1. Introduction
Long-term prognosis in patients with coronary artery disease (CAD) after coronary artery bypass grafting (CABG) depends not only on preoperative factors (initial severity of coronary disease and myocardial condition, comorbidity, level of pathological biomarkers) [
1,
2,
3], but also from further treatment and rehabilitation measures. The task of secondary prevention in this category of patients is to level out those unfavorable risk factors that led to the CAD development, and, ultimately, to the need for myocardial revascularization [
4,
5]. Not surprisingly, most studies have focused on identifying baseline factors associated with long-term prognosis after CABG. For example, recent studies have shown the prognostic impact of biomarkers such as lipoprotein (a) [
3] and LDL/HDL ratio [
2] during 10-year follow-up of patients after CABG. The presence of a large number of risk factors and biomarkers that need to be adjusted and monitored when managing this category of patients creates certain inconvenience for practitioners and can lead to excessive expenditure of resources.
Therefore, it seems attractive to use such an integral indicator that reflects the influence of various risk factors, as arterial wall stiffness. Classically, it is determined by assessing the pulse wave velocity [
6], however, this method has certain limitations (dependence on blood pressure level, inconvenience of performing the study, dependence on the qualifications of personnel) [
6], so another indicator has been proposed - cardio-ankle vascular index (CAVI) [
7]. This indicator is based on determining the rigidity parameter β, which reflects the degree of dependence of pressure on volume, therefore the CAVI index does not depend on the level of blood pressure. This makes it potentially suitable for studying the state of the vascular stack over time [
8].
Currently, prospective epidemiological studies have shown that CAVI is associated with the development of cardiovascular events [
9,
10]. Moreover, the presence of pathological CAVI is associated with an unfavorable prognosis in patients with various forms of coronary artery disease: with acute coronary syndrome [
11,
12], with stable CAD [
13], after CABG [
14]. Since there is data on the impact of treatment interventions on CAVI values [
15,
16], a logical question arises: does a change in this index (or lack of change) affect the prognosis? To date, only a few studies [
13,
17] have been conducted in this direction. This motivated the present study, which aimed to examine the long-term prognostic value of changes in CAVI within a year after CABG.
2. Material and Methods
2.1. Study Population
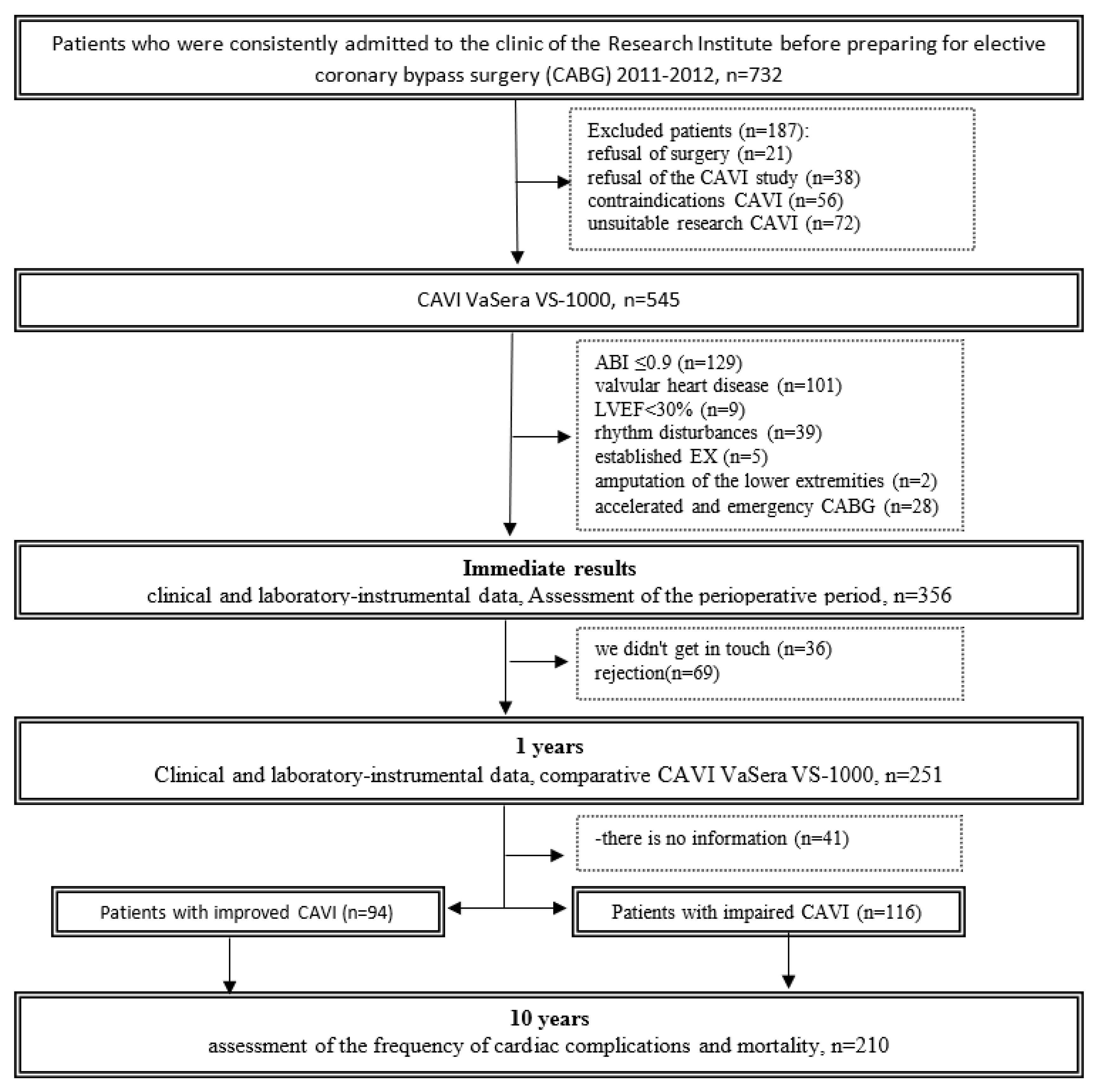
Initially, the study included 732 consecutive patients (age from 33 to 81 years) in the cardiology department of the Federal State Budgetary Institution "Research Institute of Complex Problems of Cardiovascular Diseases" (Kemerovo) for planned coronary bypass surgery (2012-2013). A cohort of 545 patients was recruited and underwent arterial stiffness testing with cardio-ankle vascular index (CAVI) using the VaSera automated device (Fukuda Denshi, Tokyo, Japan). The criteria for inclusion and exclusion of patients from the study were presented in detail in previously published articles [
14,
18]. Thus, the initial analyzed sample consisted of 356 people. At this stage, 125 patients (35.1%) had CAVI values of 9.0 or more. Before the study, written informed consent was obtained from all patients. The study was approved by the local ethics committee of the institution, and the study protocol complied with the ethical principles of the Declaration of Helsinki.
One year after surgery, patients were contacted by telephone and ensured their presence at the study center. One year after surgery, 251 patients (71%) were able to re-measure CAVI, track changes and classify them as patients with improved CAVI (CAVI decreased from pathological value to normal or the index remained within normal values) or worsened CAVI (persistent pathological index value or an increase in CAVI to pathological values or an increase of 1 unit or more). All patients were prescribed optimal drug therapy and given recommendations for lifestyle changes in accordance with the recommendations.
2.2. Evaluation of Indicators
For patients with coronary artery disease, clinical and laboratory data were assessed before surgery and one year after it. The examination consisted of determining serum cholesterol and low-density lipoprotein (LDL), fasting glucose, ultrasound examination of the heart and carotid arteries, and coronary angiography in accordance with the protocol specified in our previous articles [
14].
CAVI measurements were carried out using the VaSera VS-1000 device in a quiet room in the morning in a supine position. The index was calculated automatically on the right and left. Four occlusive cuffs were applied to the shoulders and legs on the right and left, ECG electrodes were placed on the wrists, and a PCG microphone was placed in the second intercostal space to the left of the sternum edge to obtain a PCG signal. The index is calculated automatically by the device on the right and left lower extremities [
8]
2.3. Follow-Up
After surgery, patients were followed up for 9.7±0.9 years. Data were collected through active telephone monitoring and medical information system analysis. Long-term information was collected on 210 (83.7%) patients. In the long-term period, data on the condition of patients, drug therapy were analyzed and hard endpoints were recorded, such as coronary and non-coronary death, non-fatal myocardial infarction (MI) and acute cerebrovascular accident (Stroke/TIA). Among 210 patients, two groups were formed: the first included 94 (44.8%) patients with improved CAVI one year after coronary artery bypass grafting, the second group included 116 (55.2%) patients whose CAVI status worsened one year after CABG. The flow diagram of the study is detailed in
Figure 1.
2.4. Statistical Analyses
For statistical processing, the programs "STATISTICA 8.0" (Dell Software, Inc., Round Rock, TX, USA) and SPSS 17.0 (IBM, Armonk, NY, USA) were used. To decide on the distribution of quantitative variables, the Kolmogorov-Smirnov test was used. To present quantitative variables with a distribution other than normal, the median and quartiles (lower and upper) were used. To compare two independent groups based on quantitative characteristics, the Mann-Whitney test was used. Qualitative values were presented in absolute numbers (n) and percentage (%), and comparisons between the groups were performed using χ2 tests. The binary logistic regression analysis (Forward LR method) was used to assess the relationship of binary signs (all-cause mortality; combined end point - all-cause death + non-fatal myocardial infarction + non-fatal stroke) with preoperative indicators, with the data of arterial stiffness after a year, and with dynamics CAVI at one year. Performance of arterial stiffness parameters in recognizing the risk of unfavorable prognosis (all-cause death, development of a composite endpoint) after CABG was evaluated through receiver operating characteristic curve analysis. The level of statistical significance was defined as p<0.05.
3. Results
Table 1 presents baseline characteristics comparing clinical parameters of patients with worsening/persistent abnormal CAVI and improved/persistent normal CAVI. There were no statistically significant differences in clinical variables between groups, with the exception of a history of diabetes mellitus (p=0.011).
The dynamics of clinical manifestations of coronary and heart failure, as well as laboratory and instrumental characteristics of patients, at the time of inclusion in the study and one year after surgery, are presented in
Table 2. In the group of patients with CAVI progression, clinical manifestations of stage II-III CHF initially prevailed NYHA (p=0.009). However, one year after surgery, the clinical manifestations of CHF decreased in both groups and there were no statistically significant differences between the groups.
At baseline, total cholesterol and LDL cholesterol levels did not differ, but one year after CABG, total cholesterol and LDL cholesterol levels were higher in the CAVI improved group, and the differences were statistically significant (p<0.05). Echocardiographic parameters, including left ventricular ejection fraction and E/A ratio, did not differ between groups. The dynamics of CAVI and ABI are also reflected in
Table 2. In an intergroup comparison, the initial values of the indices on the right and left were comparable in both groups; after a year, the level of CAVI was naturally higher in the group with its progression (p <0.001).
As a screening procedure, ultrasound examination of the carotid arteries was performed initially and over time. Stenosis of the carotid arteries of 30% or more was detected equally often in both groups in 18.1% of cases, however, after a year, progression of atherosclerosis of the carotid arteries ≥30% was noted for both groups (with an improvement in CAVI 24.4%, with a worsening CAVI 23.7%, p>0.05). When analyzing the initial anatomical characteristics of the coronary arteries, no statistically significant differences were found between the groups (
Table 3), which also explains the lack of differences in the number of coronary bypass grafts applied during surgery (
Table 4). The groups were comparable in terms of the total duration of the operation and the duration of artificial circulation. In both groups, combined operations with CABG were performed equally often.
Reception of optimal drug therapy (OMT) (angiotensin converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, calcium channel blockers, statins, antiplatelet agents) during the entire observation period is presented in
Table 5. In the prehospital period, the frequency of OMT use was low and comparable in both groups. During their hospital stay, all patients received standard therapy. After a year, there was a trend towards a higher frequency of taking OMT, without intergroup differences, however, in the long-term period, low adherence to the prescribed treatment was noted in all patients and the groups did not differ statistically significantly.
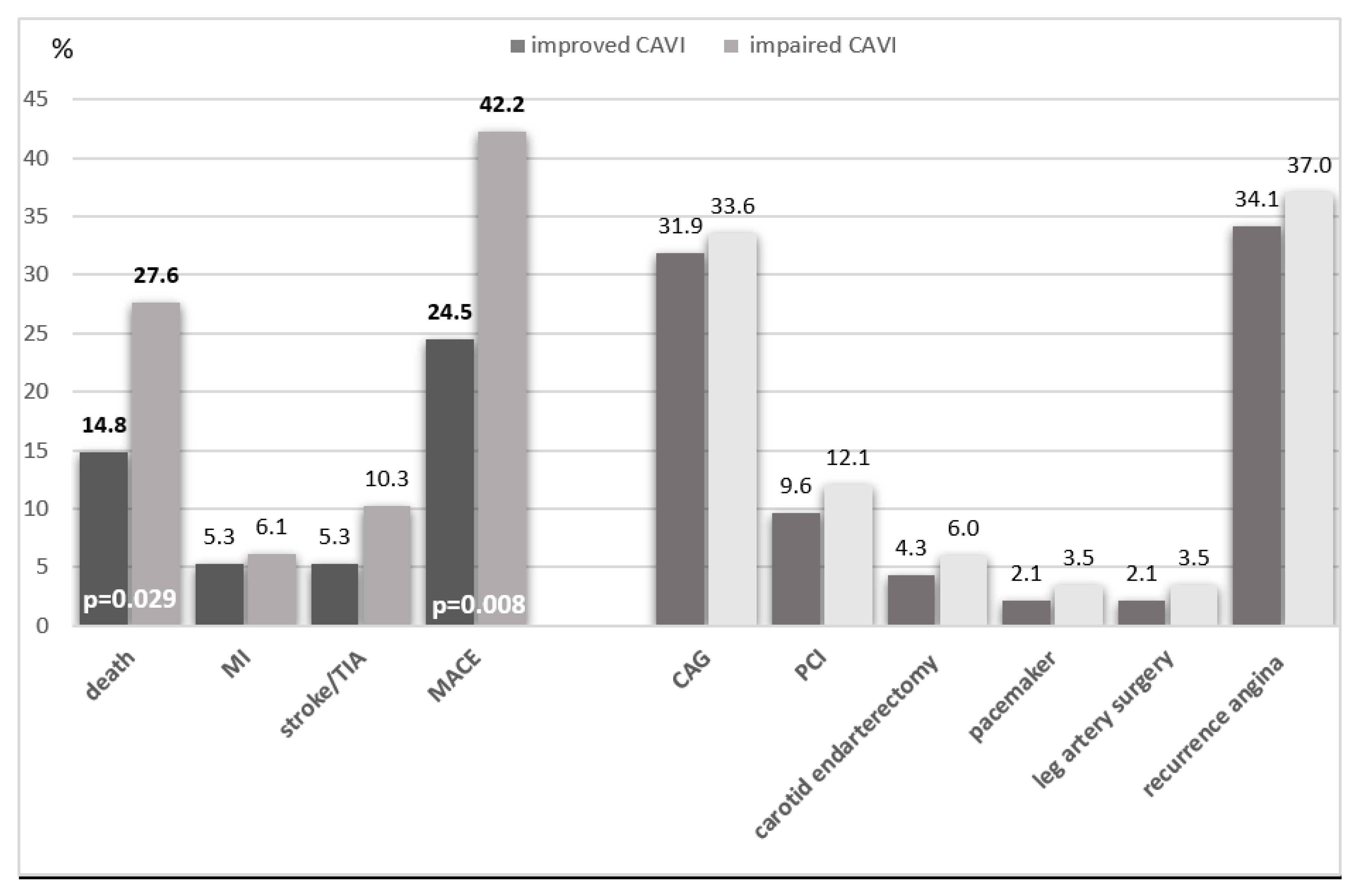
During the long-term follow-up period, 75 (35.7%) cardiovascular events were recorded, including death in 46 (21.9%) patients, non-fatal myocardial infarction in 12 (5.7%) and stroke in 17 (8.1%) patients.
Death from all causes was significantly more common in the group with CAVI progression - 32 (27.6%) than in the group with CAVI improvement - 14 (14.8%; p = 0.029). Death from cardiac causes was also more common in the worsening than improving CAVI group (14.7% vs. 9.6%, p=0.266). Patients with CAVI progression were more likely to have MACE (death, MI, stroke/TIA) in 49 (42.2%) cases, compared with patients with CAVI improvement - in 23 (24.5%) cases, p = 0.008 (
Figure 2).
In the multiple binary logistic regression model (direct LR method), the following factors had a significant relationship (χ2(3) = 16.671, p = 0.001) with death from all causes during long-term follow-up after CABG – group with negative dynamics of CAVI (B = 0.852, p = 0.024), number of shunts (B = 0.627, p = 0.006), and the presence of carotid stenosis more than 30% (B = 0.843, p = 0.051). This model explained only 12.7% (Nagelkerke R2) of the variance in all-cause death and correctly classified 77.6% of cases (
Table 5). The presence of carotid stenosis more than 30% (B = 1.208, p = 0.002) and the group with negative dynamics of CAVI after a year (B = 0.853, p = 0.008) were independent predictors of the development of the combined endpoint during long-term follow-up after CABG. For this model, the statistical significance was χ2(2) = 16.736, p = 0.001, the Nagelkerke R2 value was 0.115, and the model correctly classified 67.2% of cases (
Table 5).
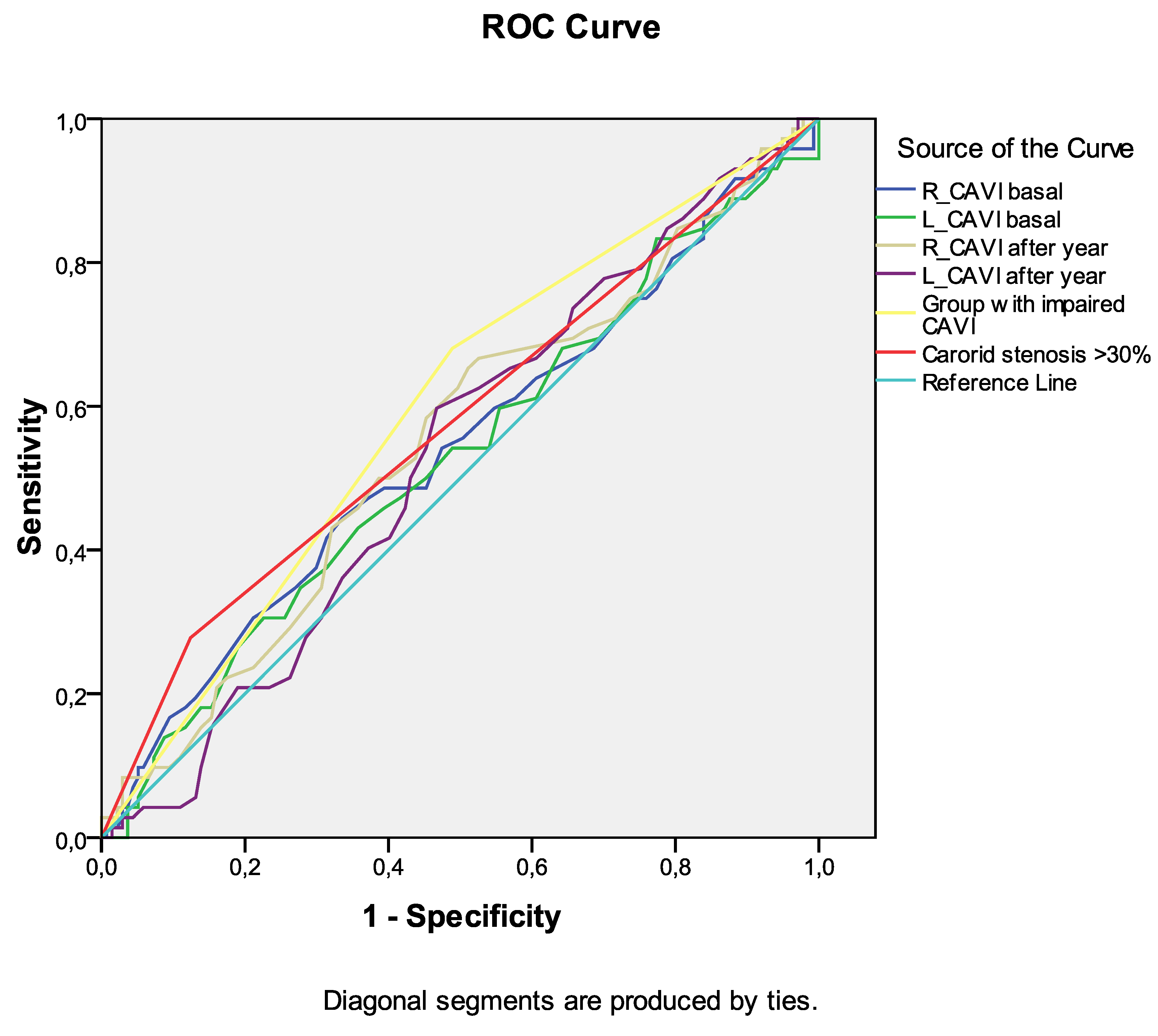
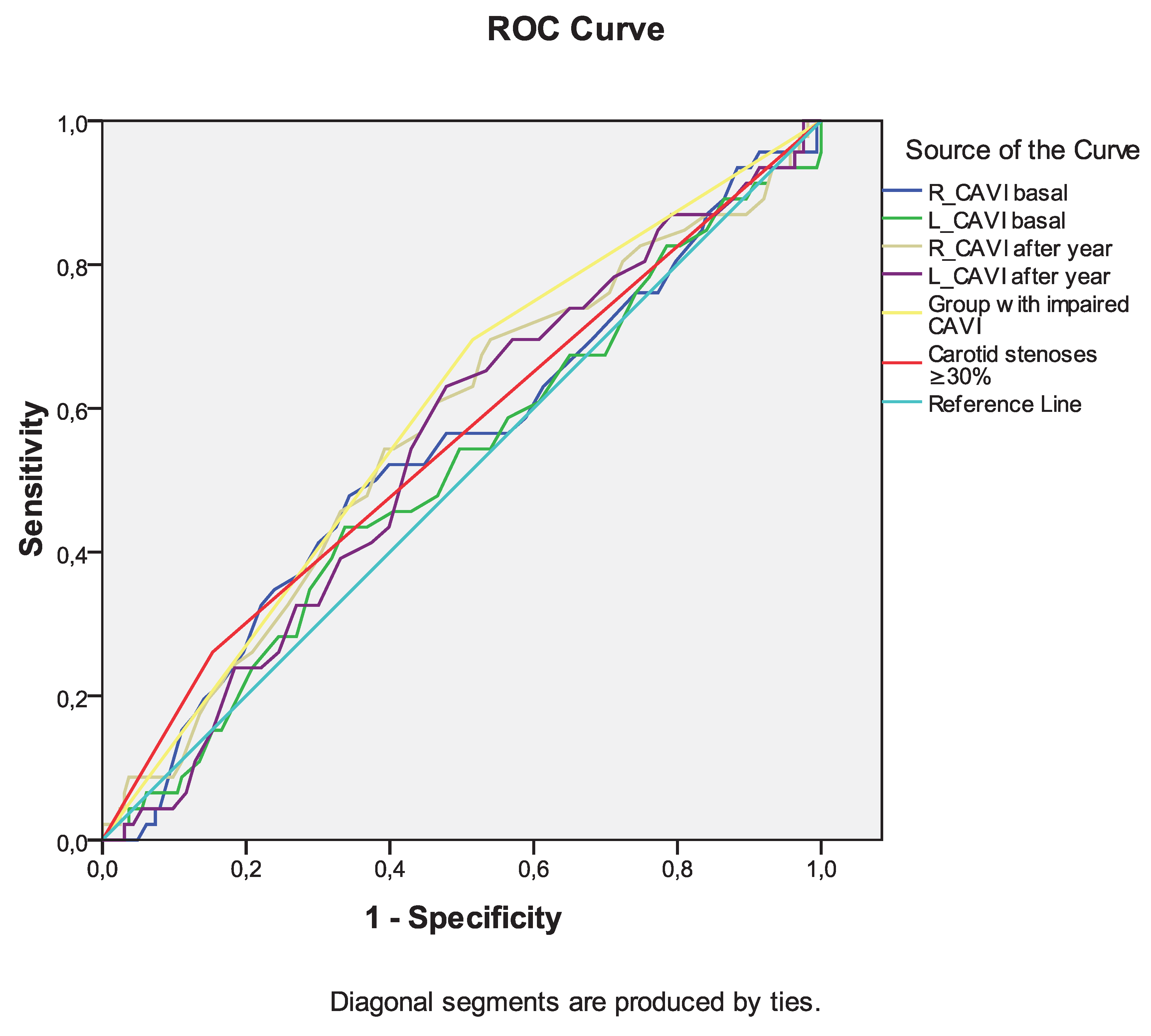
Among the indicators of arterial stiffness (R_CAVI and L_CAVI basal, R_CAVI and L_CAVI after a year, dynamics of CAVI after a year), the greatest association with the development of death from all causes during long-term follow-up was noted for the group with negative dynamics of CAVI after a year (
Table 6,
Figure 3). Similar data were obtained for the development of the combined endpoint during long-term follow-up (
Table 7,
Figure 4). However, the curve area of this variable was <0.7 in both cases, indicating unacceptable discrimination.
4. Discussion
In the present study, one year after CABG surgery, almost half of the patients showed positive dynamics or stable normal CAVI values. At ten-year follow-up, these patients, when compared with patients with negative dynamics or persistently pathological CAVI, showed a decrease in all-cause mortality and the incidence of the composite endpoint. Among the independent predictors of overall mortality and development of composite endpoint were negative dynamics of CAVI during the year and the presence of subclinical carotid stenoses during preoperative examination.
One of the advantages of CAVI over other indicators of arterial stiffness is its independence from blood pressure levels and, accordingly, the possibility of dynamic assessment. Despite this, the study of prognostic assessment of such dynamics is still infrequently used. Thus, in patients with dyslipidemia and risk factors, unfavorable dynamics of CAVI during the first year positively correlated with the development of MACE at five-year follow-up. At the same time, Cox proportional hazards regression analysis failed to show an independent effect of CAVI on the risk of developing MACE [
17]. Apparently, the initial cohort of subjects (without an established CAD diagnosis) did not allow us to identify such an effect due to the low frequency of MACE in it. To date, only one study has examined the effect of CAVI dynamics on prognosis in patients with CAD. Otsuka T et al [
13] showed that in patients with newly CAD diagnosed, when assessing CAVI after 6 months, this index improved only in half of the patients, in the rest it remained persistently elevated. At 3-year follow-up, persistently elevated CAVI was an independent predictor of future MACE. In our study, the frequency of positive changes in CAVI over the course of a year was comparable, and we were also able to confirm the association of changes in CAVI with prognosis over a longer period of observation in a cohort of patients after CABG. Although in a previous study we showed the negative impact of the pathological CAVI index before CABG on the 10-year postoperative prognosis [
18], in the present study, the persistence of pathological CAVI during the year turned out to have a more pronounced prognostic significance.
Another option for studying the association of the dynamics of CAVI with prognosis is to study it against the background of psycho-emotional stress. Dynamic observation showed that in response to severe stress (earthquake in Japan), there was a significant increase in CAVI and, as a consequence, the number of cardiovascular events in patients with coronary artery disease [
19]. Also, the association of stress level and arterial stiffness is evidenced by a study by Tajima T. et al. [
20]. In a study of healthy individuals, they showed that in women, an increase in salivary alpha-amylase activity (a biomarker for chronic psychological stress) was associated with an increase in the CAVI index. Significantly, in healthy young adults, even a 5-minute mental counting test resulted in a significant increase in arterial stiffness within 30 minutes [
21] Interesting observations are presented in the article by Shimizu K et al [
22] - immediately before MACE (myocardial infarction, cerebral hemorrhage and aortic dissection), patients tended to rapidly increase in CAVI from ~0.5 to 1.0 over several weeks immediately preceding the event. The above scientific and clinical data have led to the advancement of the “smooth muscle cell contraction hypothesis” as the cause of plaque rupture. The authors of this hypothesis proposed that MACEs occur due to plaque rupture due to ischemic injury and necrosis caused by the rapid increase in CAVI in the background of an initially elevated CAVI [
23]. It can also be assumed that increased arterial stiffness is a factor mediating the effect of psychoemotional stress on prognosis. In addition, it is the initially increased arterial stiffness that contributes to the implementation of psychoemotional stress as a trigger for the development of MACE in cardiac patients.
The clinical significance of this study seems to us to be versatile. First, it emphasizes the need to assess CAVI over time to identify the group of patients at greatest risk of developing MACE in the future. Secondly, it seems appropriate to use CAVI as an indicator of the effectiveness of therapeutic and preventive measures. Herewith the range of influences can be very wide - from educational activities [
24], physical training [
25] and lifestyle correction [
15] to the appointment of optimal drug therapy [
26]. At the same time, assessing the dynamics of CAVI may be useful in assessing the effectiveness of therapy, since even drugs of the same group can have different effects on arterial stiffness [
16].
Considering the adverse effect of stress on arterial stiffness, which mediates the development of MACE, another direction in the treatment of such patients may be behavioral therapy. Currently, there are no studies examining the effect of stress-limiting therapy on improving vascular function, as already noted [
27]. Accordingly, future research is needed to determine whether stress-reducing behavioral interventions can lead to reductions in cardiovascular events through improvements in CAVI.
5. Study limitations
When evaluating the results of this study, its limitations should be considered. First, we did not include patients with certain comorbid conditions (atrial fibrillation, low ejection fraction, ABI values less than 0.9, presence of valvular lesions) in order to be able to correctly evaluate CAVI. Thus, the predictive value of CAVI dynamics can only be attributed to this sample of patients and cannot be extended to all patients after CABG. Accordingly, our analysis did not include patients with peripheral arterial disease, which could influence the relative contribution of the presence of subclinical carotid stenosis and unfavorable changes in the CAVI index to long-term prognosis after CABG. Second, the sample size is relatively small, so our results can be considered preliminary, which should be confirmed in a study with a larger sample or in a multicenter study. Currently, such a study is already underway - the Cardiovascular Prognostic COUPLING Study [
28], which will resolve the issue of confirming our data. Third, we did not conduct targeted monitoring of the therapy of the patients included in the study; they received treatment from doctors at their place of residence in accordance with current recommendations. However, inclusion of therapy received in multiple regression models did not reveal an independent effect on prognosis.
6. Conclusions
In the present study, one year after CABG surgery, 45% of patients showed positive dynamics or stable normal CAVI values. At ten-year follow-up, these patients, when compared with patients with negative dynamics or persistently pathological CAVI, showed a decrease in all-cause mortality and the incidence of the composite endpoint. Among the independent predictors of overall mortality and development of combined endpoint were negative dynamics of CAVI during the year and the presence of subclinical carotid stenoses during preoperative examination. In further studies, it is necessary to study what interventions in patients after CABG can cause favorable dynamics of CAVI, to what extent such dynamics can improve the prognosis, and also whether behavioral interventions can improve CAVI or help reduce the development of MACE in such patients.
Funding
This study was supported by the Complex Program of Basic Research under the Siberian Branch of the Russian Academy of Sciences within the Basic Research Topic of Research Institute for Complex Issues of Cardiovascular Diseases No. 0419-2022-0002.
Conflict of Interest
All authors declare no conflicts of interest.
References
- Sattartabar, B.; Ajam, A.; Pashang, M.; Jalali, A.; Sadeghian, S.; Mortazavi, H.; Mansourian, S.; Bagheri, J.; Karimi, A.A.; Hosseini, K. Sex and age difference in risk factor distribution, trend, and long-term outcome of patients undergoing isolated coronary artery bypass graft surgery. BMC Cardiovasc. Disord. 2021, 21, 460. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Akbari, K.; Shafiee, A.; Zoroufian, A.; Jalali, A.; Samimi, S.; Pashang, M.; Hosseini, K.; Bagheri, J.; Masoudkabir, F. Time-varying effect of postoperative cholesterol profile on long-term outcomes of isolated coronary artery bypass graft surgery. Lipids Health Dis. 2023, 22, 163. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Li, F.; Zhang, H.; Zeng, J.; Su, X.; Qu, J.; Lin, S.; Gu, D.; Rao, C.; Zhao, Y.; Zheng, Z. Impact of High Lipoprotein(a) on Long-Term Survival Following Coronary Artery Bypass Grafting. J Am Heart Assoc. 2024, e031322. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Bauer, T.M.; Yaser, J.M.; Daramola, T.; Mansour, A.I.; Ailawadi, G.; Pagani, F.D.; Theurer, P.; Likosky, D.S.; Keteyian, S.J.; Thompson, M.P. Cardiac Rehabilitation Reduces 2-Year Mortality After Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2023, 116, 1099–1105. [Google Scholar] [CrossRef]
- Townsend, R.R.; Wilkinson, I.B.; Schiffrin, E.L., et al.; et al. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American Heart Association. Hypertension 2015, 66, 698–722. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K.; Utino, J.; Otsuka, K.; Takata, M. A novel blood pressure-independent arterial wall stiffness parameter: Cardio-ankle vascular index (CAVI). J. Atheroscler. Thromb. 2006, 13, 101–107. [Google Scholar] [CrossRef]
- Shirai, K.; Hiruta, N.; Song, M.; Kurosu, T.; Suzuki, J.; Tomaru, T.; Miyashita, Y.; Saiki, A.; Takahashi, M.; Suzuki, K.; et al. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: Theory, evidence and perspectives. J. Atheroscler. Thromb. 2011, 18, 924–938. [Google Scholar] [CrossRef]
- Matsushita, K.; Ding, N.; Kim, E.D.; Budoff, M.; Chirinos, J.A.; Fernhall, B.; Hamburg, N.M.; Kario, K.; Miyoshi, T.; Tanaka, H.; Townsend, R. Cardio-ankle vascular index and cardiovascular disease: Systematic review and meta-analysis of prospective and cross-sectional studies. J. Clin. Hypertens (Greenwich) 2019, 21, 16–24. [Google Scholar] [CrossRef]
- Nagayama, D. Psychological stress-induced increase in the cardio-ankle vascular index (CAVI) may be a predictor of cardiovascular events. Hypertens Res. 2022, 45, 1672–1674. [Google Scholar] [CrossRef] [PubMed]
- Gohbara, M.; Iwahashi, N.; Sano, Y.; Akiyama, E.; Maejima, N.; Tsukahara, K.; Hibi, K.; Kosuge, M.; Ebina, T.; Umemura, S.; Kimura, K. Clinical Impact of the Cardio-Ankle Vascular Index for Predicting Cardiovascular Events After Acute Coronary Syndrome. Circ. J. 2016, 80, 1420–1426. [Google Scholar] [CrossRef]
- Kirigaya, J.; Iwahashi, N.; Tahakashi, H.; Minamimoto, Y.; Gohbara, M.; Abe, T.; Akiyama, E.; Okada, K.; Matsuzawa, Y.; Maejima, N.; Hibi, K.; Kosuge, M.; Ebina, T.; Tamura, K.; Kimura, K. Impact of Cardio-Ankle Vascular Index on Long-Term Outcome in Patients with Acute Coronary Syndrome. J. Atheroscler. Thromb. 2020, 27, 657–668. [Google Scholar] [CrossRef]
- Otsuka, T.; Fukuda, S.; Shimada, K., et al.; et al. Serial assessment of arterial stiffness by cardio-ankle vascular index for prediction of future cardiovascular events in patients with coronary artery disease. Hypertens Res. 2014, 37, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Sumin, A.N.; Shcheglova, A.V.; ZHidkova, I.I., et al.; et al. Assessment of arterial stiffness by cardio-ankle vascular index for prediction of five-year cardiovascular events after coronary artery bypass surgery. Glob Heart. 2021, 16, 90. [Google Scholar] [CrossRef]
- Shirai, K.; Saiki, A.; Nagayama, D.; Tatsuno, I.; Shimizu, K.; Takahashi, M. The Role of Monitoring Arterial Stiffness with Cardio-Ankle Vascular Index in the Control of Lifestyle-Related Diseases. Pulse 2015, 3, 118–133. [Google Scholar] [CrossRef]
- Peng, F.; Pan, H.; Wang, B.; Lin, J.; Niu, W. The impact of angiotensin receptor blockers on arterial stiffness: A meta-analysis. Hypertens Res. 2015, 38, 613–620. [Google Scholar] [CrossRef]
- Saiki, A.; Watanabe, Y.; Yamaguchi, T.; Ohira, M.; Nagayama, D.; Sato, N.; Kanayama, M.; Takahashi, M.; Shimizu, K.; Moroi, M.; Miyashita, Y.; Shirai, K.; Tatsuno, I. CAVI-Lowering Effect of Pitavastatin May Be Involved in the Prevention of Cardiovascular Disease: Subgroup Analysis of the TOHO-LIP. J. Atheroscler. Thromb. 2021, 28, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Sumin, A.N.; Shcheglova, A.V.; Ivanov, S.V.; Barbarash, O.L. Long-Term Prognosis after Coronary Artery Bypass Grafting: The Impact of Arterial Stiffness and Multifocal Atherosclerosis. J. Clin. Med. 2022, 11, 4585. [Google Scholar] [CrossRef]
- Shimizu, K.; Takahashi, M.; Shirai, K. A huge earthquake hardened arterial stiffness monitored with cardio-ankle vascular index. J. Atheroscler. Thromb. 2013, 20, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Tajima, T.; Ikeda, A.; Steptoe, A.; Takahashi, K.; Maruyama, K.; Tomooka, K.; Saito, I.; Tanigawa, T. The independent association between salivary alpha-amylase activity and arterial stiffness in Japanese men and women: The Toon Health Study. Hypertens Res. 2022, 45, 1249–1262. [Google Scholar] [CrossRef]
- Kume, D.; Nishiwaki, M.; Hotta, N.; Endoh, H. Impact of acute mental stress on segmental arterial stiffness. Eur. J. Appl. Physiol. 2020, 120, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Takahashi, M.; Sato, S.; Saiki, A.; Nagayama, D.; Harada, M.; Miyazaki, C.; Takahara, A.; Shirai, K. Rapid Rise of Cardio-Ankle Vascular Index May Be a Trigger of Cerebro-Cardiovascular Events: Proposal of Smooth Muscle Cell Contraction Theory for Plaque Rupture. Vasc Health Risk Manag. 2021, 17, 37–47. [Google Scholar] [CrossRef]
- Shimizu, K.; Takahashi, M.; Sato, S.; Saiki, A.; Nagayama, D.; Hitsumoto, T.; Takahara, A.; Shirai, K. Rapid Rise in Cardio-Ankle Vascular Index as a Predictor of Impending Cardiovascular Events -Smooth Muscle Cell Contraction Hypothesis for Plaque Rupture. Vasc Health Risk Manag. 2022, 18, 879–886. [Google Scholar] [CrossRef]
- Uemura, K.; Yamada, M.; Kuzuya, M.; Okamoto, H. Effects of Active Learning Education on Arterial Stiffness of Older Adults with Low Health Literacy: A Randomized Controlled Trial. J. Atheroscler. Thromb. 2021, 28, 865–872. [Google Scholar] [CrossRef]
- Zhou, Z.; Hou, L.; Cui, M.; Mourot, L.; Zhu, W. Acute effects of low-volume intermittent versus higher-volume continuous exercise on arterial stiffness in healthy young men. Sci. Rep. 2022, 12, 1749. [Google Scholar] [CrossRef]
- Kawabata, T.; Kubozono, T.; Ojima, S.; Kawasoe, S.; Akasaki, Y.; Salim, A.A.; Ikeda, Y.; Miyata, M.; Takenaka, T.; Ohishi, M. Insufficient blood pressure control is independently associated with increased arterial stiffness. Hypertens Res. 2022, 45, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, D.; Fujishiro, K.; Suzuki, K.; Shirai, K. Comparison of Predictive Ability of Arterial Stiffness Parameters Including Cardio-Ankle Vascular Index, Pulse Wave Velocity and Cardio-Ankle Vascular Index0. Vasc Health Risk Manag. 2022, 18, 735–745. [Google Scholar] [CrossRef]
- Kario, K.; Kabutoya, T.; Fujiwara, T.; Negishi, K.; Nishizawa, M.; Yamamoto, M.; Yamagiwa, K.; Kawashima, A.; Yoshida, T.; Nakazato, J.; Matsui, Y.; Sekizuka, H.; Abe, H.; Abe, Y.; Fujita, Y.; Sato, K.; Narita, K.; Tsuchiya, N.; Kubota, Y.; Hashizume, T.; Hoshide, S. Rationale, design, and baseline characteristics of the Cardiovascular Prognostic COUPLING Study in Japan (the COUPLING Registry). J. Clin. Hypertens (Greenwich). 2020, 22, 465–474. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Flow chart of the study design from screening to completion of the trial. CAVI – cardio-ankle vascular index; ABI ankle-brachial index; LVEF left ventricular ejection fraction; CABG - coronary artery bypass graft.
Figure 1.
Flow chart of the study design from screening to completion of the trial. CAVI – cardio-ankle vascular index; ABI ankle-brachial index; LVEF left ventricular ejection fraction; CABG - coronary artery bypass graft.
Figure 2.
Complications of the ten-year period depending on the dynamics of CAVI in patients with coronary heart disease who underwent CABG. Abbreviations: CAG - Coronarography; PCI - percutaneous coronary intervention, TIA - transischemic attack, MI - myocardial infarction, MACE - major adverse cardiac events.
Figure 2.
Complications of the ten-year period depending on the dynamics of CAVI in patients with coronary heart disease who underwent CABG. Abbreviations: CAG - Coronarography; PCI - percutaneous coronary intervention, TIA - transischemic attack, MI - myocardial infarction, MACE - major adverse cardiac events.
Figure 3.
Receiver operating characteristic curve analysis. Performance of baseline parameters (values of CAVI and presence of carotid stenoses ≥30%) in discriminating сombined end-point development in ten-year period after CABG.
Figure 3.
Receiver operating characteristic curve analysis. Performance of baseline parameters (values of CAVI and presence of carotid stenoses ≥30%) in discriminating сombined end-point development in ten-year period after CABG.
Figure 4.
Receiver operating characteristic curve analysis. Performance of baseline parameters (values of CAVI and presence of carotid stenoses ≥30%) in discriminating all-cause mortality development in ten-year period after CABG.
Figure 4.
Receiver operating characteristic curve analysis. Performance of baseline parameters (values of CAVI and presence of carotid stenoses ≥30%) in discriminating all-cause mortality development in ten-year period after CABG.
Table 1.
Comparison of the initial characteristics in groups with improved or worsened CAVI one year after CABG.
Table 1.
Comparison of the initial characteristics in groups with improved or worsened CAVI one year after CABG.
| |
Group with improved CAVI (n=94) |
Group with impaired CAVI (n=116) |
P-value |
| Age, years |
57.5 [53.0; 64.0] |
59.0 [55.0; 64.5] |
0.118 |
| Male, n (%) |
66 (70.2) |
87 (75.0) |
0.437 |
| Height, cm |
169.0 [163.0; 176.0] |
170.0 [164.0; 176.0] |
0.646 |
| Weight, kg |
80.0 [72.0; 90.0] |
80.0 [70.0; 89.0] |
0.914 |
| Body mass index, kgm–2 |
28.1 [24.6; 31.6] |
28.0 [25.5; 31.1] |
0.861 |
| Myocardial infarction, (n %) |
54 (57.5) |
72 (62.1) |
0.496 |
| Hypertension, n (%) |
73 (77.7) |
104 (89.7) |
0.017 |
| Stroke, (n %) |
6 (6.4) |
9 (7.8) |
0.7 |
| Transischemic attack, (n %) |
0 |
2 (1.72) |
0.2 |
| Diabetes, n (%) |
7 (7.5) |
23 (19.8) |
0.011 |
| PCI, (n %) |
4 (4.3) |
9 (7.8) |
0.294 |
| CABG, (n %) |
1 (1.1) |
1 (0.9) |
0.881 |
| Carotid endarterectomy, (n %) |
0 |
3 (2.6) |
0.116 |
| Smoking experience, years |
30.0 [20.0; 40.0] |
30.0 [20.0; 40.0] |
0.478 |
| Smoking, (n %) |
20 (21.3) |
32 (27.6) |
0.292 |
Table 2.
Comparison of clinical and laboratory-instrumental characteristics of data in groups with improved or worsened CAVI one year after CABG.
Table 2.
Comparison of clinical and laboratory-instrumental characteristics of data in groups with improved or worsened CAVI one year after CABG.
| |
Group with improved CAVI
(n=94) |
Group with impaired CAVI
(n=116) |
P-value |
| The clinical characteristics |
| Angina basal (n %) |
73 (77.7) |
94 (81.0) |
0.546 |
| Angina after year (n %) |
9 (9.6) |
10 (8.6) |
0.812 |
| Heart failure II-III FK NYHA basal (n, %) |
18 (19.2) |
41 (35.3) |
0.009 |
| Heart failure II-III FK NYHA after year (n, %) |
12 (12.9) |
15 (13.2) |
0.956 |
| Laboratory data |
| Total cholesterol basal, mmol/L |
5.0 [4.2; 5.9] |
4.6 [4.0; 5.6] |
0.056 |
| Total cholesterol after year, mmol/L |
4.9 [4.1; 6.2] |
4.35 [3.7; 5.5] |
0.019 |
| LDL cholesterol basal, mmol/L |
3.04 [2.1; 3.9] |
2.8 [2.22; 3.5] |
0.139 |
| LDL cholesterol after year, mmol/L |
3.2 [2.3; 3.8] |
2.4 [1.9; 3.4] |
0.017 |
| Fasting glucose basal, mmol/L basal |
5.5 [5.0; 6.2] |
5.6 [5.2; ] |
0.155 |
| Fasting glucose after year, mmol/L |
5.5 [5.0; 6.2] |
5.8 [5.3; 6.9] |
0.066 |
| GFR CKD-EPI basal, ml/min/1.73 m2 |
80.9 [65.5; 102.7] |
82.1 [65.4; 97.7] |
0.966 |
| GFR CKD-EPI after year, ml/min/1.73m2 |
92.8 [70.6; 112.7] |
86.2 [71.4;106.0] |
0.553 |
| Echocardiography |
| LV ejection fraction basal, % |
60.0 [54.0; 64.0] |
60.0 [53.0; 64.0] |
0.483 |
| LV ejection fraction after year, % |
62.0 [57.0; 65.0] |
61.0 [51.0; 65.0] |
0.116 |
| E/A basal |
0.88 [0.7; 1.2] |
0.8 [0.7; 1.1] |
0.653 |
| E/A after year |
0.8 [0.51; 1.1] |
0.68 [0.5; 1.0] |
0.133 |
| Vasera-1000 |
| R-CAVI basal |
8.4 [7.6; 9.3] |
8.5 [7.9; 9.3] |
0.458 |
| R-CAVI after year |
7.7 [7.1; 8.2] |
9.1 [8.6; 9.7] |
<0.001 |
| L-CAVI basal |
7.6 [7.1; 8.1] |
8.5 [7.9; 9.4] |
0.371 |
| L-CAVI after year |
7.7 [7.1; 8.1] |
9.1 [8.3; 9.6] |
<0.001 |
| R- ABI basal |
1.12 [1.03; 1.22] |
1.14 [1.08; 1.2] |
0.351 |
| R- ABI after year |
1.08 [099; 1.17] |
1.09 [0.99; 1.19] |
0.501 |
| L- ABI basal |
1.1 [1.02; 1.16] |
1.11 [1.04; 1.18] |
0.402 |
| L-ABI after year |
1.03 [0.92; 1.11] |
1.05 [094; 1.13] |
0.117 |
Table 3.
Severity of damage to coronary arteries, non-coronary arterial basins in groups with improved or worsened CAVI one year after CABG.
Table 3.
Severity of damage to coronary arteries, non-coronary arterial basins in groups with improved or worsened CAVI one year after CABG.
| |
Group with improved CAVI
(n=94) |
Group with impaired CAVI
(n=116) |
P-value |
| Coronary angiography |
| LCA ≥50%, (n %) |
17 (18.1) |
29 (25.0) |
0.228 |
| Single-vessel coronary artery ≥70%, (n %) |
17 (18.1) |
26 (22.41) |
0.429 |
| Bicavascular coronary artery ≥70%, (n, %) |
30 (31.9) |
38 (32.8) |
0.896 |
| Tricovascular coronary artery (n, %) |
40 (42.6) |
46 (39.7) |
0.671 |
| Non-coronary atherosclerosis |
| Carotid artery stenosis ≥30%, basal (n,%) |
17 (18.1) |
21 (18.1) |
0.997 |
| Carotid artery stenosis≥30%, after year (n,%) |
22 (24.4) |
27 (23.7) |
0.899 |
| Carotid artery stenosis ≥50%, basal (n,%) |
11 (11.7) |
16 (13.8) |
0.652 |
| Carotid artery stenosis ≥50%, after year (n,%) |
7 (7.8) |
12 (10.5) |
0.502 |
Table 4.
The main characteristics of coronary artery bypass surgery in groups with improved or worsened CAVI one year after CABG.
Table 4.
The main characteristics of coronary artery bypass surgery in groups with improved or worsened CAVI one year after CABG.
| |
Group with improved CAVI (n=94) |
Group with impaired
CAVI (n=116) |
P-value |
| Euroscore (scores) |
2.5 [1.0; 4.0] |
2.0 [2.0; 4.0] |
0.630 |
| Euroscore, % |
1.33 (0.88; 2.35) |
1.54 (1.0; 2.4) |
0.464 |
| Cardiopulmonary bypass, (n %) |
84 (89.4) |
96 (82.8) |
0.174 |
| Number of shunts |
3.0 [2.0; 3.0] |
3.0 [2.0; 3.0] |
0.542 |
| Cardiopulmonary bypass time, minutes |
98.5
[77.5; 110.0] |
94.0
[84.0; 107.0] |
0.795 |
| Total operation time, minutes |
240.0 [204.0; 300.0] |
240.0 [195.0; 273.0] |
0.330 |
| Ventriculoplasty, (n %) |
5 (5.32) |
4 (3.5) |
0.505 |
| Thrombectomy, (n, %) |
4 (4.3) |
1 (0.9) |
0.108 |
| Carotid endarterectomy, (n %) |
1 (1.1) |
3 (2.6) |
0.422 |
| Radiofrequency ablation, (n %) |
1 (1.1) |
3 (2.6) |
0.422 |
Table 5.
Comparison of medication use in groups with improved or worsened CAVI one year after CABG.
Table 5.
Comparison of medication use in groups with improved or worsened CAVI one year after CABG.
| |
Group with improved CAVI (n=94) |
Group with impaired
CAVI (n=116) |
P-value |
| Preoperative medicine therapy |
| Beta-blockers, n (%) |
62 (66.0) |
74 (63.8) |
0.943 |
| CCBs, n (%) |
20 (21.3) |
25 (21.6) |
0.883 |
| Statins, n (%) |
44 (46.8) |
62 (54.9) |
0.248 |
| ARB, n (%) |
7 (7.5) |
10 (8.6) |
0.714 |
| ACEI, n (%) |
44 (46.8) |
54 (46.6) |
0.982 |
| Aspirin, n (%) |
62 (66.0) |
82 (70.7) |
0.303 |
| 1 years medicine therapy |
| Beta-blockers, n (%) |
76 (80.8) |
98 (84.5) |
0.543 |
| CCBs, n (%) |
26 (27.7) |
25 (21.6) |
0.283 |
| Statins, n (%) |
84 (89.4) |
107 (92.2) |
0.623 |
| ARB, n (%) |
62 (65.6) |
76 (65.5) |
0.861 |
| ACEI, n (%) |
7 (7.5) |
11 (9.5) |
0.616 |
| Aspirin, n (%) |
80 (85.1) |
102 (87.9) |
0.682 |
| 10 years medicine therapy |
| Beta-blockers, n (%) |
57 (60.6) |
65 (56.0) |
0.501 |
| CCBs, n (%) |
23 (24.5) |
22 (19.0) |
0.333 |
| Statins, n (%) |
62 (65.9) |
69 (59.5) |
0.335 |
| ARB, n (%) |
41 (43.6) |
51 (44.0) |
0.959 |
| ACEI, n (%) |
19 (20.2) |
14 (12.1) |
0.106 |
| Aspirin, n (%) |
60 (63.8) |
65 (56.0) |
0.252 |
Table 6.
Results of binary logistic regression (forward LR method): association of factors with the risk of unfavorable long-term prognosis development after CABG.
Table 6.
Results of binary logistic regression (forward LR method): association of factors with the risk of unfavorable long-term prognosis development after CABG.
| |
|
B |
S.E. |
Wald |
df |
Sig. |
Exp(B) |
| All-cause mortality |
| Step 1 |
Number of shunts |
0.551 |
0.213 |
6.704 |
1 |
0.010 |
1.735 |
| Constant |
-2.646 |
0.593 |
19.912 |
1 |
0.000 |
0.071 |
| Step 2 |
Group with impaired CAVI |
0.876 |
0.376 |
5.421 |
1 |
0.020 |
2.400 |
| Number of shunts |
0.609 |
0.221 |
7.580 |
1 |
0.006 |
1.839 |
| Constant |
-4.185 |
0.929 |
20.306 |
1 |
0.000 |
0.015 |
| Step 3 |
Group with impaired CAVI |
0.852 |
0.378 |
5.083 |
1 |
0.024 |
2.345 |
| Carotid stenoses ≥30% |
0.843 |
0.432 |
3.810 |
1 |
0.051 |
2.323 |
| Number of shunts |
0.627 |
0.226 |
7.685 |
1 |
0.006 |
1.873 |
| Constant |
-4.367 |
0.942 |
21.518 |
1 |
0.000 |
0.013 |
| Combined end-point (all-cause death + non-fatal myocardial infarction + non-fatal stroke) |
| Step 1 |
Carotid stenoses ≥30% |
1.186 |
0.389 |
9.293 |
1 |
0.002 |
3.274 |
| Constant |
-0.829 |
0.173 |
22.982 |
1 |
0.000 |
0.436 |
| Step 2 |
Group with impaired CAVI |
0.853 |
0.322 |
6.995 |
1 |
0.008 |
2.346 |
| Carotid stenoses ≥30% |
1.208 |
0.398 |
9.201 |
1 |
0.002 |
3.347 |
| Constant |
-2.166 |
0.549 |
15.595 |
1 |
0.000 |
0.115 |
Table 7.
Receiver operating characteristic curve analysis. Performance of baseline parameters (values of CAVI and presence of carotid stenoses ≥30%) in discriminating сombined end-point development in ten-year period after CABG. Area under the curve.
Table 7.
Receiver operating characteristic curve analysis. Performance of baseline parameters (values of CAVI and presence of carotid stenoses ≥30%) in discriminating сombined end-point development in ten-year period after CABG. Area under the curve.
| Test Result Variable(s) |
|
Asymptotic 95% Confidence Interval |
| Area |
Std. Error |
Asymptotic Sig. |
Lower Bound |
Upper Bound |
| R_CAVI basal |
0.541 |
0.043 |
0.326 |
0.457 |
0.626 |
| L_CAVI basal |
0.530 |
0.043 |
0.481 |
0.446 |
0.613 |
| R_CAVI after year |
0.550 |
0.042 |
0.235 |
0.467 |
0.633 |
| L_CAVI_after year |
0.532 |
0.041 |
0.448 |
0.452 |
0.612 |
| Group with impaired CAVI |
0.596 |
0.041 |
0.023 |
0.516 |
0.676 |
| Carotid stenoses ≥30% |
0.577 |
0.043 |
0.068 |
0.493 |
0.661 |
Table 8.
Receiver operating characteristic curve analysis. Performance of baseline parameters (values of CAVI and presence of carotid stenoses ≥30%) in discriminating all-cause mortality development in ten-year period after CABG. Area under the curve.
Table 8.
Receiver operating characteristic curve analysis. Performance of baseline parameters (values of CAVI and presence of carotid stenoses ≥30%) in discriminating all-cause mortality development in ten-year period after CABG. Area under the curve.
| Test Result Variable(s) |
|
Asymptotic 95% Confidence Interval |
| Area |
Std. Error |
Asymptotic Sig. |
Lower Bound |
Upper Bound |
| R_CAVI basal |
0.541 |
0.049 |
0.397 |
0.444 |
0.637 |
| L_CAVI basal |
0.514 |
0.049 |
0.767 |
0.419 |
0.610 |
| R_CAVI after year |
0.567 |
0.048 |
0.165 |
0.472 |
0.662 |
| L_CAVI_after year |
0.547 |
0.047 |
0.330 |
0.455 |
0.639 |
| Group with impaired CAVI |
0.590 |
0.046 |
0.062 |
0.499 |
0.681 |
| Carotid stenoses ≥30% |
0.554 |
0.050 |
0.266 |
0.456 |
0.651 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).