Submitted:
04 March 2024
Posted:
05 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Description of biological Material
2.2. Preparation of culture medium and inoculation of D. melanogaster
2.3. Growing environment and experimental metal varinates Cu2+ (CuSO4) and Pb2+ (Pb(C2H3O2)2)
- -
- normal environment with grey (control) [81];
- -
- medium with grey, supplemented with copper sulphate solution CuSO4 -5H2O (0.50 mM, 1.00 mM, 2.00 mM and 4.00 mM);
- -
- medium with grey, supplemented with Pb(C2H3O2)2.·3H2O lead acetate solution (0.50 mM, 1.00 mM, 2.00 mM and 4.00 mM);
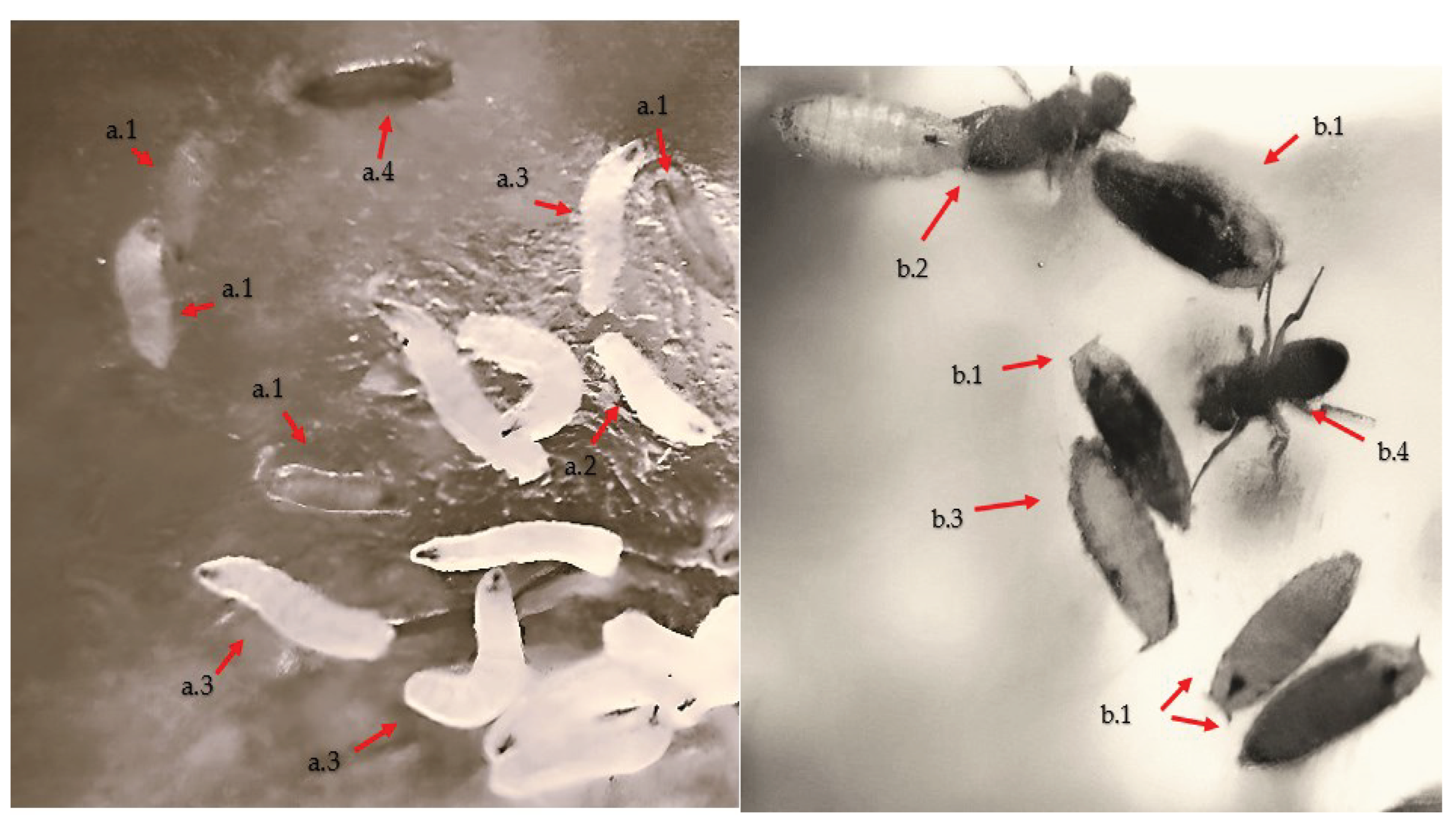
2.4. Statistical calculation methods used to determine proliferation and toxicity levels between Cu2+ and Pb2+
3. Results
3.1. Influence of genotype, concentration and type of metal Cu2+ and Pb2+ on proliferation in Drosophila melanogaster
| Analysis factor | df | SS | S2 | F | Pr(>F) | |
|---|---|---|---|---|---|---|
| Pupal stage | Concentration | 4 | 24437 | 6109 | 203.090 | < 2e-16 *** |
| Genotype | 3 | 5526 | 1842 | 61.234 | < 2e-16 *** | |
| Metal | 1 | 1687 | 1687 | 56.094 | 1.45e-13 *** | |
| Concentration: Genotype | 12 | 1132 | 94 | 3.136 | 0.000211 *** | |
| Concentration:Metal | 4 | 585 | 146 | 4.861 | 0.000691 *** | |
| Genotype:Metal | 3 | 80 | 27 | 0.884 | 0.448785 Ns | |
| Concentration: Genotype: Metal | 12 | 179 | 15 | 0.496 | 0.918151 Ns | |
| Residuals | 1060 | 31886 | 30 | |||
| Adult stage | Concentration | 4 | 12209 | 3052.3 | 135.964 | < 2e-16 *** |
| Genotype | 3 | 4569 | 1522.9 | 67.837 | < 2e-16 *** | |
| Metal | 1 | 893 | 893.5 | 39.799 | 5.55e-10 *** | |
| Concentration: Genotype | 12 | 551 | 45.9 | 2.044 | 0.0189 * | |
| Concentration:Metal | 4 | 274 | 68.5 | 3.051 | 0.0166 * | |
| Genotype:Metal | 3 | 65 | 21.7 | 0.968 | 0.4075 Ns | |
| Concentration: Genotype: Metal | 12 | 89 | 7.4 | 0.331 | 0.9836 Ns | |
| Residuals | 586 | 13155 | 22.4 |
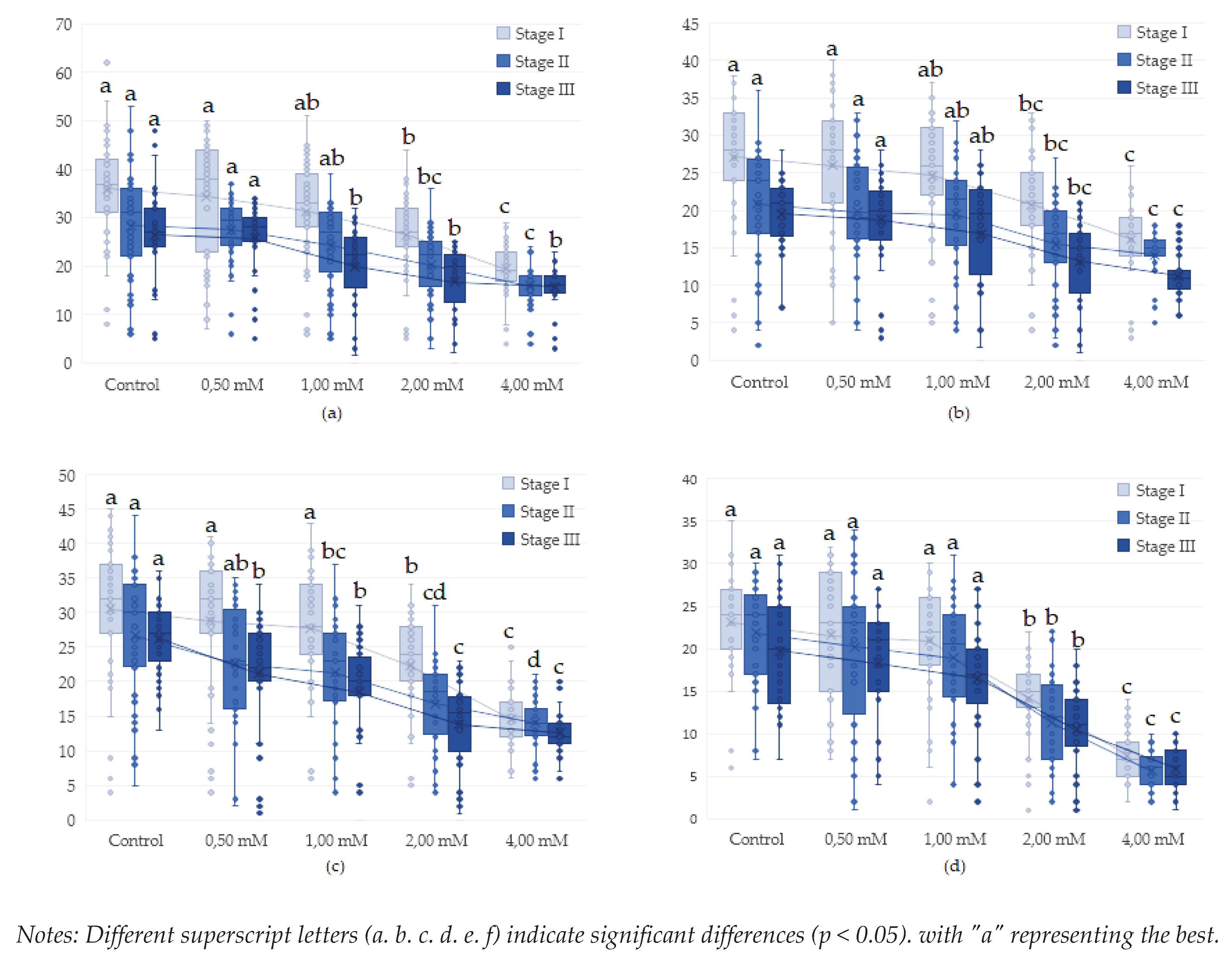
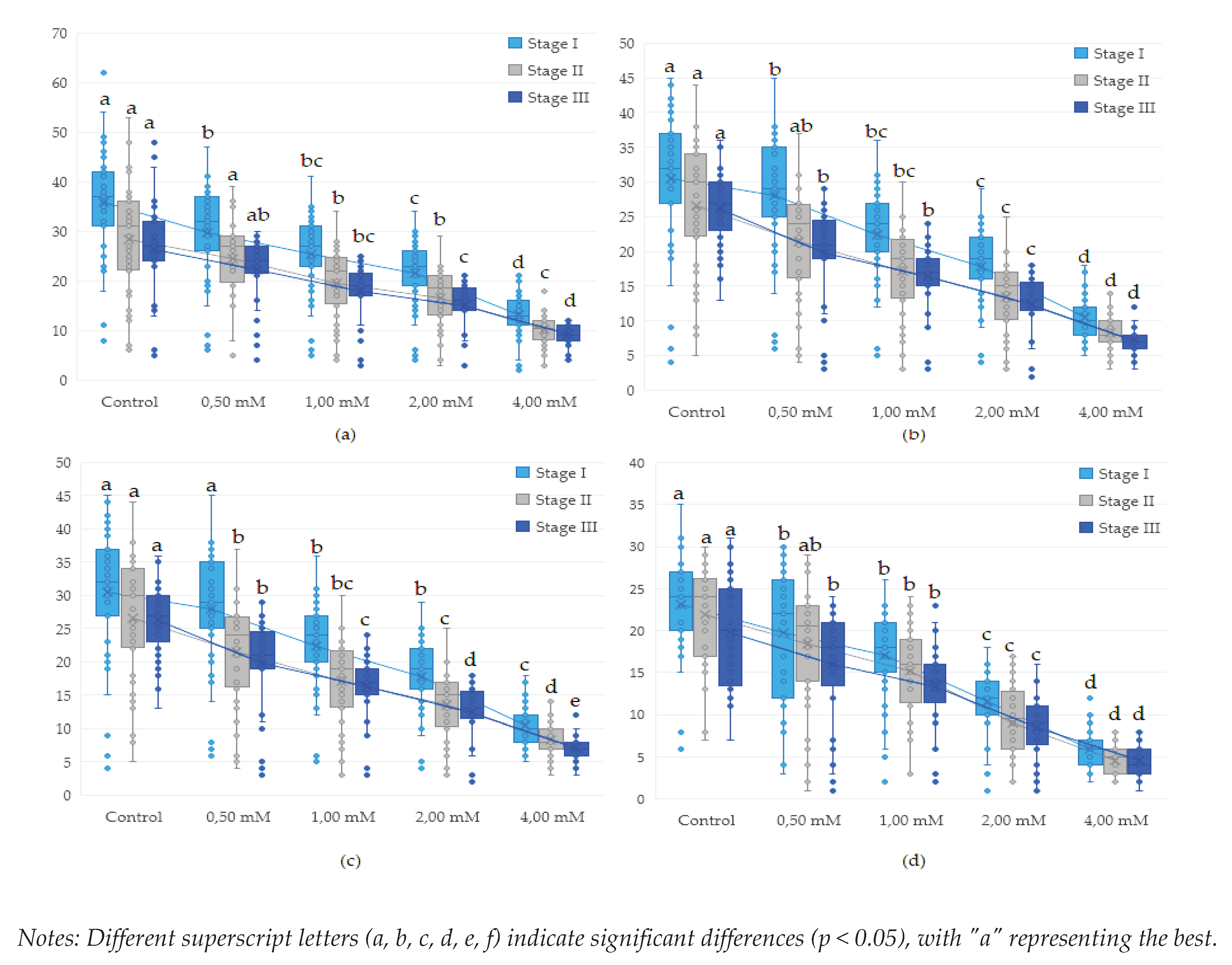
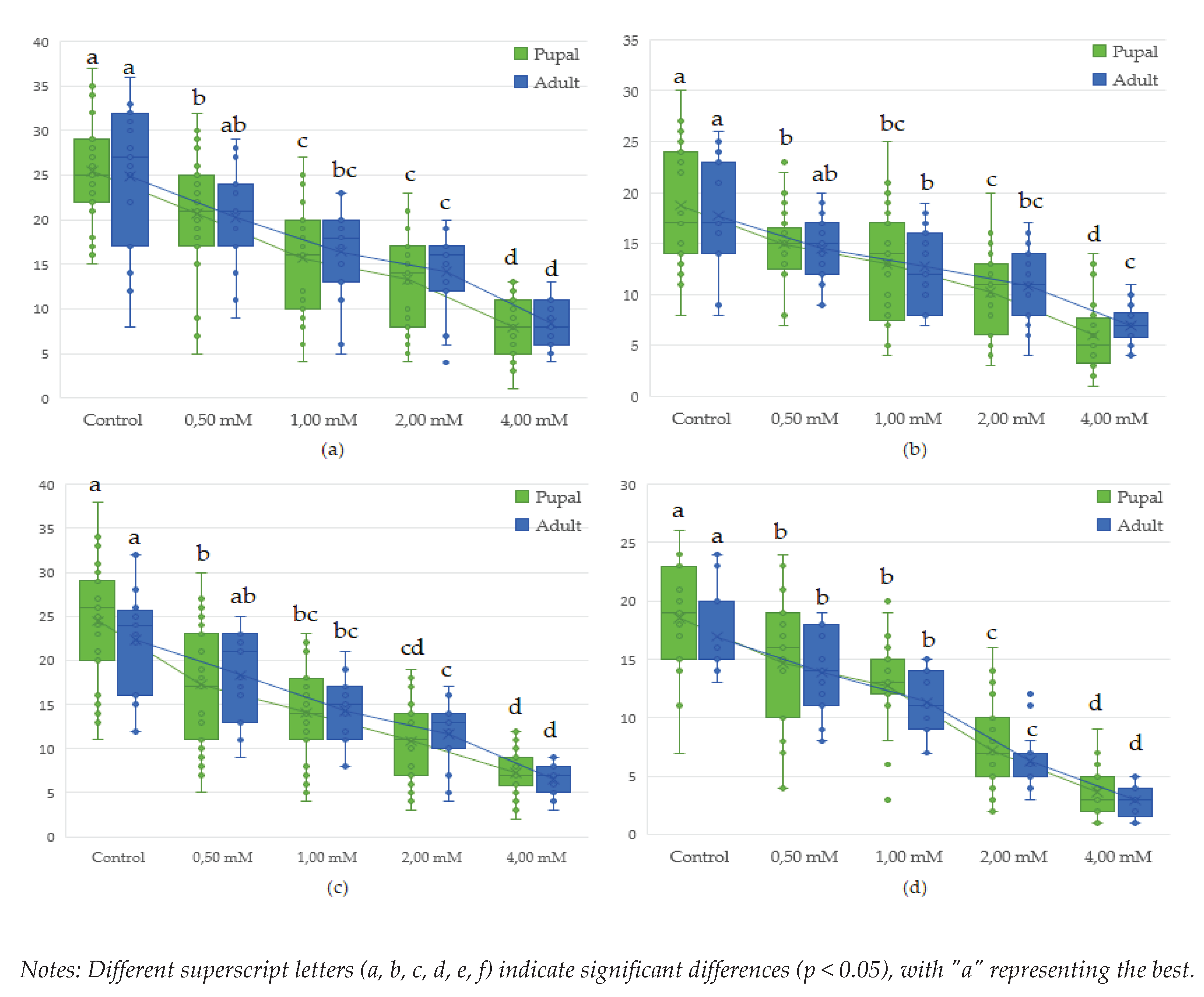
3.2. Influence of copper (CuSO4) on proliferation in Drosophila melanogaster during the life cycle (egg-adult)
| Genotype | Concentration (mM) | Copper (CuSO4) | |||||||||
| StageI | IC | StageII | IC | StageIII | IC | Pupal | IC | Adult | IC | ||
| Mean | Mean | Mean | Mean | Mean | |||||||
| wild | Control | 35.97a | 0.00 | 28.33a | 0.00 | 26.39a | 0.00 | 25.41a | 0.00 | 24.87a | 0.00 |
| 0.50 | 29.85b | 17.03 | 24.58abc | 13.24 | 22.24ab | 15.73 | 20.67ab | 18.66 | 20.27abc | 18.50 | |
| 1.00 | 25.41bcd | 29.37 | 19.58cdef | 30.88 | 17.85bcde | 32.38 | 15.67cde | 38.34 | 16.40cdef | 34.05 | |
| 2.00 | 21.56de | 40.06 | 16.58defgh | 41.47 | 15.03defg | 43.05 | 13.33def | 47.52 | 14.20def | 42.90 | |
| 4.00 | 13.23fgh | 63.22 | 10.14ijk | 64.22 | 9.24hijk | 64.98 | 7.90ghi | 68.92 | 8.39gh | 66.26 | |
| brown | Control | 27.08bc | 0.00 | 20.83cde | 0.00 | 19.52bcd | 0.00 | 18.74bc | 0.00 | 17.73bcd | 0.00 |
| 0.50 | 22.28cde | 17.71 | 17.83defgh | 14.40 | 16.39cdef | 15.99 | 14.96cdef | 20.16 | 14.47def | 18.42 | |
| 1.00 | 19.97de | 26.23 | 16.03efgh | 23.07 | 14.88efg | 23.76 | 13.04def | 30.43 | 12.73defg | 28.20 | |
| 2.00 | 16.85efg | 37.78 | 12.56hij | 39.73 | 11.67ghij | 40.22 | 10.22fgh | 45.45 | 10.80fgh | 39.10 | |
| 4.00 | 10.82hi | 60.04 | 7.03kl | 66.27 | 6.48kl | 66.77 | 6.00hi | 67.98 | 6.94hi | 60.84 | |
| white | Control | 30.54b | 0.00 | 26.64ab | 0.00 | 26.18a | 0.00 | 24.48a | 0.00 | 22.31ab | 0.00 |
| 0.50 | 28.00bc | 8.31 | 21.47bcd | 19.40 | 19.82bc | 24.31 | 17.26bcd | 29.50 | 18.33bcd | 17.83 | |
| 1.00 | 22.33cde | 26.87 | 17.14defgh | 35.66 | 16.30cdef | 37.73 | 14.07cdef | 42.51 | 14.27def | 36.06 | |
| 2.00 | 17.85ef | 41.56 | 13.53fghi | 49.22 | 12.30fghi | 53.01 | 10.85efg | 55.67 | 11.67efgh | 47.71 | |
| 4.00 | 10.46hi | 65.74 | 8.33ghij | 68.72 | 7.18jkl | 72.57 | 7.30ghi | 70.18 | 6.56hi | 70.62 | |
| white-vestigial | Control | 23.10cd | 0.00 | 21.85bcd | 0.00 | 19.70bc | 0.00 | 18.56bc | 0.00 | 16.93bcde | 0.00 |
| 0.50 | 19.79de | 14.32 | 18.42defg | 15.72 | 15.94cdefg | 19.08 | 14.63cdef | 21.16 | 13.87defg | 18.11 | |
| 1.00 | 17.05efg | 26.19 | 15.19fghi | 30.47 | 13.27efgh | 32.62 | 12.74def | 31.34 | 11.33efgh | 33.07 | |
| 2.00 | 11.51ghi | 50.17 | 9.03jkl | 58.69 | 8.45ijkl | 57.08 | 7.22ghi | 61.08 | 6.27hi | 62.99 | |
| 4.00 | 5.97i | 74.14 | 4.53l | 79.27 | 4.58l | 76.77 | 3.64i | 80.37 | 2.94i | 82.63 | |
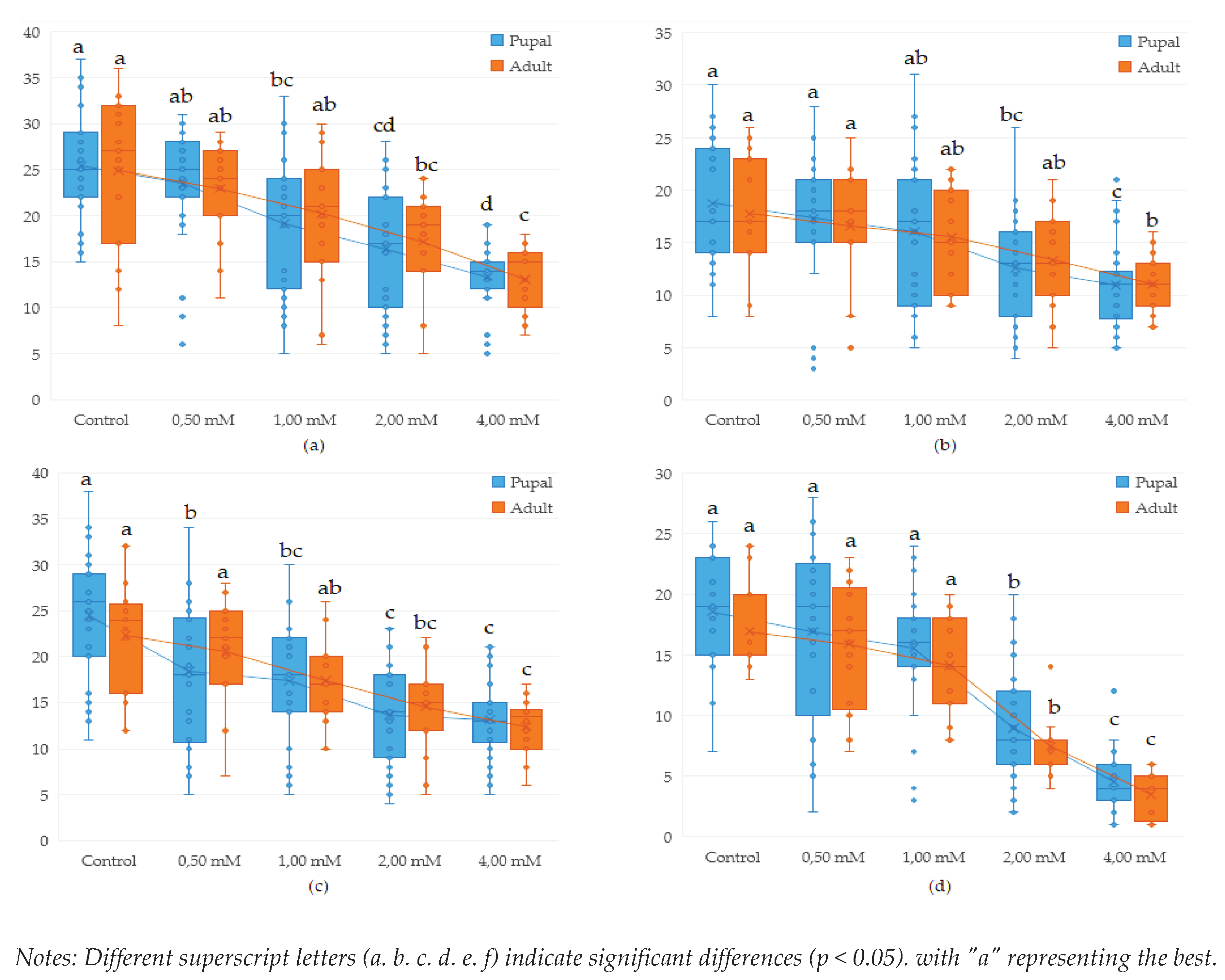
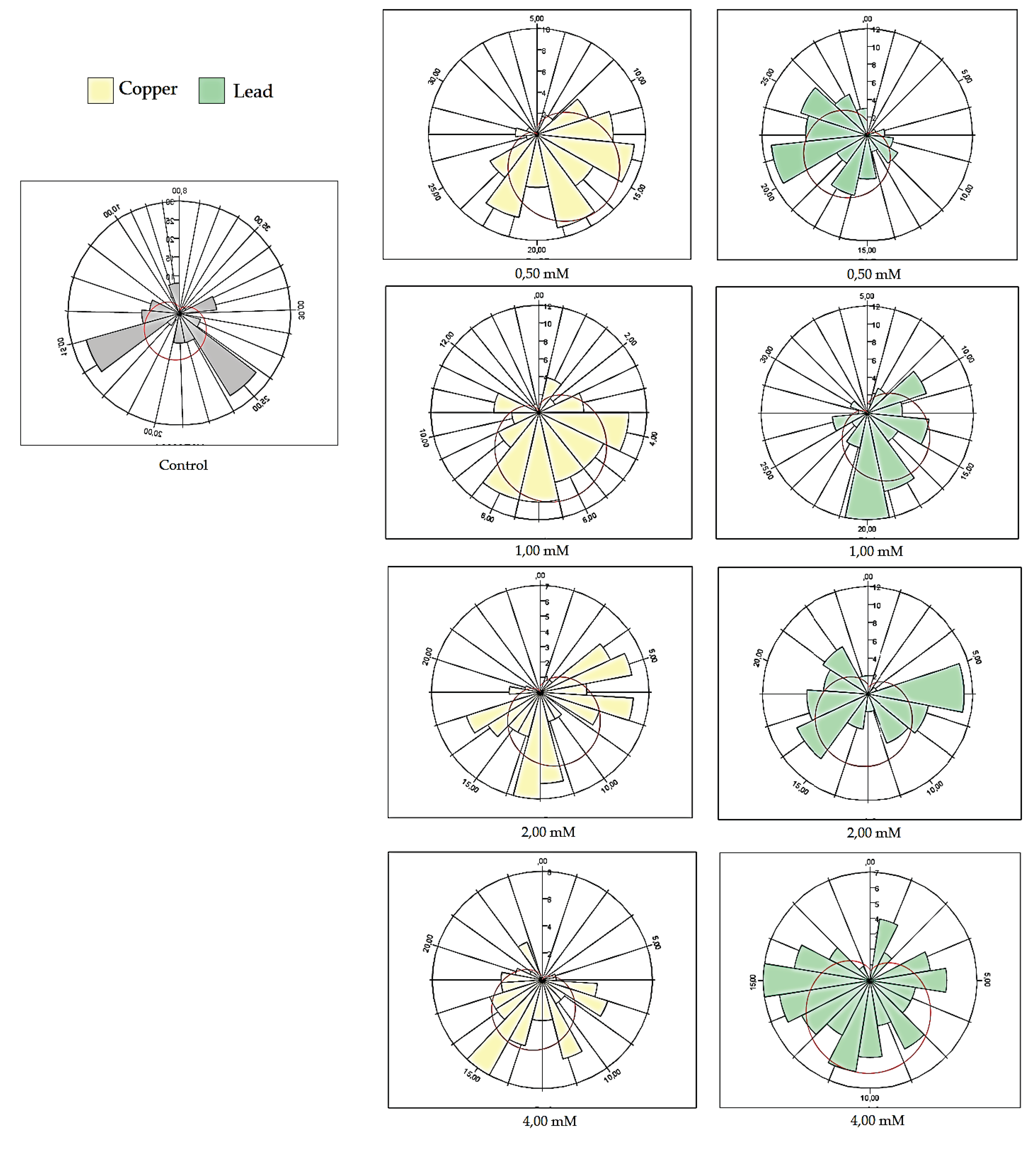
3.3. Influence of lead (Pb(C2H3O2)2 on proliferation in Drosophila melanogaster during the life cycle (egg-adult)
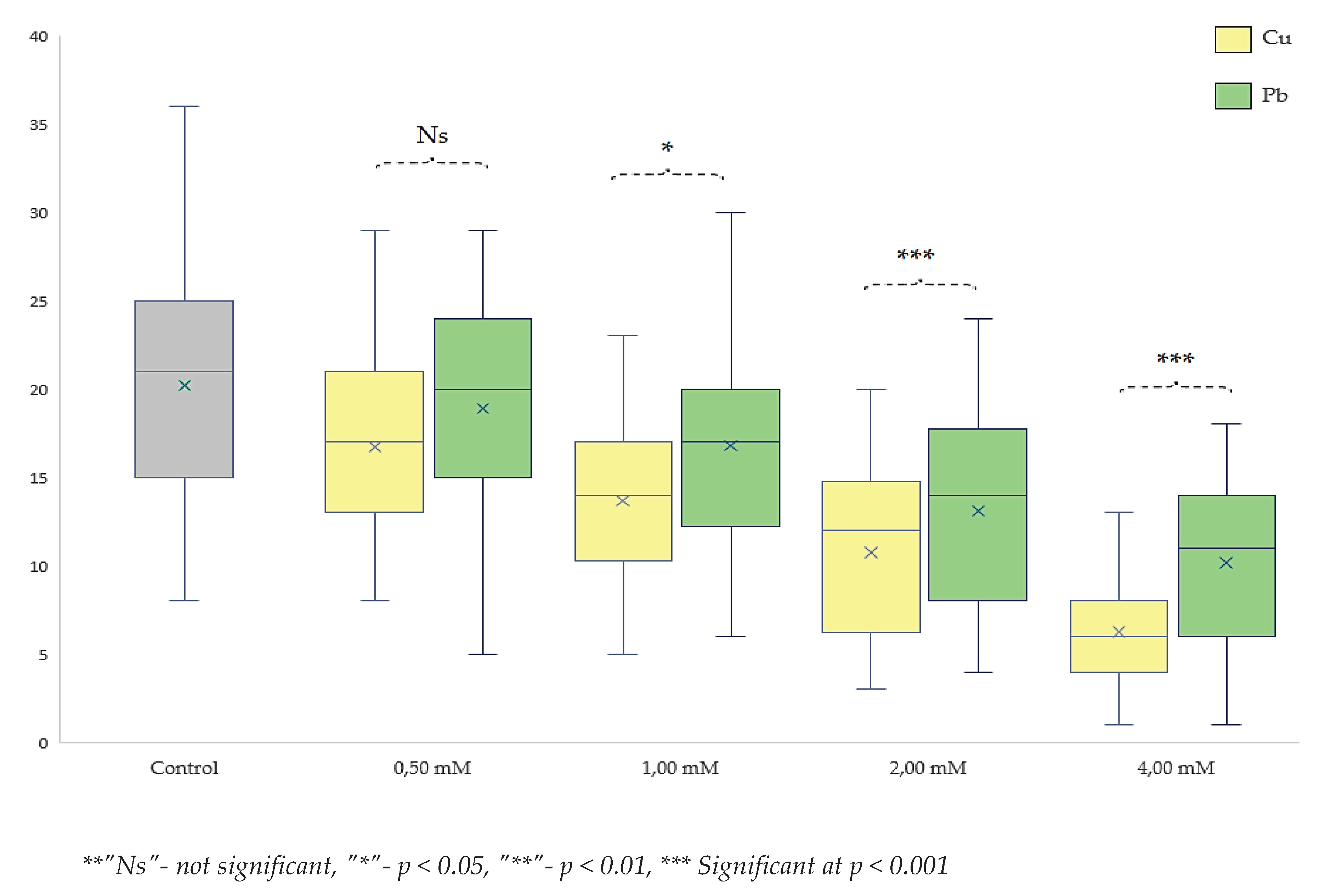
Comparative study of toxicity effects on drosophila melanogaster at various concentrations of lead and copper
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santarelli et al. ‘Drosophila melanogaster as a model to study autophagy in neurodegenerative diseases induced by proteinopathies’. Front. Neurosci. vol. 17. p. 1082047. 2023. Accessed: Dec. 01. 2023. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fnins.2023.1082047/full. [CrossRef]
- S. Bahadorani and A. J. Hilliker. ‘Biological and behavioral effects of heavy metals in Drosophila melanogaster adults and larvae’ J. Insect Behav. vol. 22. pp. 399-411. 2009. Accessed: Nov. 28. 2023. [Online]. Available: https://idp.springer.com/authorize/casa?redirect_uri=https://link.springer.com/article/10.1007/s10905-009-9181-4&casa_token=OVUFcjRGCKcAAAAA:9DkjNeV35QQ7C2M4GIWsE6dzITLw1AA536l5Rc32ReOEwAbtnpIRP2iKDcOI1xtFUOkz1LzARu9PBOFY. [CrossRef]
- C. V. Klimaczewski. A. Ecker. B. Piccoli. M. Aschner. N. V. Barbosa. and J. B. T. Rocha. ‘Peumus boldus attenuates copper-induced toxicity in Drosophila melanogaster’ Biomed. Pharmacother. vol. 97. pp. 1-8. Jan. 2018. [CrossRef]
- L. K. Venkareddy and Muralidhara. ‘Potential of casein as a nutrient intervention to alleviate lead (Pb) acetate-mediated oxidative stress and neurotoxicity: First evidence in Drosophila melanogaster’. NeuroToxicology vol. 48. pp. 142-151. May 2015. [CrossRef]
- S. Saini. L. Rani. N. Shukla. M. Banerjee. D. K. Chowdhuri. and N. K. Gautam. ‘Development of a Drosophila melanogaster based model for the assessment of cadmium and mercury mediated renal tubular toxicity’. Ecotoxicol. Environ. Saf. vol. 201. p. 110811. Sep. 2020. [CrossRef]
- F. Turna Demir. G. Akkoyunlu. and E. Demir. ‘Interactions of ingested polystyrene microplastics with heavy metals (Cadmium or Silver) as environmental pollutants: A comprehensive in vivo study using Drosophila melanogaster’. Biology vol. 11. no. 10. Art. no. 10. 2022. Accessed: Nov. 28. 2023. [Online]. Available: https://www.mdpi.com/2079-7737/11/10/1470. [CrossRef]
- Calap-Quintana. Pablo. Javier González-Fernández. Noelia Sebastiá-Ortega. José Vicente Llorens. and María Dolores Moltó. ‘Drosophila melanogaster Models of Metal-Related Human Diseases and Metal Toxicity’. Int. J. Mol. Sci. vol. 18. no. 7. Art. no. 7. 2017. [CrossRef]
- ‘IJMS | Free Full-Text | Drosophila melanogaster Models of Metal-Related Human Diseases and Metal Toxicity’. Accessed: Nov. 28. 2023. [Online]. Available: https://www.mdpi.com/1422-0067/18/7/1456. [CrossRef]
- E. GÜNEȘ and E. ȘENSOY. ‘The Effect of Turkısh Coffee and Cadmıum Acetate on Drosophila melanogaster’. J. Int. Environ. Appl. Sci. vol. 15. no. 1. Accessed: Nov. 28. 2023. [Online]. Available: https://dergipark.org.tr/en/pub/jieas/issue/53199/697665.
- L. Bonilla-Ramirez. M. Jimenez-Del-Rio. and C. Velez-Pardo. ‘Acute and chronic metal exposure impairs locomotion activity in Drosophila melanogaster: a model to study Parkinsonism’. Biometals vol. 24. pp. 1045-1057. 2011. Accessed: Jan. 16. 2024. [Online]. Available: https://idp.springer.com/authorize/casa?redirect_uri=https://link.springer.com/article/10.1007/s10534-011-9463-0&casa_token=tDZ6LaLxDuIAAAAA:G24wIqXMSR0Z1-tYE_ICt7bGN8h7A1w6PqX-CE7rWrzDRYe6Np_o9w4xCuWvBDgCWwI9uQIcaeQ6TCbS. [CrossRef]
- M. N. Arbeitman et al. ‘Gene Expression During the Life Cycle of Drosophila melanogaster’. Science vvol. 297. no. 5590. pp. 2270-2275. Sep. 2002. [CrossRef]
- M. Mishra and M. Panda. ‘Reactive oxygen species: the root cause of nanoparticle-induced toxicity in Drosophila melanogaster’. Free Radic. Res. vol. 55. no. 8. pp. 919-935. Aug. 2021. [CrossRef]
- M. Alaraby. A. Hernández. and R. Marcos. ‘Copper oxide nanoparticles and copper sulphate act as antigenotoxic agents in drosophila melanogaster’. Environ. Mol. Mutagen vol. 58. no. 1. pp. 46-55. 2017. [CrossRef]
- E. Baeg. K. Sooklert. and A. Sereemaspun. ‘Copper Oxide Nanoparticles Cause a Dose-Dependent Toxicity via Inducing Reactive Oxygen Species in Drosophila’. Nanomaterials vol. 8. no. 10. Art. no. 10. Oct. 2018. [CrossRef]
- et al. O. Emilian et al. ‘Cytological Applications of the Vacuolization Phenomenon as a Means of Determining Saline Cytotoxicity’. Appl. Sci. vol. 13. no. 14. p. 8461. 2023. Accessed: Dec. 01. 2023. [Online]. Available: https://www.mdpi.com/2076-3417/13/14/8461. [CrossRef]
- Shilpa. K. P. Anupama. A. Antony. and H. P. Gurushankara. ‘Lead (Pb) induced Oxidative Stress as a Mechanism to Cause Neurotoxicity in Drosophila melanogaster’. Toxicology vol. 462. p. 152959. Oct. 2021. [CrossRef]
- Y. Zheng. H. Zhang. X. Deng. J. Liu. and H. Chen. ‘The relationship between vacuolation and initiation of PCD in rice (Oryza sativa) aleurone cells’. Sci. Rep vol. 7. no. 1. Art. no. 1. Jan. 2017. [CrossRef]
- V. Shubin. I. V. Demidyuk. A. A. Komissarov. L. M. Rafieva. and S. V. Kostrov. ‘Cytoplasmic vacuolization in cell death and survival’. Oncotarget vol. 7. no. 34. pp. 55863-55889. Jun. 2016. [CrossRef]
- E. R. Carmona. A. Creus. and R. Marcos. ‘Genotoxicity testing of two lead-compounds in somatic cells of Drosophila melanogaster’. Mutat. Res. Toxicol. Environ. Mutagen. vol. 724. no. 1. Art. no. 1. Sep. 2011. [CrossRef]
- P. Yang et al. ‘Effects of cadmium on oxidative stress and cell apoptosis in Drosophila melanogaster larvae’. Sci. Rep. vol. 12. no. 1. Art. no. 1. 2022. Accessed: Nov. 28. 2023. [Online]. Available: https://www.nature.com/articles/s41598-022-08758-0. [CrossRef]
- G. J. T. Salazar. A. Ecker. S. A. Adefegha. and J. G. M. da Costa. ‘Advances in Evaluation of Antioxidant and Toxicological Properties of Stryphnodendron rotundifolium Mart. in Drosophila melanogaster Model’. Foods vol. 11. no. 15. Jan. 2022. [CrossRef]
- B. Skwarylo-Bednarz et al. ‘The impact of copper on catalase activity and antioxidant properties of soil under amaranth cultivation’. J. Elem. vol. 23. no. 3. 2018. Accessed: Dec. 01. 2023. [Online]. Available: https://bibliotekanauki.pl/articles/14416.pdf. [CrossRef]
- P. Duarte-Jurado et al. ‘Antioxidant Therapeutics in Parkinson’s Disease: Current Challenges and Opportunities’. Antioxidants vol. 10. no. 3. Art. no. 3. Mar. 2021. [CrossRef]
- V. Muñoz-Soriano and N. Paricio. ‘Drosophila Models of Parkinson’s Disease: Discovering Relevant Pathways and Novel Therapeutic Strategies’. Park. Dis vol. 2011. p. e520640. Mar. 2011. [CrossRef]
- J. Whitworth. ‘1 - Drosophila Models of Parkinson’s Disease’. in Advances in Genetics. vol. 73. T. Friedmann. J. C. Dunlap. and S. F. Goodwin. Eds. Academic Press. 2011. pp. 1-50. [CrossRef]
- M. B. Feany and W. W. Bender. ‘A Drosophila model of Parkinson’s disease’. Nature vol. 404. no. 6776. Mar. 2000. [CrossRef]
- R. Costa. E. Speretta. D. C. Crowther. and I. Cardoso. ‘Testing the Therapeutic Potential of Doxycycline in a Drosophila melanogaster Model of Alzheimer Disease *’. J. Biol. Chem vol. 286. no. 48. pp. 41647-41655. Dec. 2011. [CrossRef]
- K. Prüßing. A. Voigt. and J. B. Schulz. ‘Drosophila melanogaster as a model organism for Alzheimer’s disease’. Mol. Neurodegener. vol. 8. no. 1. p. 35. Nov. 2013. [CrossRef]
- X. Cheng. C. Song. Y. Du. U. Gaur. and M. Yang. ‘Pharmacological Treatment of Alzheimer’s Disease: Insights from Drosophila melanogaster’. Int. J. Mol. Sci. vol. 21. no. 13. Jan. 2020. [CrossRef]
- Z. Mirzoyan. M. Sollazzo. M. Allocca. A. M. Valenza. D. Grifoni. and P. Bellosta. ‘Drosophila melanogaster: A Model Organism to Study Cancer’. Front. Genet. vol. 10. 2019. Accessed: Jan. 21. 2024. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fgene.2019.00051. [CrossRef]
- C. Munnik. M. P. Xaba. S. T. Malindisa. B. L. Russell. and S. A. Sooklal. ‘Drosophila melanogaster: A platform for anticancer drug discovery and personalized therapies’. Front. Genet vol. 13. 2022. Accessed: Jan. 21. 2024. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fgene.2022.949241. [CrossRef]
- S. Ma et al. ‘The multisubstrate deoxyribonucleoside kinase of Drosophila melanogaster as a therapeutic suicide gene of breast cancer cells’. J. Gene Med vol. 13. no. 6. pp. 305-311. 2011. [CrossRef]
- N. A. Ramesh et al. ‘A genetic and physiological model of renal dysfunction in Lowe syndrome’. bioRxiv. p. 2024.01.15.575703. Jan. 16. 2024. [CrossRef]
- J. P. Álvarez-Rendón. R. Salceda. and J. R. Riesgo-Escovar. ‘Drosophila melanogaster as a Model for Diabetes Type 2 Progression’. BioMed Res. Int vol. 2018. p. e1417528. Apr. 2018. [CrossRef]
- N. Baenas and A. E. Wagner. ‘Drosophila melanogaster as a Model Organism for Obesity and Type-2 Diabetes Mellitus by Applying High-Sugar and High-Fat Diets’. Biomolecules vol. 12. no. 2. Art. no. 2. Feb. 2022. [CrossRef]
- Y. Miao et al. ‘Drosophila melanogaster diabetes models and its usage in the research of anti-diabetes management with traditional Chinese medicines’. Front. Med. vol. 9. 2022. Accessed: Jan. 21. 2024. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fmed.2022.953490. [CrossRef]
- Y. H. Inoue. H. Katsube. and Y. Hinami. ‘Drosophila Models to Investigate Insulin Action and Mechanisms Underlying Human Diabetes Mellitus’. in Drosophila Models for Human Diseases. M. Yamaguchi. Ed. in Advances in Experimental Medicine and Biology. Singapore: Springer. 2018. pp. 235-256. [CrossRef]
- F. A. Al-Momani and A. M. Massadeh. ‘Effect of different heavy-metal concentrations on Drosophila melanogaster larval growth and development’. Biol. Trace Elem. Res. vol. 108. no. 1. pp. 271-277. Dec. 2005. [CrossRef]
- M. R. Slobodian. J. D. Petahtegoose. A. L. Wallis. D. C. Levesque. and T. J. S. Merritt. ‘The Effects of Essential and Non-Essential Metal Toxicity in the Drosophila melanogaster Insect Model: A Review’ Toxics vol. 9. no. 10. Art. no. 10. Oct. 2021. [CrossRef]
- T. Tongesayi. P. Fedick. L. Lechner. C. Brock. A. Le Beau. and C. Bray. ‘Daily bioaccessible levels of selected essential but toxic heavy metals from the consumption of non-dietary food sources’. Food Chem. Toxicol. vol. 62. pp. 142-147. Dec. 2013. [CrossRef]
- R. Burke. ‘Molecular physiology of copper in drosophila melanogaster’ Curr. Opin. Insect Sci. vol. 51. p. 100892. 2022. Accessed: Jan. 16. 2024. [Online]. Available: https://www.sciencedirect.com/science/article/pii/S221457452200027X?casa_token=pFqqOwYOLQcAAAAA:izJoBleLYyOiZxeYDZyxGkVZEIIma9lC0A2gMfRAxgxQmU3twI5i8PwuEtme4S2YKVOKWtyCew. [CrossRef]
- J. A. Navarro and S. Schneuwly. ‘Copper and zinc homeostasis: lessons from Drosophila melanogaster’. Front. Genet. vol. 8. p. 223. 2017. [CrossRef]
- Southon. R. Burke. M. Norgate. P. Batterham. and J. Camakaris. ‘Copper homoeostasis in Drosophila melanogaster S2 cells’. Biochem. J. vol. 383. no. 2. 2004. [CrossRef]
- E. Demir and F. Turna Demir. ‘Drosophila melanogaster as a dynamic in vivo model organism reveals the hidden effects of interactions between microplastic/nanoplastic and heavy metals’. J. Appl. Toxicol. vol. 43. no. 2. pp. 212-219. 2023. [CrossRef]
- X. Li. Y. Sun. S. Gao. Y. Li. L. Liu. and Y. Zhu. ‘Taste coding of heavy metal ion-induced avoidance in Drosophila’. iScience vol. 26. no. 5. p. 106607. May 2023. [CrossRef]
- L. Mo et al. ‘Hedgehog pathway is negatively regulated during the development of Drosophila melanogaster PheRS-m (Drosophila homologs gene of human FARS2) mutants’. Hum. Cell vol. 36. no. 1. pp. 121-131. Jan. 2023. [CrossRef]
- S. N. Chan and J. W. Pek. ‘Distinct biogenesis pathways may have led to functional divergence of the human and Drosophila Arglu1 sisRNA’. EMBO Rep vol. 24. no. 2. p. e54350. Feb. 2023. [CrossRef]
- L. Willoughby. H. Chung. C. Lumb. C. Robin. P. Batterham. and P. J. Daborn. ‘A comparison of Drosophila melanogaster detoxification gene induction responses for six insecticides. caffeine and phenobarbital’. Insect Biochem. Mol. Biol vol. 36. no. 12. pp. 934-942. Dec. 2006. [CrossRef]
- K. Trinh et al. ‘Induction of the Phase II Detoxification Pathway Suppresses Neuron Loss in Drosophila Models of Parkinson’s Disease’. J. Neurosci vol. 28. no. 2. pp. 465-472. Jan. 2008. [CrossRef]
- C. M. Wood. A. P. Farrell. and C. J. Brauner. Homeostasis and Toxicology of Essential Metals. Academic Press. 2012.
- M. A. Zoroddu. J. Aaseth. G. Crisponi. S. Medici. M. Peana. and V. M. Nurchi. ‘The essential metals for humans: a brief overview’. J. Inorg. Biochem vol. 195. pp. 120-129. 2019. Accessed: Dec. 01. 2023. [Online]. Available: https://www.sciencedirect.com/science/article/pii/S0162013418306846?casa_token=R9XvW5gvzaMAAAAA:RP8DrUsKLyWE6luXgsWTFsWOiSsDWUMttt_PJkyQR9aB6075Ig477mqdoVNJvMi2V8f-vbWxFA. [CrossRef]
- K. Jomova et al. ‘Essential metals in health and disease’. Chem. Biol. Interact. vol. 367. p. 110173. Nov. 2022. [CrossRef]
- D. Raj and S. K. Maiti. ‘Sources. bioaccumulation. health risks and remediation of potentially toxic metal (loid) s (As. Cd. Cr. Pb and Hg): an epitomised review’. Environ. Monit. Assess vol. 192. no. 2. p. 108. 2020. Accessed: Dec. 01. 2023. [Online]. Available: https://idp.springer.com/authorize/casa?redirect_uri=https://link.springer.com/article/10.1007/s10661-019-8060-5&casa_token=mGZ0TObc9usAAAAA:tWWIIF3SByFfFK4M5LN843_PkcaHv9wv9vmHn7V1rfIyWJwPDi6W_KAh4r7XZzhCEP8KT78YlNtMj1PT. [CrossRef]
- Cherfi. S. Abdoun. and O. Gaci. ‘Food survey: Levels and potential health risks of chromium. lead. zinc and copper content in fruits and vegetables consumed in Algeria’. Food Chem. Toxicol vol. 70. pp. 48-53. Aug. 2014. [CrossRef]
- N. Bagdatlioglu. C. Nergiz. and P. G. Ergonul. ‘Heavy metal levels in leafy vegetables and some selected fruits’. J. Für Verbraucherschutz Leb. vol. 5. no. 3. pp. 421-428. Sep. 2010. [CrossRef]
- Pruteanu. I. Voicea. and V. Fatu. ‘Accumulation of copper in vegetables and fruits’. presented at the 21st International Scientific Conference Engineering for Rural Development. May 2022. [CrossRef]
- D. Demirezen and K. Uruç. ‘Comparative study of trace elements in certain fish. meat and meat products’. Meat Sci. vol. 74. no. 2. pp. 255-260. Oct. 2006. [CrossRef]
- ]Ľ. Harangozo et al. ‘Copper content in cereals grown in the model condition’. Potravinarstvo Slovak J. Food Sci. vol. 11. no. 1. pp. 20-25. Jan. 2017. [CrossRef]
- S. Kashian and A. A. Fathivand. ‘Estimated daily intake of Fe. Cu. Ca and Zn through common cereals in Tehran. Iran’. Food Chem. vol. 176. pp. 193-196. Jun. 2015. [CrossRef]
- B. R. Stern et al. ‘Copper and Human Health: Biochemistry. Genetics. and Strategies for Modeling Dose-response Relationships’. J. Toxicol. Environ. Health Part B vol. 10. no. 3. pp. 157-222. Apr. 2007. [CrossRef]
- L. M. Gaetke and C. K. Chow. ‘Copper toxicity. oxidative stress. and antioxidant nutrients’. Toxicology vol. 189. no. 1-2. pp. 147-163. Jul. 2003. [CrossRef]
- J. W. Spears. E. B. Kegley. and L. A. Mullis. ‘Bioavailability of copper from tribasic copper chloride and copper sulfate in growing cattle’. Anim. Feed Sci. Technol. vol. 116. no. 1. pp. 1-13. Sep. 2004. [CrossRef]
- D. Jiang et al. ‘Redox Reactions of Copper Complexes Formed with Different β-Amyloid Peptides and Their Neuropathalogical Relevance’. Biochemistry vol. 46. no. 32. pp. 9270-9282. Aug. 2007. [CrossRef]
- M. Adrees et al. ‘The effect of excess copper on growth and physiology of important food crops: a review’. Environ. Sci. Pollut. Res. vol. 22. pp. 8148-8162. 2015. Accessed: Dec. 01. 2023. [Online]. Available: https://idp.springer.com/authorize/casa?redirect_uri=https://link.springer.com/article/10.1007/s11356-015-4496-5&casa_token=3HPXX7mXHbIAAAAA:xZOl8aFLsfXIpKE95bMZq9p9XS30aWcJUWD2GSKD_-zbsZSYp2HVK1DlbYumwaY4xLXX-m_WjWOLT7L1. [CrossRef]
- D. Huang et al. ‘The effects of rice straw biochar on indigenous microbial community and enzymes activity in heavy metal-contaminated sediment’. Chemosphere vol. 174. pp. 545-553. 2017. Accessed: Dec. 01. 2023. [Online]. Available: https://www.sciencedirect.com/science/article/pii/S0045653517301492?casa_token=_pxZ_ZOXE2wAAAAA:srx6h9_BJoLLmzQgb_LkmR2GiMNJQBDbiUAt9C3b11tcbIXiekswOWMoSgK-BgFgYikUmt42tg. [CrossRef]
- M. Zhang. D.-M. Xian. N. Zhang. H.-H. Li. and Z.-L. You. ‘The construction of versatile azido-bridged Schiff base Copper (II) complexes with xanthine oxidase inhibitory activity’. Struct. Chem. vol. 23. pp. 1489-1496. 2012. Accessed: Dec. 01. 2023. [Online]. Available: https://idp.springer.com/authorize/casa?redirect_uri=https://link.springer.com/article/10.1007/s11224-012-9956-7&casa_token=9xac46Yb7YoAAAAA:LvnT_U9do9lI_6PGPsgkNX1OV1fPfez29UfPhaNhNi3aOmIlKqf1wqn5h-DVjdPCOQ06nTILNmD1VoHr. [CrossRef]
- E. Yusefi-Tanha. S. Fallah. A. Rostamnejadi. and L. R. Pokhrel. ‘Particle size and concentration dependent toxicity of copper oxide nanoparticles (CuONPs) on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar)’. Sci. Total Environ vol. 715. p. 136994. 2020. Accessed: Dec. 01. 2023. [Online]. Available: https://www.sciencedirect.com/science/article/pii/S0048969720305040?casa_token=igXo1RRiJQUAAAAA:I_IcHbkrWlsE-JfQjiCxSjigFojkQVcpW_WGIkFsav4P0diupz0TGRDv7u5fMakNXbk4YVXtnw. [CrossRef]
- S. I. Lavrentyeva. L. Y. Ivachenko. K. S. Golokhvast. and M. A. Nawaz. ‘Ribonuclease activity of Glycine max and Glycine soja sprouts as a marker adaptation to copper sulphate and zinc sulphate toxicity’. Biochem. Syst. Ecol. vol. 83. pp. 66-70. 2019. Accessed: Dec. 01. 2023. [Online]. Available: https://www.sciencedirect.com/science/article/pii/S0305197818305659?casa_token=rOJlpgSSphQAAAAA:Ydm7EWuB8RokVLCpjA83zLXCladUpoMjOPiVISMXXUN31t7_sOYzt515ElykfTGC5iTbH5eCpQ. [CrossRef]
- M. Jaishankar. T. Tseten. N. Anbalagan. B. B. Mathew. and K. N. Beeregowda. ‘Toxicity. mechanism and health effects of some heavy metals. Interdiscip Toxicol 7: 60-72’. 2014. [CrossRef]
- T. Jan. M. Azam. K. Siddiqui. A. Ali. I. Choi. and Q. M. R. Haq. ‘Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants’. Int. J. Mol. Sci. vol. 16. no. 12. pp. 29592-29630. 2015. Accessed: Dec. 01. 2023. [Online]. Available: https://www.mdpi.com/1422-0067/16/12/29592. [CrossRef]
- G. Y. Zaghloul. H. A. Eissa. A. Y. Zaghloul. M. S. Kelany. M. A. Hamed. and K. M. E. Moselhy. ‘Impact of some heavy metal accumulation in different organs on fish quality from Bardawil Lake and human health risks assessment’ Geochem. Trans. vol. 25. no. 1. p. 1. Jan. 2024. [CrossRef]
- Duruibe. Ogwuegbu. and Egwurugwu. ‘Heavy metal pollution and human biotoxic effects’. Int. J. Phys. Sci. vol. 2. no. 5. pp. 112-118. 2007. Accessed: Dec. 01. 2023. [Online]. Available: https://academicjournals.org/journal/IJPS/article-full-text-pdf/59CA35213127.
- Giannakoula. I. Therios. and C. Chatzissavvidis. ‘Effect of lead and copper on photosynthetic apparatus in citrus (Citrus aurantium L.) plants. The role of antioxidants in oxidative damage as a response to heavy metal stress’. Plants vol. 10. no. 1. p. 155. 2021. Accessed: Dec. 01. 2023. [Online]. Available: https://www.mdpi.com/2223-7747/10/1/155. [CrossRef]
- L. Shvachiy. V. Geraldes. Â. Amaro-Leal. and I. Rocha. ‘Persistent effects on cardiorespiratory and nervous systems induced by long-term lead exposure: results from a longitudinal study’. Neurotox. Res. vol. 37. pp. 857-870. 2020. Accessed: Nov. 28. 2023. [Online]. Available: https://idp.springer.com/authorize/casa?redirect_uri=https://link.springer.com/article/10.1007/s12640-020-00162-8&casa_token=Nm8RaX-XldkAAAAA:hZWncHlznxWnNUmj10kjlzGSmkVrHAbrhHnuYU2VhPfq5LFQUat6S148LlU-OoueMLlUpAN00Scg72iD. [CrossRef]
- E. J. Morley. H. V. Hirsch. K. Hollocher. and G. A. Lnenicka. ‘Effects of chronic lead exposure on the neuromuscular junction in Drosophila larvae’. Neurotoxicology vol. 24. no. 1. pp. 35-41. 2003. Accessed: Dec. 01. 2023. [Online]. Available: https://www.sciencedirect.com/science/article/pii/S0161813X02000955. [CrossRef]
- F. A. Al-Momani and A. M. Massadeh. ‘Effect of different heavy-metal concentrations on Drosophila melanogaster larval growth and development’. Biol. Trace Elem. Res. vol. 108. pp. 271-277. 2005. Accessed: Dec. 01. 2023. [Online]. Available: https://idp.springer.com/authorize/casa?redirect_uri=https://link.springer.com/article/10.1385/BTER:108:1-3:271&casa_token=oopytYw7h_gAAAAA:Z85BaCAO0WIhan06sS0RFPOQFV8aTFiPRcwVT4bxvDOH56oURaoE31dhlkNELWNl0BfmtCRC4mkK0Ff5. [CrossRef]
- M. S. Abdullahi. ‘Toxic effects of lead in humans: an overview’. Glob. Adv. Res. J. Environ. Sci. Toxicol. vol. 2. no. 6. pp. 157-162. 2013. Accessed: Dec. 01. 2023. [Online]. Available: http://trusting-shtern.139-59-89-2.plesk.page/toxic-effects-of-lead-in-humans-an-overview_YTozNTo5.pdf.
- Q. Jamal et al. ‘Heavy metals accumulation and their toxic effects’. J. Bio-Mol. Sci. JBMS vol. 1. no. 1. pp. 27-36. 2013. Accessed: Dec. 01. 2023. [Online]. Available: https://www.researchgate.net/profile/Qaiser-Jamal/publication/276921553_Heavy_Metals_Accumulation_and_Their_Toxic_Effects_Review/links/56f12e9a08aedbe21877222d/Heavy-Metals-Accumulation-and-Their-Toxic-Effects-Review.pdf.
- R. A. Festa and D. J. Thiele. ‘Copper: an essential metal in biology’. Curr. Biol. vol. 21. no. 21. pp. R877-R883. 2011. Accessed: Dec. 01. 2023. [Online]. Available: https://www.cell.com/current-biology/pdf/S0960-9822(11)01079-7.pdf.
- L. Frat et al. ‘Single and mixed exposure to cadmium and mercury in Drosophila melanogaster: Molecular responses and impact on post-embryonic development’. Ecotoxicol. Environ. Saf.. vol. 220. p. 112377. Sep. 2021. [CrossRef]
- X. Zeng. C. Deng. Y. Liang. J. Fu. S. Zhang. and T. Ni. ‘Ecological risk evaluation and sensitivity analysis of heavy metals on soil organisms under human activities in the Tibet Plateau. China’. Plos One vvol. 18. no. 8. Accessed: Nov. 28. 2023. [Online]. Available: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0285116. [CrossRef]
- et al. ‘Lead Toxicity: Health Hazards. Influence on Food Chain. and Sustainable Remediation Approaches’. Int. J. Environ. Res. Public. Health vol. 17. no. 7. Art. no. 7. Jan. 2020. [CrossRef]
- V. Mohod and J. Dhote. ‘Review of heavy metals in drinking water and their effect on human health’. Int. J. Innov. Res. Sci. Eng. Technol. vol. 2. no. 7. Art. no. 7. 2013. Accessed: Nov. 28. 2023. [Online]. Available: https://www.researchgate.net/profile/Jayashree-Dhote/publication/251237189_Review_of_heavy_metals_in_drinking_water_and_their_effect_on_human_health/links/00b4951ef99e1d1f97000000/Review-of-heavy-metals-in-drinking-water-and-their-effect-on-human-health.pdf.
- Y. Finkelstein. M. E. Markowitz. and J. F. Rosen. ‘Low-level lead-induced neurotoxicity in children: an update on central nervous system effects’. Brain Res. Rev vol. 27. no. 2. Art. no. 2. Jul. 1998. [CrossRef]
- C. S. Mendes. I. Bartos. T. Akay. S. Márka. and R. S. Mann. ‘Quantification of gait parameters in freely walking wild type and sensory deprived Drosophila melanogaster’elife vol. 2. p. e00231. 2013. Accessed: Dec. 01. 2023. [Online]. Available: https://elifesciences.org/articles/231. [CrossRef]
- W. Jin. R. M. Riley. R. D. Wolfinger. K. P. White. G. Passador-Gurgel. and G. Gibson. ‘The contributions of sex. genotype and age to transcriptional variance in Drosophila melanogaster’. Nat. Genet vol. 29. no. 4. pp. 389-395. 2001. Accessed: Dec. 01. 2023. [Online]. Available: https://www.nature.com/articles/ng766z. [CrossRef]
- E. H. Morrow. A. Leijon. and A. Meerupati. ‘Hemiclonal analysis reveals significant genetic. environmental and genotype × environment effects on sperm size in Drosophila melanogaster’. J. Evol. Biol vol. 21. no. 6. pp. 1692-1702. Nov. 2008. [CrossRef]
- B. Gallia. S. Ioan. P. Sorina. T. Gheorghe. and I. Stefanescu. ‘The involvement of deuterium presence in the Drosophila melanogaster evolution: diverse effects of deuterium concentrations upon the phenotype of different strains and Romanian ecotypes’. Smart Energy Sustain. Environ. vol. 16. no. 2. p. 133. 2013. Accessed: Dec. 01. 2023. [Online]. Available: https://search.proquest.com/openview/48f43fa6fdfeaf86a57bc2167b055d40/1?pq-origsite=gscholar&cbl=2045793.
- L. Everson da Silva et al. ‘In Vitro Antiprotozoal Evaluation of Zinc and Copper Complexes Based on Sulfonamides Containing 8-Aminoquinoline Ligands’. Lett. Drug Des. Discov vol. 7. no. 9. pp. 679-685. Nov. 2010. [CrossRef]
- N. Asif et al. ‘Toxicological assessment of Phormidium sp. derived copper oxide nanoparticles for its biomedical and environmental applications’. Sci. Rep. vol. 13. no. 1. Art. no. 1. Apr. 2023. [CrossRef]
- O. Abolaji. K. D. Fasae. C. E. Iwezor. M. Aschner. and E. O. Farombi. ‘Curcumin attenuates copper-induced oxidative stress and neurotoxicity in Drosophila melanogaster’. Toxicol. Rep vol. 7. pp. 261-268. 2020. Accessed: Jan. 16. 2024. [Online]. Available: https://www.sciencedirect.com/science/article/pii/S2214750019306043. [CrossRef]
- Southon. A. Farlow. M. Norgate. R. Burke. and J. Camakaris. ‘Malvolio is a copper transporter in Drosophila melanogaster’. J. Exp. Biol. vol. 211. no. 5. pp. 709-716. 2008. Accessed: Jan. 16. 2024. [Online]. Available: https://journals.biologists.com/jeb/article-abstract/211/5/709/18125. [CrossRef]
- P. T. Halmenschelager and J. B. T. da Rocha. ‘Biochemical CuSO 4 Toxicity in Drosophila melanogaster depends on sex and developmental stage of exposure’. Biol. Trace Elem. Res. vol. 189. pp. 574-585. 2019. Accessed: Nov. 28. 2023. [Online]. Available: https://idp.springer.com/authorize/casa?redirect_uri=https://link.springer.com/article/10.1007/s12011-018-1475-y&casa_token=CO_xdDrjXpQAAAAA:qWTtFM6KyMOKFrHo4FSavQgZZinXbzNtimb2Qq9q6F5heS76xjFnCvkRR-COGXC4dDtJC8vhCNJHSjgE. [CrossRef]
- M. A. Balinski and R. C. Woodruff. ‘Differential sexual survival of Drosophila melanogaster on copper sulfate’. Genetica vol. 145. pp. 131-137. 2017. Accessed: Nov. 28. 2023. [Online]. Available: https://idp.springer.com/authorize/casa?redirect_uri=https://link.springer.com/article/10.1007/s10709-017-9951-4&casa_token=lwJNGLeE7ZcAAAAA:6loOri92nj1csDLftBbi1DopNcTIbYn-Enpz1XnN6oRjmvY0gp6IQXT9eWGGc1iM0N9K92NASZTycypk. [CrossRef]
- ‘Copper and its role in the human body - the importance of establishing copper concentrations in the body’. Romanian J. Mil. Med. vol. 124. no. 2. May 2021. [CrossRef]
- M. Angelova. S. Asenova. V. Nedkova. and R. Koleva-Kolarova. ‘COPPER IN THE HUMAN ORGANISM’. Trakia J. Sci. vol. 9. no. 1. 2011.
- Hyre. K. Casanova-Hampton. and S. Subashchandrabose. ‘Copper Homeostatic Mechanisms and Their Role in the Virulence of Escherichia coli and Salmonella enterica’. EcoSal Plus vol. 9. no. 2. p. eESP-0014-2020. Dec. 2021. [CrossRef]
- E. Santo. N. Taudte. D. H. Nies. and G. Grass. ‘Contribution of Copper Ion Resistance to Survival of Escherichia coli on Metallic Copper Surfaces’. Appl. Environ. Microbiol vol. 74. no. 4. pp. 977-986. Feb. 2008. [CrossRef]
- P. Saenkham-Huntsinger et al. ‘Copper Resistance Promotes Fitness of Methicillin-Resistant Staphylococcus aureus during Urinary Tract Infection’. mBio vol. 12. no. 5. pp. e02038-21. Oct. 2021. [CrossRef]
- W. B. Wang and J. C. Clapper. ‘Antibacterial activity of electrospun polyacrylonitrile copper nanoparticle nanofibers on antibiotic resistant pathogens and methicillin resistant Staphylococcus aureus (MRSA)’. Nanomaterials vol. 12. no. 13. p. 2139. 2022. Accessed: Jan. 16. 2024. [Online]. Available: https://www.mdpi.com/2079-4991/12/13/2139. [CrossRef]
- Y. Zhuang. L. Ren. S. Zhang. X. Wei. K. Yang. and K. Dai. ‘Antibacterial effect of a copper-containing titanium alloy against implant-associated infection induced by methicillin-resistant Staphylococcus aureus’. Acta Biomater. vol. 119. pp. 472-484. 2021. Accessed: Jan. 16. 2024. [Online]. Available: https://www.sciencedirect.com/science/article/pii/S1742706120306152?casa_token=jmF9O7-xdvsAAAAA:8rFT2nSP8lXqhxmHV20-b9HVEj38foo_kyJoTrvuYIlUBAVToHIutNWYJjnQ-PgJ0DopbPtfHA. [CrossRef]
- Andreou. S. Trantza. D. Filippou. N. Sipsas. and S. Tsiodras. ‘COVID-19: The Potential Role of Copper and N-acetylcysteine (NAC) in a Combination of Candidate Antiviral Treatments Against SARS-CoV-2’. In Vivo vol. 34. no. 3 suppl. pp. 1567-1588. 2020. [CrossRef]
- S. Raha. R. Mallick. S. Basak. and A. K. Duttaroy. ‘Is copper beneficial for COVID-19 patients?’. Med. Hypotheses vol. 142. p. 109814. Sep. 2020. [CrossRef]
- A. Cortes and J. M. Zuñiga. ‘The use of copper to help prevent transmission of SARS-coronavirus and influenza viruses. A general review’. Diagn. Microbiol. Infect. Dis vol. 98. no. 4. p. 115176. Dec. 2020. [CrossRef]
- Y. Fujimori et al. ‘Novel Antiviral Characteristics of Nanosized Copper(I) Iodide Particles Showing Inactivation Activity against 2009 Pandemic H1N1 Influenza Virus’. Appl. Environ. vol. 78. no. 4. pp. 951-955. Feb. 2012. [CrossRef]
- K. Imai et al. ‘Inactivation of high and low pathogenic avian influenza virus H5 subtypes by copper ions incorporated in zeolite-textile materials’. Antiviral Res. vol. 93. no. 2. pp. 225-233. Feb. 2012. [CrossRef]
- M. Rai et al. ‘Copper and copper nanoparticles: role in management of insect-pests and pathogenic microbes’. Nanotechnol. Rev vol. 7. no. 4. pp. 303-315. Aug. 2018. [CrossRef]
- L. Green. M. Coronado-Zamora. S. Radío. G. E. Rech. J. Salces-Ortiz. and J. González. ‘The genomic basis of copper tolerance in Drosophila is shaped by a complex interplay of regulatory and environmental factors’. BMC Biol. vol. 20. no. 1. Art. no. 1. Dec. 2022. [CrossRef]
- Z.-H. Liu et al. ‘Oxidative stress caused by lead (Pb) induces iron deficiency in Drosophila melanogaster’. Chemosphere. vol. 243. p. 125428. Mar. 2020. [CrossRef]
- D. Fatmawati. D. Khoiroh. S. Zubaidah. H. Susanto. M. Agustin. and A. Fauzi. ‘Wing morphological changes of Drosophila melanogaster exposed with lead in nine generations’. in AIP Conference Proceedings. AIP Publishing. 2023. Accessed: Nov. 28. 2023. [Online]. Available: https://pubs.aip.org/aip/acp/article-abstract/2634/1/020041/2871056ip. [CrossRef]
- N. L. Cooper. J. R. Bidwell. and A. Kumar. ‘Toxicity of copper. lead. and zinc mixtures to Ceriodaphnia dubia and Daphnia carinata’. Ecotoxicol. Environ. Saf. vol. 72. no. 5. Jul. 2009. [CrossRef]
- M. T. S. D. Vasconcelos and M. F. C. Leal. ‘Antagonistic interactions of Pb and Cd on Cu uptake. growth inhibition and chelator release in the marine algae Emiliania huxleyi’. Mar. Chem. vol. 75. no. 1. Art. no. 1. Jul. 2001. [CrossRef]
- D. Z. An et al. ‘Influence of Iron Supplementation on DMT1 (IRE)-induced Transport of Lead by Brain Barrier Systems in vivo’. Biomed. Environ. Sci. vol. 28. no. 9. Art. no. 9. Sep. 2015. [CrossRef]
- E. R. Everman. K. M. Cloud-Richardson. and S. J. Macdonald. ‘Characterizing the genetic basis of copper toxicity in Drosophila reveals a complex pattern of allelic. regulatory. and behavioral variation’. Genetics vol. 217. no. 1. Art. no. 1. Jan. 2021. [CrossRef]
| Analysis factor | df | SS | S2 | F | Pr(>F) | |
|---|---|---|---|---|---|---|
| Larval stage I | Concentration | 4 | 55264 | 13816 | 240.429 | < 2e-16 *** |
| Genotype | 3 | 24097 | 8032 | 139.782 | < 2e-16 *** | |
| Metal | 1 | 3936 | 3936 | 68.499 | 2.74e-16 *** | |
| Concentration: Genotyp | 12 | 1240 | 103 | 1.798 | 0.043 * | |
| Concentration:Metal | 4 | 1200 | 300 | 5.219 | 0.000356 *** | |
| Genotype:Metal | 3 | 290 | 97 | 1.680 | 0.169 Ns | |
| Concentration: Genotip: Metal | 12 | 232 | 19 | 0.337 | 0.982 Ns | |
| Residuals | 1520 | 87344 | 57 | |||
| Larvar stage II | Concentration | 4 | 37452 | 9363 | 200.948 | < 2e-16 *** |
| Genotype | 3 | 9527 | 3176 | 68.159 | < 2e-16 *** | |
| Metal | 1 | 2763 | 2763 | 59.292 | 2.56e-14 *** | |
| Concentration: Genotyp | 12 | 1617 | 135 | 2.892 | 0.000583 *** | |
| Concentration:Metal | 4 | 1037 | 259 | 5.563 | 0.000192 *** | |
| Genotype:Metal | 3 | 130 | 43 | 0.933 | 0.423 Ns | |
| Concentration: Genotip: Metal | 12 | 284 | 24 | 0.508 | 0.910 Ns | |
| Residuals | 1392 | 64859 | 47 | |||
| Larvar stage III | Concentration | 4 | 31284 | 7821 | 248.496 | < 2e-16 *** |
| Genotype | 3 | 8277 | 2759 | 87.665 | < 2e-16 *** | |
| Metal | 1 | 2266 | 2266 | 71.992 | < 2e-16 *** | |
| Concentration: Genotyp | 12 | 1084 | 90 | 2.870 | 0.000646 *** | |
| Concentration:Metal | 4 | 748 | 187 | 5.943 | 9.72e-05 *** | |
| Genotype:Metal | 3 | 114 | 38 | 1.209 | 0.304 Ns | |
| Concentration: Genotip: Metal | 12 | 199 | 17 | 0.526 | 0.899 Ns | |
| Residuals | 1278 | 40223 | 31 |
| Genotype | Concentration (mM) | Lead (C2H3O2)2 | |||||||||
| StageI | IC | StageII | IC | StageIII | IC | Pupal | IC | Adult | IC | ||
| Mean | Mean | Mean | Mean | Mean | |||||||
| wild | Control | 35.9a | 0.00 | 28.33a | 0.00 | 26.39a | 0.00 | 25.41a | 0.00 | 24.87a | 0.00 |
| 0.50 | 34.3ab | 4.63 | 27.47ab | 3.04 | 25.76a | 2.41 | 23.48abc | 7.58 | 22.93ab | 7.77 | |
| 1.00 | 31.3abc | 12.97 | 24.19abcd | 14.61 | 19.92b | 24.54 | 19.11bcd | 24.78 | 20.20abc | 18.77 | |
| 2.00 | 26.4cdefg | 26.37 | 20.31defg | 28.33 | 16.72bcde | 36.64 | 16.37defg | 35.57 | 17.13bcde | 31.10 | |
| 4.00 | 19.4hijk | 46.04 | 15.89fghi | 43.92 | 15.70bcdef | 40.53 | 13.66defgh | 46.26 | 13.28def | 46.60 | |
| brown | Control | 27.08cdefg | 0.00 | 20.83cdefg | 0.00 | 19.52b | 0.00 | 18.74cd | 0.00 | 17.73bcd | 0.00 |
| 0.50 | 25.97cdefg | 4.07 | 19.83defgh | 4.80 | 18.70bc | 4.19 | 17.30def | 7.71 | 16.60bcde | 6.39 | |
| 1.00 | 24.6defgh | 8.90 | 19.44defgh | 6.67 | 16.81bcde | 13.60 | 16.07defg | 14.23 | 15.53cde | 12.41 | |
| 2.00 | 20.7ghij | 23.30 | 15.47ghi | 25.73 | 13.17cdef | 32.53 | 12.59fgh | 32.81 | 13.33def | 24.81 | |
| 4.00 | 16.18ijk | 40.25 | 14.06hi | 32.53 | 11.24efg | 42.39 | 10.97gh | 41.48 | 11.06ef | 37.66 | |
| white | Control | 30.54abcd | 0.00 | 26.64abc | 0.00 | 26.18a | 0.00 | 24.48ab | 0.00 | 22.31ab | 0.00 |
| .50 | 28.7bcde | 5.71 | 22.61abcde | 15.12 | 21.12ab | 19.33 | 18.42cde | 24.75 | 20.53abc | 7.97 | |
| 1.00 | 27.72cdef | 9.24 | 21.28cdefg | 20.13 | 18.42bc | 29.66 | 17.33def | 29.20 | 17.40bcd | 22.02 | |
| 2.00 | 22.26fghi | 27.12 | 16.75efghi | 37.12 | 13.83cdef | 47.16 | 13.59defgh | 44.48 | 14.53cde | 34.86 | |
| 4.00 | 14.62jk | 52.14 | 14.03hi | 47.34 | 12.67def | 51.62 | 13.13efgh | 46.35 | 12.39def | 44.48 | |
| white-vestigial | Control | 23.10efgh | 0.00 | 21.85bcdef | 0.00 | 19.70b | 0.00 | 18.56cde | 0.00 | 16.93bcde | 0.00 |
| 0.50 | 21.54fghi | 6.77 | 20.22defg | 7.46 | 18.29bcd | 7.14 | 16.97def | 8.57 | 15.88cde | 6.21 | |
| 1.00 | 20.92ghij | 9.43 | 18.89defgh | 13.56 | 16.42bcde | 16.62 | 15.56defg | 16.17 | 14.13cde | 16.54 | |
| 2.00 | 14.13k | 38.85 | 11.19ij | 48.77 | 10.48fg | 46.77 | 8.93hi | 51.90 | 7.47fg | 55.91 | |
| 4.00 | 7.41ll | 67.92 | 5.65j | 74.16 | 5.82g | 70.46 | 4.69i | 74.73 | 3.59g | 78.81 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





