1. Introduction
Probiotics are live bacteria that, when administered in sufficient quantities, provide health benefits to the host [
1,
2]. Consumers typically ingest probiotics from dairy, pharmaceutical, and fermented foods. On the other hand, dairy products do not correspond to the dietary needs of specific customer categories, such as vegetarians and lactose-intolerant consumers [
3]. As a result, the development of non-dairy alternatives based on fruit and vegetables, such as probiotic fresh-cut fruits, could serve as a preference vector for these consumers [
4]. ’Minimally processed‘, ‘ready-to-eat’, ‘fresh-cut’, and some other designations indicate fruits ready for consumption [
5], categories that have growing popularity attributable to their convenience, sensory quality, excellent nutritional value, and ability to retain freshness [
6]. Recently, probiotic addition was proposed for a growing number of fresh-cut fruits, such as apples, pears, cantaloupes, pineapples, carrots and blueberries [
7,
8,
9,
10,
11,
12]. However, several authors reported that specific features of the fruit matrices could affect the probiotic viability during shelf life and that the metabolic activity of the strain utilized could have a detrimental effect on the sensory characteristics of the fruit [
8]. In this light, the use of edible coating based on biopolymers could act as an immobilizing agent to limit the metabolic activity and the proliferation of the probiotic cells added [
13,
14], but also for extending the shelf life of fresh-cut fruits [
15], as it could act as a barrier to water and gas, and reduce the water loss and oxidative reactions of the fruits [
16,
17,
18]. Among the most appreciated features of the probiotic strains selected for the application in the fresh-cut sector, there is the ability to control harmful microorganisms and decay agents. In this context, the use of antagonistic microorganisms, such as Lactic Acid Bacteria (LAB), has assumed international relevance as a promising eco-friendly alternative to chemical interventions [
19], with the aim of sustainable improvement of the shelf-life, quality, and safety of fruits and vegetables [
20,
21,
22].
Lactiplantibacillus plantarum is a lactic acid bacterium belonging to the heterogenous class of lactobacilli. This bacterium is an extremely widespread species in the agro-food sector due to its outstanding biological versatility. Among the most interesting characteristics of this microorganism it is possible to include the probiotic features and antimicrobial properties, both strain-related characteristics [
23,
24,
25]. These are two interconnected attributes, as among the selection criteria of a probiotic microorganism, there are antimicrobial properties with respect to pathogenic bacteria, with the aim of supporting intestinal health. Different strains of
L. plantarum have found application in the postharvest of fruits and vegetables [
21,
26,
27,
28], with particular reference to the issues of biocontrol and improvement of functional quality, supporting the interest in this microorganism as a model lactic acid bacteria for this kind of applications. Table grapes are non-climacteric fruits, particularly perishable after harvest, which tend to deteriorate due to water loss, oxidation, and fungal colonization. Several treatments were proposed to maintain the postharvest quality of table grapes [
29] and to limit the decay induced by its most important fungal spoilage,
Botrytis cinerea [
29,
30]. However, colonisation by other fungal species was reported for table grapes [
31]. Among those,
Aspergillus genus is one of the most dangerous filamentous fungi because they can colonize a wide range of food commodities and can spread and propagate in the storage phase. This genus is also responsible for safety concerns regarding allergenic reactions and the production of mycotoxins, such as aflatoxins and ochratoxin A [
32].
In this work, five Lactiplantibacillus plantarum strains, previously characterised for their probiotic and antifungal activity, have been incorporated into a sodium alginate coating to develop edible probiotic coatings for table grapes cv. Italia. In addition, the ability of functionalized coatings to counteract the decay development was evaluated on table grapes berries artificially contaminated with Aspergillus niger.
2. Materials and Methods
2.1. Microbial Strains and Growth Conditions
Five
L. plantarum strains isolated from fruits and vegetables previously characterized for their probiotic and antimicrobial activity were selected for this study [
33,
34]. Lactic Acid Bacteria were routinely cultured in MRS broth (Oxoid, Basingstoke, United Kingdom) at 30 °C.
The filamentous fungus Aspergillus niger CECT 2805 from cryopreserved cultures was propagated on Potato Dextrose Agar at 24 °C for five days. Fungal spores suspension was prepared by brushing the plate surface with saline solution (0.86% NaCl) supplemented with 0.01% Tween 80 using a sterile swab, storing the suspension at 4 °C for short-term uses. Fungal spores concentration was determined by plating serial dilution on PDA plates and adjusted to approximately 1 x 106 spores/mL.
2.2. Preparation of the Probiotic Coating-Forming Solutions
The probiotic coating-forming solutions were obtained as reported by Alvarez et al. [
7]. The coating-forming solution consisted of 2% (
w/v) alginate (Sigma-Aldrich, St. Louis, MO, USA) with 1.5% (
w/v) glycerol as a plasticizer. After complete dissolving, the coating-forming solution was autoclaved at 121 °C for 15 min and cooled down until room temperature.
L. plantarum strains were grown overnight in MRS broth, washed twice and resuspended in coating forming solution to obtain a final concentration of ~ 10
9 CFU/mL. Inoculum concentration was checked by plating appropriate dilutions onto MRS agar.
2.3. Preparation of Probiotic-Coated Table Grape Berries
Healthy table grapes (cv. Italia) were purchased from a local retailer, sanitized by dipping for 1 minute in 0.01% (w/v) sodium hypochlorite (NaOCl), rinsed twice with sterile demineralized water, and dried under a laminar flow hood. Berries were dipped into the probiotic coating solution and then in the hardening solution (2% w/v CaCl2) both for 30 sec. Uncoated grapes were dipped in saline solution containing the same amount of probiotics and then in hardening solution. After drying, probiotic table grapes berries were packed in plastic containers (five berries each) under passive atmosphere and stored at 4 °C for 14 days to simulate commercial shelf-life. Each treatment was performed in triplicate.
2.4. Probiotic Viability
The survival of the probiotic strains in coated and uncoated berries during the simulated shelf-life was evaluated. Analysis was performed after 0, 7 and 14 days of storage at 4 °C. Samples were mixed (1/10 w/v) with sterile saline solution and homogenized for three minutes in a stomacher blender. Then, serial 10-fold dilutions were plated on MRS agar. Viable counts expressed as log of colony forming units per gram (LogCFU/g) were determined after incubation of the plates at 30 °C for 48 hours.
2.5. Fruit Decay Assay
Probiotic-coated table grape berries were prepared as described above. Then, artificial wounds were performed using a sterile needle to make 3 mm deep and 3 mm wide wounds (four wounds for each acinus) along the equatorial areas of the berries. Each wound was inoculated with 10 µL of A. niger spores suspension (~ 106 spores/mL). After drying in a laminar flow hood for about 1 hour, table grapes berries were packed as above. Five berries were wound-inoculated for each probiotic treatment. The plastic containers were maintained at 24 °C for 3 days, in order to create favorable conditions for the onset of post-harvest pathology. The development of the fungus was monitored daily by visual analysis.
2.6. Sensorial Quality Analysis
A group of ten trained panellists performed the sensory evaluations of artificially contaminated table grape berries after 3 days of shelf life at 24 °C. Panellists received training in order to identify and rate the non-tasting quality attributes prior to evaluations. Positive descriptors, such as appearance, colour, freshness, firmness, and overall acceptance, were evaluated using a hedonic scale from 1 to 5, where 1 = not present/very low/not typical, to 5 = very pronounced/very typical of fresh fruits. Negative descriptors, such as off odour and mould occurrence, were ranked from 1 = 0% mould presence/odour to 5 = 100% mould presence/odour. In both cases, the value 3 was fixed as the limit of marketability.
2.7. Statistical Analysis
One-way analysis of variance (ANOVA) was performed by using SAS statistical computer package (SAS Institute, Cary, NC, USA). Significant differences in bacterial viability were determined using Fisher’s Least Significant Difference (LSD) test with p < 0.05 as the minimal level of significance. Significant differences in lesion diameter were determined by post hoc Tukey’s Honestly Significant Difference (HSD) test with p < 0.05 as the minimal level of significance.
3. Results and Discussion
LAB belonging to the genus
Lactiplantibacillus are typically found in association with fruits and vegetables [
35], and they are considered natural competitors of the undesired microflora responsible for spoilage and decay of fruits and vegetables, such as phytopathogenic bacteria and filamentous fungi [
36]. In particular, the species
Lactiplantibacillus plantarum also assumed a relevant role as biocontrol agent because it is already adapted to fruit environments and their related stressors, thus simplifying its application for industrial purposes [
36]. The competition between LAB and filamentous fungi is mediated by a plethora of mechanisms, including the competition for nutrients, the secretion of metabolic byproducts (i.e. organic acids) and the production of active compounds such as peptides and VOCs, but also for synergistic mechanisms among those [
37,
38]. In this paper, five strains of
Lactiplantibacillus plantarum isolated from wild plant matrices of the Mediterranean area (i.e. aloe, carob, blackthorn), considered as unconventional ecological niches due to their restricted use in food industries and already characterized for probiotic [
33] and antimicrobial activity ([
34]; De Simone et al.
unpublished results), were selected for their ability to inhibit the growth of
Aspergillus niger CECT 2805. Different modes of action and synergies among the antimicrobial features were previously identified as responsible for the detected activity. In fact, the five strains are able to produce different organic acids, including lactic, acetic and 3-phenyllactic acid, but also volatile organic compounds, such as high amounts of 2-undecanone and 2-nonanone, which are well known for their antifungal activity [
34]. For these reasons, the strains were chosen for probiotic fortification and for application as biopreservatives on ready-to-eat perishable fruits using table grapes cv. Italia berries as a model fruit. In this context, the use of coating was chosen as an immobilizing agent with the aim of increasing the number of probiotic cells delivered by the berries and uniformly distributing the biocontrol strains on the surface of the fruits, but also as a combined treatment to ameliorate the effect of bioprotection. In this work, an edible coating based on sodium alginate and glycerol was chosen because these chemicals are already used as food additives, with the codes E401 and E422, respectively.
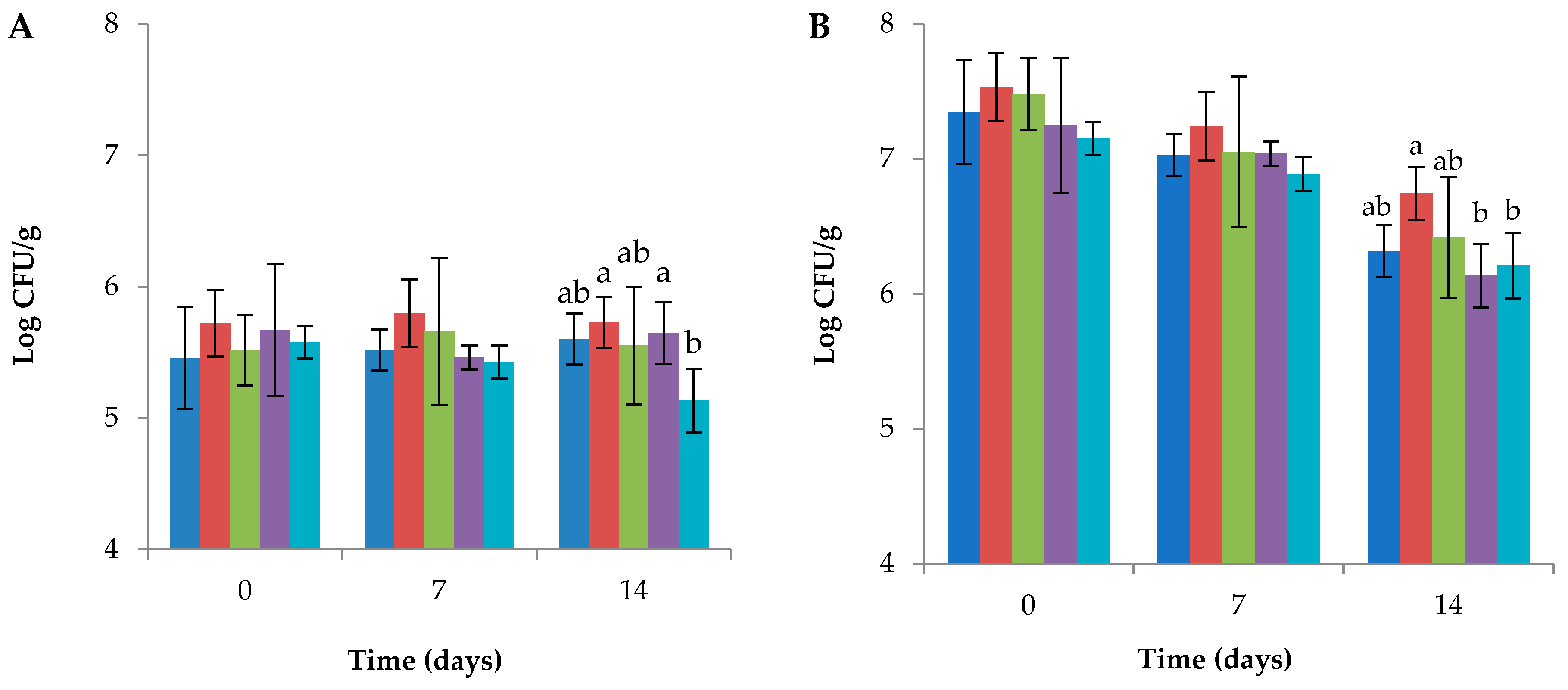
3.1. Probiotic Viability on Table Grapes Berries during Shelf Life
The viability of probiotic
L. plantarum strains on table grapes berries included or not into edible coating during 14 days of shelf life at 4 °C is shown in
Figure 1. At day 0, the alginate edible coating increased the number of probiotic cells transferred to the surface of the berries, which was generally increased from about 5 to more than 7 LogCFU/g for all the strains used in this work. A greater number of probiotic living cells for coated berries with respect to uncoated berries was recorded on all sampling days. However, in uncoated berries, the number of living cells remained stable during the 14 days, whereas a reduction of about 1 Log CFU/g was detected in the coated berries. No difference was found between the
L. plantarum strains at sampling days 0 and 7 in both conditions. At 14 days,
L. plantarum 11-A and CZ-97 showed higher viability in uncoated grapes, with about 5,7Log CFU/g, whereas, in coated berries,
L. plantarum 11-A was found significantly different, with 6,7 Log CFU/g.
Without edible coating, Lappa et al. [
39] could transfer a slightly lower quantity of
L. plantarum than what we observed in uncoated grapes. Whereas, in our precedent work,
L. plantarum MEP3 had better adhesion ability on uncoated table grapes berries, with approximately 7 Log CFU/g of viable cells transferred to the surface of the berries, that remained stable during shelf life [
40], indicating the need to develop new strategies to increase the adhesion of probiotic bacteria with limited ability to colonize the surface of the fruits. In addition, the LAB viability observed in ready-to-eat table grapes was slightly lower than in other fresh-cut fruits [
8,
9,
40,
41]. For this reason, it should be considered that the decreased ability to colonize the fruit's surface may be due to the structural and chemical characteristics of the cuticle of grape berries, which may prevent microorganisms from adhering to and remaining on the fruit [
40]. However, probiotic intake advantages can be gained from foods that contain at least 6-7 Log CFU of viable bacteria per gram of product [
42]. Thus, the use of edible coatings could be considered an advantageous option to obtain probiotic ready-to-eat fruits. In fact, the content of viable probiotic cells in uncoated table grapes berries does not reach the requirement limit to be considered as beneficial. In this light, the use of edible coating gave the possibility to enhance of about 2 Log the quantity of probiotic bacteria transferred to the berries' surface, thus fulfilling the requirement limit of 6-7 LogCFU/g also at the end of shelf life. In addition, assuming that the mean weight of one grape berry is between 8 and 12 grams on average, and considering that an estimated portion of fresh fruit in a single meal could be represented by 5 - 10 berries, with a quantity between 80 – 100 g of product, the concentrations of probiotic for a single portion of ready-to-eat table grapes berries should be estimated to more than 8 LogCFU also at the end of shelf life, tailoring this matrix as a functional food.
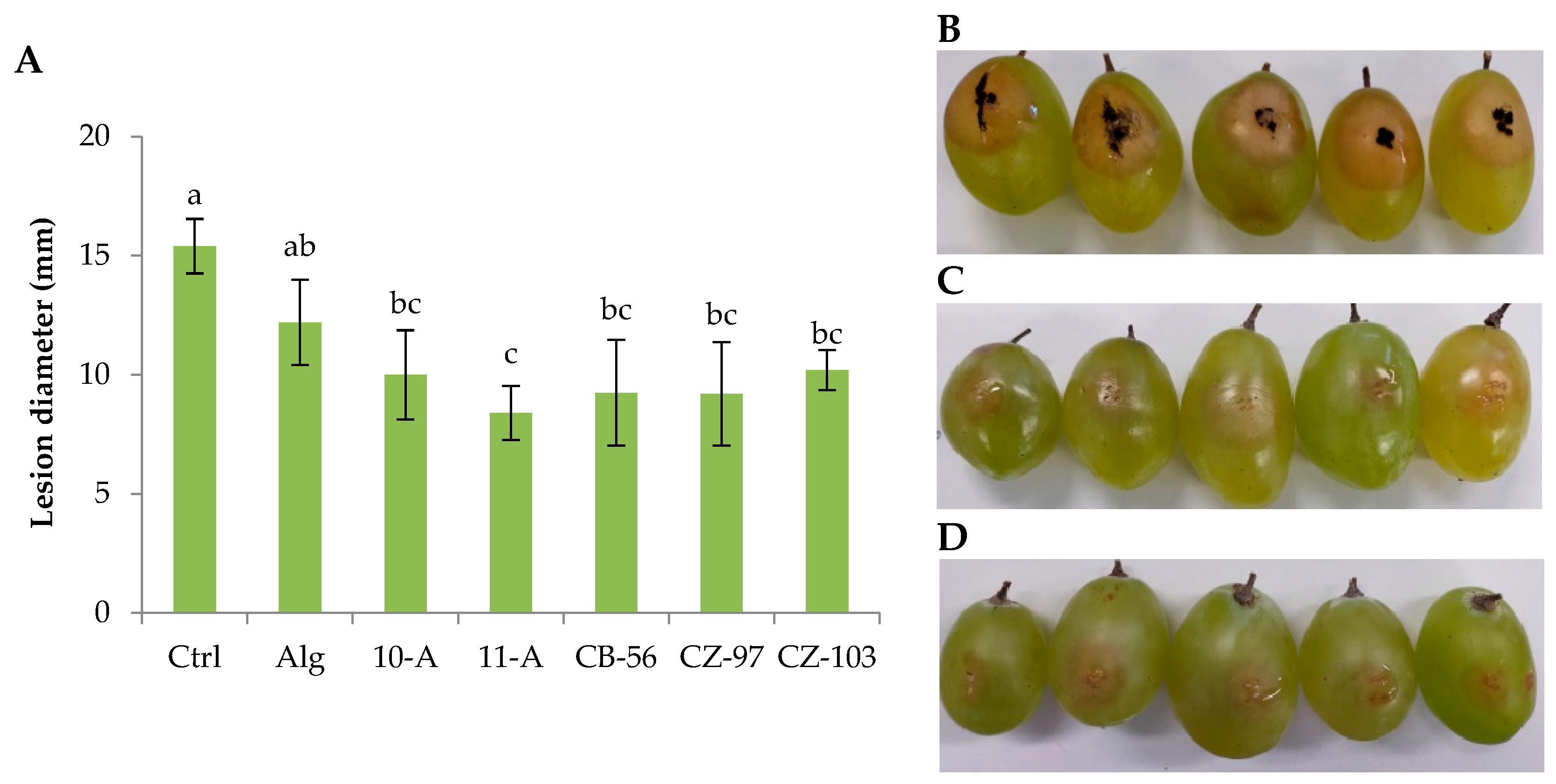
3.2. Fruit Decay Assay
Based on previous characterization of antifungal activity against
Aspergillus niger CECT 2805 [
34], it was also hypothesized that probiotic
L. plantarum strains could act as a preventive treatment against fungal contamination in table grapes berries. For this reason, the bioprotective potential of the probiotic strains applied to edible coatings was further investigated against the same target when artificially wound-inoculated. The lesion diameter and the symptoms of infection of the table grape berries subjected to different probiotic edible coating treatments are shown in
Figure 2.
As reported in previous studies, the alginate coating treatments effectively reduced postharvest deterioration and reduced quality parameters, such as weight and firmness losses, total soluble solids, titratable acidity, and colour [
43,
44]. Our work only investigated the potential to reduce the lesion diameter caused by fungal inoculation in artificially performed wounds. In this case, when alginate coating alone (without probiotic cells) was compared to uncoated control, a significant effect on lesion diameter reduction was observed, with a lesion diameter from 15,40 ± 1,14 to 12,20 ± 1,79 mm for uncoated and alginate coated berries, respectively (
Figure 2A). The lesion diameter reduction was also accompanied by the reduction of the symptoms of infection, such as browning around the wound and sporulation (
Figure 2B,C). This is consistent with previous literature in this topic and confirms the positive effect of edible coatings to limit the physiological damage of the berries. However, among the probiotic coatings, most of the strains showed the same behaviour, and only slight differences were found between most of them and the alginate coating alone. In fact, four out of five strains showed the same statistical significance level, with values of lesion diameter ranging from 10,00 ± 1,87 to 9,20 ± 2,05 mm, for
L. plantarum strain 10-A and CZ-103, respectively. Differently, the coating treatment with
L. plantarum 11-A showed a higher reduction in lesion diameter, with values of 8,40 ± 1,14 mm, which was significantly different from the other strains and from the alginate coating alone. In addition, the symptoms of infection were also lower, with the absence of sporulation and only a limited browning around the wound, which partially healed (
Figure 2D). Different authors have previously reported the protective effect of living cells of L. plantarum against fungal decay caused by
Aspergillus species [
39,
40]. At the same time, the inhibitory effect of the CFS of
L. plantarum strains was assessed in table grapes against
Pseudomonas syringae pv.
syringae, and
Botrytis cinerea [
45]. This suggests the high potential of this LAB species as a broad-spectrum biocontrol agent for table grapes. However, to the best of our knowledge, the biocontrol effect of edible coatings functionalized with antifungal strains of
L. plantarum against fungal decay has not been previously investigated.
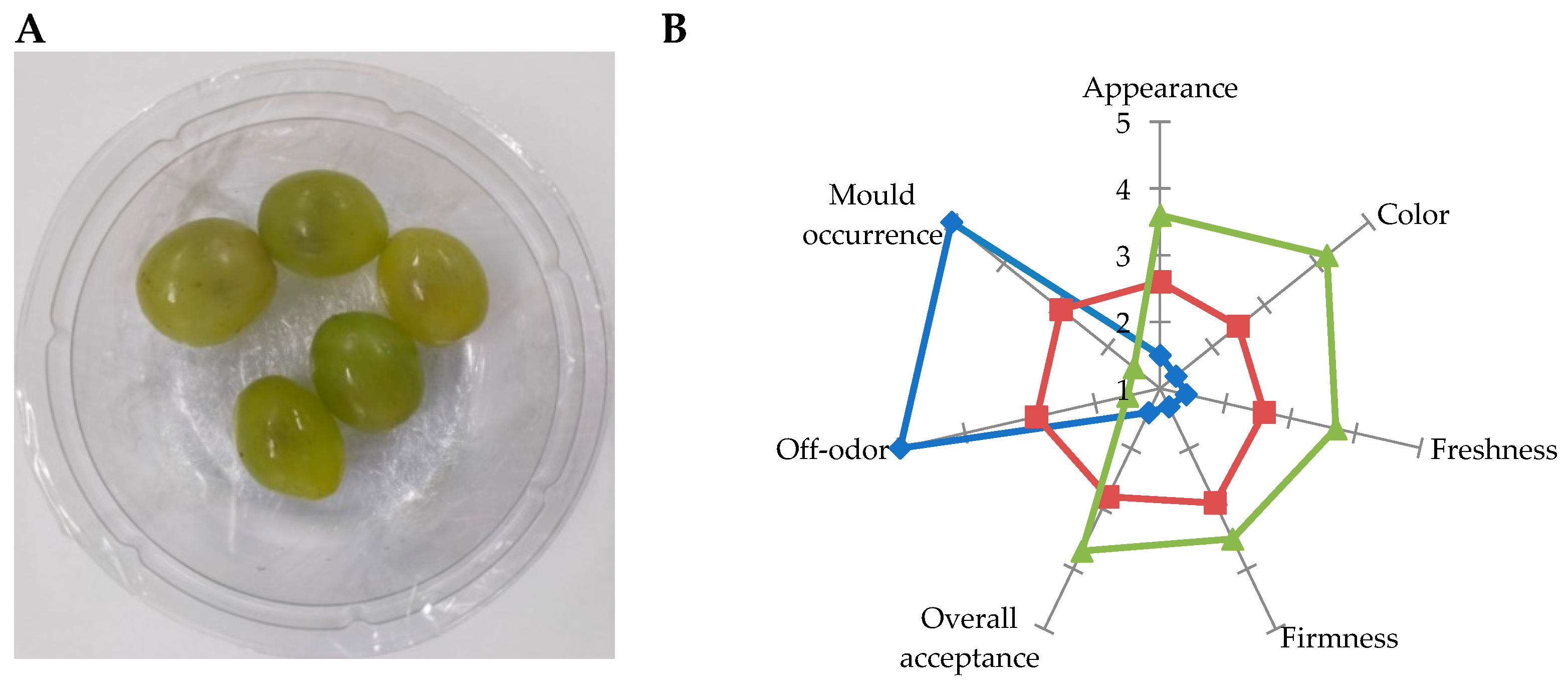
3.3. Sensorial Analysis
Figure 3 represents variations in the sensory characteristics of table grape berries at 3 days of shelf life at 24 °C. Sensorial analysis was performed only in uncoated, coated and probiotic
L. plantarum 11-A coated berries. As expected, artificial contamination of berries was found to impact the product's quality significantly negatively. In fact, among the parameters evaluated, those related to positive features, such as appearance, colour, freshness, firmness, and overall acceptance (
Figure 3B, right side), were all ranked plenty lower than the limit of marketability for uncoated berries. At the same time, mould occurrence and off-odour (
Figure 3B, left side), which were both related to fungal contamination, fermentations, and fruit tissue necrosis, were ranked at the top.
Alginate-coated berries showed intermediate values of all the descriptors evaluated, with those related to positive features slightly below the limit of marketability. On the contrary, mould occurrence and off odour were ranked higher than the limit and considered unacceptable for marketing. Table grapes berries coated with L. plantarum 11-A showed the best performance, with positive feature values still higher than the limit of marketability and with low impact of mould occurrence and off odour even after fungal artificial contamination. For these reasons, table grape berries coated with the probiotic strain L. plantarum 11-A were considered to be of sufficient quality for marketing.
4. Conclusions
Fresh fruit and vegetables represent crucial factors in a balanced diet aimed at maintaining a healthy state. Due to their water and nutritional content, fruit and vegetables are very perishable foods, particularly due to the development of mould. In light of lifestyle changes, in order to maximize the presence of fruit and vegetables in the diet, it becomes crucial to optimize post-harvest management to improve the shelf life, quality and safety of ready-to-eat products. There is growing interest in postharvest applications of lactic acid bacteria for the production of ready-to-eat fruits and vegetables with selected LAB strains. In particular, these solutions enhance the antimicrobial and probiotic properties of selected strains to improve hygienic quality and functional characteristics. Edible packaging solutions represent further useful solutions to improve the characteristics of the finished product appreciated by the market, protecting the product from a physical, chemical and biological point of view. This scientific work proposes a synergy between these two categories of innovations, using L. plantarum, alginate coating and table grapes as model factor for the experimental design. Alginate coating was used to improve the transfer of probiotic cells on table grape berry surfaces. With this strategy, probiotic cell transfer increased by about 2 Log CFU/g, and higher viability was maintained until the end of shelf-life. Significant differences in terms of decay prevention were also found in the alginate coating containing the probiotic strain L. plantarum 11-A. All the strains displayed the same behaviour after inclusion in alginate in terms of improvement of the number of cells. On the opposite, a strain-dependent effect was underlined for the properties linked to biocontrol properties after inclusion in the alginate matrix, suggesting that the application of antimicrobial microbes as bio-tools in edible packaging may represent an innovative criterion in the selection of lactobacilli to be exploited postharvest.
Author Contributions
Nicola De Simone: Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Angela Scauro: Methodology, Investigation, Data curation, Conceptualization. Danial Fatchurrahman: Investigation, Data curation. Pasquale Russo: Writing – review & editing, Supervision, Conceptualization. Vittorio Capozzi: Writing – review & editing, Supervision, Resources, Conceptualization. Mariagiovanna Fragasso: Resources, Conceptualization. Giuseppe Spano: Writing – review & editing, Supervision, Resources, Funding acquisition, Conceptualization.
Funding
This work was partially supported by PON project ‘Conservabilità, qualità e sicurezza dei prodotti ortofrutticoli ad alto contenuto di servizio’-POFACS-CUP B74I20000120005. Mariagiovanna Fragasso is supported by the European Union Next-Generation EU [Piano Nazionale di Ripresa e Resilienza (PNRR) – Missione 4 Componente 2, Investimento 1.4 – D.D. 1032 17/06/2022, CN00000022] within the Agritech National Research Centre for Agricultural Technologies". Vittorio Capozzi is supported by the Next-Generation EU [PNRR], in the framework of the Component 2 Investment 1.3- Award Number: Project code PE00000003, Project title: “ON Foods-Research and innovation network on food and nutrition Sustainability, Safety and Security–Working ON Foods”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guo, Z.; Liu, X.M.; Zhang, Q.X.; Shen, Z.; Tian, F.W.; Zhang, H.; Sun, Z.H.; Zhang, H.P.; Chen, W. Influence of Consumption of Probiotics on the Plasma Lipid Profile: A Meta-Analysis of Randomised Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; de Vos, W.M. Intestinal Microbiota in Human Health and Disease: The Impact of Probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef] [PubMed]
- Vijaya Kumar, B.; Vijayendra, S.V.N.; Reddy, O.V.S. Trends in Dairy and Non-Dairy Probiotic Products - a Review. J. Food Sci. Technol. 2015, 52, 6112–6124. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.M.F.; Ramos, A.M.; Vanzela, E.S.L.; Stringheta, P.C.; de Oliveira Pinto, C.L.; Martins, J.M. Products of Vegetable Origin: A New Alternative for the Consumption of Probiotic Bacteria. Food Res. Int. 2013, 51, 764–770. [Google Scholar] [CrossRef]
- Gross, K.C.; Wang, C.Y.; Saltveit, M.E. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks. In United States Department of Agriculture (USDA) - Agricultural Research Service -Agriculture Handbook No. 66; Gross, K.C., Wang, C.Y., Saltveit, M.E., Eds.; U.S. Department of Agriculture: Washington, DC, USA, 2016; p. 780. [Google Scholar]
- Remize, F.; Garcia, C. Fresh-Cut Vegetables and Fruits: Do They Really Meet Sustainability and Nutritional Benefits? Curr. Food Sci. Technol. Rep. 2024, 2, 37–44. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Bambace, M.F.; Quintana, G.; Gomez-Zavaglia, A.; Moreira, M. del R. Prebiotic-Alginate Edible Coating on Fresh-Cut Apple as a New Carrier for Probiotic Lactobacilli and Bifidobacteria. LWT 2021, 137, 110483. [Google Scholar] [CrossRef]
- Russo, P.; Peña, N.; de Chiara, M.L.V.; Amodio, M.L.; Colelli, G.; Spano, G. Probiotic Lactic Acid Bacteria for the Production of Multifunctional Fresh-Cut Cantaloupe. Food Res. Int. 2015, 77, 762–772. [Google Scholar] [CrossRef]
- Russo, P.; de Chiara, M.L.V.; Vernile, A.; Amodio, M.L.; Arena, M.P.; Capozzi, V.; Massa, S.; Spano, G. Fresh-Cut Pineapple as a New Carrier of Probiotic Lactic Acid Bacteria. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Bambace, M.F.; Alvarez, M.V.; Moreira, M. del R. Novel Functional Blueberries: Fructo-Oligosaccharides and Probiotic Lactobacilli Incorporated into Alginate Edible Coatings. Food Res. Int. 2019, 122, 653–660. [Google Scholar] [CrossRef]
- Iglesias, M.B.; Abadias, M.; Anguera, M.; Sabata, J.; Viñas, I. Antagonistic Effect of Probiotic Bacteria against Foodborne Pathogens on Fresh-Cut Pear. LWT - Food Sci. Technol. 2017, 81, 243–249. [Google Scholar] [CrossRef]
- Shigematsu, E.; Dorta, C.; Rodrigues, F.J.; Cedran, M.F.; Giannoni, J.A.; Oshiiwa, M.; Mauro, M.A. Edible Coating with Probiotic as a Quality Factor for Minimally Processed Carrots. J. Food Sci. Technol. 2018, 55, 3712–3720. [Google Scholar] [CrossRef]
- Tapia, M.S.; Rojas-Graü, M.A.; Rodríguez, F.J.; Ramírez, J.; Carmona, A.; Martin-Belloso, O. Alginate- and Gellan-Based Edible Films for Probiotic Coatings on Fresh-Cut Fruits. J. Food Sci. 2007, 72, E190–E196. [Google Scholar] [CrossRef]
- Temiz, N.N.; Özdemir, K.S. Microbiological and Physicochemical Quality of Strawberries (Fragaria × Ananassa) Coated with Lactobacillus Rhamnosus and Inulin Enriched Gelatin Films. Postharvest Biol. Technol. 2021, 173, 111433. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent Developments in Shelf-Life Extension of Fresh-Cut Fruits and Vegetables by Application of Different Edible Coatings: A Review. LWT 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. The Use of Packaging Techniques to Maintain Freshness in Fresh-Cut Fruits and Vegetables: A Review. Int. J. Food Sci. Technol. 2009, 44, 875–889. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Lindner, M.; Rothkopf, I.; Schmid, M.; Müller, K. The Development of a Uniform Alginate-Based Coating for Cantaloupe and Strawberries and the Characterization of Water Barrier Properties. Foods 2019, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.Z.; Chen, W.; Gurtler, J.B.; Fan, X. Effectiveness of Edible Coatings to Inhibit Browning and Inactivate Foodborne Pathogens on Fresh-Cut Apples. J. Food Saf. 2020, 40, e12802. [Google Scholar] [CrossRef]
- Sellitto, V.M.; Zara, S.; Fracchetti, F.; Capozzi, V.; Nardi, T. Microbial Biocontrol as an Alternative to Synthetic Fungicides: Boundaries between Pre- and Postharvest Applications on Vegetables and Fruits. Fermentation 2021, 7, 60. [Google Scholar] [CrossRef]
- Capozzi, V.; Fragasso, M.; Bimbo, F. Microbial Resources, Fermentation and Reduction of Negative Externalities in Food Systems: Patterns toward Sustainability and Resilience. Fermentation 2021, 7, 54. [Google Scholar] [CrossRef]
- De Simone, N.; Capozzi, V.; de Chiara, M.L.V.; Amodio, M.L.; Brahimi, S.; Colelli, G.; Drider, D.; Spano, G.; Russo, P. Screening of Lactic Acid Bacteria for the Bio-Control of Botrytis Cinerea and the Potential of Lactiplantibacillus Plantarum for Eco-Friendly Preservation of Fresh-Cut Kiwifruit. Microorganisms 2021, 9, 773. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic Acid Bacteria as Antibacterial Agents to Extend the Shelf Life of Fresh and Minimally Processed Fruits and Vegetables: Quality and Safety Aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus Plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res. Int. 2018, 2018, e9361614. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, X.; Fu, H.; Wang, X.; Guo, X.; Wang, M. Lactiplantibacillus Plantarum: A Comprehensive Review of Its Antifungal and Anti-Mycotoxic Effects. Trends Food Sci. Technol. 2023, 136, 224–238. [Google Scholar] [CrossRef]
- Rocchetti, M.T.; Russo, P.; Capozzi, V.; Drider, D.; Spano, G.; Fiocco, D. Bioprospecting Antimicrobials from Lactiplantibacillus Plantarum: Key Factors Underlying Its Probiotic Action. Int. J. Mol. Sci. 2021, 22, 12076. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Shi, M.; Jiang, Y.; Hu, B.; Guo, X.; Gong, D.; Zhang, Y. Compositional Shifts in Fresh-Cut Apples Microbiome in Response to Application of Lactiplantibacillus Plantarum Assessed by next-Generation Sequencing. LWT 2024, 191, 115627. [Google Scholar] [CrossRef]
- Li, K.; Zhang, W.; Kwok, L.-Y.; Menghe, B. Screening of Lactobacillus Plantarum with Broad-Spectrum Antifungal Activity and Its Application in Preservation of Golden-Red Apples. 2020.
- Islam, S.; Biswas, S.; Jabin, T.; Moniruzzaman, M.; Biswas, J.; Uddin, M.S.; Akhtar-E-Ekram, M.; Elgorban, A.M.; Ghodake, G.; Syed, A. Probiotic Potential of Lactobacillus Plantarum DMR14 for Preserving and Extending Shelf Life of Fruits and Fruit Juice. Heliyon 2023. [Google Scholar] [CrossRef]
- De Simone, N.; Pace, B.; Grieco, F.; Chimienti, M.; Tyibilika, V.; Santoro, V.; Capozzi, V.; Colelli, G.; Spano, G.; Russo, P. Botrytis Cinerea and Table Grapes: A Review of the Main Physical, Chemical, and Bio-Based Control Treatments in Post-Harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef]
- De Simone, N.; Capozzi, V.; Amodio, M.L.; Colelli, G.; Spano, G.; Russo, P. Microbial-Based Biocontrol Solutions for Fruits and Vegetables: Recent Insight, Patents, and Innovative Trends. Recent Pat. Food Nutr. Agric. 2021, 12, 1. [Google Scholar] [CrossRef]
- Serra, R.; Lourenço, A.; Alípio, P.; Venâncio, A. Influence of the Region of Origin on the Mycobiota of Grapes with Emphasis on Aspergillus and Penicillium Species. Mycol. Res. 2006, 110, 971–978. [Google Scholar] [CrossRef]
- Lasram, S.; Oueslati, S.; Mliki, A.; Ghorbel, A.; Silar, P.; Chebil, S. Ochratoxin A and Ochratoxigenic Black Aspergillus Species in Tunisian Grapes Cultivated in Different Geographic Areas. Food Control 2012, 25, 75–80. [Google Scholar] [CrossRef]
- Rocchetti, M.T.; Russo, P.; De Simone, N.; Capozzi, V.; Spano, G.; Fiocco, D. Immunomodulatory Activity on Human Macrophages by Cell-Free Supernatants to Explore the Probiotic and Postbiotic Potential of Lactiplantibacillus Plantarum Strains of Plant Origin. Probiotics Antimicrob. Proteins 2023. [Google Scholar] [CrossRef] [PubMed]
- De Simone, N.; López, L.; Ciudad, C.S.; Scauro, A.; Russo, P.; Rodríguez, J.; Spano, G.; Martínez, B. Antifungal Activity of Lactiplantibacillus Plantarum Isolated from Fruit and Vegetables and Detection of Novel Antifungal VOCs from Fungal-LAB Co-Cultures. Food Biosci. 2024, 103824. [Google Scholar] [CrossRef]
- Plessas, S. Advancements in the Use of Fermented Fruit Juices by Lactic Acid Bacteria as Functional Foods: Prospects and Challenges of Lactiplantibacillus (Lpb.) Plantarum Subsp. Plantarum Application. Fermentation 2022, 8, 6. [Google Scholar] [CrossRef]
- Shi, C.; Chen, Y.; Li, C.; Al-Asmari, F.; Cui, H.; Lin, L. Potential Application of Lactiplantibacillus Plantarum in Food Bio-Preservation – A Comprehensive Review with a Focus on the Antibacterial and Anti-Virulence Effects on Foodborne Pathogens. Food Rev. Int. 2024, 0, 1–27. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, M.; Xue, R.; Liu, T.; Yang, Z.; Yang, Z. Purification and Characterization of a Novel Low-Molecular-Weight Antimicrobial Peptide Produced by Lactiplantibacillus Plantarum NMGL2. Int. J. Biol. Macromol. 2023, 248, 125932. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, S.; Weng, P.; Wu, Z.; Liu, Y. Purification and Antimicrobial Mechanism of a Novel Bacteriocin Produced by Lactiplantibacillus Plantarum FB-2. LWT 2023, 185, 115123. [Google Scholar] [CrossRef]
- Lappa, I.K.; Mparampouti, S.; Lanza, B.; Panagou, E.Z. Control of Aspergillus Carbonarius in Grape Berries by Lactobacillus Plantarum: A Phenotypic and Gene Transcription Study. Int. J. Food Microbiol. 2018, 275, 56–65. [Google Scholar] [CrossRef]
- De Simone, N.; Rocchetti, M.T.; la Gatta, B.; Spano, G.; Drider, D.; Capozzi, V.; Russo, P.; Fiocco, D. Antimicrobial Properties, Functional Characterisation and Application of Fructobacillus Fructosus and Lactiplantibacillus Plantarum Isolated from Artisanal Honey. Probiotics Antimicrob. Proteins 2022. [Google Scholar] [CrossRef]
- Alegre, I.; Viñas, I.; Usall, J.; Anguera, M.; Abadias, M. Microbiological and Physicochemical Quality of Fresh-Cut Apple Enriched with the Probiotic Strain Lactobacillus Rhamnosus GG. Food Microbiol. 2011, 28, 59–66. [Google Scholar] [CrossRef]
- Romano, N.; Tavera-Quiroz, M.J.; Bertola, N.; Mobili, P.; Pinotti, A.; Gómez-Zavaglia, A. Edible Methylcellulose-Based Films Containing Fructo-Oligosaccharides as Vehicles for Lactic Acid Bacteria. Food Res. Int. 2014, 64, 560–566. [Google Scholar] [CrossRef]
- Konuk Takma, D.; Korel, F. Impact of Preharvest and Postharvest Alginate Treatments Enriched with Vanillin on Postharvest Decay, Biochemical Properties, Quality and Sensory Attributes of Table Grapes. Food Chem. 2017, 221, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Aloui, H.; Khwaldia, K.; Sánchez-González, L.; Muneret, L.; Jeandel, C.; Hamdi, M.; Desobry, S. Alginate Coatings Containing Grapefruit Essential Oil or Grapefruit Seed Extract for Grapes Preservation. Int. J. Food Sci. Technol. 2014, 49, 952–959. [Google Scholar] [CrossRef]
- Petkova, M.; Gotcheva, V.; Dimova, M.; Bartkiene, E.; Rocha, J.M.; Angelov, A. Screening of Lactiplantibacillus Plantarum Strains from Sourdoughs for Biosuppression of Pseudomonas Syringae Pv. Syringae and Botrytis Cinerea in Table Grapes. Microorganisms 2022, 10, 2094. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).