Submitted:
05 March 2024
Posted:
05 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Soil Characterization
2.2. Phenological and Agronomical Traits
2.3. Biomass Yield
2.4. Heavy Metal Concentration and Uptake
2.4.1. Cd Concentration and Uptake
2.4.2. Ni Concentration and Uptake
2.4.3. Cu Concentration and Uptake
2.4.4. Pb Concentration and Uptake
2.4.5. Zn Concentration and Uptake
3. Discussion
4. Materials and Methods
4.1. Soil Analysis
4.2. Agronomic Practices and Experimental Set Up
- (i)
- 0 kg N ha-1, which referred to as N0
- (ii)
- 30 kg N ha-1, which referred to as N1
- (iii)
- 60 kg N ha-1, which referred to as N2
4.3. Plants Sampling and Measurements
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
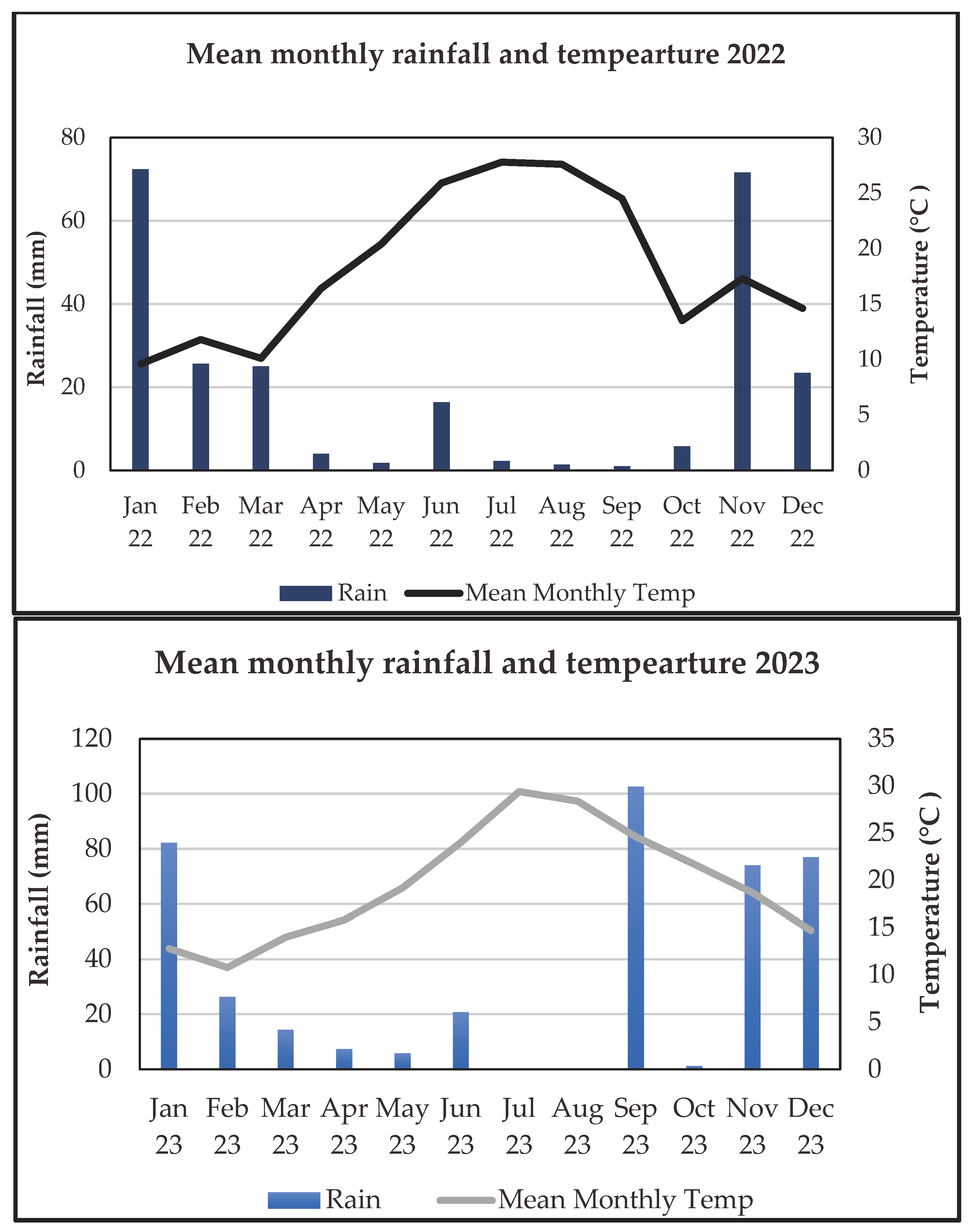
Climatic Conditions
References
- European Environment Agency. Available online: https://www.eea.europa.eu/data-and-maps/indicators/progress-in-management-of-contaminated-sites-3/assessment (accessed on 23.1.24).
- Saleem, M.H.; Ali S.; Hussain, S.; Kamran, M.; Chattha, M.S.; Ahmad, S.; Aqeel, M.; Rizwan, M.; Aljarba, N.H.; Alkahtani, S.; Abdel- Daim, M.M. Flax (Linum usitatissumum L.): A Potential Candidate for Phytoremediation? Biological and Economical Points of View. Plants 2020, 9, 496. [CrossRef]
- Wuanna, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. International Scholarly Research Notices 2011, 2011, 402647. [CrossRef]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: An approach for a sustainable environment. Frontiers in Plant Science 2023, 14. [CrossRef]
- Cleophas, F.N.; Zahari, N.Z.; Murugayah, P.; Rahim, S.A.; Yatim, A. N. M. Phytoremediation: A Novel Approach of bast Fiber Plants (Hemp, Kenaf, Jute and Flax) for Heavy Metals Decontamination in Soil- Review. Toxics 2022, 11. [CrossRef]
- Zhao, X.; Guo, Y.; Papazoglou, E.G. Screening flax, kenaf and hemp varieties for phytoremediation of trace element- contaminated soils. Industrial Crops & Products 2022, 185, 115121. [CrossRef]
- Heller, K.; Sheng, Q. C.; Guan, F.; Alexopoulou, E.; Hua, L.S.; Wu, G.W.; Jankauskiene, Z.; Fu, W.Y. A comparative study between Europe and China crop management of two types of flax: linseed and fiber flax. Industrial crops and products 2015, 68, 24-31. [CrossRef]
- Ceh, B.; Straus, S.; Hladnik, A.; Krusar, A. Impact of linseed variety, location and production year on seed yield, oil content and its composition. Agronomy 2020, 10. [CrossRef]
- Emam, S.M. Cultivars response of flax (Linum usitatissimum L.) to different nitrogen resources in dry environment. Egypt Journal of Agronomy 2019, 41, 119- 131. [CrossRef]
- Dordas, A.C. Variation of physiological determinants of yield in linseed in response to nitrogen feralization. Industrial Crops and products 2010, 31, 455- 465. [CrossRef]
- Angelova, V.; Ivanova, R.; Delibaltova, V.; Ivanov K. Bio-accumulation and distribution of heavy metals in fibre crops (flax, cotton and hemp). Industrial crops and products 2004, 19, 197-205. [CrossRef]
- Hosman, E.M.; El- Feky, S.S.; Elshahawy, M.I.; Shaker, M.E. Mechanism of phytoremediation potential of flax (Linum usitissimum L.) to Pb, Cd and Zn. Asian journal of plant science and research 2017, 7, 30-40.
- Douchiche, O.; Chaibi, W.; Morvan, C. Cadmium tolerance and accumulation characteristics of mature flax, cv. Hermes: contribution of the basal stem compared to the root. Journal of Hazardous Materials 2012, 101, 235-236. [CrossRef]
- Bjelkova, M.; Gencurova, V.; Griga, M. Accumulation of cadmium by flax and linseed cultivars in field- simulated conditions: A potential for phytoremediation of Cd- contaminated soils. Industrial crops and products 2011, 33, 761-774. [CrossRef]
- Guo, Y.; Qiu, C.; Long, S.; Wang, H.; Wang, Y. Cadmium accumulation, translocation, and assessment of eighteen Linum usitatissimum L. cultivars growing in heavy metal contaminated soil. International Journal of Phytoremediation 2020, 490-496. [CrossRef]
- Zuk, M.; Richter, D.; Matuła, J.; Szopa, J. Linseed, the multipurpose plant. Industrial Crops and Products 2015, 75, 165-177. [CrossRef]
- Ma, H.; Guna, V.; Raju, T.; Narasimha Murthy, A.; Reddy, N. Converting flax processing waste into value added biocomposites. Industrial Crops and Products 2023, 195. [CrossRef]
- Stavropoulos, P.; Mavroeidis, A.; Papadopoulos, G.; Roussis, I.; Bilalis, D.; Kakabouki, I. On the Path towards a “Greener” EU: A Mini Review on Flax (Linum usitatissimum L.) as a Case Study. Plants 2023, 12, 1102. [CrossRef]
- Kypritidou, Z.; Koourgia, P.M.; Argyraki, A.; Demetriades, A. Do humans take good care of their offsprig as animals do…! The Lavreotiki ‘sagas’, Hellenic Republic- Part1: historical outline and mapping of lead contamination. Environmental Geochemistry Health 2023, 45, 1107- 1116. [CrossRef]
- Kalyvas, G.; Gasparatos, D.; Papassiopi, N.; Massa, I. Topsoil pollution as ecological footprint of historical mining activities in Greece. Land degradation & Development 2017, 29, 2025-2035. [CrossRef]
- Kontopoulos, A.; Komnitsas, K.; Xenidis, A.; Papassiopi, N. ENVIRONMENTAL CHARACTERISATION OF THE SULPHIDIC TAILINGS IN LAVRION. Minerals Engineering 1995, 8, 1209-1219. [CrossRef]
- El- Bohramy, A.M.A.; Khedr, R.A.; El- Mansoury, M.A.M. Physiological, Biochemical and Agronomic response of some flax cultivars to water deficit under clay soil conditions in North Delta. Journal of the advances in agricultural researches 2022, 351- 365, 27. [CrossRef]
- Gabiana, C.; McKenzie, B.A.; Hill, G.D. The influence of plant population, nitrogen and irrigation on yield and yield components of linseed. Agronomy Society of New Zeland 2005, 35, 45-56. https://www.agronomysociety.org.nz/files/2005_6._Pop_N_irrigation_effects_on_linseed.pdf.
- Goudenhooft, C.; Bourmaud, A.; Baley, C. Flax (Linum usitissimum L.) fibers for composite reinforcement: Exploring the link between plant growth, cell walls development, and fiber properties. Frontiers in Plant science 2019, 10. [CrossRef]
- Rossi, A.; Clemente, C.; Tavarini, S.; Angelini, L.G. Variety and sowing date affect seed yield and chemical composition if linseed grown under organic production system in a semiarid Mediterranean environment. Agronomy 2022, 13. [CrossRef]
- Soethe, G.; Feiden, A.; Bassegio, D.; Santos, R.F.; Melegari de Souza, S.N.; Secco, D. Sources and rates of nitrogen in the cultivation of flax. African Journal of Agricultural Research 2013, 8, 2249-2253. [CrossRef]
- Arslanoglu, S. F.; Sert, S.; Sahin, H.A.; Aytaç, S.; El Sabagh, A. Yield and Yield Criteria of Flax Fiber (Linum Usititassimum L.) as Influenced by Different Plant Densities. Sustainability 2022, 14. [CrossRef]
- Casa, R.; Russel, G.; Lo Cascio, B.; Rossini, F. Environmental effects on linseed (Linum Usititassimum L.) yield and growth of flax at different stand densities. European journal of Agronomy 1999, 11, 267-278. [CrossRef]
- Erdogdu, Y.; Yaver, S.; Onemli, F. The effect of different seeding rates on gain yield and yield components in some flax (Linum usitatissimum L.) varieties. International journal of environmental & agriculture research 2018, 4, 1-9.
- Tavarini, S.; Castagna, A.; Conte, G.; Foschi, L.; Sanmartin, C.; Incrocci, L.; Ranieru, A.; Serra, A.; Angelini, L.G. Evaluation of chemical composition if two linseeds varieties as sources if health- beneficial substances. Molecules 2019, 24. [CrossRef]
- El- Shimy, K.S.S.; Hammam, G.Y.M.; Allam, S.A.H.; Mostafa, S.H.A.; El- Gedwy, E.S.M.M. Flax yield potential affected by irrigation intervals and nitrogen fertilizer rates. Annals of Agricultural Science 2017, 55, 817- 824. [CrossRef]
- Siedelka, A. Some aspects of interaction between heavy metals and plant mineral nutrients. Acta societatis botanicorum Poloniae 1995, 64, 265-272. [CrossRef]
- Petrova, S.; Benesova, D.; Soudek, P.; Vanek, T. Enhancement of metal(loid)s phytoextraction by Cannabis sativa L. Journal of food, agriculture and environment 2012, 10, 631-641.
- Shehata, S.M.; Badawy, R.K.; Aboulsoud, Y.I.E. Phytoremediation of some heavy metals in contaminated soil. Bulletin of the National Research Center 2019, 43. [CrossRef]
- Kiran; Bharti, S.; Sharma, R. Effect of heavy metals: An overview. Material Today: Proceedings 2022, 51, 880-885. [CrossRef]
- Brutch, E.; Zabegaeva, O.; Nozkova, J.; Brutch, N. Cadmium tolerance and its absorption ability in fibre flax and linseed varieties. Turkish Journal of Agriculture and Forestry 2022, 46, 83-89. [CrossRef]
- Kakabouki, I.; Mavroeidis, A.; Tatridas, A.; Roussis, I.; Katsenios, N.; Efthimiadou, A.; Tiga, E.L.; Karydoyianni, S.; Zisi, C.; Folina, A.; Bilalis, D. Reintroducing flax (Linum usitatissimum L.) to the Mediterranean Basin: The importance of nitrogen fertilization. Plants 2021, 10. [CrossRef]
- Abdel- Kader, E.M.A.; Mousa, A.M.A. Effect of nitrogen fertilizer on some flax varieties under two different location conditions. Journal of plant production 2019, 10, 37-44. [CrossRef]
- Taddese, G.; Tenaye, S. Effect of nitrogen on flax (Linum usitissimum L.) fiber yield at debre behran area, Ethiopia. Forestry research and engineering: International Journal 2018, 2, 284- 286. [CrossRef]
- Istanbulluoglu, A.; Konukcu, F.; Kocaman, I.; Sener, M. The effect of deficit irrigation regimes in yield and growth components of linseed (Linum usitissimum L.). Journal of Agricultural science and engineering 2015, 1, 108-113.
- Patel, R.K.; Tomar, G.S.; Dwivedi, S.K. Effect of nitrogen scheduling and nitrogen level on growth, yield and water productivity of linseed (Linum usitissimum L.) under Vertisols. Journal Applied and natural science 2017, 9, 698-705. [CrossRef]
- Rahimi, M.M.; Zarei, M.A.; Arminian, A. Selection criteria if flax (Linum usitissimum L.) for seed yield, yield components and biochemical compositions under various planting dates and nitrogen. African journal of agricultural research 2011, 6, 3167-3175. [CrossRef]
- Zhang, Q.; Gao, Y.; Yan, B.; Cui, Z.; Wu, B.; Yang, K.; Ma, J. Perspective on oil flax and dry biomass with reduced nitrogen supply. Oil crop science, 5 2020, 42-46. [CrossRef]
- Pudelko, K.; Mankowski, J.; Kolodziej, J. Cultivation of fiber and oil flax (Linum usitissimum L.) in No- tillage and conventional systems. Part II. Influence of No- tillage and use of herbicides on yield and weed infestation of oil flax and the physical and biological properties of the soil. Journal of natural fibers 2015, 12, 72-83. [CrossRef]
- Chai, M.; Li, R.; Shen, X.; Tam, F.Y.N.; Zan, Q.; Li, R. Does ammonium nitrogen affect subcellular distribution and chemical factors of cadmium in Kandelia obovata? Ecotoxicology and environmental safety 2018, 162, 430-437. [CrossRef]
- Grant, A.C.; Dribnenki, P. J.C.; Bailey, D.L. Cadmium and zinc concentrations and ratios in seed and tissue of solin (cv Linola TM 947) and flax (cvs McGregor and Vimy) as affected by nitrogen and phosphorous fertilizer and Provide (Panicillium bilaji). Journal of science of food and agriculture 2000, 80, 1735-1743. [CrossRef]
- Tang, G.; Zhang, X.; Qi, L.; Wang, C.; Li, L.; Guo, J.; Dou, X.; Lu, M.; Huang, J. Nitrogen and phosphorus fertilizer increases the uptake of soil heavy metals pollutants by plant community. Bulletin of environmental contamination and toxicology 2022, 109, 1059- 1066. [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Aamer, M.; Nawaz, M.; Ali, A.; Khan, M.A.U.; Khan, T.A. Nickel toxicity in plants: reasons, toxic effects, tolerance mechanisms, and remediation possibilities- a review. Environmental science and pollution research 2019, 26, 12673-12688. [CrossRef]
- Ahmad, M.; Ashraf, M. Essential roles and hazardous effects of nickel in plants. In: Reviews of Environmental Contamination and Toxicology, Whitcane D., Eds.; Springer: New York, 2011, pp. 125-167.
- Chauhan, S.S.; Thakurand, R.; Sharma, G. NICKEL: ITS AVAILABILITY AND REACTIONS IN SOIL. Journal of Industrial Pollution Control 2008, 24, 1-8.
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Natasha; Murtaza, G.; Dumat, C.; Shadid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil- plant environment. Chemosphere 2020, 259, 127436. [CrossRef]
- Wei, B.; Yu, J.; Cao, Z.; Meng, M.; Yang, L.; Chen, Q. The Availability and Accumulation of Heavy Metals in Greenhouse Soils Associated with Intensive Fertilizer Application. International Journal of Environmental Research and Public Health 2020, 17, 5359. [CrossRef]
- Olivares, A.R.; Carrillo- Gonzalez, R.; Gonzalez- Chavez, M.C.A.; Hernadez, R.M.S. Potential of castor bean (Ricinus communis L.) for phytoremediation of mine tailings and oil production. Journal of environmental management 2013, 114, 316-323. [CrossRef]
- Shrestha, P.; Belliturk, K.; Gorres, H.J. Phytoremediation of heavy metal- contaminated soil by switchgrass: A comparative study utilizing different composts and coir fiber on pollution remediation, plant productivity and nutrient leaching. International journal of environmental research and public health 2019, 16. [CrossRef]
- Balafrej, H.; Bogusz, D.; Triqui, Z.-E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc hyperaccumulation in plants: a review. Plants 2020, 9, 562. [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New phytologist 2017, 173, 677-702. [CrossRef]
- Griga, M.; Bjelková, M.; Tejklová, E. Potential of flax (Linum usitatissimum L.) for heavy metal phytoextraction and industrial processing of contaminated biomass—a review. In Risk Assessment and Sustainable Land Management Using Plants in Trace Element-contaminated Soils COST Action 837. 4th WG2 Workshop, Bordeaux—Aquitaine, Villenave dˇıOrnon: France, 25th- 27th April, 2002.
- Food and Agriculture organization of the United Nations. Available online: https://www.fao.org/publications/card/en/c/ed82a8fe-eab5-5880-bebb-5e22f342f55e/ (accessed on 8.12.2023).
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, part 2 Chemical and Microbiological Properties, 2nd ed.; Page, A. L., Miller, R.H.; Keeney, D.R., Eds.; American Society of Agronomy and the Soil Science Society of America: Madison, Wisconsin, USA, 1982; pp. 539-579.
- U.S. EPA. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils. Available online: https://www.epa.gov/esam/epa-method-3050b-acid-digestion-sediments-sludges-and-soils (accessed on 12.12.2023).
- FAO. Available online: https://www.fao.org/3/cc0048en/cc0048en.pdf (accessed on 8.12.2023).
- Flax Council of Canada. Available online: https://www.flaxcouncil.ca (accessed on 10.9.2023).
- METEOSEARCH. Available online https://meteosearch.meteo.gr (accessed on 25.2.2024).
| Properties | |
|---|---|
| pH | 8.2 ± 0 |
| Organic matter (%) | 2.75 ± 0 |
| CEC (%) | 28.9 ± 0.99 |
| CaCO3 (%) | 5.30 ± 0.30 |
| Conductivity (μS cm-1) | 220.5 ± 9.20 |
| Total N (%) | 0.2 ± 0 |
| Available P (mg kg-1) | 6.8 ± 0.21 |
| Available K (mg kg-1) | 564.7 ± 57.30 |
| Mechanical analysis | |
| Clay (%) | 36.0 ± 2.83 |
| Silt (%) | 47.0 ± 2.83 |
| Sand (%) | 17.0 ± 0 |
| Texture | Silt-clay |
| Total Content (mg /kg-1) | |
|---|---|
| Cd | 6.45 ± 0.2 |
| Ni | 114.5 ± 14.8 |
| Cu | 149.0 ± 11.3 |
| Pb | 3279.5 ± 362.7 |
| Zn | 2238.0 ± 148.5 |
| Bioavailable Content (mg /kg-1) | |
| Cd | 1.95 ± 0.1 |
| Ni | 0.8 ± 0 |
| Pb | 525.5 ± 77.10 |
| Zn | 79.5 ± 6.4 |
| Agricultural Period | Nitrogen Level | Height (cm) | Shoot Diameter (mm) | No. of Branches |
|---|---|---|---|---|
| Spring cultivation (2022) | N0 | 59.02± 12.0 a | 3.74± 1.0 a | 0.50± 0.8 a |
| N1 | 50.73± 6.8 a | 3.62± 0.9 a | 0± 0 a | |
| N2 | 54.58± 6.7 a | 4.53± 0.8 a | 1.00± 1.3 a | |
| Winter cultivation (2023) | N0 | 76.71± 7.7 a | 2.07± 0.6 a | 1.50± 1.0 a |
| N1 | 77.44± 9.1 a | 2.29± 0.5 ab | 1.61± 0.8 a | |
| N2 | 81.39± 6.2 b | 2.48± 0.5 b | 1.61± 0.6 a |
| Agricultural period | Nitrogen level | Biomass yields (tn ha-1) |
|---|---|---|
| Spring cultivation (2022) | N0 | 3.81± 0.9 ab |
| N1 | 5.27± 1.0 b | |
| N2 | 2.88± 0.5 a | |
| Winter cultivation (2023) | N0 | 2.21± 0.6 a |
| N1 | 2.09± 0.7 a | |
| N2 | 2.30± 0.6 a |
| Agricultural period |
Nitrogen level | Cd (mg kg-1) |
Ni (mg kg-1) | Cu (mg kg-1) | Pb (mg kg-1) | Zn (mg kg-1) |
|---|---|---|---|---|---|---|
| Spring cultivation (2022) | N0 | 8.46± 0.9a | <D.L. | 6.35±1.1a | 18.15±3.9a | 51.85±11.1b |
| N1 | 6.95±0.8a | 0.22±0.1 | 5.39±1.5a | 16.04±2.4a | 40.06±5.1ab | |
| N2 | 7.14±1.2a | <D.L. | 5.14±1.5a | 17.87±0.9a | 35.16±1.0a | |
| Winter cultivation (2023) | N0 | 6.73±1.0a | <D.L. | 6.76±0.5a | 7.21±0.6a | 48.60±11.7a |
| N1 | 5.82±1.2a | <D.L. | 6.47±0.3a | 7.48±0.7a | 50.89±17.0a | |
| N2 | 5.84±1.9a | <D.L. | 7.21±0.1 a | 13.02±4.9 b | 49.00±8.8a |
| Agricultural period | Nitrogen level | Cd (g ha-1) |
Ni (g ha-1) |
Cu (g ha-1) |
Pb (g ha-1) |
Zn (g ha-1) |
|---|---|---|---|---|---|---|
| Spring cultivation (2022) | N0 | 31.81±6.1b | <D.L. | 24.53±9.2a | 71.28±32.5a | 190.68±8.9b |
| N1 | 36.15±3.1 b | 1.10±0.3 | 27.47±2.5a | 85.70±27.7a | 212.10±56.1b | |
| N2 | 20.38±3.8 a | <D.L. | 15.30±6.9a | 51.69±10.8a | 101.38±18.5a | |
| Winter cultivation (2023) | N0 | 14.59±2.8a | <D.L. | 14.81±3.1 a | 15.72±2.9a | 103.43±11.3a |
| N1 | 12.51±6.4a | <D.L. | 13.48±4.6a | 15.29±3.6a | 109.15±63.2a | |
| N2 | 12.78±2.4a | <D.L. | 16.61±4.2 a | 28.10±2.1b | 110.22±21.2a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





