1. Introduction
Probiotics including lactic acid bacteria are beneficial microorganisms that are present in the digestive tract, oral cavity, and reproductive systems of both humans and animals [
1]. The bacteria in the genus
Lactobacillus such as
Lactobacillus paracasei that belong to the normal mucosal microbiota of humans and animals is a well-documented probiotic and e.g. often used in dairy product fermentation [
2]. Recently, the name
L. paracasei was changed to
Lacticaseibacillus paracasei because the genome diverged from
Lactobacillus [
2]. It has been demonstrated that bacteria in the genus
Lacticaseibacillus are capable of creating bacteriocins, antimicrobial peptides utilized in the food and healthcare industries [
3,
4]. Unfortunately, factors in the gastrointestinal tract such as low pH, low enzyme levels, and low bile salts make it difficult for free probiotic cells to survive in the body after oral administration. Hence, the food and feed industry need to implement a strategy to increase the probiotics' survival rate.
Microencapsulation entails the process of encasing probiotics in microcapsules made of polymers and other organic and inorganic materials [
5]. Microcapsules may be beneficial as delivery system supporting minimal damage to the live probiotic bacteria by the various conditions in the gastrointestinal tract [
5]. Additionally, microencapsulation might make it easier to regulate release and successfully deliver probiotics to the action site [
6]. In addition, the encapsulation could maintain the viability of probiotics during the food manufacturing process and long-term storage [
6]. Several strategies to prepare microcapsules exist and we have earlier reported encapsulation of probiotics using sodium alginate in combination with calcium chloride [
6]. Sodium alginate is a natural polysaccharide which is biodegradable and commonly used in industry. Sodium alginate can be extracted from brown algae cell walls but the extraction process needs several chemical reagents such as alkaline medium, sodium carbonate, and sodium hydroxide [
7]. Therefore, alternative natural materials which can be applied for encapsulation of probiotics are of interest.
This study focused on
C. pareira leaf extract. The plant species belonging to the Menispermaceae family is common in many Southeast Asia countries including Thailand where it is locally known as Khruea-ma-noi. The plant has been extensively used in the traditional medicinal system for the treatment of numerous diseases such as ulcers and wounds [
8,
9]. In addition, the leaves of the plant have been used as raw materials for Thai food and importantly they contain hydrocolloids, which may act as gelling agents as they possess solidifying properties [
10]. In addition, pectin, a high molecular weight polysaccharide, was detected in
C. pareira leaves [
9]. Recently, a preparation of biodegradable films containing pectin extracted from
C. pareira leaves has been documented [
11].
Therefore, this study aimed to investigate the development of microencapsulation of L. paracasei using C. pareira extract as a natural encapsulating material. Furthermore, the characterization, the properties, and the storage of the microcapsules under adverse conditions were determined. In addition, the phytochemical present in the plant extract was investigated.
2. Materials and Methods
2.1. Ethical Approval
The study did not involve any live animals or humans, so ethical approval was not necessary. All the experiments were performed under the regulation of biosafety for scientific experiments (Ref. No. WU-IBC-66-042) of Walailak University, Nakhon Si Thammarat, Thailand.
2.2. Plant Collection and Chemical Reagents
C. pareira leaves were harvested in Nakhon Phanom province, Northeast Thailand in December 2023. The plant materials were kept at 4 °C until used. All the reagents containing tween 80, oil, porcine bile extract, pancreatin, lipase from porcine pancreas, pepsin from porcine gastric mucosa, and trypsin from bovine pancreas were purchased from Sigma-Aldrich, Co. Ltd. (St. Louis, MO, USA). Calcium chloride (CaCl2) and succinic acid (C4H6O4) were purchased from Merck (Darmstadt, Germany).
2.3. Bacterial Strain and Bacterial Culture Conditions
Lacticaseibacillus paracasei WU2502, a probiotic candidate, was used in this study. The bacterium was isolated from Palmyra palm sugar as described earlier by our research team [
2]. In brief, the bacterium was cultured in 10 mL of Mann Rogosa Sharpe (MRS) broth (HiMedia, India) and MRS agar (HiMedia, India), incubated at 37°C for 24 h [
12]. The bacterial culture was kept in MRS broth containing 20% glycerol at -80 °C until used.
2.4. Chemical Composition and Characterization of C. pareira
The Official Methods of Analysis (AOAC) techniques were used to assess the chemical composition of
C. pareira leaf extract [
13]. Briefly, the leaves were dried at 60°C in order to measure the levels of dry matter (DM), crude protein (CP), ether extract (EE), crude fiber (CF), ash, and nitrogen free extract. The moisture content was evaluated by drying the sample in a hot air oven at 100 °C until it achieved a consistent weight. Ash was determined after burning in a muffle furnace at 550 °C. Fat was extracted using petroleum ether and determined using a Soxtec System 1043 (Tecator, Hoganas, Sweden) after the petroleum ether was removed. Protein was calculated using the Kjeldahl technique with a multiplication factor of 6.25, and carbohydrate was calculated by subtracting the quantities of the above components from 100. The enzymatic gravimetric technique was used to determine soluble and insoluble dietary fibers. The analysis of metabolized energy was conducted using a bomb calorimeter (Mode AC-500 model manufactured by Leco in St. Joseph, MI, USA). Benzoic acid was used as the reference standard.
The chemical structure of the extracted C. pareira was determined by attenuated total reflection infrared (ATR-FTIR) spectroscopy using a Bruker Alpha ATR technique. Spectra were detected in the range of 4000–400 cm−1.
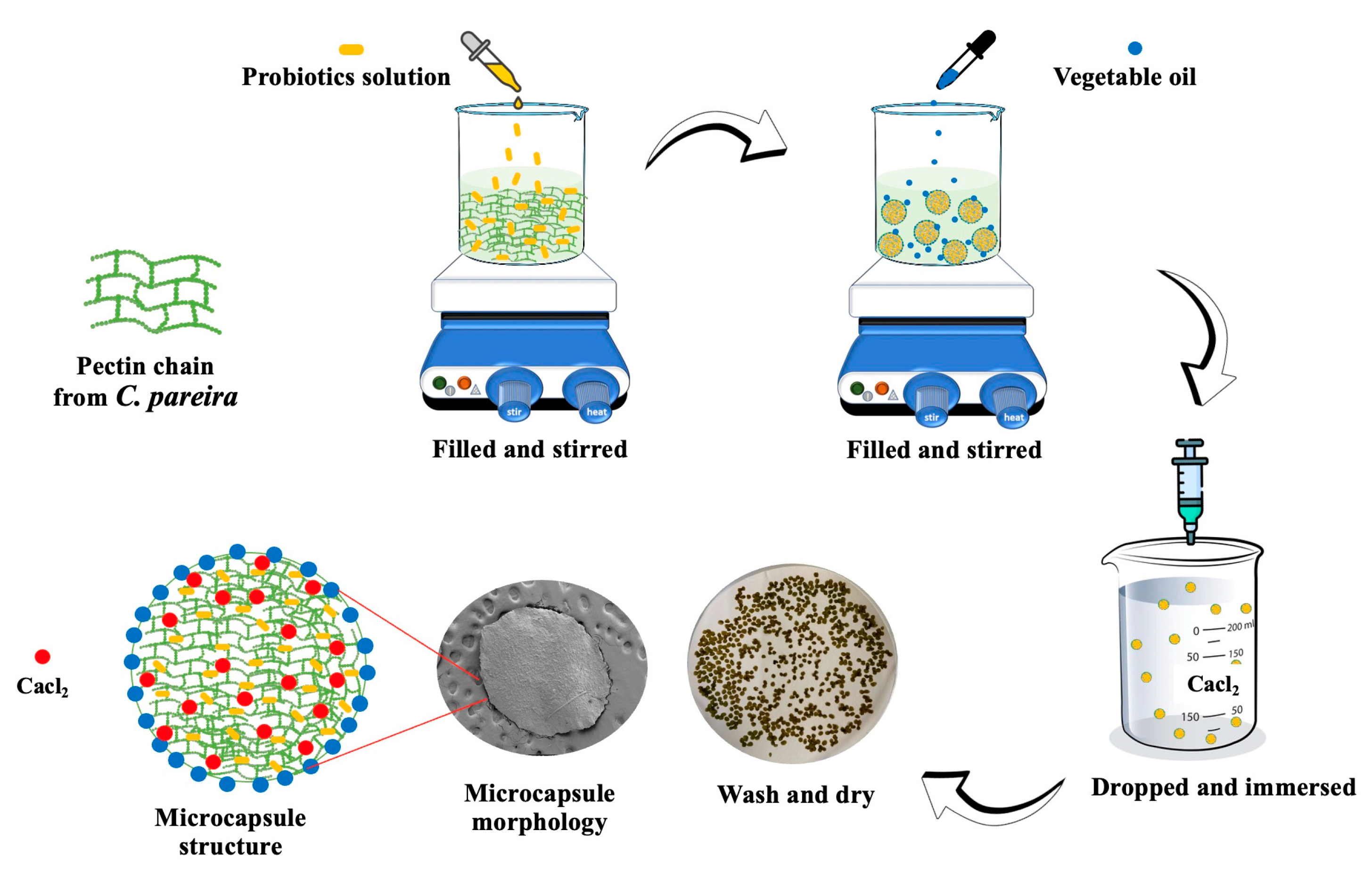
2.5. Preparation of L. paracasei Encapsulated C. Pareira Microcapsules (LP-CP)
Fifteen grams of fresh
C. pareira leaves were mixed in deionized water (DI) (100 mL), followed by adding tween 80 (2 mL) and stirring for 20 min at room temperature. The solution was maintained under constant stirring for 20 min followed by adding the overnight culture of
L. paracasei (1 × 10
10 CFU/mL, 10 mL) and soybean oil (10 mL). The solution was added dropwise through a needle (diameter 1.8 mm) into CaCl
2 (0.1 mol/L) aqueous solution to obtain the crude
L. paracasei encapsulated
C. pareira microcapsules. The distance between the syringe and CaCl
2 solution was around 4-5 cm. The microcapsules were sieved by a mesh (diameter 0.053 mm.) and washed with DI water twice before drying in a hot air oven at 37°C overnight to obtain LP-CP microcapsules. A schematic diagram of LP-CP preparation is shown in
Figure 1. Alginate microcapsules was used as the positive control, was prepared as previously described by our research team [
6].
2.6. Determination of Physico-Chemical Characteristics of LC-LP Microcapsules
2.6.1. Structural Analysis
The morphologies of LC-LP microcapsules as well as sodium alginate microcapsules were identified by scanning electron microscopy (SEM-Zeiss, Munich, Germany). The microcapsules of sodium alginate and LP-CP were cut across by a razor blade to observe the cross-section. The chemical structures of LP-CP were evaluated by a Bruker Alpha Fourier transform infrared spectrometer using ATR technique in the range of 4000 cm
−1 to 400 cm
−1 [
14].
L. paracasei viability was evaluated after heating LP-CP at 75 °C, 80 °C, 85 °C, 90 °C, 95 °C, and 100 °C for 3 min.
2.6.2. Encapsulation Efficiency
The enumeration of the viable cells was determined in triplicate using a drop plate technique in an MRS agar as previously described [
15]. Briefly, dilutions of the culture were prepared by adding the samples (1 mL) into a tube containing peptone water (9 mL, 0.1 g/100 mL). The mixture was vortexed for 10 min until homogenization. The sample (20 μL) was cultured on MRS agar and incubated at 37 °C for 48 h. The bacterial viability was investigated and represented as CFU/mL. The viability of
L. paracasei in LP-CP was carried out by homogenization LP-CP (1 g) in sodium citrate (10 mL) to dissolve the encapsulating material. Then, the samples were serial diluted and cultured on MRS agar as described above. The encapsulation efficiency (EY, %) was calculated as described by Vimon et al. [
16].
where N is the number of viable entrapped cells released from the microcapsules, and N
0 is the number of free cells added to the LP-CP.
2.6.3. Swelling Properties
The swelling of LP-CP was evaluated in the simulated poultry digestive tract following the procedure described by Azad et al. [
17], with a few modifications. Simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) were freshly prepared according to Chitprasert & Ngamekaue [
18]. SGF was made by dissolving sodium chloride (2 g) in deionized water (100 mL) and then adding 0.1 N HCl until the pH reached 1.2. The final volume was increased to 1 L. SIF was prepared by dissolving dipotassium phosphate (6.8 g) in sodium hydroxide (0.1 M, 190 mL). The pH of the solution was adjusted to 7.4 before increasing the final volume to 1 L.
For simulated gastric stage study, LP-CP (1.0 g ± 0.05 g) was added into SGF (100 mL). The pH of the solution was adjusted from 1.2 to 2.0 and LP-CP was incubated at 39.5 °C ± 0.5 °C in a Memmert WNB 14 thermostat water bath for 60 min, 120 min and 180 min, respectively, to represent the time of the microcapsules in the stomach in vivo. After incubation at each point, the LP-CP was filtered and weighed whereas the SGF solution was collected for use in the intestinal stage.
For simulated intestinal stage study, trypsin solution (2 mg/mL, 1 mL,), bile solution (40 mg/mL, 14 mL), pancreatic solution (3.2 mg/mL, 7.5 mL,), and SIF (7.5 mL) were mixed with the above SGF solution. The pH of SIF was adjusted from 7.4 to 5.5 whereas LP-CP was incubated at 39.5 °C ± 0.5 °C in each pH for 220 min and 240 min, respectively, to represent the time in the intestinal tract
in vivo. The treated LP-CP was collected and weighed at each incubation time. The results were calculated by equation (2) to indicate the percentages of swelling [
18].
where W
0 and W
s are the weights of the dry and the swollen microcapsules after 4 h, respectively.
2.6.4. Release Performances
The release performance of LP-CP was carried out as described by Azad et al. [
17]. After incubation at each point, the supernatant (1 mL) was collected, and the bacterium released was determined using the pour plate technique in MRS agar. The index of cell release was calculated by equation (3).
2.6.5. Impact of Storage Condition
The storage stability of
L. paracasei and LP-CP was studied in three replicates according to Mitsuwan et al. [
6]. Briefly, free
L. paracasei (10 mL) and LP-CP (10.0 g ± 0.5 g) were separately sealed in glass vials and wrapped with aluminum foil. The containers were stored at room temperature for 90 days. Samples were taken after 30 days, 60 days, and 90 days of storage to determine the viability in terms of tolerance characteristics.
2.6.6. Effect of Acids, Enzymes and Temperature on the Viability of Free Cells and LP-CP
Tolerance characteristics were measured thrice with 5 mL of free cells and 5.0 ± 0.5 g of LP-CP according to Mitsuwan et al. [
6]. Acid tolerance was investigated by soaking the sample in citrate phosphate buffer pH 2 (0.2 M, 20 mL). Bile salt tolerance was carried out by dissolving 3.0 g of porcine bile extract into DI (100 mL) with the sample. Trypsin tolerance was tested by preparing trypsin from bovine pancreas (1.0 g) in DI (100 mL) containing the sample. All tubes were incubated in a thermostat water bath vibrator at 39.5 °C ± 0.5 °C for 30 min. For thermal treatment, the sample was tested at 85 °C for 1 min (simulation of feed pelleting condition). The treated samples were immediately removed to measure the viability as in the previous procedures.
2.7. Statistical Analysis
All samples were statistically analyzed in triplicate by one-way analysis of variance (ANOVA) in a completely randomized design. The mean was further evaluated using Duncan’s new multiple-range post-hoc test. The statistical significance was considered at P < 0.05. Results were presented as mean ± standard deviation.
3. Results and Discussion
3.1. Property and Phytochemical Composition of C. pareira Leaf Extract
C. pareira leaf was mixed and crushed with deionized water to prepare the plant extract. It was noticed that the extraction procedures did not degrade the extract's gelling characteristics. The extract preserved its original pH of 3.4 and formed a gel that could be reversed by temperature adjustments. The extract was a deep shade of impenetrable green. The extraction procedure may remove a large proportion of insoluble fiber and protein while keeping fiber-free carbohydrates, which include phenolic compounds, organic acids, starch, sugars, and calcium. The proximate composition of the extract is shown in
Table 1. The presence of divalent cations may significantly affect the interactions between soluble fiber molecules. It is well-known that soluble fiber, divalent cations, and fiber-free carbohydrates are the most significant components of freshly extracted (manually prepared)
C. pareira for gel production [
19]. The presence of substances that release green color, such as phenolic compounds, significantly influences the process [
20].
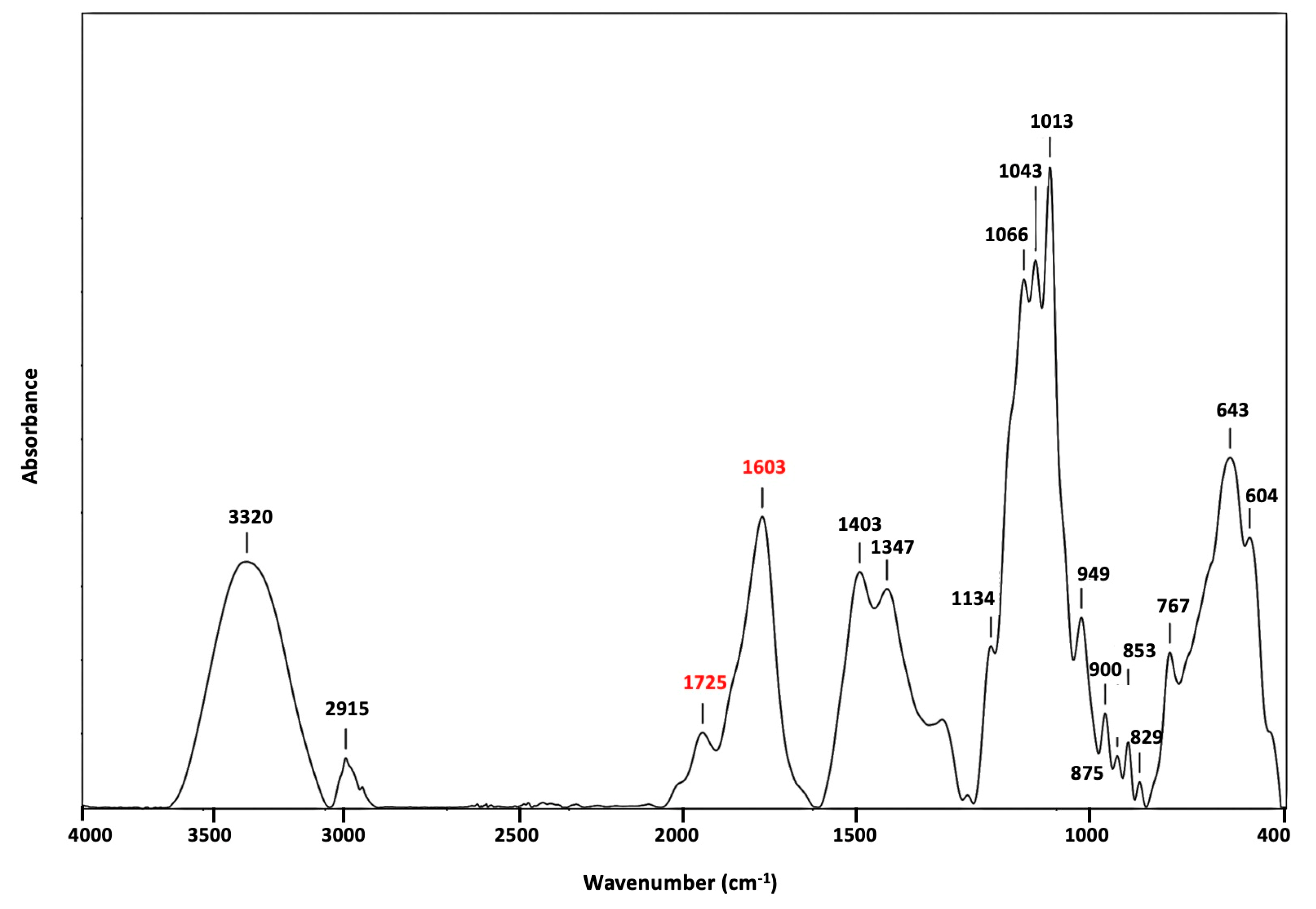
The chemical structure of
C. pareira was verified using FTIR spectroscopy data (
Figure 2). The absorb peak at 3320 cm
−1 was due to O-H stretching vibrations. The peak at 2915 cm
−1 was attributed to C-H absorption bands of CH
2, CH
3, and O-CH
3 stretching vibrations. Peak at 1403 cm
−1 was due to asymmetric C-H vibrations of CH
3. The characteristic peaks at 1603 cm
−1 (COO− asymmetric stretching) and 1347 cm
−1 (COO− symmetric stretching) could correspond to carboxylic acid vibrations, while esterified C=O vibrations were found at 1725 cm
−1 (symmetric stretching) [
20]. In general, the absorb peak at 1603 cm
−1 and 1725 cm
−1 could be classified as pectin. Pectin that exhibits low levels of esterification was earlier reported to have a higher peak intensity of around 1603 cm
−1 [
21]. In contrast, Pectin with greater esterification has a higher peak intensity at 1725 cm
−1 (ester carbonyl band). The isolated
C. pareira pectin exhibited a high peak intensity at 1635 cm
−1, implying low levels of esterification. The "finger print" area for pectin, located at 829-1134 cm
−1, includes C=O stretching and C-H bending modes [
21]. This is qualitatively consistent with prior research, which revealed the characteristics of pectin with FTIR [
11].
3.2. Characterization and Performance of Microcapsules
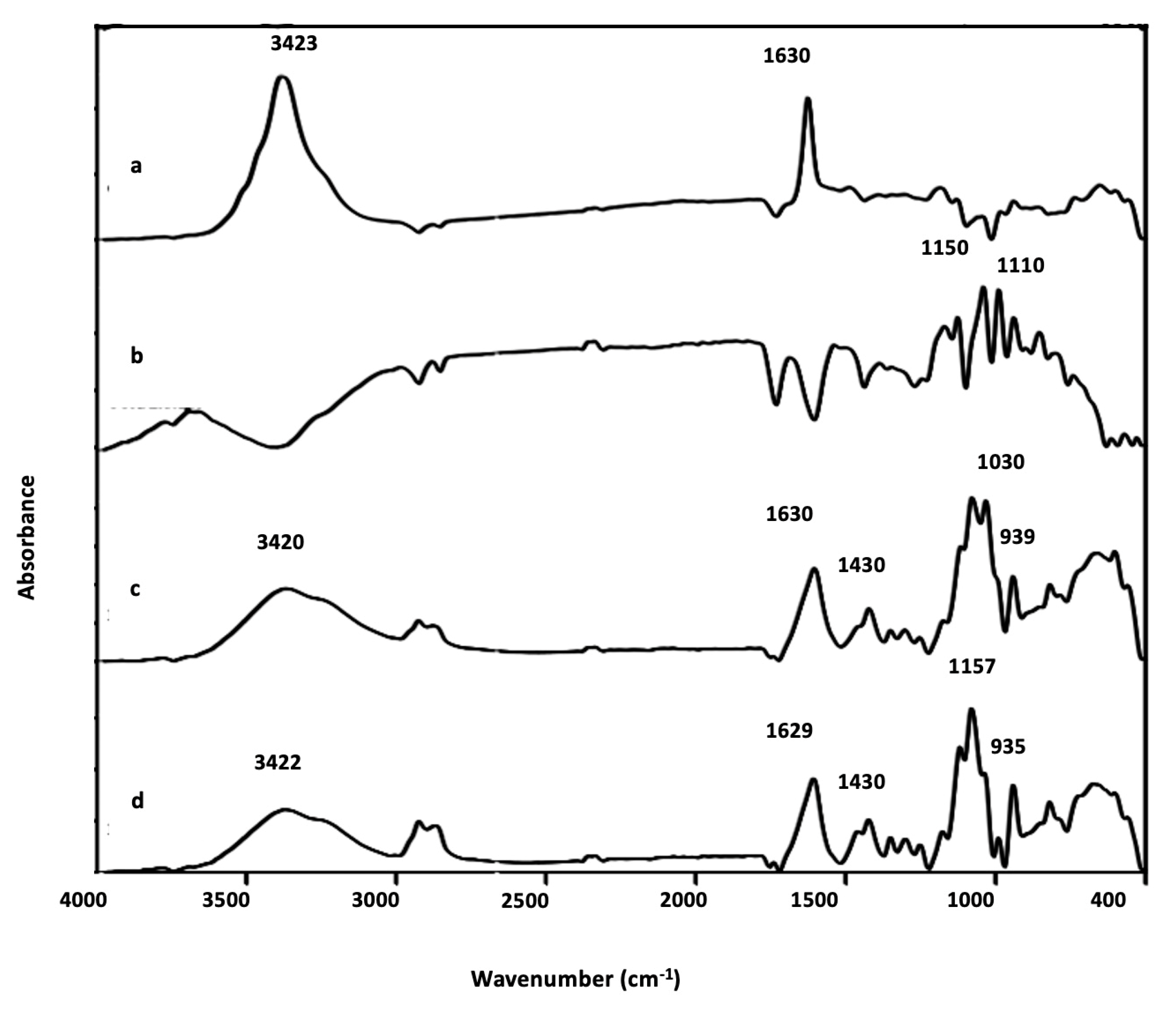
3.2.1. Structural Analysis
The structural characterization of the microcapsule was carried out by FTIR technique to analyze the functional groups and chemical interactions (
Figure 3). The spectrum of
C. pareira revealed the typical pyranose ring at 1630 cm
-1 apart from hydroxyl group (OH stretching at 3423 cm
-1) (
Figure 3a) [
21,
22]. In case of
L. paracasei which are proteins, the amino groups (NH
2) and acidic carboxyl groups (COOH) can be confirmed at 1150 cm
-1 (amide I), and 1010 cm
-1 (amines) respectively (
Figure 3b). For
C. pareira microcapsule, in the absence of Ca
2+, the O-C-O stretching vibrations of these carboxylates give two characteristic infrared absorption bands near 1400 cm
−1 and 1597 cm
−1. When Ca
2+ ions were present, the bands were shifted to 1430 cm
−1 and 1630 cm
−1, respectively (
Figure 3c). Changes in the position of the carboxylate bands typically indicate that significant interaction has occurred between the carboxylates and the metal ion [
16]. In the case of LP-CP (
Figure 3d), the characteristic peaks of carboxyl, hydroxyl, amino groups and calcium chloride were shifted implying the successful preparation.
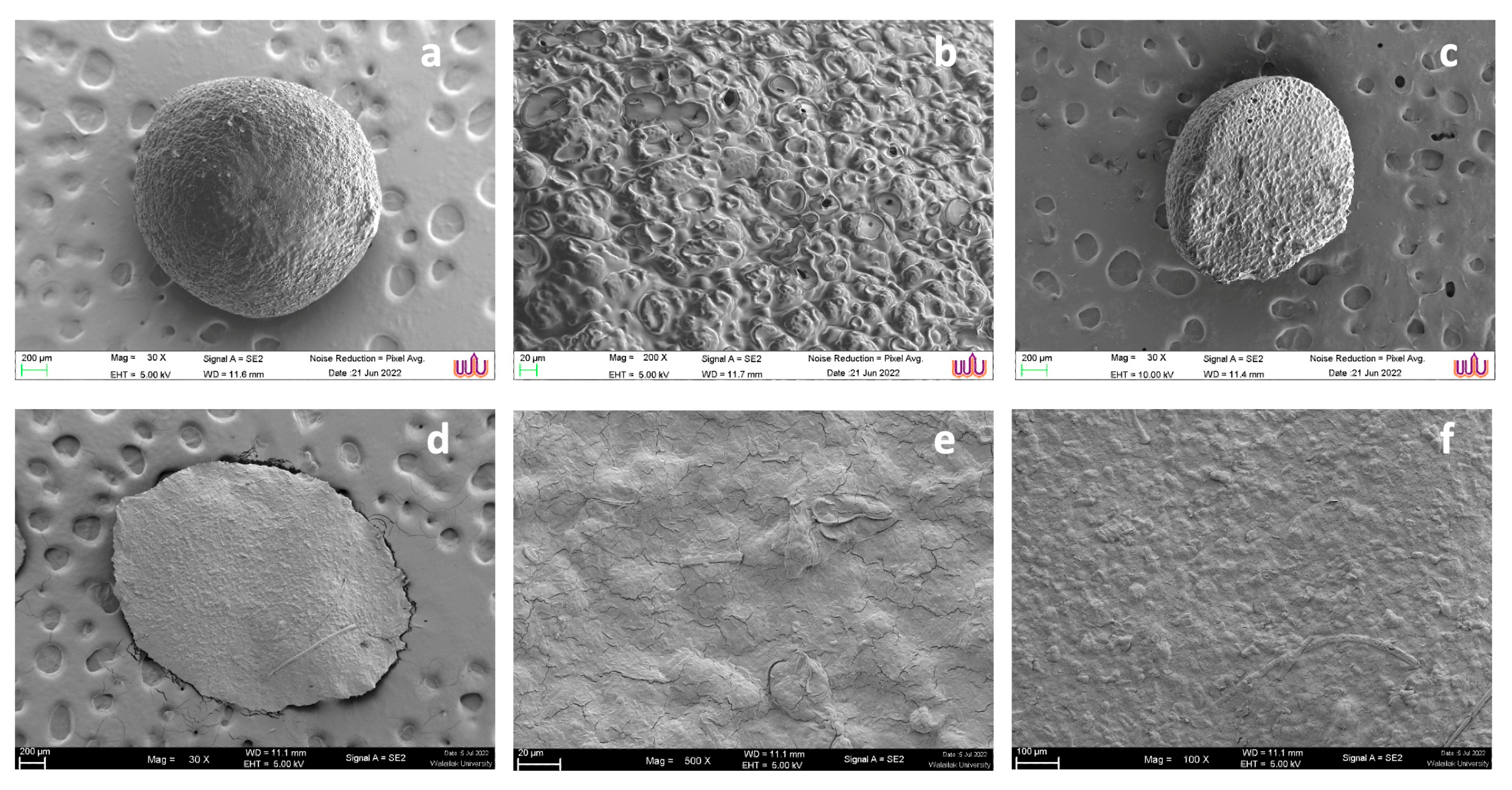
3.2.2. Morphology Analysis
The morphologies of sodium alginate and LP-CP were observed by SEM. On the sodium alginate surface, there are notable porous networks, as seen in
Figure 4 (a)–(b). The diameter of the pores ranged from 45 to 65 µm (
Figure 4c). CaCl
2 generates interconnected structures by ionic gelation with sodium alginate. In the case of LP-CP microcapsules, the surface of microcapsules became smooth (
Figure 4d,e). However, the drying process caused the shrinkage of the capsules and resulted in flat shapes, as shown in
Figure 3d. The flat and irregular surface was due to water loss during the drying process [
22]. On the surface, the porous structure of LP-CP was identifiable. (
Figure 4f), demonstrating that the porous networks of
C. pareira were preserved. The surface reveals a dense network, particularly on the surface, that might be related to the
C. pareira-CaCl
2 chains formed through crosslinking. The average diameter of LP-CP was 300 μm which is within the range of the feed ingredients or feedstuffs (approximately, 400 μm - 2,000 μm) as reported by Abdollahi et al. [
23]. The LP-CP exhibited high encapsulation efficiency at 90.5 ± 0.1%, indicating successful encapsulation.
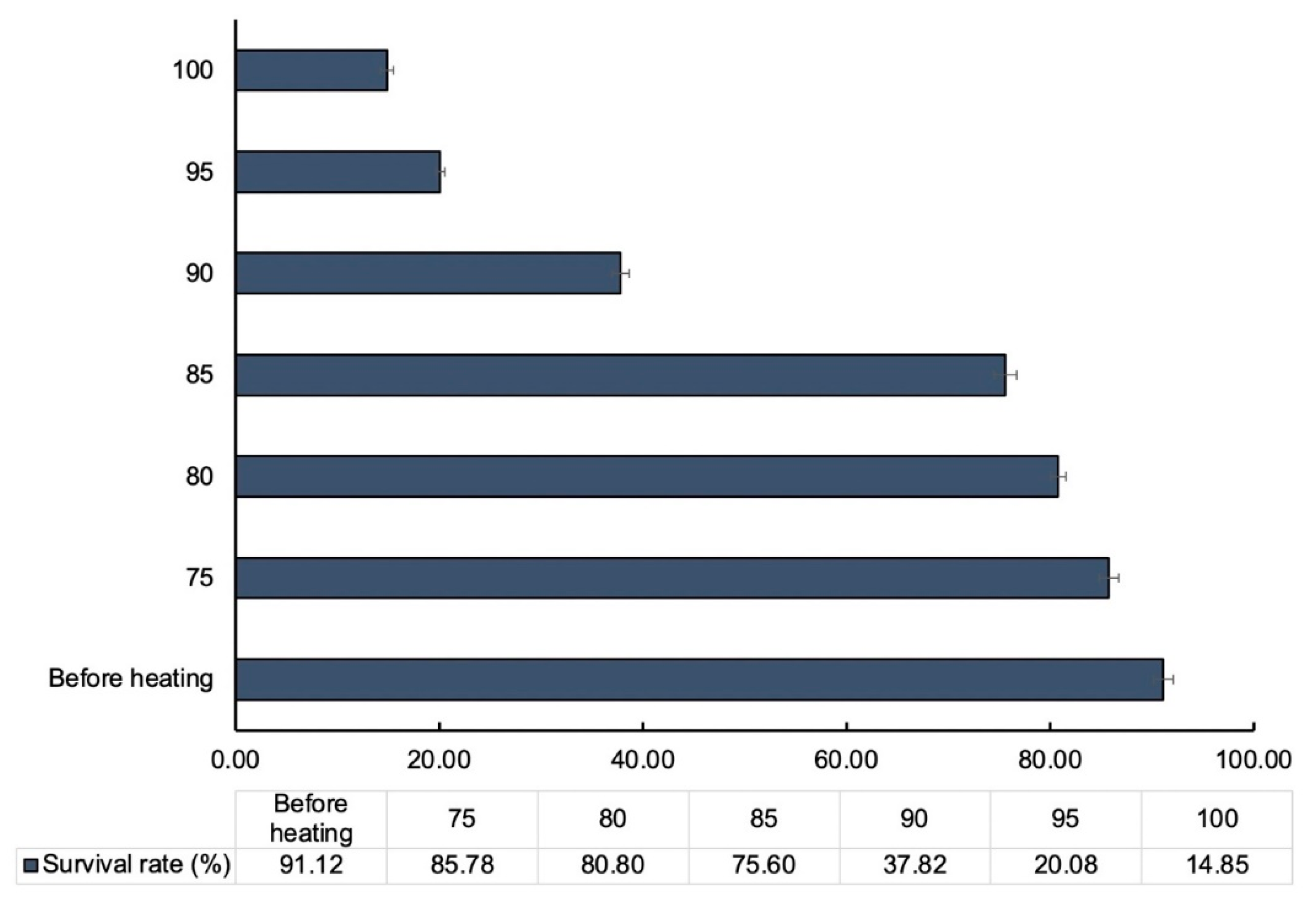
3.2.3. Viability of LP-CP Under Pelleting Temperature
In order to produce pelleted animal feed, the pelleting process requires temperatures between 75 °C and 85 °C for 2-3 minutes [
23]. The question is whether
L. paracasei encapsulation by
C. pareira maintains cell viability at these temperatures. According to Su et al. [
24], the viability of free cells (
L. plantarum) after heating at 85 °C was as low as 70-80 % from the initial. Similarly, Santos et al. [
25] showed that after treating at 80 °C, the vitality of free
Lactobacillus spp. was between 20% and 30%. The survivability of LP-CP microcapsules after 3 minutes of heating at various temperatures is summarized in
Figure 5. In the beginning of the experiment, 91.12% of
L. paracasei was determined to be viable. After three minutes of isothermal treatment at 75 °C and 80 °C, the viability of
L. paracasei remained constant. (P<0.05). When subjecting the microcapsules to heat treatment at 85 °C, the viability of
L. paracasei slightly declined (75.60%). It demonstrates that the LP-CP microencapsulation technology may be compatible with feed pelleting. The results were corresponding with data from the components of microcapsules. (
Figure 3c).
3.3. In Vitro Studies of Microcapsules
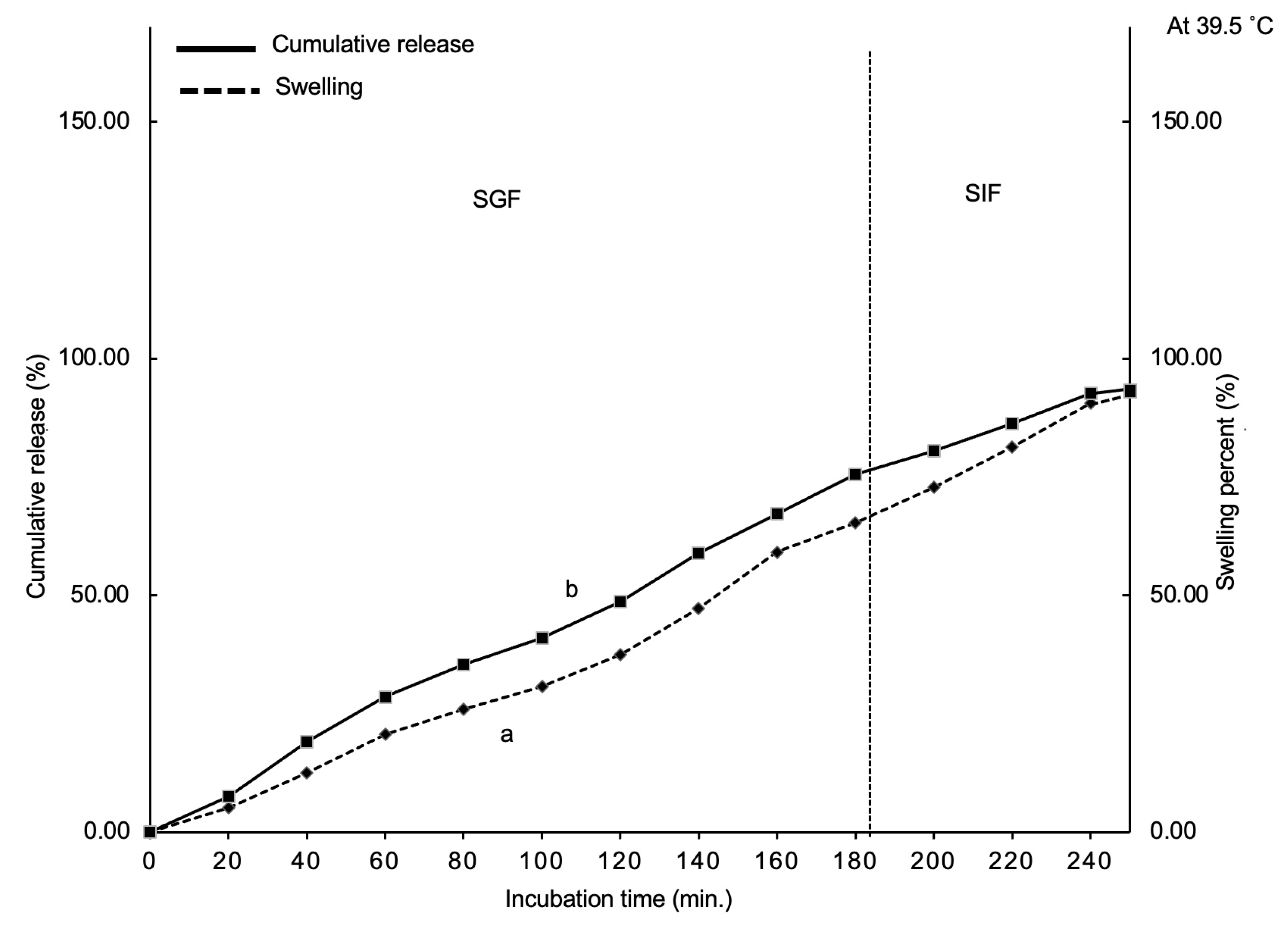
3.3.1. Swelling and Cumulative Release Studies
To assess the release of
L. paracasei in the animal digestive tract based on SIF and SGF, swelling and cumulative release tests were conducted. The swelling at different incubation periods is shown in
Figure 6a. For the intervals of 60, 120, and 180 minutes in SGF, the swelling values were 17.63% ± 1.0%, 35.23 ± 1.5%, and 58.04% ± 2.0%, respectively. The total weight of LP-CP microcapsules rapidly increased to double times of the original weight. After 180 minutes of SIF treatment, LP-CP microcapsules swelled by 89% ± 2.5%.
The swelling indicates how
C. pareira responds to digestive fluid. During SGF treatment, gastric acidic pH (pH 2-3) is expected to induce CaCl
2 protonation, allowing water penetration and swelling. Continuous swelling is initiated by deprotonation of
C. pareira due to treatment in SIF, which is moderately neutral (pH 6.5) but higher than the pKa of
C. pareira (3.6). Anionic hydrogels have a special characteristic whereby an increase in pH causes an increase in swelling [
17].
There is a definite interaction between LP-CP swelling (
Figure 6a) and
L. paracasei cumulative release (
Figure 6b). At the initial stage of the incubation (60 minutes), the release rate was approximately 27%. The first burst of release may be related to
L. paracasei present on the
C. pareira microcapsule surface. The cumulative release of
L. paracasei in SGF was approximately 66% at 180 minutes. The release in SGF is almost 38% if the pretreatment to exclude burst release (28%) was taken into consideration. The cumulative release in SIF reaches 90% at 240 minutes. The controlled release of core matter is known to depend on the type of encapsulation material and its characteristics, such as cross-linking amount, pH, chemical interaction, and incubation period. [
26]. In this study, controlled release by
C. pareira might function synergistically in the following approaches. The degradation of the CaCl
2 cross-linker was initially interrupted because of an ion exchange between calcium and chloride ions in SGF. The structural destruction of microcapsules facilitated SGF penetration of
C. pareira micropores. Although variations in pH and endogenous enzymes in the intestinal tract, the carboxylate groups of
C. pareira chains and their networks in SGF retained
L. paracasei. In the last stage, the
C. pareira crosslink network began to break down as
C. pareira deprotonated in the SIF, leading to diffusion in
L. paracasei. Corstens et al. [
27] reported that carboxyl groups swelled during the ion exchange between calcium and phosphate ions in SIF. In this work, calcium ion crosslink network was disrupted, allowing
L. paracasei to be slowly released. According to the above-mentioned about the release mechanism, structure and ionic crosslinks of
C. pareira with CaCl
2 resulting in the successful release of
L. paracasei. This is supposed to occur in the lower region of the gut.
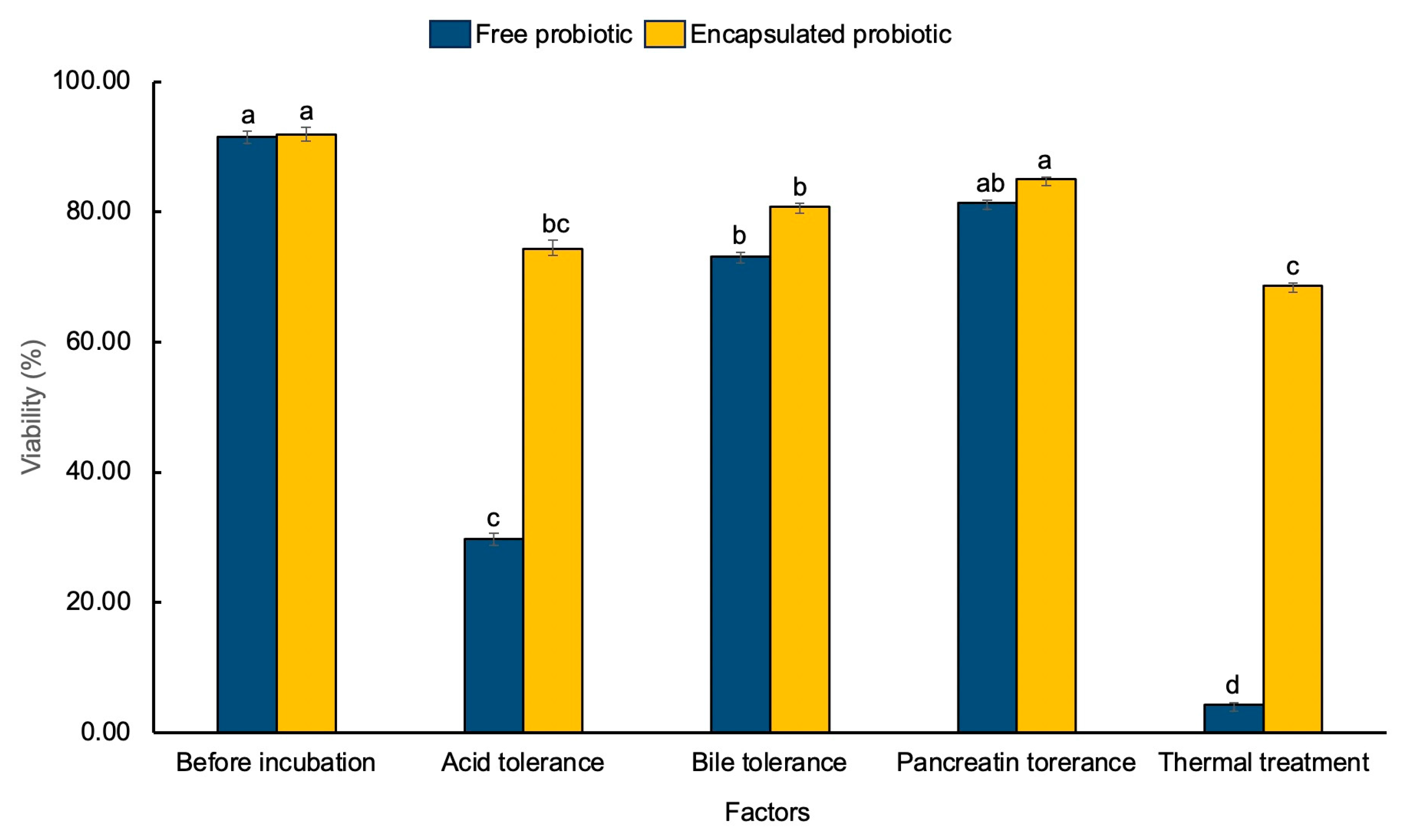
3.3.2. Effect of Acids, Enzymes and Temperature on the Viability of Free Cells and LP-CP
When probiotics are encapsulated, it is important to make sure that the barrier of protection is effective in a substance that simulates gastrointestinal fluid, including bile, acid, and enzyme [
28]. Based on the number of bacteria in the sample, the functions of
C. pareira in retaining the survival of
L. paracasei were examined. At the beginning of the experiment, viability amounts for
L. paracasei and LP-CP were 91.48 ± 0.1 and 91.83% ± 0.2%, respectively (
Figure 7). Apparently, acid and heat are the worst conditions. Microencapsulated
L. paracasei still able to maintain the viability as high as 69%. In contrast, free cell viability decreased significantly to 18%-95% (p < 0.05). According to Mitsuwan et al [
6] reported that under acid conditions, microcapsules of probiotics showed a significantly enhanced survival rate of probiotic bacteria by approximately 80% when compared with the non-encapsulated cells. Likewise, another previous research demonstrated that a decrease in survival of free cells was found to be nearly twice when compared with encapsulated cells (at pH 2) [
28]. Our results reflect that the structure of
C. pareira, which consists of pectin, may provide probiotics with protection from the harmful effects of severely unsuitable conditions.
3.4. Impact of Storage Condition
Probiotics need to be kept for a certain amount of time before usage in typical applications. During that period, the probiotics may lose viability due to storage conditions such as temperature, humidity, oxygen content, and light exposure [
29]. These main issues harm bacteria cell walls, reducing their effectiveness and leading to shorter shelf life. Therefore, the viability of
L. paracasei during storage was investigated in this study.
Table 2 demonstrated that
L. paracasei survival gradually decreased over the first 30 days and significantly declined by almost 100% after 90 days (P < 0.05) at both 4°C and 32°C. Regarding LP-CP, almost no decrease in viability was observed in any storage durations at 4 °C or 32 °C (P < 0.05) at 30 days. The viability declined (40%–50%) when the storage time was extended to 60 and 90 days. This supports our previous study [
16] where also sodium alginate encapsulation was reported to extend probiotic viability under storage conditions when compared with the free cells. This agrees with Silva et al. [
29], revealing that encapsulation
L. acidophilus was viable for a storage period of 45 days at 5 °C and had a count greater than 8 log CFU/g. The loss in viability of free cells and microcapsules at high temperatures is probably caused by the oxidation of lipids membrane and the denaturation of proteins, which leads to the destruction of macromolecules in bacterial cells [
30]. The reason might be that probiotic microorganisms contained in protective carriers preserve cellular structures, reducing external stress by preventing molecular mobility.
It is established that microcapsules can be used as the ingredients to produce human food and animal food. Our results could suggest that the microcapsules prepared by C. pareira extract may be applied to animal feed or human food production. The plant extract acted as the barrier to protect from various conditions simulating the GI tract as well as the high temperature during the process of feed production. Although, the microcapsules exhibited a flat rather than a sphere shape, but they could maintain L. paracasei cells.
4. Conclusions
The present study revealed the microencapsulation of L. paracasei using C. pareira extract as the natural encapsulating material forming gelatin like structure with CaCl2. The microcapsules showed a desirable result of encapsulation efficiency (90.5%) which was confirmed by the analyses of their chemical structures. Under extreme acid and thermal conditions, bacterial viability in the microcapsules was significantly increased when compared to non-encapsulated bacteria. During storage conditions of 90 days, LP-CP viability remained at 50%, whereas the survival rate of free cells significantly decreased by 100%. This information suggests that C. pareira is a potent polymer to be used as an eco-friendly material for the microencapsulation of probiotics.
Author Contributions
Conceptualization, S.V. and W.M.; methodology, S.V., W.M., C.R., R.C., S.B., F.M., S.S., P.S.; formal analysis, S.V. and W.M.; investigation, S.V. and W.M.; writing—original draft preparation, S.V. and W.M.; writing—review and editing, S.V., W.M., T.S.D., C.N., V.N.; visualization, S.V.; supervision, W.M.; project administration, W.M.; funding acquisition, S.V. All authors have read and agreed to the published version of the manuscript.”
Funding
This research was financially supported by Walailak University (Grant no. WU-66291), Center of Excellence in Innovation of Essential Oil and Bioactive Compounds (Grant no. WU-COE-65-05).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors acknowledge Akkhraratchakumari Veterinary College, One Health Research Center, and The Research Institute for Health Sciences, Walailak University for their support in conducting the entire research work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ale, E.C.; Binetti, A.G. Role of probiotics, prebiotics, and synbiotics in the elderly: Insights into their applications. Frontiers in Microbiology. 2021, 12–19. [Google Scholar] [CrossRef]
- Mitsuwan, W.; Sornsenee, P.; Romyasamit, C. Lacticaseibacillus spp.; Probiotic candidates from Palmyra palm sugar possesses antimicrobial and anti-biofilm activities against methicillin-resistant Staphylococcus aureus. Veterinary World. 2022, 15, 299–308. [Google Scholar] [CrossRef]
- Ren, S.; Yuan, X.; Liu, F.; Fang, F.; Iqbal, HM.; Zahran, SA.; Bilal, M. Bacteriocin from Lacticaseibacillus rhamnosus sp. A5: isolation, purification, characterization, and antibacterial evaluation for sustainable food processing. Sustainability. 2022, 14, 9571. [Google Scholar] [CrossRef]
- Belguesmia, Y.; Hazime, N.; Kempf, I.; Boukherroub, R.; Drider, D. New bacteriocins from Lacticaseibacillus paracasei CNCM I-5369 adsorbed on alginate nanoparticles are very active against Escherichia coli. International Journal of Molecular Sciences. 2020, 21, 8654. [Google Scholar] [CrossRef]
- Sun, W.; Nguyen, QD.; Süli, BK.; Alarawi, F.; Szécsi, A.; Gupta, V.K.; Friedrich, L.F.; Gere, A.; Bujna, E. Microencapsulation and Application of Probiotic Bacteria Lactiplantibacillus plantarum 299v Strain. Microorganisms. 2023, 11, 947. [Google Scholar] [CrossRef]
- Mitsuwan, W.; Saengsawang, P.; Jeenkeawpieam, J.; Nissapatorn, V.; de Lourdes Pereira, M.; Kitpipit, W.; Thomrongsuwannakij, T.; Poothong, S.; Vimon, S. Development of a microencapsulated probiotic containing Pediococcus acidilactici WU222001 against avian pathogenic Escherichia coli. Veterinary World, 2023, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Frent, O.D.; Vicas, L.G.; Duteanu, N.; Morgovan, C.M.; Jurca, T.; Pallag, A.; Muresan, ME.; Filip, S.M.; Lucaciu, R-L.; Marian, E. Sodium alginate—Natural microencapsulation material of polymeric microparticles. International Journal of Molecular Sciences. 2022, 23, 12108. [Google Scholar] [CrossRef] [PubMed]
- Samanta, J.; Bhattacharya, S.; Rayat, R. Phytochemical investigation and pharmacognostic standardization of Cissampelos pareira root. Ancient Science of Life. 2012, 31, 181. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Bhatt, V.; Suresh, P.S.; Sharma, U. Cissampelos pareira L.: a review of its traditional uses, phytochemistry, and pharmacology. Journal of Ethnopharmacology. 2021, 274, 113850. [Google Scholar] [CrossRef] [PubMed]
- Vardhanabhuti, B.; Ikeda, S. Isolation and characterization of hydrocolloids from monoi (Cissampelos pareira) leaves. Food hydrocolloids. 2006, 20, 885–891. [Google Scholar] [CrossRef]
- Linn, K.S.; Kasemsiri, P.; Jetsrisuparb, K.; Iamamornphan, W.; Chindaprasirt, P.; Knijnenburg, J.T. Development of biodegradable films with antioxidant activity using pectin extracted from Cissampelos pareira leaves. Journal of Polymers and the Environment. 2022, 1–12. [Google Scholar] [CrossRef]
- Kabploy, K.; Saengsawang, P.; Romyasamit, C.; Sangkanu, S.; Kitpipit, W.; Thomrongsuwannakij, T.; Wongtawan, T.; Daus, M.; Pereira, M.L.; Mitsuwan, W. Sangyod rice bran extract enhances Lacticaseibacillus paracasei growth during the exponential phase and antibacterial activity of L. paracasei supernatant against zoonotic and foodborne pathogens. Veterinary World. 2022, 15, 2466–2474. [Google Scholar] [CrossRef]
- AOAC International. Official methods of analysis of association of official analytical chemists International, 17th ed; AOAC Inter: Rockville, MD, USA, 2016. [Google Scholar]
- Lei, S.; Juan, F. G. M.; Qing-An, Z. Encapsulation of Benzaldehyde Produced by the Eco-Friendly Degradation of Amygdalin in the Apricot Kernel Debitterizing Wastewater. Foods. 2024, 13, 437. [Google Scholar]
- Gbassi, G. K.; Vandamme, T.; Yolou, F. S.; Marchioni, E. In vitro effects of pH, bile salts and enzymes on the release and viability of encapsulated Lactobacillus plantarum strains in a gastrointestinal tract model. International Dairy Journal. 2011, 21, 97–102. [Google Scholar] [CrossRef]
- Sasi, V.; Thanit, K.; Suwabun, C.; Kris, A.; Chackrit, N. Matrices-charges of agar-alginate crosslinked microcapsules via o/w microemulsion: A non-spore forming probiotic bacteria encapsulation system for extensive viability. Carbohydrate Polymers. 2023, 321, 121302. [Google Scholar]
- Azad, A.K.; Al-Mahmood, S.M.A.; Chatterjee, B.; Wan, S. W.M.A.; Elsayed, T.M.; Doolaanea, A.A. Encapsulation of black seed oil in alginate beads as a pH-sensitive carrier for intestine-targeted drug delivery: in vitro, in vivo and ex vivo study. Pharmaceutics. 2020, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Chitprasert, P.; Ngamekaue, N. Stability enhancement of Ocimum sanctum Linn. essential oils using stearic acid in aluminum carboxymethyl cellulose film-coated gelatin microcapsules. Journal of Food Science. 2017, 82, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent trends in the use of pectin from agro-waste residues as a natural- based biopolymer for food packaging applications. Materials. 2020, 13, 673. [Google Scholar] [CrossRef]
- Singthong, J.; Cui, S.W.; Ningsanond, S.; Goff, H.D. Structural characterization, degree of esterification and some gel- ling properties of Krueo Ma Noy (Cissampelos pareira) pectin. Carbohydr Polym. 2004, 58, 391–400. [Google Scholar] [CrossRef]
- Singthong, J.; Ningsanond, S.; Cui, S.W.; Goff, H.D. Extraction and physicochemical characterization of Krueo Ma Noy pectin. Food Hydrocoll. 2005, 19, 793–801. [Google Scholar] [CrossRef]
- Mendes, d. S.; Cunha, W.E.M.; Rodrigues, F.F.G.; Silveira, E.R.; Lima, R.D.P.; Costa, J.G.M. Cissampelos genus: biological activities, ethnobotanical and phytochemical aspects. Phytochem Rev. 2020, 19, 955–982. [Google Scholar] [CrossRef]
- Abdollahi, M.R.; Ravindrana, V.; Svihus, B. Influence of grain type and feed form on performance, apparent metabolisable energy and ileal digestibility of nitrogen, starch, fat, calcium and phosphorus in broiler starters. Animal Feed Science and Technology. 2013, 186, 193–203. [Google Scholar] [CrossRef]
- Su, J.; Wang, X.; Li, W.; Chen, L.; Zeng, X.; Huang, Q. Enhancing the viability of Lactobacillus plantarum as probiotics through encapsulation with high in- ternal phase emulsions stabilized with whey protein isolate microgels. Journal of Agricultural and Food Chemistry 2018, 66, 12335–12343. [Google Scholar] [CrossRef]
- Santos, B.C.S.; Pires, A.S.; Yamamoto, C.H.; Couri, M.R.C.; Taranto, A.G.; Alves, M.S.; Araujo, A.; Sousa, O.V. Methyl Chavicol and its synthetic analogue as possible antioxidant and antilipase agents based on the in vitro and in silico assays. Oxid. Med. Cell. Longev 2018, 1–12. [Google Scholar]
- Bhopatkar, D.; Anal, A.K.; Stevens, W.F. Ionotropic alginate beads for controlled intestinal protein delivery: Effect of chitosan and barium counter-ions on entrapment and release. J. Microencapsul 2005, 22, 91–100. [Google Scholar] [CrossRef]
- Corstens, M.N.; Berton-Carabin, C.C.; Elichiry-Ortiz, P.T.; Hol, K.; Troost, F.J.; Masclee, A.A.; Schroën, K. Emulsion-alginate beads designed to control in vitro intestinal lipolysis: Towards appetite control. J. Funct. Foods 2017, 34, 319–328. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Liu, B.; Meng, X. Microencapsulation of Lactobacillus bulgaricus and survival assays under simulated gastrointestinal conditions. Journal of Functional Foods 2017, 29, 248–255. [Google Scholar] [CrossRef]
- Maritiele Naissinger da Silva a, *, Bruna Lago Tagliapietra b, Neila Silvia Pereira dos Santos Richards. Encapsulation, storage viability, and consumer acceptance of probiotic butter. LWT - Food Science and Technology 2021, 139, 110536. [Google Scholar] [CrossRef]
- Conrad, P. B., Miller, D. P., Cienlenski, P. R., & Pablo, J. J. Stabilizations and preservation of Lactobacillus acidophilus in saccharide matrices. Cryobiology 2000, 41, 17–24. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).