1. Introduction
Infections with the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) have declined because of successful vaccination campaigns and the lower lethality of omicron variants compared to the former ones, and hospitalization rates have dropped significantly. Nevertheless, the virus is still responsible for more than 20,000,000 current infections, including severe and life-threatening diseases [
1]. Especially in low and middle-income countries worldwide coronavirus disease 2019 (COVID-19) remains a serious health problem [
2]. Although several vaccines have been approved within an impressively short time, public opposition against vaccination and an increasing vaccine fatigue are a major obstacle in maintaining immunity in the worldwide population [
3]. Furthermore, newly emerging SARS-CoV-2 variants accompanied by an increasing number of breakthrough infections require developing prophylactic and therapeutic treatment options against SARS-CoV-2 infections [
4,
5,
6]. Antidepressants represent a promising class of cheap and globally available drugs with proven antiviral activity against SARS-CoV-2 [
7].
Several pre-clinical and clinical studies demonstrated that antidepressants belonging to the group of functional inhibitors of the acid sphingomyelinase (FIASMA) prevent infection of human cells with SARS-CoV-2 [
7,
8,
9,
10,
11,
12,
13]. Furthermore, patients treated with such antidepressants showed less severe COVID-19 symptoms, a less frequent requirement of ventilation and most importantly, showed less often a lethal course of disease [
14,
15,
16,
17].
We have previously demonstrated that pre-treatment of cultured Vero E6 epithelial cells or freshly isolated human nasal epithelial cells with antidepressants such as amitriptyline, desipramine, imipramine, fluoxetine, sertraline, maprotiline or escitalopram prevented infections with SARS-CoV-2 spike pseudotyped vesicular stomatitis virus (VSV) particles and an early isolate of SARS-CoV-2 D614G [
18].
Mechanistically, we demonstrated that binding of SARS-CoV-2 spike to ACE2, the cellular receptor of SARS-CoV-2, induces activation of the acid sphingomyelinase, the subsequent conversion of sphingomyelin to ceramide and formation of ceramide-enriched membrane platforms at the outer leaflet of the plasma membrane [
18,
19]. Inhibition of the acid sphingomyelinase by antidepressants prevented these biochemical changes and therefore blocked the infection of target cells with SARS-CoV-2 [
18].
Thus, antidepressants targeting the acid sphingomyelinase represent a promising strategy for the treatment and prevention of severe COVID-19 [
20,
21]. Although initial studies demonstrated potent antiviral activity against SARS-CoV-2 D614G, it remains unclear if antidepressants such as fluoxetine and sertraline can interfere with the replication of newly emerged SARS-CoV-2 variants of concern.
In the present study, we investigated the antiviral activity of fluoxetine and sertraline against the SARS-CoV-2 variants D614G, alpha, delta, omicron BA.1, and omicron BA.5.
2. Materials and Methods
2.1. Cells and Viruses
All cell lines were incubated at 37°C and 5 % CO2 in a humidified atmosphere in a standard tissue incubator unless specifically indicated otherwise. HEK293T (human kidney cell line) cells were obtained from Merck (Darmstadt, Germany) via the lab of Ulf Brockmeier (Department of Neurology and Center for Translational Neuro- and Behavioral Sciences(C-TNBS), University Hospital Essen, Germany) and maintained in Dulbecco’s modified Eagle medium (DMEM; Thermo Fisher Scientific, Waltham, Massachusetts, USA) supplemented with 10 % fetal bovine serum (FBS) (GE Healthcare, Chicago, Illinois, USA) 100 U/mL penicillin and 0.1 mg/mL streptomycin (Thermo Fisher Scientific). Vero E6 (African green monkey kidney cell line) cells were obtained from ATCC and maintained in DMEM supplemented with 10 % FBS (GE Healthcare), 100 U/mL penicillin, 0.1 mg/mL streptomycin, 100 µM non-essential amino acids, 1 mM sodium pyruvate (each Thermo Fisher Scientific) and 10 mM HEPES, pH7.3 (Carl Roth, Karlsruhe, Germany).
A549-AT cells (human lung cells stably transfected with ACE2 and TMPRSS2 [
22] were cultured in minimal essential medium (MEM, Thermo Fisher Scientific) supplemented with 10 % FBS, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 100 µM non-essential amino acids, 1 mM sodium pyruvate and 10 mM HEPES, pH 7.3 (each Thermo Fisher Scientific).
The clinical SARS-CoV-2 isolates of wild type (D614G), alpha, delta, omicron BA.1, and BA.5 were obtained from nasopharyngeal swabs of COVID-19 patients hospitalized at the University Hospital Essen, Germany, as previously described [
4,
23]. The SARS-CoV-2 variants were identified after sequencing the spike gene and correlating with the corresponding variants according to the list of variants of concern from the WHO [World Health Organization Tracking SARS-CoV-2 Variants [
24] (accessed on 09 January 2024)];. The viruses were propagated on A549-AT cells and stored at −80 °C. Viral titers were determined by a standard endpoint dilution assay and calculated as 50 % tissue culture infective dose (TCID
50)/mL, as previously described [
25].
2.2. Plasmids
The initial neutralization experiments were performed using pp-VSV-SARS-CoV-2 spike pseudotyped virus like particles. These were generated by using pCG1_SARS-2-S-del18 plasmid, which was kindly provided by Markus Hoffmann and Stefan Pöhlmann (Infection Biology Unit, German Primate Centre – Leibniz Institute for Primate Research, Göttingen, Germany) and previously described [
26]. SARS-CoV-2 spike variants with point mutations in the receptor-binding domain (RBD) were generated sequentially by using the Q5 site-directed mutagenesis kit (New England Biolabs, Ipswich, Massachusetts, USA) according to the manufacturer’s instructions and confirmed by sequencing (Table S1).
Expression plasmids carrying all mutations of the respective variants of concern were a gift from David Nemazee (Wt: pCDNA3.3_CoV2_D18, Addgene plasmid #170442; alpha: pCDNA3.3_CoV2_B.1.1.7, Addgene plasmid #170451, beta: pCDNA3.3_CoV2_501V2, Addgene plasmid #170449; gamma: pCDNA3.3_CoV2_P1, Addgene plasmid number #170450; delta: pCDNA3.3-SARS2-B.1.617.2, Addgene plasmid #172320) or obtained from GenScript (omicron: SARS-CoV-2 Omicron Strain S gene Human codon_pcDNA3.1(+), #MC_0101274) (Table S2). Plasmids were amplified in E.coli DH5α competent cells (New England Biolabs) and isolated and purified using Maxiprep Kits according to the manufacturer’s instructions (Qiagen, Hilden, Germany, and Macherey-Nagel, Düren, Germany).
2.3. Preparation of VSV*∆G-Fluc spike pseudotype virus like particles
The replication-restricted VSV system for pseudoviral particles with enhanced green fluorescent protein (eGFP) and firefly luciferase (Fluc) reporters (VSV*∆G-FLuc) was kindly provided by Gert Zimmer (Institute of Virology and Immunology, Mittelhäusern/Switzerland), passed on by the lab of Stefan Pöhlman and previously described by Berger Rensch and Zimmer [
27]. We followed the protocol for pseudoviral particle generation described by Becker et al. [
28]. VSV*ΔG-Fluc (VSV-G) stocks and anti-VSV-G antibody supernatants were prepared and titrated as described. The SARS-CoV-2 pseudotyped VSV particles were prepared according to the described protocol, but with slight modifications: Low-passage HEK293T cells were seeded into 10 cm cell culture dishes (at 1.8 x 10
6 cells/dish) and grown for 24 h at 37°C with 5 % CO
2 in a standard cell culture incubator. On the next day, the cells received fresh medium (9 mL/dish) and the transfection mixtures were prepared by mixing 42 µg of the respective plasmid DNA per dish and sterile ultrapure water to a final volume of 400 µl. 100 µl sterile-filtered CaCl
2 (stock conc. 2.5 M, Thermo Fisher Scientific) were added and mixed. 500 µl 2x HBS buffer (280 mM NaCl, 50 mM HEPES, 20 mM KCl, 1.5 mM Na
2HPO
4, pH 7.1, each Thermo Fisher Scientific) was added dropwise while bubbling the solution with an electronic pipettor. The mixture was then immediately vortexed and incubated for 20 min at room temperature. The transfection complexes were added dropwise to the cells and incubated for 28-30 h at 33°C, 5 % CO
2. Cells were inoculated with VSV*ΔG-FLuc at a multiplicity of infection of five for one hour at 33°C and 5 % CO
2 in a standard tissue incubator. The supernatant was then removed and cells washed two times with PBS (Thermo Fisher Scientific). Fresh medium containing anti-VSV-G antibodies were added and cells were incubated overnight (18 h) at 33°C with 5 % CO
2 in a standard tissue incubator. The supernatant containing the pseudoviral particles was then harvested, cellular debris was removed by centrifugation at 2,000 × g for 10 min and the clarified supernatant was used immediately for experiments.
2.4. Transduction in vitro experiments
Vero E6 cells were seeded at 1x104 cells/well in 48-well plates and grown for 48 h. Cells were then pre-treated for 4 h with antidepressants in DMEM (Thermo Fisher Scientific) with only 1 % FBS (Thermo Fisher Scientific) prior to infection. Fluoxetine hydrochloride and sertraline hydrochloride (each Sigma Aldrich, St. Louis, Missouri, USA) were dissolved in dimethylsulfoxide (DMSO, Carl Roth) and diluted to a final concentration of 25 µM fluoxetine or 10 µM sertraline. Mock treated cells were assessed as controls. The medium was removed and the cells were incubated with medium containing VSV*ΔG-Fluc spike and 25 µM fluoxetine or 10 µM sertraline. Medium containing VSV*∆G-Fluc spike and corresponding DMSO concentrations without the addition of antidepressants served as control. Cells were infected for 1 h at 33°C with 5 % CO2 in a standard tissue incubator. Pseudoviral supernatants were discarded and the cells overlaid with fresh medium and incubated for 18 h, 33 °C with 5 % CO2. The effect of antidepressants on the infection of the cells was assessed by qualitative and quantitative analysis of the eGFP expression. The cell cultures were scanned for eGFP fluorescence on a Typhoon FLA biomolecular imager (GE Healthcare) and microscopic fluorescence images were obtained (EVOS, Thermo Fisher Scientific). For quantitative analysis, cells were harvested for flow cytometric analysis (Attune NxT, Thermo Fisher Scientific). The infection efficacy in the mock controls was set as 100 % and the effect of the antidepressants was reported relative to these controls.
2.5. Neutralization assay
The antiviral activity of fluoxetine and sertraline was determined by a cell-culture-based endpoint dilution assay. Therefore, confluent A549-AT cells grown in a 24-well-plate were pre-incubated with different concentrations of fluoxetine (0 µM, 5 µM, 10 µM, 20 µM, 25 µM or 30 µM) or sertraline (0 µM, 5 µM or 10 µM) for two hours at 37°C, 5 % CO2. Subsequently, the A549-AT cells were infected with 100 TCID50 of SARS-CoV-2 and incubated for three days at 37°C, 5 % CO2. Untreated A549-AT cells served as a negative control. Thereafter, the supernatants were harvested and the viral titers were determined by end-point dilution. Therefore, serial dilutions of the cell culture media (1:10 – 1:108) were incubated on A549-AT cells grown on a 96-well microtiter plate for three days at 37°C, 5 % CO2. The inoculation medium was then removed, the cells stained with 0.5 % crystal violet (Roth), solved in 20 % methanol (Merck), and evaluated for cytopathic effects (CPE) by light microscopy. The experiment was performed in triplicates.
2.6. Quantification of SARS-CoV-2 RNA
Total RNA was purified from cell culture supernatants using the RNeasy Mini Kit (Qiagen) according to manufacturer’s instructions with preceding DNase I digestion with the RNase-Free DNase Set (Qiagen). 250 ng of total RNA were reverse transcribed using the PrimeScript RT Master Mix (Takara) for relative determination of SARS-CoV-2 M- or N-gene expression. For RT-qPCR, GoTaq Probe qPCR Master Mix (Promega) was used according to the manufacturer’s instructions, with gene-specific primers and probes (Table S3). RT-qPCR was performed on a LightCycler 480 II (Roche) instrument, with the following conditions: Initial denaturation for 2 min at 95°C with a ramp rate of 4.4°C/s, followed by 40 cycles of denaturation for 15 s at 95°C with a ramp rate of 4.4 °C/s, and amplification for 60 s at 60°C with a ramp rate of 2.2 °C/s. To assess M- and N-gene copy numbers, the M- and N-genes were partially cloned into pCR2.1 (Thermo Fisher Scientific) or pMiniT 2.0 (NEB), respectively. A 1:10 plasmid dilution series was used as a reference.
2.7. Cytotoxicity Assay
Potential cytotoxicity of various concentrations of fluoxetine and sertraline towards A549-AT cells was determined using the Orangu™ cell counting solution (Cell guidance systems, Cambridge, UK) according to the manufacturer’s protocol. Briefly, increasing fluoxetine or sertraline concentrations (0 µM, 5 µM, 10 µM, 20 µM, 25 µM or 30 µM) in cell culture medium were incubated with 1 × 104 A549-AT cells per well of a 96-well-plate at 37°C, 5 % CO2. At four distinct time points (2 h, 24 h, 48 h, and 72 h), the inoculation medium was removed and replaced by medium containing 10 % of Orangu™ cell counting solution. After 60 min of incubation (37°C, 5 % CO2), cell viability was measured at an absorbance of 450 nm using Tristar 3 (Berthold Technologies, Oak Ridge, Tennessee, USA) and normalized to untreated control cells. The experiment was performed in triplicates.
3. Results
3.1. Initial investigation of the antiviral activity of fluoxetine and sertraline against SARS-CoV-2
In a first step, the ability of fluoxetine and sertraline to inhibit the SARS-CoV-2 infection of cells was evaluated with VSV*∆G-Fluc spike pseudotyped virus like particles. Therefore, VeroE6 cells were pre-treated for 4 h with 25 µM fluoxetine or 10 µM sertraline and subsequently infected with different VSV*∆G-Fluc spike variant pseudotyped virus like particles for 1 h and the antiviral efficiency was assessed by fluorescence microscopy as well as quantitatively by flow cytometry the next day.
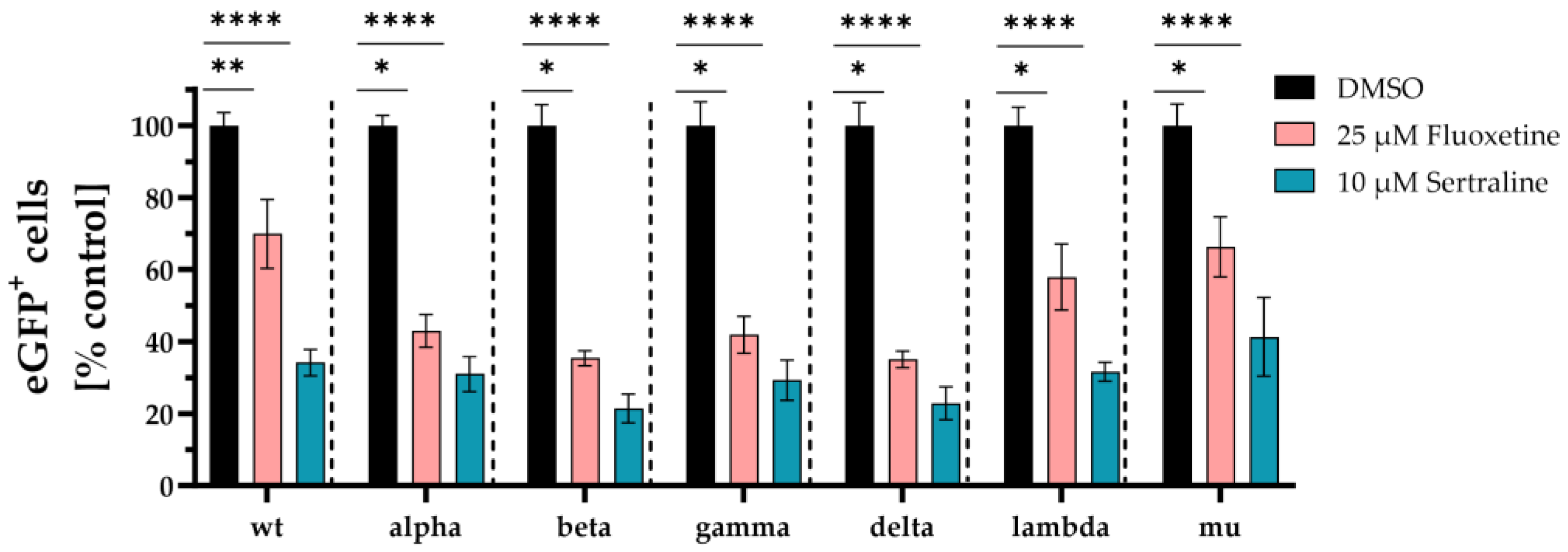
First, VSV*∆G-Fluc spike pseudotyped virus like particles with mutations only in the spike RBD region were used. Fluoxetine treatment led to significantly lower infection rates with wild type (p < 0.006), alpha (p < 0.02), beta (p < 0.03), gamma (p < 0.02), delta (p < 0.0001), lambda (p < 0.03), and mu (p < 0.02) compared to the DMSO control. The mean percentage infection of cells treated with fluoxetine was 48 % for wild type, 43 % for alpha, 35 % for beta, 42 % for delta, 35 % for gamma, 58 % for lambda and 66 % for mu.
Accordingly, treatment with sertraline led to significantly lower infection rates with wild type (p < 0.0001), alpha (p < 0.0001), beta (p < 0.0001), gamma (p < 0.0001), delta (p < 0.0001), lambda (p < 0.0001), and mu (p < 0.0001). In case of sertraline treatment, the mean percentage infection of cells was observed with 37 % for wild type, 31 % for alpha, 21 % for beta, 29 % for gamma, 23 % for delta, 32 % for lambda and 41 % for mu. Overall, sertraline treatment resulted in a greater reduction of infection compared to fluoxetine (
Figure 1).
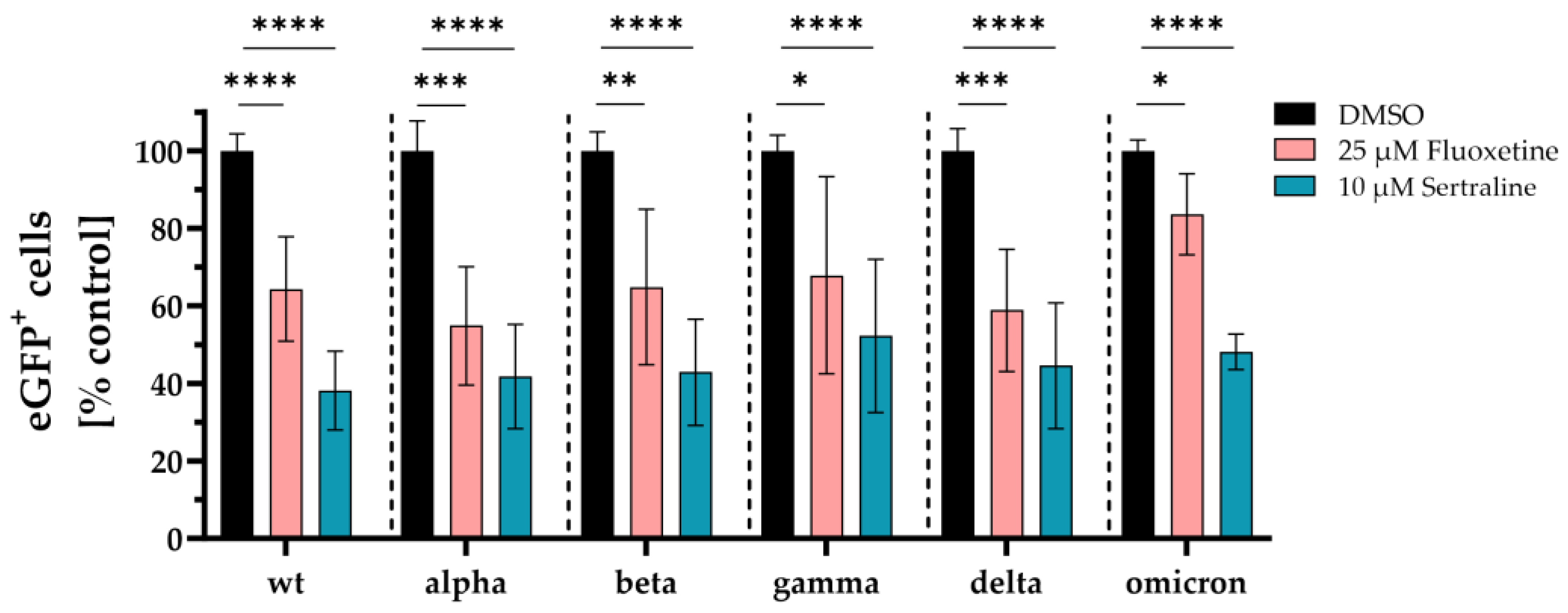
Next, expression plasmids coding for spike proteins containing all mutations of the respective variants of concern were used to generate VSV*∆G-Fluc pseudotyped virus like particles and the inhibitory effect of fluoxetine and sertraline against infection of cells with SARS-CoV-2 was analyzed. Fluoxetine significantly reduced the infection of the cells with VSV*∆G-Fluc spike pseudotyped virus like particles of wild type D614G to 64 % (p < 0.0001), of alpha to 55 % (p < 0.0005), of beta to 65 % (p < 0.009), of gamma to 68 % (p = 0.01), of delta to 59 % (p < 0.0006), and of omicron to 84 % (p < 0.04) compared to the DMSO control. Sertraline also led to a significant reduction of the infection with VSV*∆G-Fluc spike pseudotyped virus like particles of wild type D614G to 38 % (p < 0.0001), alpha to 42 % (p < 0.0001), beta to 43 % (p < 0.0001), gamma to 52 % (p <0.0001), delta to 45 % (p < 0.0001), and omicron to 48 % (p < 0.0001) compared to the DMSO control. Fluoxetine and sertraline showed broad antiviral activity against VSV*ΔG-Fluc spike pseudotyped virus like particles displaying distinct SARS-CoV-2 spike protein variants. Interestingly, sertraline more potently inhibited the infection with VSV*ΔG-Fluc spike pseudotyped virus like particles compared to fluoxetine (
Figure 2).
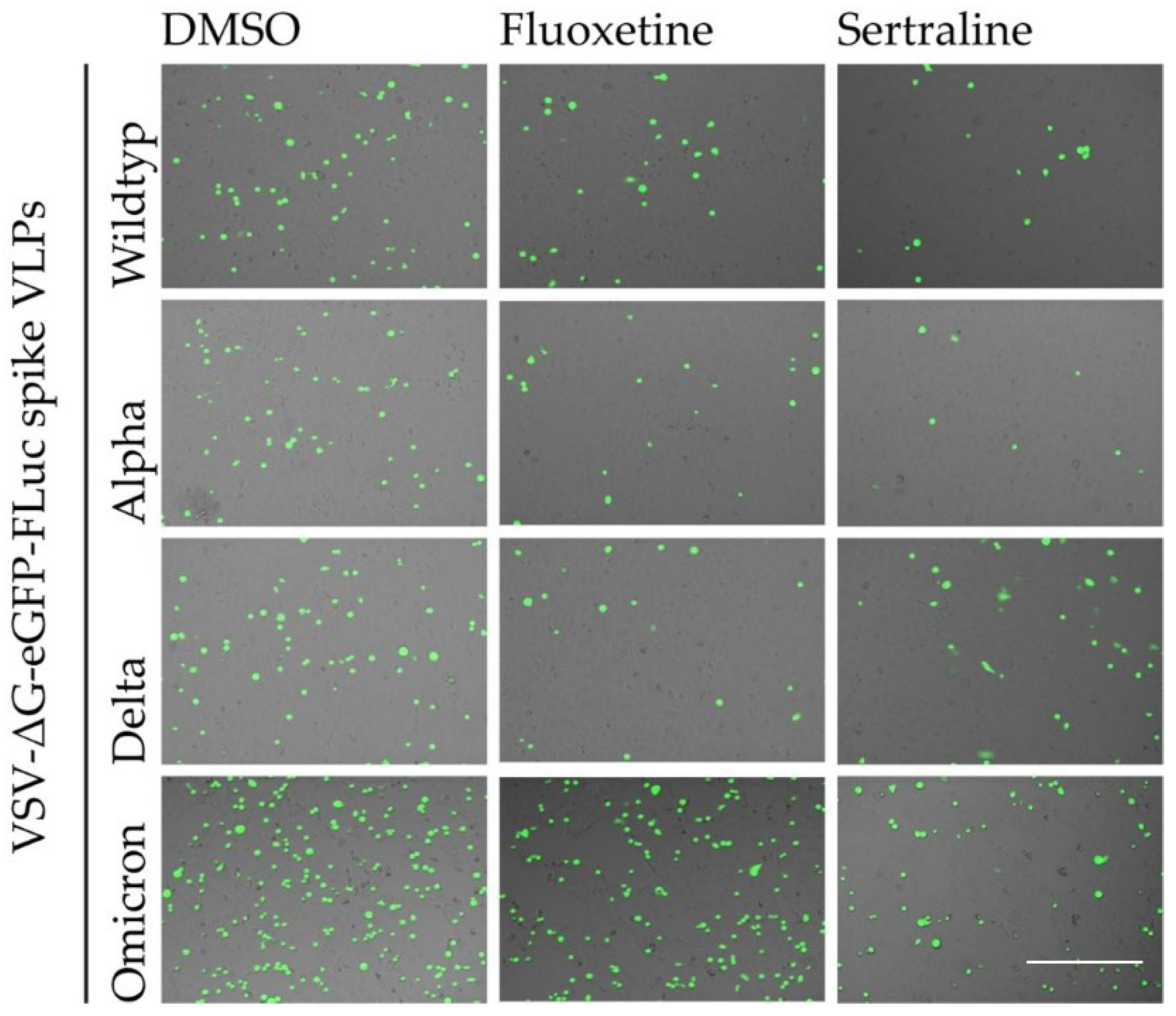
The inhibitory effect of fluoxetine and sertraline on the SARS-CoV-2 infection of Vero E6 cels with different VSV*ΔG-Fluc spike pseudotype virus like particles was confirmed by fluorescence microscopy (
Figure 3).
3.2. Fluoxetine and sertraline effectively blocks SARS-CoV-2 infection
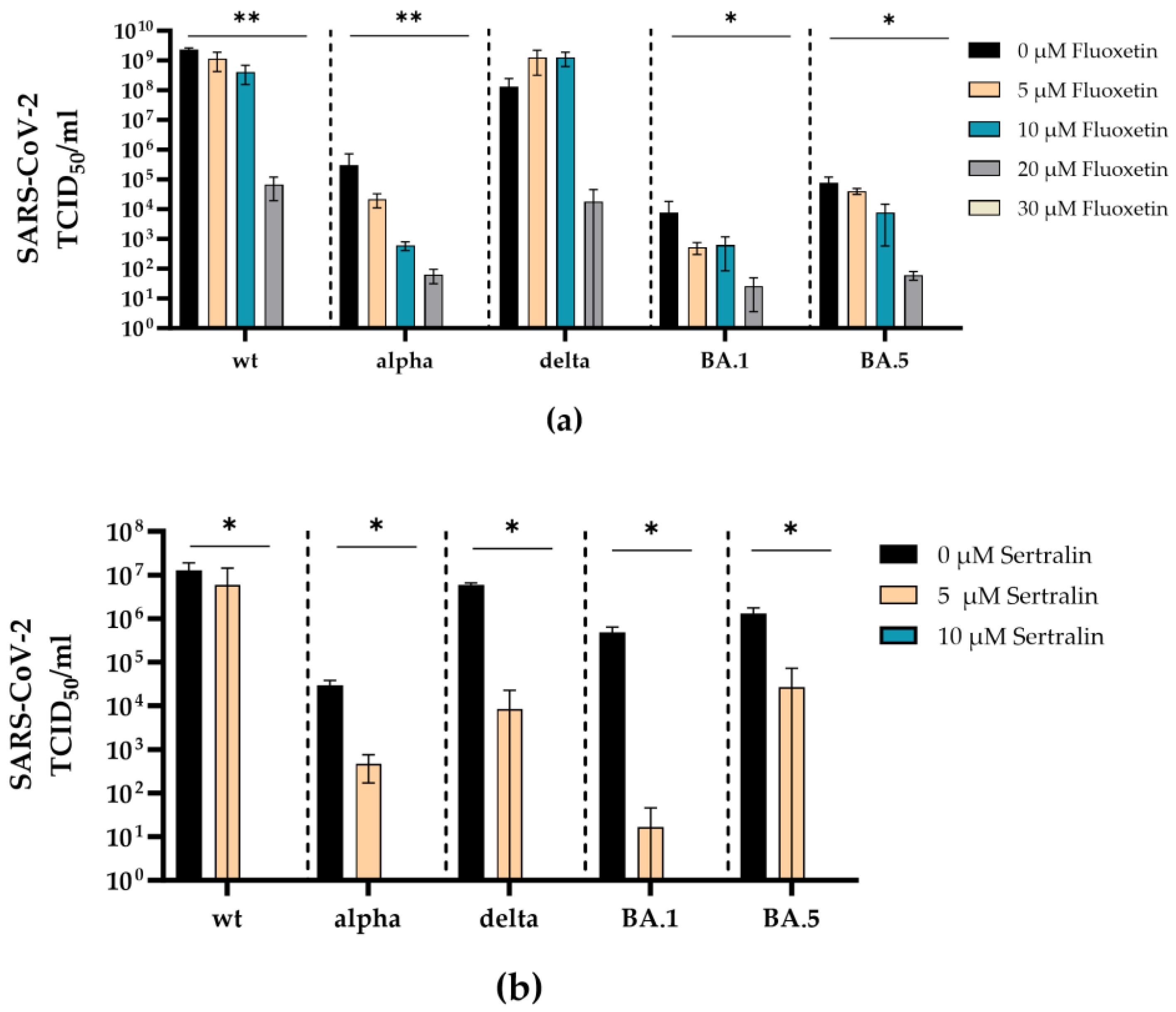
To examine whether the antidepressants fluoxetine and sertraline can inhibit infection with a real virus, the antiviral efficacy of fluoxetine (0-30 µM) or sertraline (0-10 µM) was investigated against clinical isolates SARS-CoV-2 D614G, alpha, delta, omicron BA.1, omicron BA.5.
Fluoxetine inhibited the replication of SARS-CoV-2 D614G, alpha, delta, omicron BA.1, and omicron BA.5 in a dose-dependent manner. Complete inhibition of all SARS-CoV-2 variants was reached at a concentration of 30 µM. Significant reduction of the viral load was found for SARS-CoV-2 D614G (p = 0.0095), alpha (p = 0.0096), omicron BA.1 (p = 0.016), and omicron BA.5 (p = 0.015) at a concentration of 30 µM compared to the medium control (p = 0.03) (
Figure 4a).
Accordingly, sertraline inhibited the replication of SARS-CoV-2 D614G, alpha, delta, omicron BA.1 and omicron BA5 in a dose-dependent manner, where all SARS-CoV-2 variants were completely neutralized at a concentration of 10 µM sertraline. The incubation with sertraline significantly decreased the viral replication of SARS-CoV-2 D614G (p = 0.044), alpha (p = 0.01), delta (p = 0.041), omicron BA.1 (p = 0.042), and omicron BA.5 (p = 0.02) at a concentration of 10 µM compared to the control (
Figure 4b).
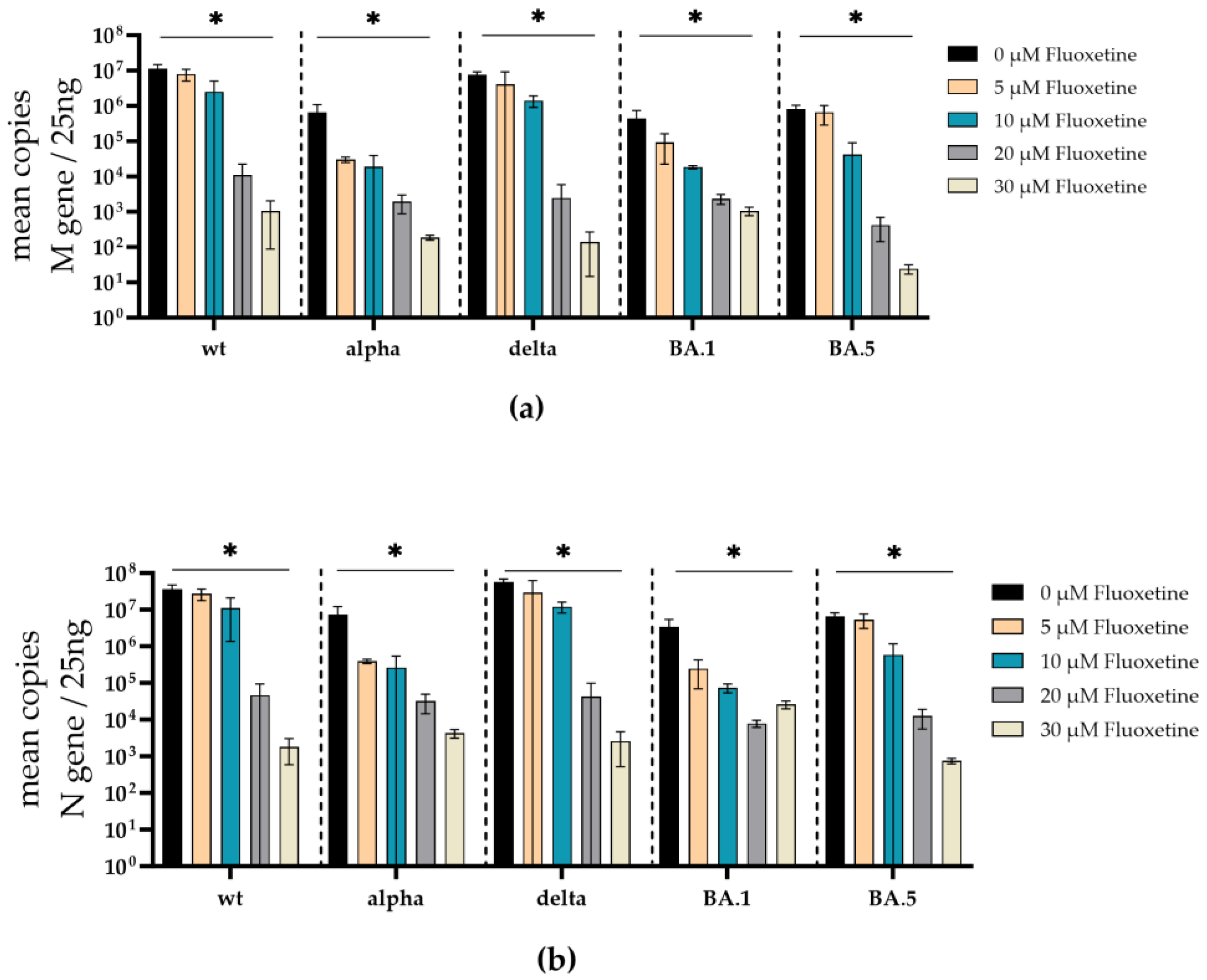
To additionally validate the antiviral effect of fluoxetine and sertraline against SARS-CoV-2 variants, the SARS-CoV-2 RNA from cell culture supernatants of infected and fluoxetine- or sertraline-treated cells was quantified. Therefore, the RNA of the respective supernatant was isolated, reverse transcribed, and the M- and N-gene of SARS-CoV-2 were quantified by RT-qPCR. Fluoxetine treatment reduced the copy-number of SARS-CoV-2 M- and N-gene RNA in a dose-dependent manner. There was a significant reduction of M-gene RNA of SARS-CoV-2 D614G (p = 0.02), alpha (p = 0.01), delta (p = 0.03), omicron BA.1 (p = 0.01), and omicron BA.5 (p = 0.02) at a fluoxetine concentration of 30 µM (
Figure 5a). Furthermore, a significant reduction of N-gene RNA was found in cell culture supernatants from SARS-CoV-2 D614G (p = 0.014), alpha (p = 0.010), delta (p = 0.02), omicron BA.1 (p = 0.010), and omicron BA.5 (p = 0.019) infected cells treated with 30 µM fluoxetine (
Figure 5b). The M-gene RNA decreased slightly more than the N-gene RNA after treatment with fluoxetine.
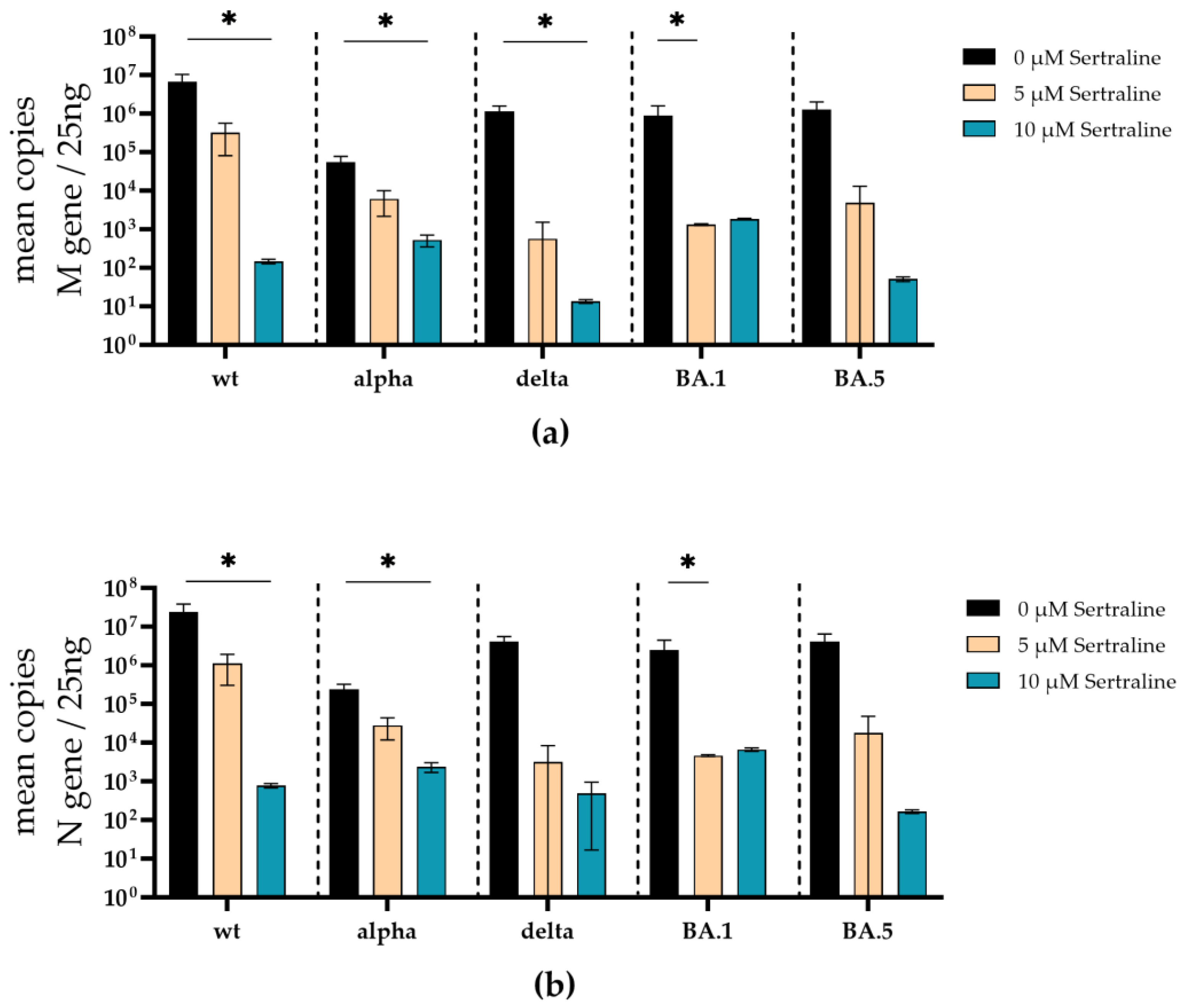
Similar to the treatment with fluoxetine, the total amounts of SARS-CoV-2 M- and N-gene RNA was substantially decreased under sertraline treatment. Significant reduction of M-gene RNA was found in the supernatants of SARS-CoV-2 D614G (p = 0.02), alpha (p = 0.02), delta (p = 0.04) infected sertraline (10 µM) treated cells. M-gene RNA was significantly reduced in the supernatant of omicron BA.1 (p = 0.02) infected cells at 5 µM sertraline. SARS-CoV-2 M-gene RNA of the omicron BA.5 variant was decreased by several log levels but did not achieve a significant level (
Figure 6a). Furthermore, a significant decrease of N-gene RNA was found in cell culture supernatants from SARS-CoV-2 D614G (p = 0.02), and alpha (p = 0.02) infected cells treated with 10 µM sertraline (
Figure 6b). In the cell culture supernatant from BA.1 (p = 0,04) infected cells, the N-gene RNA was significantly reduced at a concentration of 5 µM sertraline. Moreover, SARS-CoV-2 N-gene RNA levels of delta, and omicron BA.5 were reduced by several log levels but did not reach a significant level (
Figure 6b).
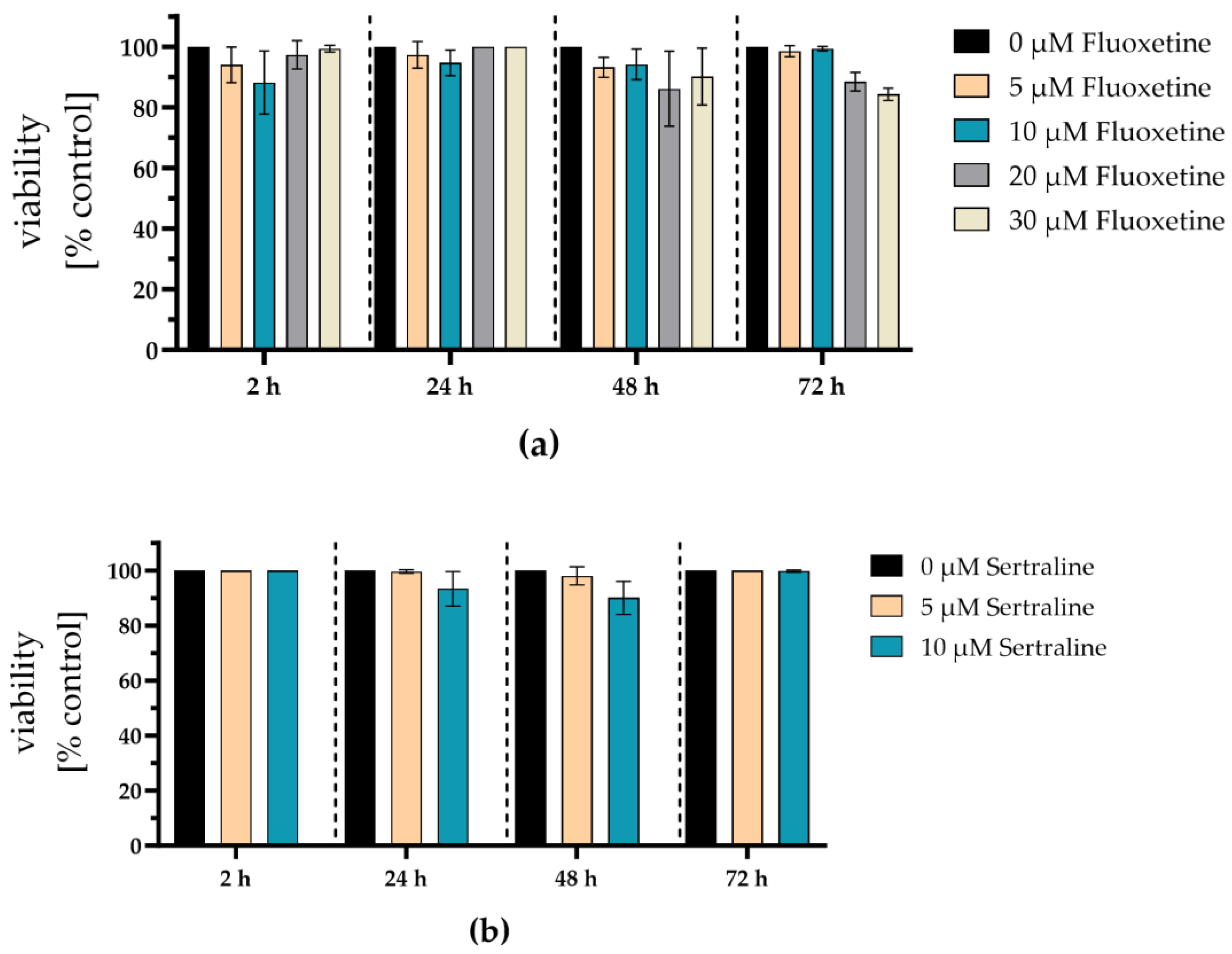
3.3. Fluoxetine and sertraline showed no toxic effects at SARS-CoV-2 neutralizing concentrations
Potential cytotoxic effects of the antidepressants fluoxetine and sertraline were analyzed by determining cell viability. Therefore, A549-AT cells were incubated with different concentrations of fluoxetine (0 – 30 µM) or sertraline (0 – 10 µM) and tested for viability after 2, 24, 48, and 72 hours of incubation at 37°C and 5% CO
2. After 72 hours, no substantial toxicity could be detected even if the cells were exposed to the highest fluoxetine (30 µM) or sertraline (10 µM) concentrations. Thus, fluoxetine and sertraline potently neutralize SARS-CoV-2 D614G and the variants alpha, delta, omicron BA.1, and omicron BA5 at subtoxic concentrations (
Figure 7).
4. Discussion
After three years of the pandemic, SARS-CoV-2 remains a global health burden marked by the persistence of severe courses of disease with substantial numbers of hospitalizations and deaths. Furthermore, in many cases, there are long-lasting symptoms, known as long-COVID-19. With increasing vaccination fatigue and a growing number of vaccine breakthrough infections, there is an urgent need for rapidly available drugs to treat or prevent SARS-CoV-2 infections, in particular such with newly emerging variants. Various antidepressants were described as potent entry inhibitors of early-emerged SARS-CoV-2 variants and other coronaviruses [
11,
12]. However, it remained unclear whether antidepressants that target the SARS-CoV-2 spike S1 domain may inhibit newly emerging SARS-CoV-2 variants despite mutations that additionally occur during SARS-CoV-2 evolution.
In the present study, we investigated the antiviral efficacy of fluoxetine and sertraline against distinct SARS-CoV-2 variants, including D614G, alpha, delta, omicron BA.1 and omicron BA.5. In a first step, the ability of fluoxetine and sertraline to inhibit SARS-CoV-2 infection was examined by using VSV*ΔG-Fluc spike pseudotyped virus like particles with mutations within the RBD or the full spike S1 domain. This method offers the advantage of rapid testing of active substances against different SARS-CoV-2 variants without the need for an S3 laboratory [
29,
30]. In line with previous studies, fluoxetine and sertraline effectively blocked the infection with VSV*ΔG-Fluc spike pseudotyped virus like particles [
7,
31]. The antiviral activity of fluoxetine and sertraline was shown for the first time on this broad variety of variants.
Notably, fluoxetine and sertraline completely neutralized the replication of all used clinical SARS-CoV-2 isolates (D614G, alpha, delta, micron BA.1 and omicron BA.5) at a subtoxic concentration of 30 µM (fluoxetine) or 10 µM (sertraline). The neutralizing effect of these antidepressants was unaffected by the respective mutations within the SARS-CoV-2 spike protein. This is an interesting finding, as fluoxetine and sertraline were described to interfere with SARS-CoV-2 entry, and at least sertraline directly targeted the SARS-CoV-2 spike protein [
7]. Besides targeting the spike protein, fluoxetine and sertraline were described as functional inhibitors of the acid sphingomyelinase (ASM), which plays an important role during SARS-CoV-2 entry into the host cell [
32,
33]. ASM is a glycoprotein that catalyzes sphingomyelin degradation to phosphorylcholine and ceramide, which is known to facilitate viral entry into the host cell [
34,
35,
36]. SARS-CoV-2 activates the ASM/ceramide system, resulting in the formation of ceramide-enriched membrane domains that cluster ACE2 and thereby, facilitate viral entry and infection [
33,
34,
37]. Thus, inhibiting ASM represents a SARS-CoV-2 spike mutation-independent mechanism to interfere with viral entry and infection.
In line with prior studies, our findings suggest that fluoxetine and sertraline use in early-stage of SARS-CoV-2 infections with recent variants may reduce the risk of a severe course of COVID-19 or death [
38,
39]. Randomized clinical trials and retrospective studies have already demonstrated that the treatment with antidepressants result in a milder course of COVID-19 and a reduced mortality rate [
14,
40,
41,
42,
43]. Furthermore, there was a lower infestation rate among patients using antidepressants, indicating that fluoxetine and sertraline may have a protective effect against SARS-CoV-2 infections [
44].
In conclusion, the antidepressants fluoxetine and sertraline are promising candidates for supportive treatment of SARS-CoV-2 infections, in particular during the early phase of infection. Especially in developing countries with a restricted medical health care system these cheap and generally available drugs may contribute to improve the treatment of COVID-19 patients. Since their antiviral efficacy is unaffected by SARS-CoV-2 spike mutations, they may be used in new or even breakthrough infections with newly emerging SARS-CoV-2 variants.
Author Contributions
Conceptualization, A.K. and K.A.B.; methodology, N.B., C.S., L.T., P.B., C.E., L.B., M.B., S.K., M.S., and K.A.B.; software, L.T.; A.K., and K.A.B., validation, A.K., K.A.B. and L.T.; formal analysis, L.T., A.K., and K.A.B.; investigation, L.T., N.B., A.K., and K.A.B.; resources, N.H., C.C., S.C., M.W., and U.D.; data curation, L.T., A.K., K.A.B., and C.S.; writing—original draft preparation, L.T., N.B., A.K., and K.A.B.; writing—review and editing, L.T., A.K., and K.A.B.; and M.L visualization, L.T., A.K., and K.A.B.; supervision, A.K., K.A.B., and E.G.; project administration, M.L., P.A.H., and O.W.; funding acquisition, A.K., K.A.B., E.G., M.K., and O.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Stiftung Universitätsmedizin Essen (awarded to A. Krawczyk) the Rudolf Ackermann Foundation (awarded to O. Witzke) and the German Research Foundation (DFG, awarded to A. Krawczyk, E. Gulbins, K. A. Becker(-Flegler) and M. Kamler).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Ulf Brockmeier from Dirk Hermann’s lab for providing the HEK293-T cells, Markus Hoffmann and Stefan Pöhlmann for passing on the VSV system, originally designed by Gerd Zimmer, and providing the pCG1_SARS-2-S-del18 plasmid.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- worldometers. Available from: https://www.worldometers.info/coronavirus/.
- Panneer, S., et al., The Great Lockdown in the Wake of COVID-19 and Its Implications: Lessons for Low and Middle-Income Countries. Int J Environ Res Public Health, 2022. 19(1).
- Stamm, T.A., et al., Determinants of COVID-19 vaccine fatigue. Nature Medicine, 2023. 29(5): p. 1164-1171.
- Bormann, M., et al., Immune responses in COVID-19 patients during breakthrough infection with SARS-CoV-2 variants Delta, Omicron-BA.1 and Omicron-BA.5. Front Immunol, 2023. 14: p. 1150667.
- Bergwerk, M., et al., Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med, 2021. 385(16): p. 1474-1484.
- Dash, N.R., et al., COVID-19 Breakthrough Infection Among Vaccinated Population in the United Arab Emirates. J Epidemiol Glob Health, 2023. 13(1): p. 67-90.
- Chen, Y., et al., Sertraline Is an Effective SARS-CoV-2 Entry Inhibitor Targeting the Spike Protein. Journal of Virology, 2022. 96(24): p. e01245-22.
- Albouz, S., et al., Lipid and lysosomal enzymes in human fibroblasts cultured with perhexiline maleate. Naunyn Schmiedebergs Arch Pharmacol, 1981. 317(2): p. 173-7.
- Hurwitz, R., K. Ferlinz, and K. Sandhoff, The Tricyclic Antidepressant Desipramine Causes Proteolytic Degradation of Lysosomal Sphingomyelinase in Human Fibroblasts. Biological Chemistry Hoppe-Seyler, 1994. 375(7): p. 447-450.
- Kornhuber, J., et al., Functional Inhibitors of Acid Sphingomyelinase (FIASMAs): a novel pharmacological group of drugs with broad clinical applications. Cell Physiol Biochem, 2010. 26(1): p. 9-20.
- Zimniak, M., et al., The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue. Scientific Reports, 2021. 11(1): p. 5890.
- Kutkat, O., et al., Robust antiviral activity of commonly prescribed antidepressants against emerging coronaviruses: in vitro and in silico drug repurposing studies. Scientific Reports, 2022. 12(1): p. 12920.
- Péricat, D., et al., Antiviral and Anti-Inflammatory Activities of Fluoxetine in a SARS-CoV-2 Infection Mouse Model. Int J Mol Sci, 2022. 23(21).
- Lenze, E.J., et al., Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial. Jama, 2020. 324(22): p. 2292-2300.
- Calusic, M., et al., Safety and efficacy of fluvoxamine in COVID-19 ICU patients: An open label, prospective cohort trial with matched controls. Br J Clin Pharmacol, 2022. 88(5): p. 2065-2073.
- Seftel, D. and D.R. Boulware, Prospective Cohort of Fluvoxamine for Early Treatment of Coronavirus Disease 19. Open Forum Infect Dis, 2021. 8(2): p. ofab050.
- Reis, G., et al., Effect of Early Treatment with Ivermectin among Patients with Covid-19. N Engl J Med, 2022. 386(18): p. 1721-1731.
- Carpinteiro, A., et al., Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells. Cell Rep Med, 2020. 1(8): p. 100142.
- Carpinteiro, A., et al., Inhibition of acid sphingomyelinase by ambroxol prevents SARS-CoV-2 entry into epithelial cells. Journal of Biological Chemistry, 2021. 296: p. 100701.
- Hoertel, N., Do the Selective Serotonin Reuptake Inhibitor Antidepressants Fluoxetine and Fluvoxamine Reduce Mortality Among Patients With COVID-19? JAMA Network Open, 2021. 4(11): p. e2136510-e2136510.
- Hoertel, N., et al., Repurposing antidepressants inhibiting the sphingomyelinase acid/ceramide system against COVID-19: current evidence and potential mechanisms. Mol Psychiatry, 2021. 26(12): p. 7098-7099.
- Widera, M., et al., Generation of a Sleeping Beauty Transposon-Based Cellular System for Rapid and Sensitive Screening for Compounds and Cellular Factors Limiting SARS-CoV-2 Replication. Front Microbiol, 2021. 12: p. 701198.
- Thümmler, L., et al., Cellular and Humoral Immunity against Different SARS-CoV-2 Variants Is Detectable but Reduced in Vaccinated Kidney Transplant Patients. Vaccines (Basel), 2022. 10(8).
- WHO. 2023; Available from: https://www.who.int/activities/tracking-SARS-CoV-2-variants.
- Heilingloh, C.S., et al., Susceptibility of SARS-CoV-2 to UV irradiation. Am J Infect Control, 2020. 48(10): p. 1273-1275.
- Hoffmann, M., et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell, 2020. 181(2): p. 271-280.e8.
- Berger Rentsch, M. and G. Zimmer, A vesicular stomatitis virus replicon-based bioassay for the rapid and sensitive determination of multi-species type I interferon. PLoS One, 2011. 6(10): p. e25858.
- Becker, K.A., et al., Ex vivo assay to evaluate the efficacy of drugs targeting sphingolipids in preventing SARS-CoV-2 infection of nasal epithelial cells. STAR Protoc, 2021. 2(1): p. 100356.
- Zettl, F., et al., Rapid Quantification of SARS-CoV-2-Neutralizing Antibodies Using Propagation-Defective Vesicular Stomatitis Virus Pseudotypes. Vaccines, 2020. 8(3): p. 386.
- Hoffmann, M., et al., SARS-CoV-2 variant B.1.617 is resistant to bamlanivimab and evades antibodies induced by infection and vaccination. Cell Rep, 2021. 36(3): p. 109415.
- Fred, S.M., et al., Antidepressant and Antipsychotic Drugs Reduce Viral Infection by SARS-CoV-2 and Fluoxetine Shows Antiviral Activity Against the Novel Variants in vitro. Front Pharmacol, 2021. 12: p. 755600.
- Geiger, N., et al., The Acid Ceramidase Is a SARS-CoV-2 Host Factor. Cells, 2022. 11(16): p. 2532.
- Schloer, S., et al., Targeting the endolysosomal host-SARS-CoV-2 interface by clinically licensed functional inhibitors of acid sphingomyelinase (FIASMA) including the antidepressant fluoxetine. Emerging Microbes & Infections, 2020. 9(1): p. 2245-2255.
- Kornhuber, J., N. Hoertel, and E. Gulbins, The acid sphingomyelinase/ceramide system in COVID-19. Molecular Psychiatry, 2022. 27(1): p. 307-314.
- Törnquist, K., et al., Sphingolipids as Modulators of SARS-CoV-2 Infection. Front Cell Dev Biol, 2021. 9: p. 689854.
- Beckmann, N. and K.A. Becker, Ceramide and Related Molecules in Viral Infections. International Journal of Molecular Sciences, 2021. 22(11): p. 5676.
- Hashimoto, Y., T. Suzuki, and K. Hashimoto, Mechanisms of action of fluvoxamine for COVID-19: a historical review. Molecular Psychiatry, 2022. 27(4): p. 1898-1907.
- Hoertel, N., et al., Medications Modulating the Acid Sphingomyelinase/Ceramide System and 28-Day Mortality among Patients with SARS-CoV-2: An Observational Study. Pharmaceuticals, 2023. 16(8): p. 1107.
- Bormann, M., et al., Recent Antiviral Treatment and Vaccination Strategies Against SARS-CoV-2. Klin Monbl Augenheilkd, 2021. 238(5): p. 569-578.
- Reis, G., et al., Oral Fluvoxamine With Inhaled Budesonide for Treatment of Early-Onset COVID-19: A Randomized Platform Trial. Ann Intern Med, 2023. 176(5): p. 667-675.
- Reis, G., et al., Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health, 2022. 10(1): p. e42-e51.
- Wang, H., et al., Relationship between antidepressants and severity of SARS-CoV-2 Omicron infection: a retrospective cohort study using real-world data. Lancet Reg Health West Pac, 2023. 34: p. 100716.
- Jittamala, P., et al., Antiviral efficacy of fluoxetine in early symptomatic COVID-19: an open-label, randomised, controlled, adaptive platform trial (PLATCOV). medRxiv, 2024: p. 2024.01.16.24301337.
- Oskotsky, T., et al., Mortality Risk Among Patients With COVID-19 Prescribed Selective Serotonin Reuptake Inhibitor Antidepressants. JAMA Netw Open, 2021. 4(11): p. e2133090.
Figure 1.
Inhibition of SARS-CoV-2 infection with distinct VSV*∆G-Fluc spike pseudotyped virus like particles. Vero E6 cells were pre-treated with either DMSO, 25 µM fluoxetine or 10 µM sertraline and subsequently infected with VSV*∆G-Fluc spike pseudotyped virus like particles with RBD mutations of different SARS-CoV-2 variants. The effect of antidepressants on the infection of the cells was assessed by quantitative analysis of the eGFP expression. Data are shown as mean and standard deviation (SD). Differences between fluoxetine/sertraline and the DMSO control were compared by Kruskal-Wallis test (*p < 0.05; **p < 0.01; ****p < 0.0001).
Figure 1.
Inhibition of SARS-CoV-2 infection with distinct VSV*∆G-Fluc spike pseudotyped virus like particles. Vero E6 cells were pre-treated with either DMSO, 25 µM fluoxetine or 10 µM sertraline and subsequently infected with VSV*∆G-Fluc spike pseudotyped virus like particles with RBD mutations of different SARS-CoV-2 variants. The effect of antidepressants on the infection of the cells was assessed by quantitative analysis of the eGFP expression. Data are shown as mean and standard deviation (SD). Differences between fluoxetine/sertraline and the DMSO control were compared by Kruskal-Wallis test (*p < 0.05; **p < 0.01; ****p < 0.0001).
Figure 2.
Inhibition of SARS-CoV-2 infection with distinct commercial VSV*ΔG-Fluc spike pseudotype virus like particles. Vero E6 cells were pre-treated with either DMSO, 25 µM fluoxetine or 10 µM sertraline and subsequently infected with VSV*ΔG-Fluc spike pseudotyped virus like particles with mutations of different SARS-CoV-2 variants. The effect of antidepressants on the infection of the cells was assessed by quantitative analysis of the eGFP expression. Data are shown as mean and standard deviation (SD). Differences between fluoxetine/sertraline and the DMSO control were compared by Kruskal-Wallis test (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001).
Figure 2.
Inhibition of SARS-CoV-2 infection with distinct commercial VSV*ΔG-Fluc spike pseudotype virus like particles. Vero E6 cells were pre-treated with either DMSO, 25 µM fluoxetine or 10 µM sertraline and subsequently infected with VSV*ΔG-Fluc spike pseudotyped virus like particles with mutations of different SARS-CoV-2 variants. The effect of antidepressants on the infection of the cells was assessed by quantitative analysis of the eGFP expression. Data are shown as mean and standard deviation (SD). Differences between fluoxetine/sertraline and the DMSO control were compared by Kruskal-Wallis test (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001).
Figure 3.
Qualitative assessment of the antiviral activity of fluoxetine and sertraline against SARS-CoV-2. Vero E6 cells were pre-treated with either DMSO, 25 µM fluoxetine or 10 µM sertraline and subsequently infected with VSV*ΔG-Fluc spike pseudotyped virus like particles with mutations of different SARS-CoV-2 variants. The effect of antidepressants on the infection of the cells was assessed by fluorescence microscopy. Scale bar represents 400 µm.
Figure 3.
Qualitative assessment of the antiviral activity of fluoxetine and sertraline against SARS-CoV-2. Vero E6 cells were pre-treated with either DMSO, 25 µM fluoxetine or 10 µM sertraline and subsequently infected with VSV*ΔG-Fluc spike pseudotyped virus like particles with mutations of different SARS-CoV-2 variants. The effect of antidepressants on the infection of the cells was assessed by fluorescence microscopy. Scale bar represents 400 µm.
Figure 4.
Fluoxetine and sertraline completely inhibit SARS-CoV-2 infection. A549-AT cells were infected with 100 TCID50 of different clinical SARS-CoV-2 isolates (D614G, alpha, delta, omicron BA.1, and omicron BA.5) in the presence of different concentrations of fluoxetine (0 – 30 µM) (a) or sertraline (0 – 10 µM) (b). After three days of incubation, the viral loads in the respective cell culture supernatants were determined by endpoint dilution in triplicates. Data are shown as mean and standard deviation (SD). Differences between fluoxetine/sertraline and the control were compared by Kruskal-Wallis test (*p < 0.05; **p < 0.01).
Figure 4.
Fluoxetine and sertraline completely inhibit SARS-CoV-2 infection. A549-AT cells were infected with 100 TCID50 of different clinical SARS-CoV-2 isolates (D614G, alpha, delta, omicron BA.1, and omicron BA.5) in the presence of different concentrations of fluoxetine (0 – 30 µM) (a) or sertraline (0 – 10 µM) (b). After three days of incubation, the viral loads in the respective cell culture supernatants were determined by endpoint dilution in triplicates. Data are shown as mean and standard deviation (SD). Differences between fluoxetine/sertraline and the control were compared by Kruskal-Wallis test (*p < 0.05; **p < 0.01).
Figure 5.
Treatment with fluoxetine significantly reduces SARS-CoV-2 M- and N-gene levels. A549-AT cells were infected with 100 TCID50 of different SARS-CoV-2 variants in the presence of different concentrations of fluoxetine (0 – 30 µM). After incubation for 72 h, supernatants were harvested. Total RNA was isolated, reverse transcribed, and the SARS-CoV-2 M- (a) and N-gene (b) were quantified by RT-qPCR. Data are shown as mean and standard deviation (SD). Differences between fluoxetine/sertraline and the control were compared by Kruskal-Wallis test (*p < 0.05).
Figure 5.
Treatment with fluoxetine significantly reduces SARS-CoV-2 M- and N-gene levels. A549-AT cells were infected with 100 TCID50 of different SARS-CoV-2 variants in the presence of different concentrations of fluoxetine (0 – 30 µM). After incubation for 72 h, supernatants were harvested. Total RNA was isolated, reverse transcribed, and the SARS-CoV-2 M- (a) and N-gene (b) were quantified by RT-qPCR. Data are shown as mean and standard deviation (SD). Differences between fluoxetine/sertraline and the control were compared by Kruskal-Wallis test (*p < 0.05).
Figure 6.
Treatment with sertraline significantly reduces SARS-CoV-2 M- and N-gene levels. A549-AT cells were infected with 100 TCID50 of different SARS-CoV-2 variants in the presence of different sertraline concentrations (0 – 10 µM). After 72 h of incubation, the supernatants were harvested and the total RNA was isolated, reverse transcribed, and the M- (a) and N-gene (b) of SARS-CoV-2 were quantified by RT-qPCR. Data are shown as mean and standard deviation (SD). Differences between fluoxetine/sertraline and the control were compared by Kruskal-Wallis test (*p < 0.05).
Figure 6.
Treatment with sertraline significantly reduces SARS-CoV-2 M- and N-gene levels. A549-AT cells were infected with 100 TCID50 of different SARS-CoV-2 variants in the presence of different sertraline concentrations (0 – 10 µM). After 72 h of incubation, the supernatants were harvested and the total RNA was isolated, reverse transcribed, and the M- (a) and N-gene (b) of SARS-CoV-2 were quantified by RT-qPCR. Data are shown as mean and standard deviation (SD). Differences between fluoxetine/sertraline and the control were compared by Kruskal-Wallis test (*p < 0.05).
Figure 7.
Fluoxetine and sertraline showed no toxic effects on A549-AT cells. Potential cytotoxic effects of fluoxetine or sertraline were tested towards A549-AT cells using “Orangu cell counting solution”. Different concentrations of (a) fluoxetine or (b) sertraline were incubated with a confluent layer of A549-AT cells and the cell viability was evaluated after 2, 24, 48, and 72 hours. All experiments were performed as triplicates. Data are shown as mean and standard deviation (SD).
Figure 7.
Fluoxetine and sertraline showed no toxic effects on A549-AT cells. Potential cytotoxic effects of fluoxetine or sertraline were tested towards A549-AT cells using “Orangu cell counting solution”. Different concentrations of (a) fluoxetine or (b) sertraline were incubated with a confluent layer of A549-AT cells and the cell viability was evaluated after 2, 24, 48, and 72 hours. All experiments were performed as triplicates. Data are shown as mean and standard deviation (SD).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).