Submitted:
04 March 2024
Posted:
05 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
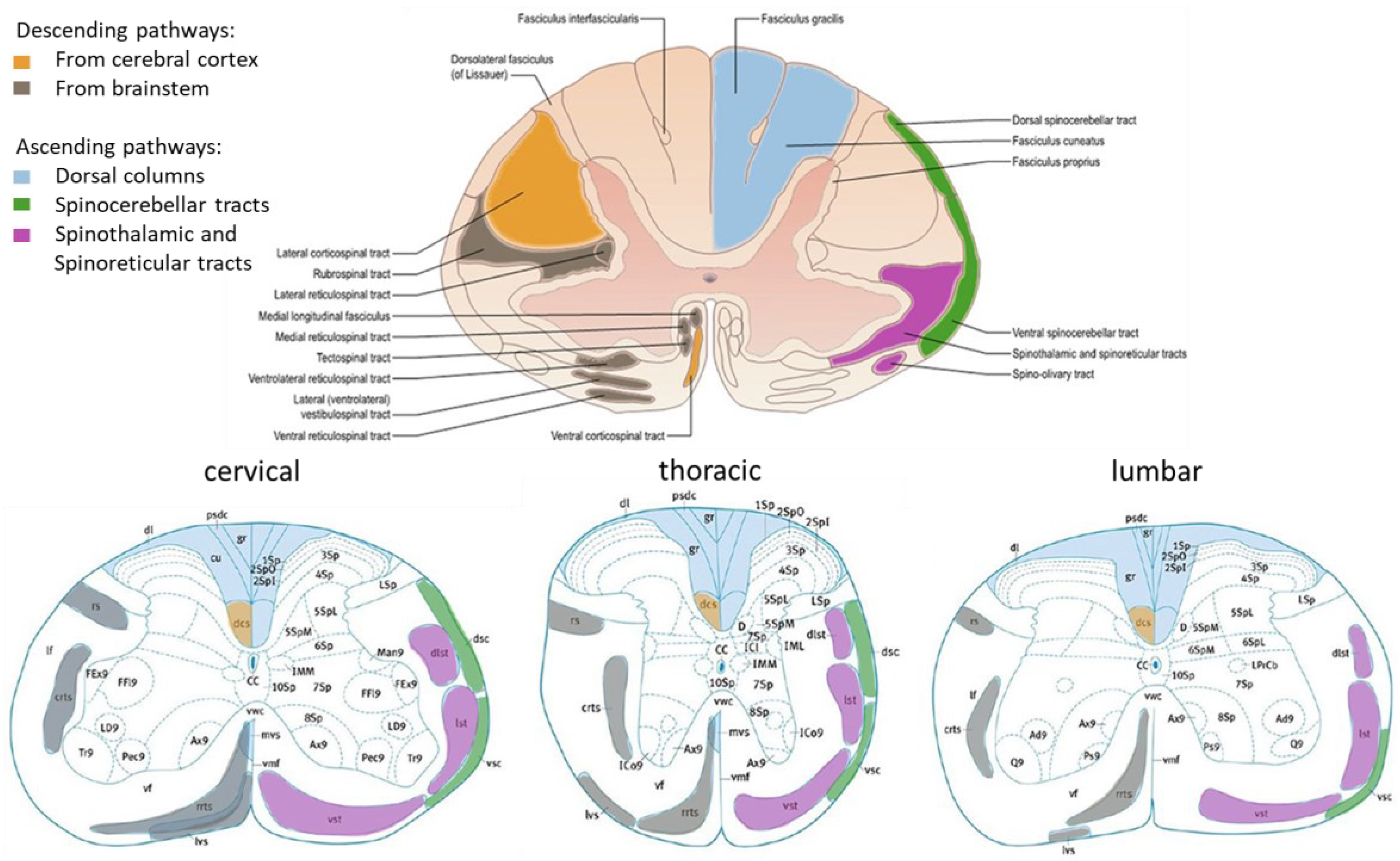
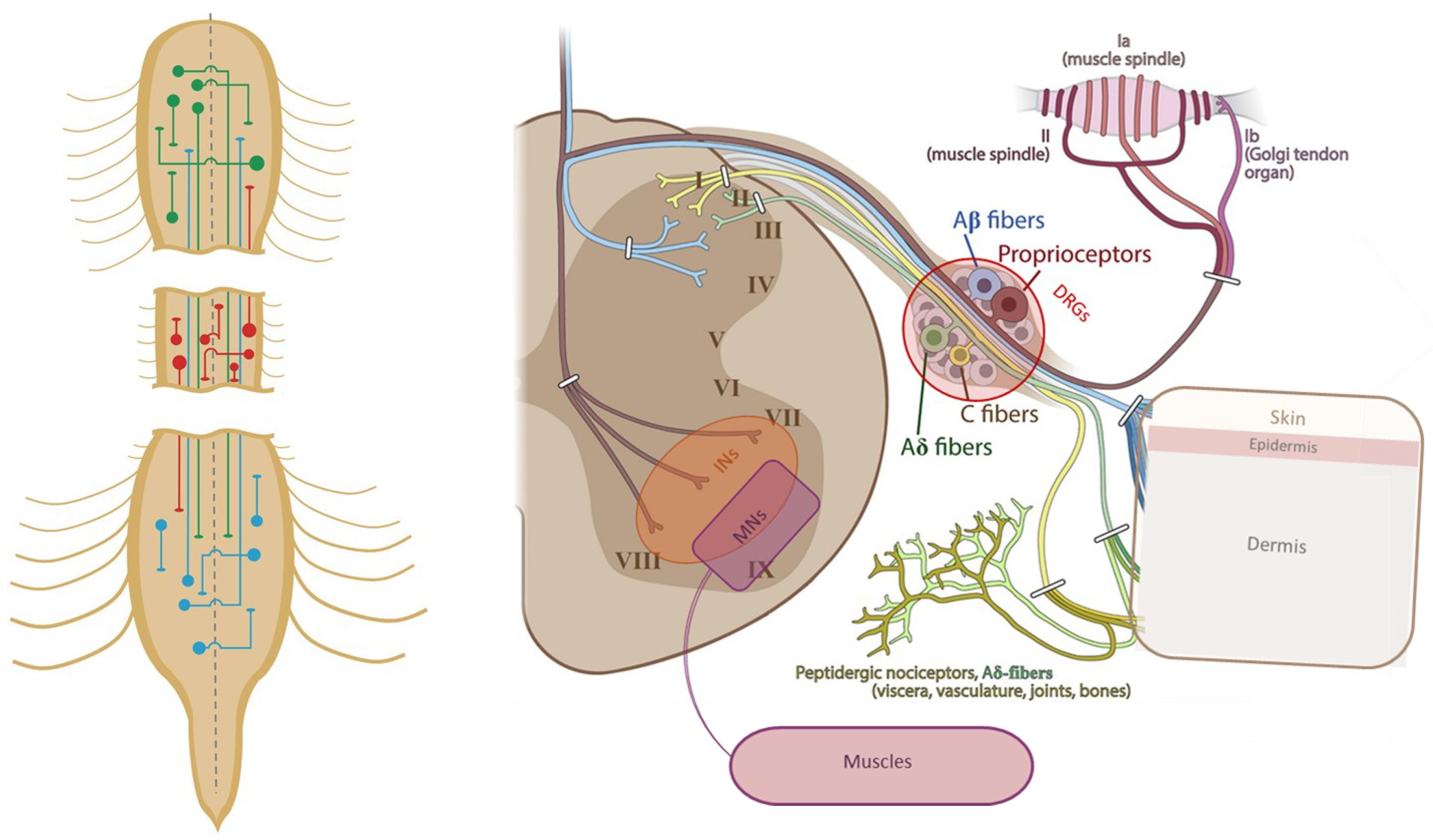
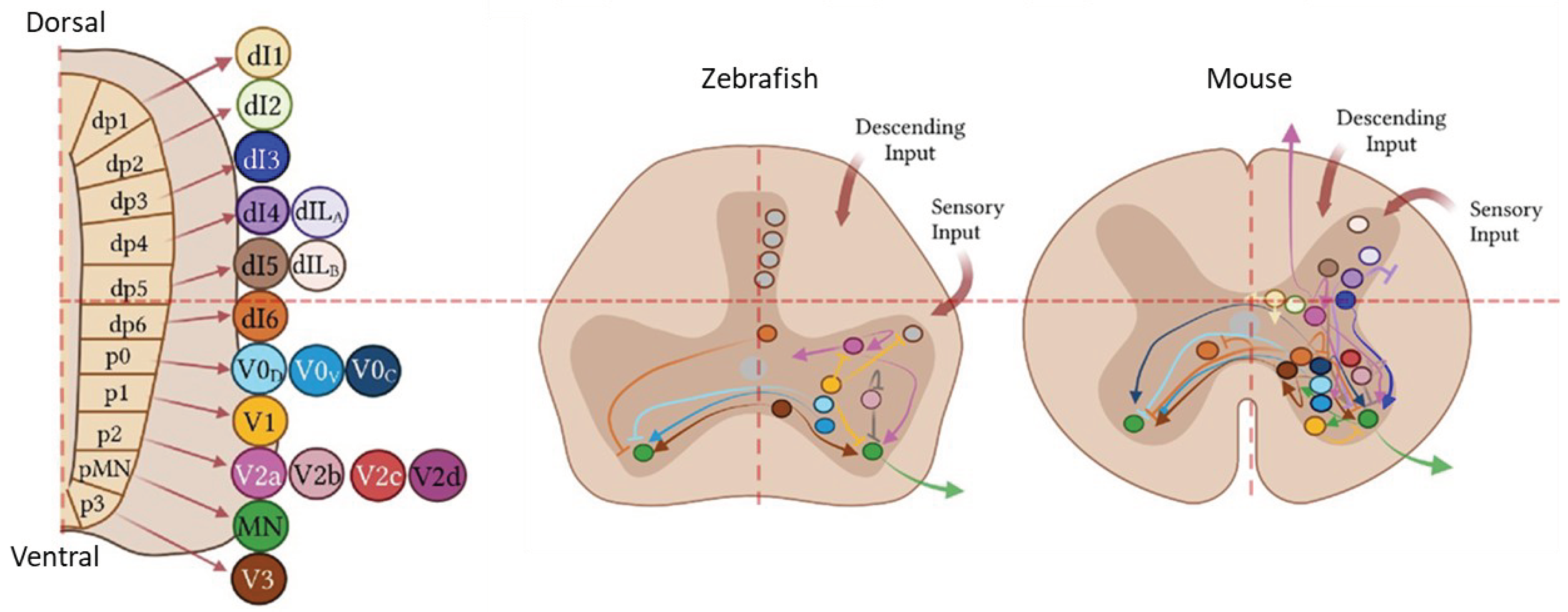
2. Current Challenges to Characterize Spinal Premotor INTERNEURON populations in the Adult Ventral Spinal Cord
3. Limitations in Defining the Potential Roles of INs for Spinal Tissue Recovery after a TSCI
4. Ventral INs Contribution to Spinal Tissue Restoration in Different TSCI Models
4.1. Cervical TSCI
4.2. Thoracic TSCI
4.3. Lumbar TSCI
5. Conclusions, Limitations, and Perspectives for the Study of the Role of INs in Intraspinal Tissue Remodelling after a TSCI
Acknowledgments
Conflicts of Interest
References
- National Spinal Cord Injury Statistical Center, Facts and figures at a glance. Birmingham, AL: University of Alabama at Birmingham, 2023. https://www.nscisc.uab.edu/public/Facts%20and%20Figures%202023%20-%20Final.pdf.
- Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017 Apr 27;3:17018. PMID: 28447605. [CrossRef]
- Kirshblum S, Snider B, Eren F, Guest J. Characterizing Natural Recovery after Traumatic Spinal Cord Injury. J Neurotrauma. 2021 May 1;38(9):1267-1284. Epub 2021 Jan 22. PMID: 33339474; PMCID: PMC8080912. [CrossRef]
- Guérout N. Plasticity of the Injured Spinal Cord. Cells. 2021 Jul 26;10(8):1886. PMID: 34440655; PMCID: PMC8395000. [CrossRef]
- Huang CX, Wang Z, Cheng J, Zhu Z, Guan NN, Song J. De novo establishment of circuit modules restores locomotion after spinal cord injury in adult zebrafish. Cell Rep. 2022 Oct 25;41(4):111535. PMID: 36288693. [CrossRef]
- Filli L, Engmann AK, Zörner B, et al. Bridging the gap: a reticulo-propriospinal detour bypassing an incomplete spinal cord injury. J Neurosci. 2014;34(40):13399-13410. [CrossRef]
- Asboth L, Friedli L, Beauparlant J, Martinez-Gonzalez C, Anil S, Rey E, Baud L, Pidpruzhnykova G, Anderson MA, Shkorbatova P, Batti L, Pagès S, Kreider J, Schneider BL, Barraud Q, Courtine G. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat Neurosci. 2018 Apr;21(4):576-588. Epub 2018 Mar 19. PMID: 29556028. [CrossRef]
- Zörner B, Bachmann LC, Filli L, Kapitza S, Gullo M, Bolliger M, Starkey ML, Röthlisberger M, Gonzenbach RR, Schwab ME. Chasing central nervous system plasticity: the brainstem’s contribution to locomotor recovery in rats with spinal cord injury. Brain. 2014 Jun;137(Pt 6):1716-32. Epub 2014 Apr 15. PMID: 24736305. [CrossRef]
- Roussel M, Lafrance-Zoubga D, Josset N, Lemieux M, Bretzner F. Functional contribution of mesencephalic locomotor region nuclei to locomotor recovery after spinal cord injury. Cell Rep Med. 2023 Feb 21;4(2):100946.PMID: 36812893; PMCID: PMC9975330. [CrossRef]
- Kiehn O. Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci. 2016 Apr;17(4):224-38. Epub 2016 Mar 3. PMID: 26935168; PMCID: PMC4844028. [CrossRef]
- Leiras R, Cregg JM, Kiehn O. Brainstem Circuits for Locomotion. Annu Rev Neurosci. 2022 Jul 8;45:63-85. Epub 2022 Jan 5. PMID: 34985919. [CrossRef]
- Zholudeva LV, Abraira VE, Satkunendrarajah K, McDevitt TC, Goulding MD, Magnuson DSK, Lane MA. Spinal Interneurons as Gatekeepers to Neuroplasticity after Injury or Disease. J Neurosci. 2021 Feb 3;41(5):845-854. Epub 2021 Jan 20. PMID: 33472820; PMCID: PMC7880285. [CrossRef]
- Francius C, Harris A, Rucchin V, Hendricks TJ, Stam FJ, Barber M, Kurek D, Grosveld FG, Pierani A, Goulding M, Clotman F. Identification of multiple subsets of ventral interneurons and differential distribution along the rostrocaudal axis of the developing spinal cord. PLoS One. 2013 Aug 15;8(8):e70325. PMID: 23967072; PMCID: PMC3744532. [CrossRef]
- Hayashi M, Hinckley CA, Driscoll SP, Moore NJ, Levine AJ, Hilde KL, Sharma K, Pfaff SL. Graded Arrays of Spinal and Supraspinal V2a Interneuron Subtypes Underlie Forelimb and Hindlimb Motor Control. Neuron. 2018 Feb 21;97(4):869-884.e5. Epub 2018 Feb 1. PMID: 29398364; PMCID: PMC8601153. [CrossRef]
- Matson KJE, Russ DE, Kathe C, Hua I, Maric D, Ding Y, Krynitsky J, Pursley R, Sathyamurthy A, Squair JW, Levi BP, Courtine G, Levine AJ. Single cell atlas of spinal cord injury in mice reveals a pro-regenerative signature in spinocerebellar neurons. Nat Commun. 2022 Sep 26;13(1):5628. PMID: 36163250; PMCID: PMC9513082. [CrossRef]
- Squair JW, Milano M, de Coucy A, Gautier M, Skinnider MA, James ND, Cho N, Lasne A, Kathe C, Hutson TH, Ceto S, Baud L, Galan K, Aureli V, Laskaratos A, Barraud Q, Deming TJ, Kohman RE, Schneider BL, He Z, Bloch J, Sofroniew MV, Courtine G, Anderson MA. Recovery of walking after paralysis by regenerating characterized neurons to their natural target region. Science. 2023 Sep 22;381(6664):1338-1345. Epub 2023 Sep 21. PMID: 37733871. [CrossRef]
- Takeoka A, Arber S. Functional Local Proprioceptive Feedback Circuits Initiate and Maintain Locomotor Recovery after Spinal Cord Injury. Cell Rep. 2019 Apr 2;27(1):71-85.e3. PMID: 30943416. [CrossRef]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol. 1986 May;92(2):421-35. PMID: 3956672. [CrossRef]
- Sherrington CS, Laslett EE. Observations on some spinal reflexes and the interconnection of spinal segments. J Physiol. 1903 Feb 23;29(1):58-96. PMID: 16992657; PMCID: PMC1540608. [CrossRef]
- Côté MP, Murray LM, Knikou M. Spinal Control of Locomotion: Individual Neurons, Their Circuits and Functions. Front Physiol. 2018 Jun 25;9:784. PMID: 29988534; PMCID: PMC6026662. [CrossRef]
- Ronzano R, Skarlatou S, Barriga BK, Bannatyne BA, Bhumbra GS, Foster JD, Moore JD, Lancelin C, Pocratsky AM, Özyurt MG, Smith CC, Todd AJ, Maxwell DJ, Murray AJ, Pfaff SL, Brownstone RM, Zampieri N, Beato M. Spinal premotor interneurons controlling antagonistic muscles are spatially intermingled. Elife. 2022 Dec 13;11:e81976. PMID: 36512397; PMCID: PMC9844990. [CrossRef]
- Dougherty KJ. Distinguishing subtypes of spinal locomotor neurons to inform circuit function and dysfunction. Curr Opin Neurobiol. 2023 Oct;82:102763. Epub 2023 Aug 21. PMID: 37611531; PMCID: PMC10578609. [CrossRef]
- El Manira A. Modular circuit organization for speed control of locomotor movements. Curr Opin Neurobiol. 2023 Oct;82:102760. Epub 2023 Aug 17. PMID: 37597455. [CrossRef] [PubMed]
- Gosgnach S. Spinal inhibitory interneurons: regulators of coordination during locomotor activity. Front Neural Circuits. 2023 Apr 20;17:1167836. PMID: 37151357; PMCID: PMC10159059. [CrossRef]
- Sengupta M, Bagnall MW. Spinal Interneurons: Diversity and Connectivity in Motor Control. Annu Rev Neurosci. 2023 Jul 10;46:79-99. Epub 2023 Feb 28. PMID: 36854318. [CrossRef]
- Flynn JR, Graham BA, Galea MP, Callister RJ. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology. 2011 Apr;60(5):809-22. Epub 2011 Jan 18. PMID: 21251920. [CrossRef]
- Laliberte AM, Goltash S, Lalonde NR, et al. Propriospinal neurons: Essential elements of locomotor control in the intact and possibly the injured spinal cord. Front Cell Neurosci. 2019;13:512. [CrossRef]
- Deska-Gauthier D, Zhang Y. The functional diversity of spinal interneurons and locomotor control. Current Opinion in Physiology. 8; 2019, 99-108. [CrossRef]
- Deska-Gauthier D, Borowska-Fielding J, Jones C, Zhang H, MacKay CS, Michail R, Bennett LA, Bikoff JB, Zhang Y. Embryonic temporal-spatial delineation of excitatory spinal V3 interneuron diversity. Cell Rep. 2024 Jan 23;43(1):113635. Epub 2023 Dec 29. PMID: 38160393; PMCID: PMC10877927. [CrossRef]
- Osseward PJ 2nd, Amin ND, Moore JD, Temple BA, Barriga BK, Bachmann LC, Beltran F Jr, Gullo M, Clark RC, Driscoll SP, Pfaff SL, Hayashi M. Conserved genetic signatures parcellate cardinal spinal neuron classes into local and projection subsets. Science. 2021 Apr 23;372(6540):385-393. PMID: 33888637; PMCID: PMC8612134. [CrossRef]
- Roome RB, Levine AJ. The organization of spinal neurons: Insights from single cell sequencing. Curr Opin Neurobiol. 2023 Oct;82:102762. Epub 2023 Aug 30. PMID: 37657185; PMCID: PMC10727478. [CrossRef]
- Lu DC, Niu T, Alaynick WA. Molecular and cellular development of spinal cord locomotor circuitry. Front Mol Neurosci. 2015 Jun 16;8:25. PMID: 26136656; PMCID: PMC4468382. [CrossRef]
- Bikoff JB, Gabitto MI, Rivard AF, Drobac E, Machado TA, Miri A, Brenner-Morton S, Famojure E, Diaz C, Alvarez FJ, Mentis GZ, Jessell TM. Spinal Inhibitory Interneuron Diversity Delineates Variant Motor Microcircuits. Cell. 2016 Mar 24;165(1):207-219. Epub 2016 Mar 3. PMID: 26949184; PMCID: PMC4808435. [CrossRef]
- Delile J, Rayon T, Melchionda M, Edwards A, Briscoe J, Sagner A. Single cell transcriptomics reveals spatial and temporal dynamics of gene expression in the developing mouse spinal cord. Development. 2019 Mar 27;146(12):dev173807. PMID: 30846445; PMCID: PMC6602353. [CrossRef]
- Hayashi M, Hinckley CA, Driscoll SP, Moore NJ, Levine AJ, Hilde KL, Sharma K, Pfaff SL. Graded Arrays of Spinal and Supraspinal V2a Interneuron Subtypes Underlie Forelimb and Hindlimb Motor Control. Neuron. 2018 Feb 21;97(4):869-884.e5. Epub 2018 Feb 1. PMID: 29398364; PMCID: PMC8601153. [CrossRef]
- Rosenberg AB, Roco CM, Muscat RA, Kuchina A, Sample P, Yao Z, Graybuck LT, Peeler DJ, Mukherjee S, Chen W, Pun SH, Sellers DL, Tasic B, Seelig G. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science. 2018 Apr 13;360(6385):176-182. Epub 2018 Mar 15. PMID: 29545511; PMCID: PMC7643870. [CrossRef]
- Sathyamurthy A, Johnson KR, Matson KJE, Dobrott CI, Li L, Ryba AR, Bergman TB, Kelly MC, Kelley MW, Levine AJ. Massively Parallel Single Nucleus Transcriptional Profiling Defines Spinal Cord Neurons and Their Activity during Behavior. Cell Rep. 2018 Feb 20;22(8):2216-2225. PMID: 29466745; PMCID: PMC5849084. [CrossRef]
- Nielson JL, Guandique CF, Liu AW, Burke DA, Lash AT, Moseanko R, Hawbecker S, Strand SC, Zdunowski S, Irvine KA, Brock JH, Nout-Lomas YS, Gensel JC, Anderson KD, Segal MR, Rosenzweig ES, Magnuson DS, Whittemore SR, McTigue DM, Popovich PG, Rabchevsky AG, Scheff SW, Steward O, Courtine G, Edgerton VR, Tuszynski MH, Beattie MS, Bresnahan JC, Ferguson AR. Development of a database for translational spinal cord injury research. J Neurotrauma. 2014 Nov 1;31(21):1789-99. Epub 2014 Jul 31. PMID: 25077610; PMCID: PMC4186058. [CrossRef]
- Russ DE, Cross RBP, Li L, Koch SC, Matson KJE, Yadav A, Alkaslasi MR, Lee DI, Le Pichon CE, Menon V, Levine AJ. A harmonized atlas of mouse spinal cord cell types and their spatial organization. Nat Commun. 2021 Sep 29;12(1):5722. Erratum in: Nat Commun. 2022 Feb 18;13(1):1033. Erratum in: Nat Commun. 2022 Oct 19;13(1):6184. PMID: 34588430; PMCID: PMC8481483. [CrossRef]
- Alstermark B, Lundberg A, Pettersson LG, et al. Motor recovery after serial spinal cord lesions of defined descending pathways in cats. Neurosci Res. 1987;5(1):68-73. [CrossRef]
- Bareyre FM, Kerschensteiner M, Raineteau O, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7(3):269-277. [CrossRef]
- Courtine G, Song B, Roy RR, et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14(1):69-74. [CrossRef]
- May Z, Fenrich KK, Dahlby J, et al. Following spinal cord injury transected reticulospinal tract axons develop new collateral inputs to spinal interneurons in parallel with locomotor recovery. Neural Plast. 2017;2017:1932875. [CrossRef]
- Meehan CF, Ford TW, Kirkwood PA. Plasticity of thoracic interneurones rostral to a lateral spinal cord lesion. Exp Neurol. 2020 Sep;331:113361. Epub 2020 May 26. PMID: 32464119. [CrossRef]
- Domínguez-Bajo A, González-Mayorga A, Guerrero CR, Palomares FJ, García R, López-Dolado E, Serrano MC. Myelinated axons and functional blood vessels populate mechanically compliant rGO foams in chronic cervical hemisected rats. Biomaterials. 2019 Feb;192:461-474. Epub 2018 Nov 19. PMID: 30502723. [CrossRef]
- Li Y, Tran A, Graham L, Brock J, Tuszynski MH, Lu P. BDNF guides neural stem cell-derived axons to ventral interneurons and motor neurons after spinal cord injury. Exp Neurol. 2023 Jan;359:114259. Epub 2022 Oct 26. PMID: 36309123 Wang et al., 2023. [CrossRef]
- Wang Z, Duan H, Hao F, Hao P, Zhao W, Gao Y, Gu Y, Song J, Li X, Yang Z. Circuit reconstruction of newborn neurons after spinal cord injury in adult rats via an NT3-chitosan scaffold. Prog Neurobiol. 2023 Jan;220:102375. Epub 2022 Nov 21. PMID: 36410665. [CrossRef]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195-218. PMID: 18558853. [CrossRef]
- Wilson AC, Sweeney LB. Spinal cords: Symphonies of interneurons across species. Front Neural Circuits. 2023 Apr 26;17:1146449. PMID: 37180760; PMCID: PMC10169611. [CrossRef]
- Fouad K, Popovich PG, Kopp MA, Schwab JM. The neuroanatomical-functional paradox in spinal cord injury. Nat Rev Neurol. 2021 Jan;17(1):53-62. Epub 2020 Dec 11. Erratum in: Nat Rev Neurol. 2023 Oct;19(10):635. PMID: 33311711; PMCID: PMC9012488. [CrossRef]
- Cheng J, Guan NN. A fresh look at propriospinal interneurons plasticity and intraspinal circuits remodeling after spinal cord injury. IBRO Neurosci Rep. 2023 Apr 3;14:441-446. PMID: 37388491; PMCID: PMC10300475. [CrossRef]
- Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006 Aug;7(8):644-53. PMID: 16858392. [CrossRef]
- Kathe C, Skinnider MA, Hutson TH, Regazzi N, Gautier M, Demesmaeker R, Komi S, Ceto S, James ND, Cho N, Baud L, Galan K, Matson KJE, Rowald A, Kim K, Wang R, Minassian K, Prior JO, Asboth L, Barraud Q, Lacour SP, Levine AJ, Wagner F, Bloch J, Squair JW, Courtine G. The neurons that restore walking after paralysis. Nature. 2022 Nov;611(7936):540-547. Epub 2022 Nov 9. PMID: 36352232; PMCID: PMC9668750. [CrossRef]
- Van Steenbergen V, Burattini L, Trumpp M, Fourneau J, Aljović A, Chahin M, Oh H, D’Ambra M, FM. Coordinated neurostimulation promotes circuit rewiring and unlocks recovery after spinal cord injury. J Exp Med. 2023 Mar 6;220(3):e20220615. Epub 2022 Dec 26. PMID: 36571760; PMCID: PMC9794600. [CrossRef]
- Aljović A, Jacobi A, Marcantoni M, Kagerer F, Loy K, Kendirli A, Bräutigam J, Fabbio L, Van Steenbergen V, Pleśniar K, Kerschensteiner M, Bareyre FM. Synaptogenic gene therapy with FGF22 improves circuit plasticity and functional recovery following spinal cord injury. EMBO Mol Med. 2023 Feb 8;15(2):e16111. Epub 2023 Jan 5. PMID: 36601738; PMCID: PMC9906383. [CrossRef]
- Satkunendrarajah K, Karadimas SK, Laliberte AM, Montandon G, Fehlings MG. Cervical excitatory neurons sustain breathing after spinal cord injury. Nature. 2018 Oct;562(7727):419-422. Epub 2018 Oct 10. PMID: 30305735. [CrossRef]
- Anderson MA, Squair JW, Gautier M, Hutson TH, Kathe C, Barraud Q, Bloch J, Courtine G. Natural and targeted circuit reorganization after spinal cord injury. Nat Neurosci. 2022 Dec;25(12):1584-1596. Epub 2022 Nov 17. PMID: 36396975. [CrossRef]
- Barra B, Conti S, Perich MG, Zhuang K, Schiavone G, Fallegger F, Galan K, James ND, Barraud Q, Delacombaz M, Kaeser M, Rouiller EM, Milekovic T, Lacour S, Bloch J, Courtine G, Capogrosso M. Epidural electrical stimulation of the cervical dorsal roots restores voluntary upper limb control in paralyzed monkeys. Nat Neurosci. 2022 Jul;25(7):924-934. Epub 2022 Jun 30. PMID: 35773543. [CrossRef]
- Vandeweerd JM, Hontoir F, De Knoop A, De Swert K, Nicaise C. Retrograde Neuroanatomical Tracing of Phrenic Motor Neurons in Mice. J Vis Exp. 2018 Feb 22;(132):56758. PMID: 29553523; PMCID: PMC5931327. [CrossRef]
- Zholudeva LV, Karliner JS, Dougherty KJ, Lane MA. Anatomical Recruitment of Spinal V2a Interneurons into Phrenic Motor Circuitry after High Cervical Spinal Cord Injury. J Neurotrauma. 2017 Nov 1;34(21):3058-3065. Epub 2017 Jun 29. PMID: 28548606; PMCID: PMC5661864. [CrossRef]
- Zholudeva LV, Iyer N, Qiang L, Spruance VM, Randelman ML, White NW, Bezdudnaya T, Fischer I, Sakiyama-Elbert SE, Lane MA. Transplantation of Neural Progenitors and V2a Interneurons after Spinal Cord Injury. J Neurotrauma. 2018 Dec 15;35(24):2883-2903. Epub 2018 Aug 10. PMID: 29873284; PMCID: PMC6306689. [CrossRef]
- Ford TW, Anissimova NP, Meehan CF, Kirkwood PA. Functional plasticity in the respiratory drive to thoracic motoneurons in the segment above a chronic lateral spinal cord lesion. J Neurophysiol. 2016 Jan 1;115(1):554-67. Epub 2015 Oct 21. PMID: 26490290; PMCID: PMC4760466. [CrossRef]
- Streeter KA, Sunshine MD, Patel SR, Gonzalez-Rothi EJ, Reier PJ, Baekey DM, Fuller DD. Mid-cervical interneuron networks following high cervical spinal cord injury. Respir Physiol Neurobiol. 2020 Jan;271:103305. Epub 2019 Sep 22. PMID: 31553921; PMCID: PMC6864252. [CrossRef]
- Granier C, Schwarting J, Fourli E, Laage-Gaupp F, Hennrich AA, Schmalz A, Jacobi A, Wesolowski M, Conzelmann KK, Bareyre FM. Formation of somatosensory detour circuits mediates functional recovery following dorsal column injury. Sci Rep. 2020 Jul 2;10(1):10953. PMID: 32616790; PMCID: PMC7331809. [CrossRef]
- Lorach H, Galvez A, Spagnolo V, Martel F, Karakas S, Intering N, Vat M, Faivre O, Harte C, Komi S, Ravier J, Collin T, Coquoz L, Sakr I, Baaklini E, Hernandez-Charpak SD, Dumont G, Buschman R, Buse N, Denison T, van Nes I, Asboth L, Watrin A, Struber L, Sauter-Starace F, Langar L, Auboiroux V, Carda S, Chabardes S, Aksenova T, Demesmaeker R, Charvet G, Bloch J, Courtine G. Walking naturally after spinal cord injury using a brain-spine interface. Nature. 2023 Jun;618(7963):126-133. Epub 2023 May 24. PMID: 37225984; PMCID: PMC10232367. [CrossRef]
- Shepard CT, Brown BL, Van Rijswijck MA, Zalla RM, Burke DA, Morehouse JR, Riegler AS, Whittemore SR, Magnuson DSK. Silencing long-descending inter-enlargement propriospinal neurons improves hindlimb stepping after contusive spinal cord injuries. Elife. 2023 Dec 15;12:e82944. PMID: 38099572; PMCID: PMC10776087. [CrossRef]
- Khalki L, Sadlaoud K, Lerond J, Coq JO, Brezun JM, Vinay L, Coulon P, Bras H. Changes in innervation of lumbar motoneurons and organization of premotor network following training of transected adult rats. Exp Neurol. 2018 Jan;299(Pt A):1-14. Epub 2017 Sep 14. PMID: 28917641. [CrossRef]
- Bertels H, Vicente-Ortiz G, El Kanbi K, Takeoka A. Neurotransmitter phenotype switching by spinal excitatory interneurons regulates locomotor recovery after spinal cord injury. Nat Neurosci. 2022 May;25(5):617-629. Epub 2022 May 6. PMID: 35524138; PMCID: PMC9076533. [CrossRef]
- Sartori AM, Hofer AS, Scheuber MI, Rust R, Kessler TM, Schwab ME. Slow development of bladder malfunction parallels spinal cord fiber sprouting and interneurons’ loss after spinal cord transection. Exp Neurol. 2022 Feb;348:113937. Epub 2021 Nov 24. PMID: 34826427. [CrossRef]
- Garcia-Ramirez DL, Ha NTB, Bibu S, Stachowski NJ, Dougherty KJ. Spinal cord injury alters spinal Shox2 interneurons by enhancing excitatory synaptic input and serotonergic modulation while maintaining intrinsic properties in mouse. J Neurosci. 2021 May 13;41(27):5833–48. Epub ahead of print. PMID: 34006587; PMCID: PMC8265802. [CrossRef]
- Husch A, Van Patten GN, Hong DN, Scaperotti MM, Cramer N, Harris-Warrick RM. Spinal cord injury induces serotonin supersensitivity without increasing intrinsic excitability of mouse V2a interneurons. J Neurosci. 2012 Sep 19;32(38):13145-54. PMID: 22993431; PMCID: PMC3506248. [CrossRef]
- Zheng X, Zhu B, Xu J, Liu D, Huang Y, Chen D, Liu Z, Guo F, Dong Y, Zhu W, Pan D, Zhang SC, Chen H, Wang W. Human spinal GABA neurons survive and mature in the injured nonhuman primate spinal cord. Stem Cell Reports. 2023 Feb 14;18(2):439-448. Epub 2023 Jan 19. PMID: 36669493; PMCID: PMC9969075. [CrossRef]
- Vasudevan D, Liu YC, Barrios JP, Wheeler MK, Douglass AD, Dorsky RI. Regenerated interneurons integrate into locomotor circuitry following spinal cord injury. Exp Neurol. 2021 Aug;342:113737. Epub 2021 May 4. PMID: 33957107. [CrossRef]
- Danner SM, Shepard CT, Hainline C, Shevtsova NA, Rybak IA, Magnuson DSK. Spinal control of locomotion before and after spinal cord injury. bioRxiv [Preprint]. 2023 Jun 1:2023.03.22.533794. Update in: Exp Neurol. 2023 Jul 25;:114496. PMID: 36993490; PMCID: PMC10055332. [CrossRef]
- Lin S, Li Y, Lucas-Osma AM, Hari K, Stephens MJ, Singla R, Heckman CJ, Zhang Y, Fouad K, Fenrich KK, Bennett DJ. Locomotor-related V3 interneurons initiate and coordinate muscles spasms after spinal cord injury. J Neurophysiol. 2019 Apr 1;121(4):1352-1367. Epub 2019 Jan 9. PMID: 30625014; PMCID: PMC6485742. [CrossRef]
- Lizen B, Claus M, Jeannotte L, Rijli FM, Gofflot F. Perinatal induction of Cre recombination with tamoxifen. Transgenic Res. 2015 Dec;24(6):1065-77. Epub 2015 Sep 22. PMID: 26395370. [CrossRef]
- Punjani N, Deska-Gauthier D, Hachem LD, Abramian M, Fehlings MG. Neuroplasticity and regeneration after spinal cord injury. N Am Spine Soc J. 2023 Jun 8;15:100235. PMID: 37416090; PMCID: PMC10320621. [CrossRef]
- Poplawski GHD, Kawaguchi R, Van Niekerk E, Lu P, Mehta N, Canete P, Lie R, Dragatsis I, Meves JM, Zheng B, Coppola G, Tuszynski MH. Injured adult neurons regress to an embryonic transcriptional growth state. Nature. 2020 May;581(7806):77-82. Epub 2020 Apr 15. PMID: 32376949. [CrossRef]
- Saliani A, Perraud B, Duval T, Stikov N, Rossignol S, Cohen-Adad J. Axon and Myelin Morphology in Animal and Human Spinal Cord. Front Neuroanat. 2017 Dec 22;11:129. PMID: 29311857; PMCID: PMC5743665. [CrossRef]
- Meltzer S, Santiago C, Sharma N, Ginty DD. The cellular and molecular basis of somatosensory neuron development. Neuron. 2021 Dec 1;109(23):3736-3757. Epub 2021 Sep 29. PMID: 34592169; PMCID: PMC8639614. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




