic Materials. Biomass and Bioenergy 2012, 46, 70–78. [Google Scholar] [CrossRef]
- Zabed, H. M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from Microalgae: Technologies, Challenges and Opportunities. Renew. Sustain. Energy Rev. 2020, 117, 109503. [Google Scholar] [CrossRef]
- Tian, L.; Shen, F.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Li, X. Reducing Agitation Energy-Consumption by Improving Rheological Properties of Corn Stover Substrate in Anaerobic Digestion. Bioresour. Technol. 2014, 168, 86–91. [Google Scholar] [CrossRef]
- Lu, D.; Xiao, K.; Chen, Y.; Soh, Y. N. A.; Zhou, Y. Transformation of Dissolved Organic Matters Produced from Alkaline-Ultrasonic Sludge Pretreatment in Anaerobic Digestion: From Macro to Micro. Water Res. 2018, 142, 138–146. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Zieliński, M.; Bartkowska, I.; Dębowski, M. Effect of Acid Whey Pretreatment Using Ultrasonic Disintegration on the Removal of Organic Compounds and Anaerobic Digestion Efficiency. Int. J. Environ. Res. Public Heal. 2022, Vol. 19, Page 11362 2022, 19(18), 11362. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kisielewska, M.; Nowicka, A.; Rokicka, M.; Szwarc, K. Cavitation-Based Pretreatment Strategies to Enhance Biogas Production in a Small-Scale Agricultural Biogas Plant. Energy Sustain. Dev. 2019, 49, 21–26. [Google Scholar] [CrossRef]


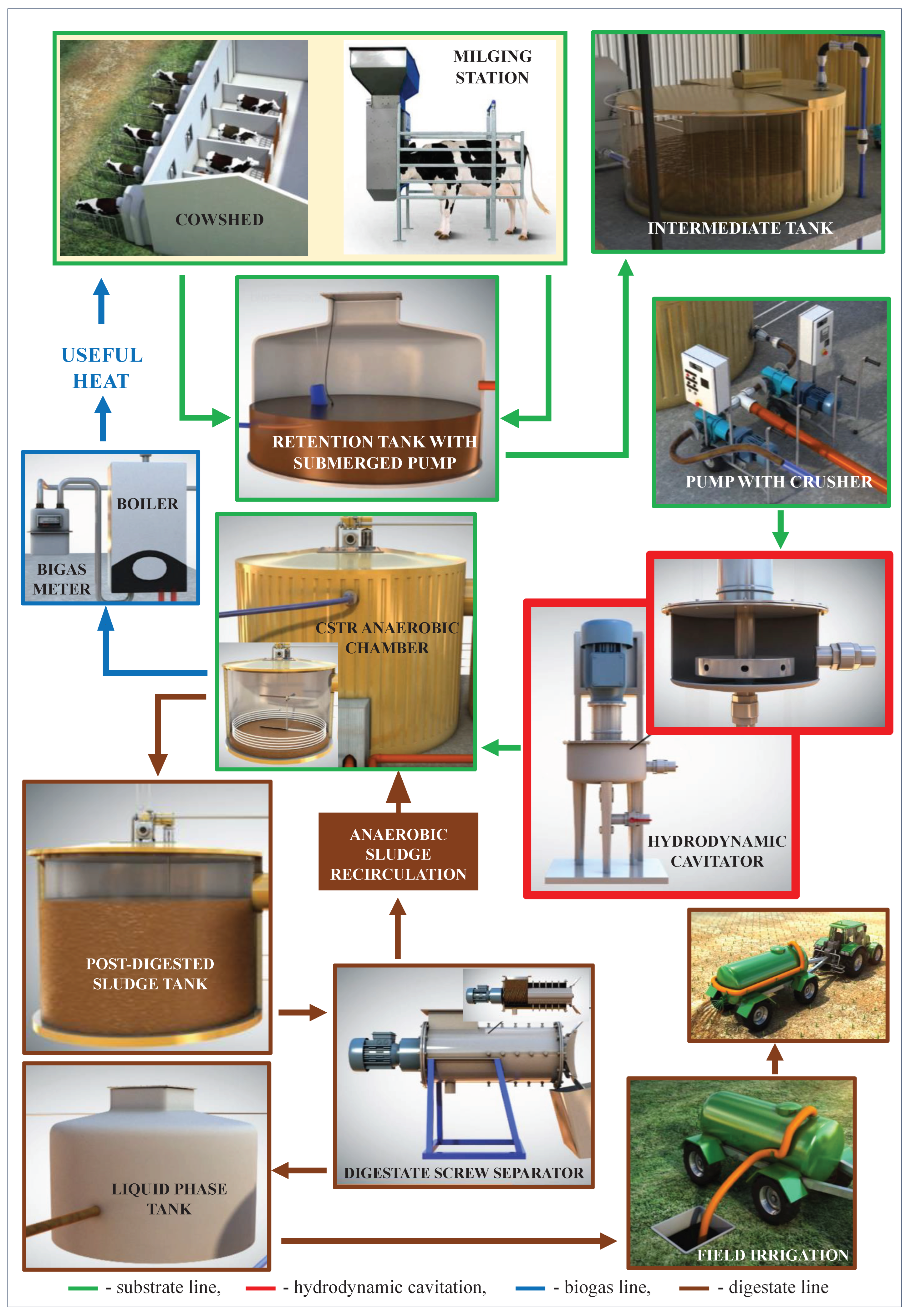

| Stage | Variant | HC Duration [min] |
Power [kW] | Volume [L] |
Energy Demand [Wh/L] |
Energy Demand [Wh] |
|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 1.2 | 5 | - | 0 |
| 1 | 2 | 8 | 50 | |||
| 2 | 4 | 16 | 80 | |||
| 3 | 6 | 24 | 120 | |||
| 4 | 8 | 32 | 160 | |||
| 5 | 10 | 40 | 200 | |||
| 6 | 12 | 48 | 240 | |||
| 7 | 14 | 56 | 280 | |||
| 2 | 0 | 0 | 16 | 100 | - | 0 |
| 1 | 10 | 26.7 | 2670 | |||
| 2 | 8 | 21.3 | 2130 | |||
| 3 | 6 | 16.0 | 1600 |
| Parameter | Unit | CS+MSS | AS |
|---|---|---|---|
| Total solids (TS) | g/L | 13.73±2.95 | 31.32±1.72 |
| Mineral solids (MS) | g/L | 11.82±1.13 | 7.21±1.10 |
| Volatile solids (VS) | g/L | 1.91±0.61 | 24.11±1.53 |
| Total nitrogen (TN) | g/L | 1.08±0.22 | 1.02±0.09 |
| Total phosphorus (TP) | g/L | 0.64±0.17 | 0.43±0.06 |
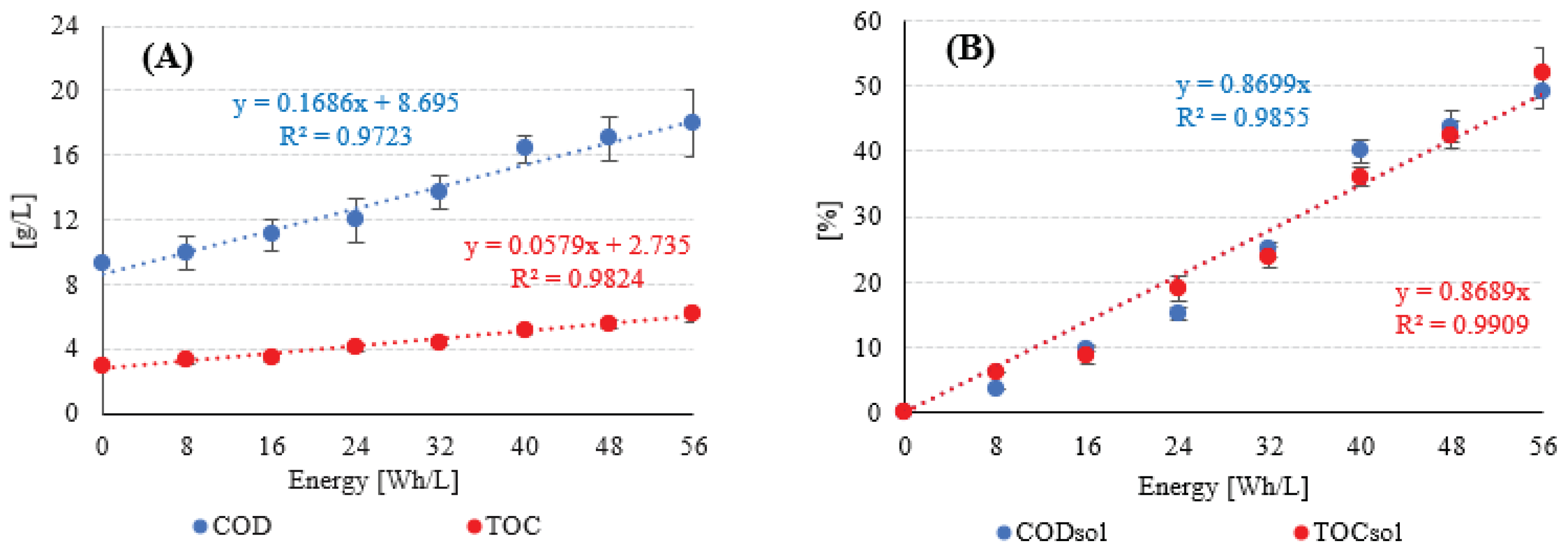
| Chemical oxygen demand (COD) | g/L | 26.92±3.40 | 41.12±4.99 |
| Total carbon (TC) | g/L | 13.47±2.11 | 18.92±1.97 |
| Total organic carbon (TOC) | g/L | 10.52±1.40 | 12.70±1.63 |
| Total inorganic carbon (IC) | g/L | 2.95±0.47 | 6.22±0.85 |
| Dissolved phase COD (CODD) | g/L | 9.31±1.02 | 5.79±0.96 |
| Dissolved phase TOC (TOCD) | g/L | 2.91±0.77 | 1.48±0.32 |
| C/N (COD/TN) | - | 24.92±2.92 | 7.06±0.72 |
| C/N (TOC/TN) | - | 5.84±0.81 | 40.31±3.78 |
| pH | - | 7.44±0.27 | 12.45±1.42 |
| Variant | HC duration [min] |
Biogas [mL/gCOD] |
CH4 [mL/gCOD] | Biogas [mL/gTOC] |
CH4 [mL/gTOC] | Biogas [mL] | CH4 [mL] |
CH4 [%] |
|---|---|---|---|---|---|---|---|---|
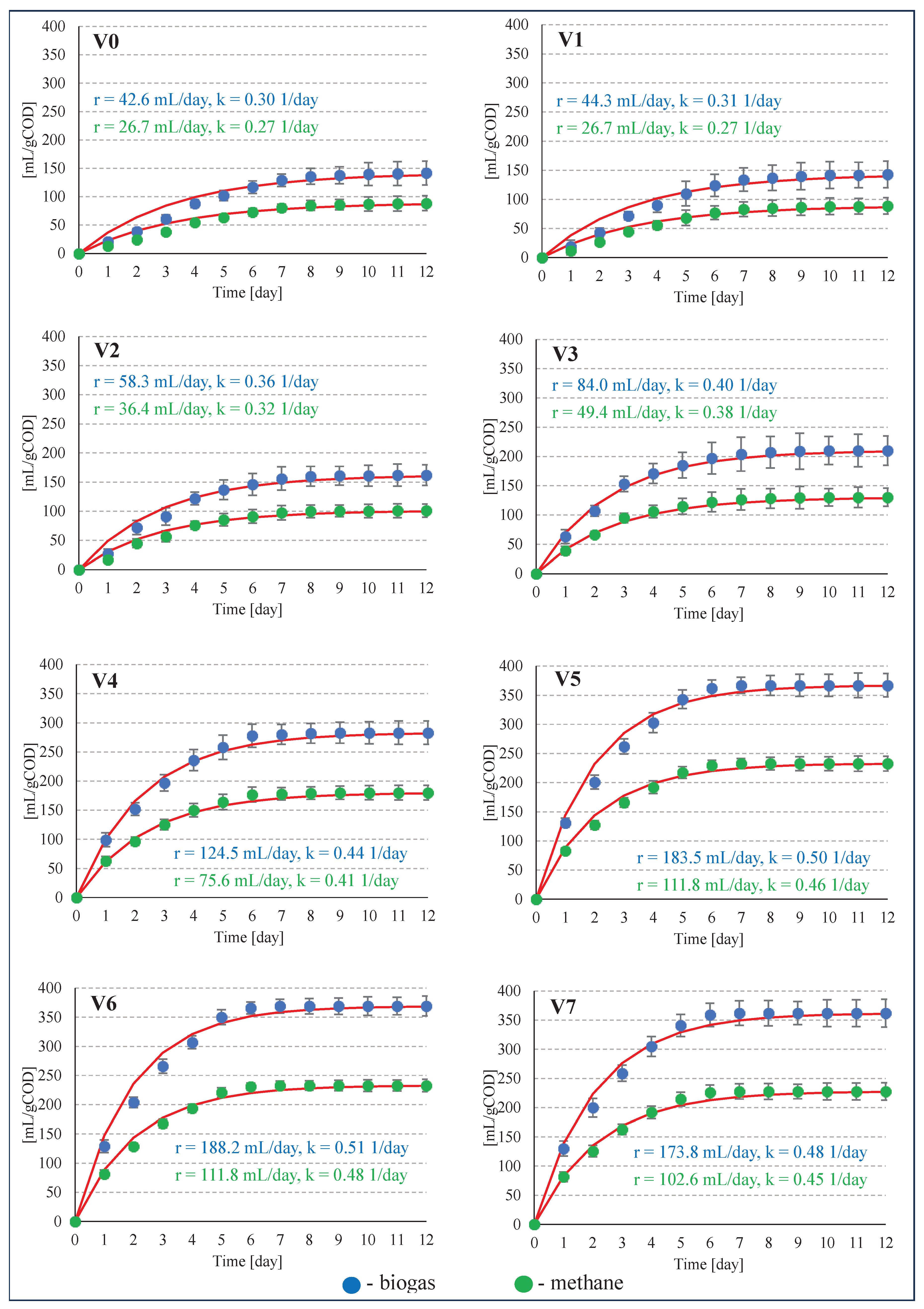
| 0 | 0 | 140±20 | 87±10 | 416±59 | 259±29 | 350±50 | 218±25 | 62.4±1.4 |
| 1 | 2 | 142±23 | 88±11 | 422±68 | 262±32 | 355±57 | 220±27 | 62.1±2.3 |
| 2 | 4 | 161±16 | 100±9 | 478±48 | 298±27 | 402±40 | 251±22 | 62.4±1.1 |
| 3 | 6 | 209±31 | 130±13 | 621±92 | 385±38 | 522±77 | 324±32 | 62.0±1.9 |
| 4 | 8 | 282±17 | 179±7 | 838±50 | 533±21 | 705±42 | 448±17 | 63.6±3.2 |
| 5 | 10 | 367±14 | 233±5 | 1090±43 | 691±15 | 917±35 | 582±12 | 63.4±1.7 |
| 6 | 12 | 369±12 | 233±6 | 1096±36 | 692±18 | 922±30 | 582±15 | 63.1±2.2 |
| 7 | 14 | 362±21 | 228±9 | 1075±62 | 676±27 | 905±52 | 569±22 | 62.9±2.1 |
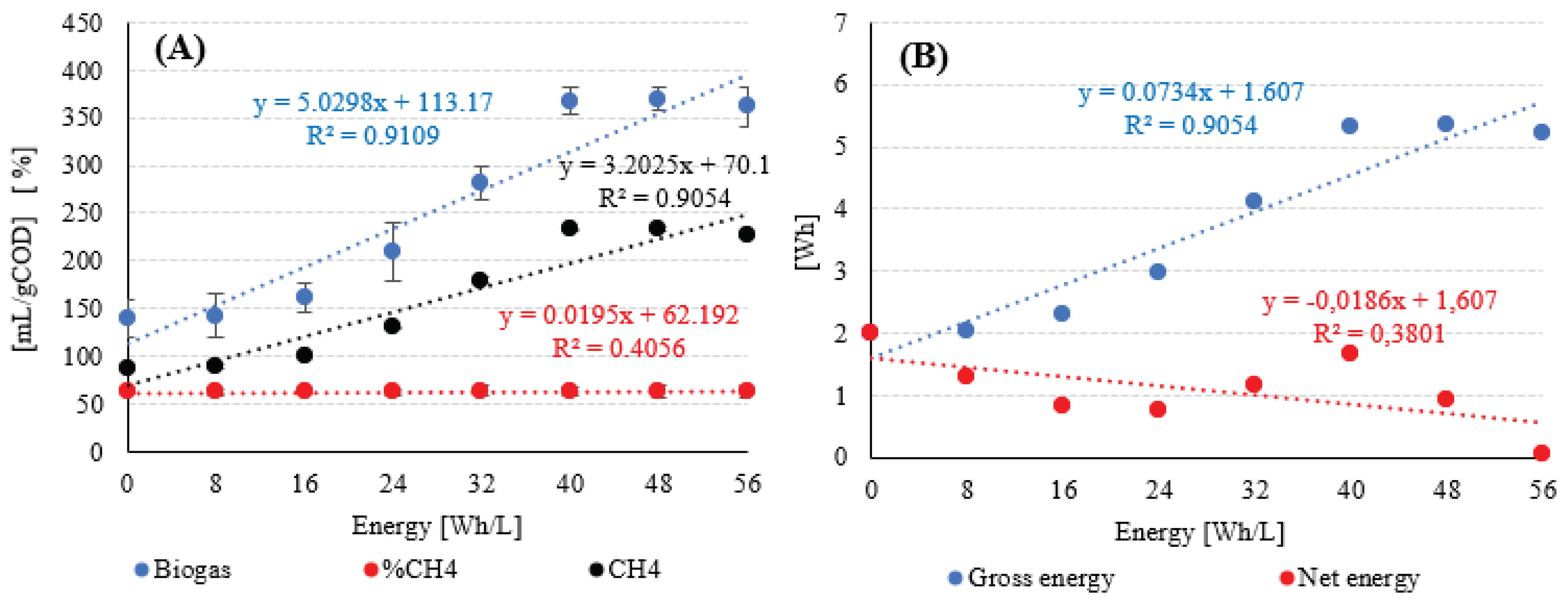
| Variant | HC Duration [min] |
Energy Demand (Ed) [Wh] |
CH4 [L] |
CH4 Energy Value (YCH4) [Wh/L] |
Energy Gross (Egross) [Wh] |
Energy Netto (Enet) [Wh] |
|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 2.18 | 9.17 | 2.00 | 2.00 |
| 1 | 2 | 0.74 | 2.20 | 2.02 | 1.28 | |
| 2 | 4 | 1.47 | 2.51 | 2.30 | 0.83 | |
| 3 | 6 | 2.21 | 3.24 | 2.97 | 0.76 | |
| 4 | 8 | 2.94 | 4.48 | 4.11 | 1.17 | |
| 5 | 10 | 3.68 | 5.82 | 5.33 | 1.65 | |
| 6 | 12 | 4.42 | 5.82 | 5.34 | 0.92 | |
| 7 | 14 | 5.15 | 5.69 | 5.22 | 0.07 |
| Variant | HC Power [kW] | V substrate [m3/day] |
HC Time [min] |
Ed [kWh/day] |
Biogas [m3/d] | CH4 [%] |
CH4 [m3/day] |
CH4 [kWh/m3] |
Egross [kWh/day] |
Enet [kWh/day] |
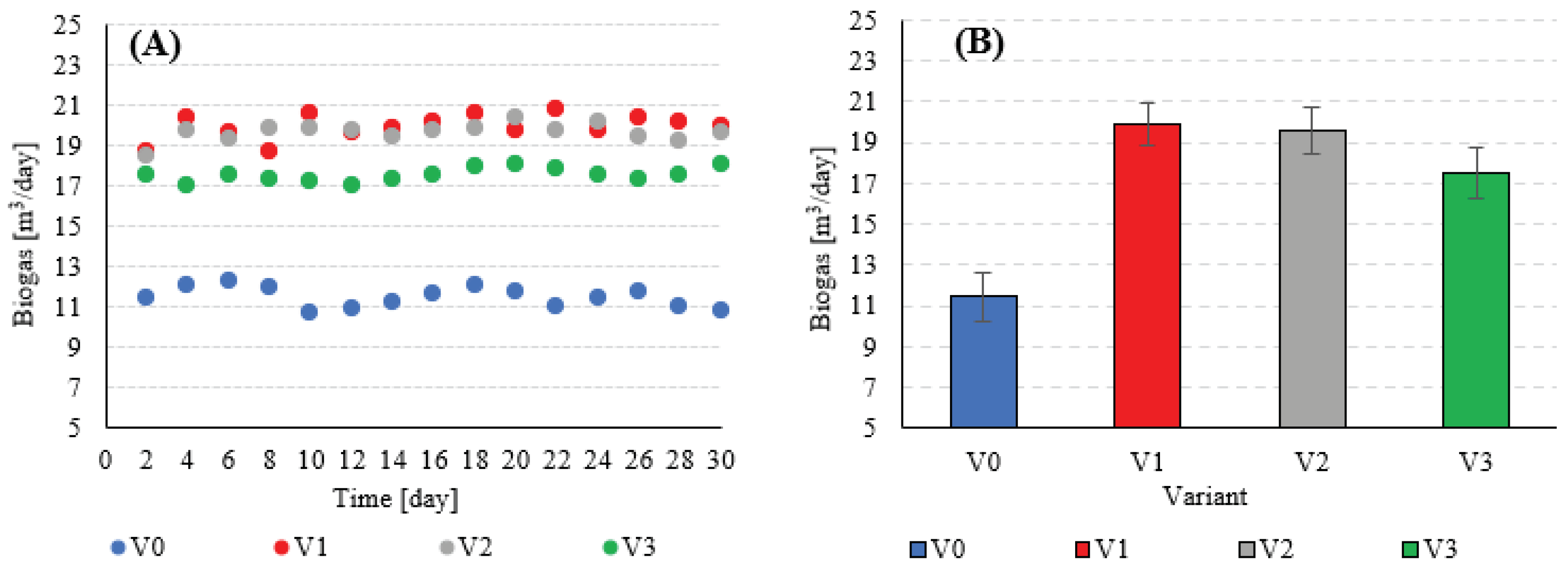
|---|---|---|---|---|---|---|---|---|---|---|
| V0 | - | 2.2 | 0 | 0 | 11.5±1.2 | 61.3±1.8 | 7.0±1.0 | 9.17 | 64.5±5.9 | 64.5±5.9 |
| V1 | 16 kW | 220 | 58.7 | 19.9±1.0 | 63.5±1.7 | 12.6±1.3 | 115.9±3.2 | 57.2±3.2 | ||
| V2 | 176 | 46.9 | 19.6±1.1 | 62.9±1.9 | 12.3±1.1 | 113.2±2.6 | 66.4±2.6 | |||
| V3 | 132 | 35.2 | 17.5±1.3 | 62.2±2.1 | 10.9±1.2 | 99.9±3.0 | 64.6±3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





