Submitted:
05 March 2024
Posted:
05 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of M. javanica inoculum
2.2. Preparation of bacterial SynComs
2.3. Pot experiment
2.4. Plant manipulation for nematode extraction and growth parameter assessment
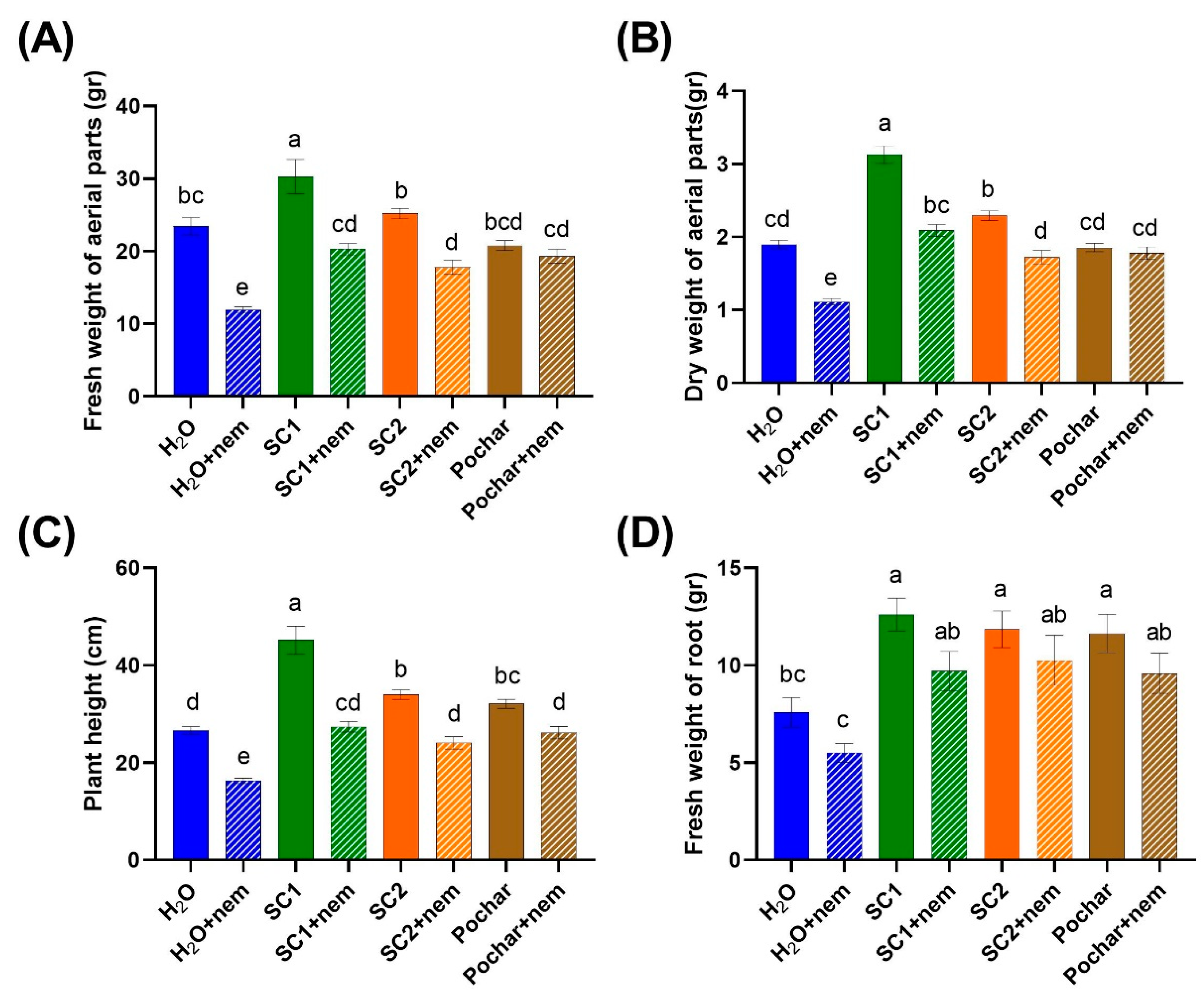
- Assessment of plant height: the plant height, i.e. the height from the plant collar up to its top was measured with a tape measure in cm.
- Assessment of the fresh and dry weights of the aerial parts: the aerial parts were separated from the roots, their fresh weight weighed on a 2-digit scale, then placed in a paper bag and left in an oven @50oC until completely dry, and subsequently the dry weight was recorded.
- Assessment of the fresh weight of roots: The roots of each plant were separately washed thoroughly but carefully, under running tap water, tap-dried with a paper towel and their fresh weight weighed on a 2-digit scale.
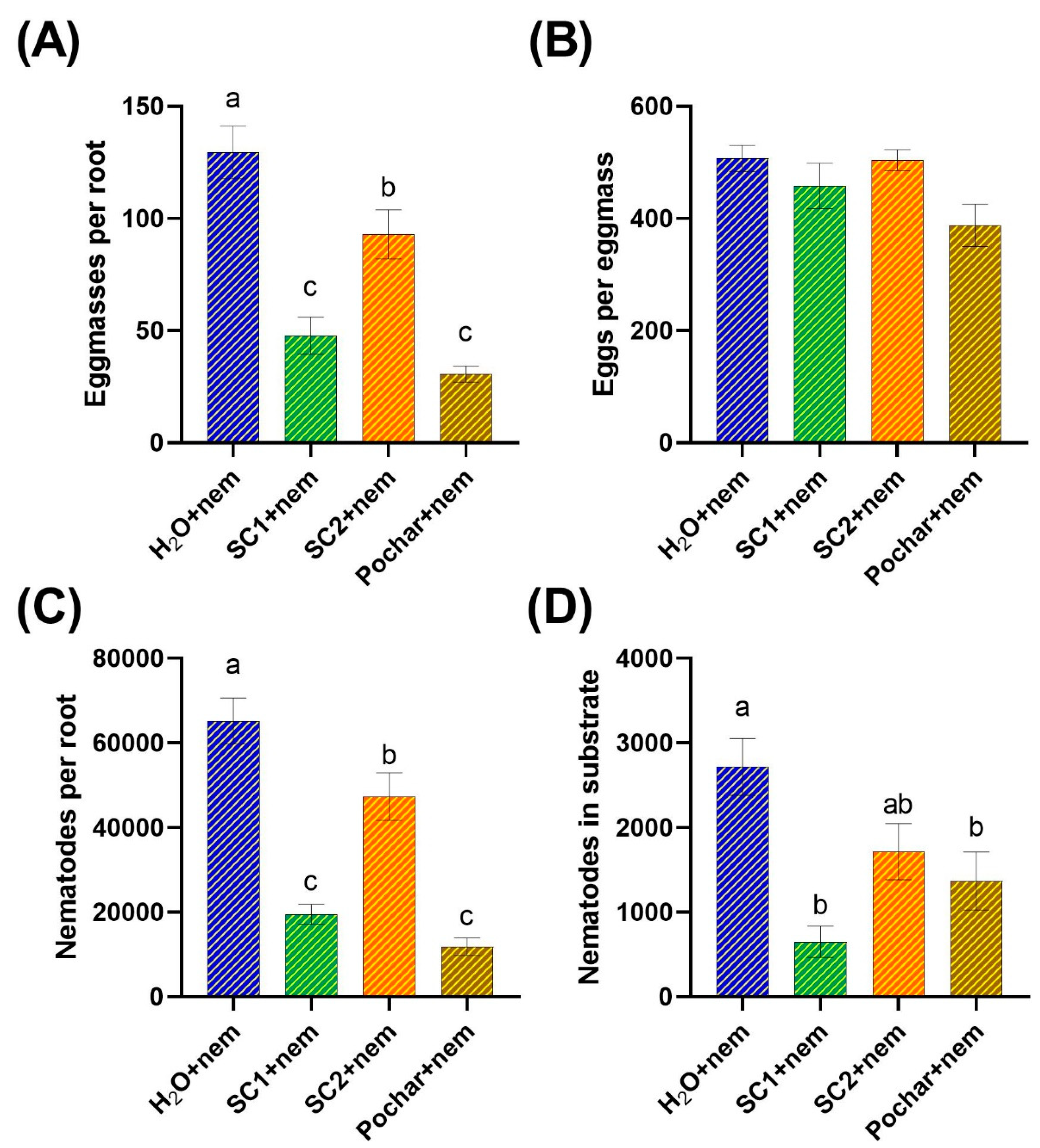
- Assessment of the total number of egg masses and eggs: The total number of egg masses per plant was counted under a stereomicroscope. Subsequently, for each plant, ten egg masses were randomly selected, placed in 1% sodium hypochlorite, shaked for 10 minutes to disperse the eggs, then the number of eggs per egg mass was estimated under the stereomicroscope.
- Assessment of the nematode population in the soil: the soil of each pot was handled following the conventional Baermann funnel technique [48] to estimate RKN soil population.
2.5. Statistical analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talavera-Rubia, M.; Vela-Delgado, M.D.; Verdejo-Lucas, S. A Cost-Benefit Analysis of Soil Disinfestation Methods against Root-Knot Nematodes in Mediterranean Intensive Horticulture. Plants 2022, 11, 2774. [CrossRef]
- Gharabadiyan, F.; Jamali, S.; Komeili, R.H. Determining of root-knot nematode (Meloidogyne javanica) damage function for tomato cultivars, J Agric Sci Belgrade 2013, 58 (2), 147-157. [CrossRef]
- Tzortzakakis, E.A.; da Conceicao, I.L.P.M.; dos Santos, M.C.V.; Abrantes, I.M.D.O. Root-knot nematodes (Meloidogyne spp.) in Greece. Hell Plant Prot J 2011, 4, 25-30.
- Nyczepir, A.P.; Thomas, S.H. Current and future management strategies in intensive crop production systems. In Root-knot nematodes Wallingford UK: CABI, 2009. 412-443.
- Riyaz, M.; Mathew, P.; Zuber, S.M.; Rather, G.A. Botanical pesticides for an eco-friendly and sustainable agriculture: New challenges and prospects,” In Sustainable agriculture (Cham: Springer) 2022, 69–96.
- Abd-Elgawad, M.M.M. Biological control agents of plant-parasitic nematodes. Egypt J Biol Pest Control 2016, 26(2), 423–429.
- Almaghrabi, O.A.; Massoud, S.I.; Abdelmoneim, T.S. Influence of inoculation with plant growth promoting rhizobacteria (PGPR) on tomato plant growth and nematode reproduction under greenhouse conditions. Saudi J Biol Sci 2013, 20(1), 57–61. [CrossRef]
- Khan, F.; Asif, M.; Khan, A.; Tariq, M.; Siddiqui, M.A. Screening of carrot cultivars against root-knot nematode Meloidogyne incognita. Ind Phytopath 2018, 71, 415–421. [CrossRef]
- Ali, W.M.; Abdel-Mageed, M.A.; Hegazy, M.G.A.; Abou-Shlell, M.K.; Sultan, S.M.E.; Salama, E.A.A.; Yousef, A.F. Biocontrol agent of root-knot nematode Meloidogyne javanica and root-rot fungi, Fusarium solani in okra morphological, anatomical characteristics and productivity under greenhouse conditions. Sci Rep 2023, 13, 11103. [CrossRef]
- Khalil, M.S.E.D.H.; Allam, A.F.G.; Barakat, A.S.T. Nematicidal activity of some biopesticide agents and microorganisms against root-knot nematode on tomato plants under greenhouse conditions. J Plant Prot Res 2012, 52(1), 47-52. [CrossRef]
- Khan, R.A.A.; Najeeb, S; Mao, Z.; Ling, J.; Yang, Y.; Li, Y.; Xie, B. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic bacteria and root-knot nematode. Microorganisms 2020, 8, 401. [CrossRef]
- Naz, I.; Khan, R.A.A.; Masood, T.; Baig, A.; Siddique, I.; Haq, S. Biological control of root knot nematode, Meloidogyne incognita, in vitro, greenhouse and field in cucumber. Biocontrol 2021, 152, 104429. [CrossRef]
- Affokpon, A.; Coyne, D.L.; Lawouin, L.; Tossou, C.; Agbede, R.D.; Coosemans J. Effectiveness of native West African arbuscular mycorrhizal fungi in protecting vegetable crops against root-knot nematodes. Biol Fertil Soils 2021, 47(2), 207–217. [CrossRef]
- Campos, M.A.S. Bioprotection by arbuscular mycorrhizal fungi in plants infected with Meloidogyne nematodes: A sustainable alternative. Crop Prot 2020, 135(105203):105203. article. [CrossRef]
- Kolawole, G.O.; Haastrup, T.M.; Olabiyi, T.I. Can arbuscular mycorrhiza fungi and NPK fertilizer suppress nematodes and improve tuber yield of yam (Dioscorea rotundata ‘cv’ ewuru)? Eurasian J Soil Sci 2018, 7(2), 181–186. [CrossRef]
- Odeyemi, I.S.; Afolami, S.O.; Sosanya, O.S. Effect of Glomus mosseae (arbuscular mycorrhizal fungus) on host-parasite relationship of Meloidogyne incognita (Southern root-knot nematode) on four improved cowpea varieties. J Plant Prot Res 2010, 50(3), 320–325. [CrossRef]
- Rouphael, Y.; Franken, P. ; Schneider, C. ; Schwarz, D.; Giorannetti, M.; Agnolucci, M.; Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci Hortic 2015, 196, 91–108. [CrossRef]
- Udo, I.A.; Uguru, M.I.; Ogbuji; R.O. Pathogenicity of Meloidogyne incognita race 1 on tomato as influenced by different arbuscular mycorrhizal fungi and bioformulated Paecilomyces lilacinus in a dysteric cambisol soil. J Plant Prot Res 2013, 53(1), 71–78. [CrossRef]
- Ahmad, G.; Nishat, Y.; Ansari, M.; Khan, A.; Haris, M.; Khan, A.A. Eco-friendly approaches for the alleviation of root-knot nematodes. In: Plant growth-promoting microbes for sustainable biotic and abiotic stress management. Springer, Cham, 2021, 557–575.
- Li, J.; Zou, C.; Xu, J.; Ji, X.; Niu, X.; Yang, J.; Zhang, K.Q. Molecular mechanisms of nematode-nematophagous microbe interactions: basis for biological control of plant-parasitic nematodes. Annu Rev Phytopathol 2015, 53, 67–95. [CrossRef]
- Tiwari, S.; Pandey, S.; Singh Chauhan, P.; Pandey, R. Biocontrol agents in co-inoculation manages root knot nematode [Meloidogyne incognita (Kofoid & White) Chitwood] and enhances essential oil content in Ocimum basilicum L. J Ind Crop Prod 2017, 97, 292–301. [CrossRef]
- Lee, Y.S.; Kim, K.Y. Antagonistic potential of Bacillus pumilus L1 against root-knot nematode, Meloidogyne arenaria. J Phytopathol 2016, 164, 29–39. [CrossRef]
- Mendoza, A.R.; Kiewnick, S.; Sikora, R.A. In vitro activity of Bacillus firmus against the burrowing nematode Radopholus similis, the root-knot nematode Meloidogyne incognita and the stem nematode Ditylenchus dipsaci. Biocontrol Sci Technol 2008, 18, 377–389. [CrossRef]
- Turatto, M.F.; Dourado, F.D.S.; Zilli, J.E.; Botelho, G.R. Control potential of Meloidogyne javanica and Ditylenchus spp. using fluorescent Pseudomonas and Bacillus spp. Braz J Microbiol 2018, 49, 54–59. [CrossRef]
- Xiang, N.; Lawrence, K.S.; Kloepper, J.W.; Donald, P.A.; McInroy, J.A.; Lawrence, G.W. Biological control of Meloidogyne incognita by spore-forming plant growth-promoting rhizobacteria on cotton. Plant Dis 2017, 101, 774–784. [CrossRef]
- Abbasi, M.; Ahmed, N.; Zaki, M.; Shuakat, S.; Khan, D. Potential of Bacillus species against Meloidogyne javanica parasitizing eggplant (Solanum melongena L.) and induced biochemical changes. Plant Soil 2014, 375, 159–173. [CrossRef]
- Metwally, W.E.; Mostafa, F.A.M.; Refaei, A.R. In vitro study on the antagonistic activity of different native isolates of rhizobacteria against Meloidogyne incognita. Egypt J Agronematol 2015, 14, 1–9. [CrossRef]
- Mnif, I.; Ghribi, D. Review lipopeptides biosurfactants: Mean classes and new insights for industrial, biomedical, and environmental applications. Biopolymers 2015, 104, 129-47. [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The Role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 2006, 57, 233–66. [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci 2012, 17, 478–86. [CrossRef]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 2010, 34, 1037–62. [CrossRef]
- Ling, N.; Zhu, C.; Xue, C.; Chen, H.; Duan, Y.; Peng, C.; Guo, S.; Shen, Q. Insight into how organic amendments can shape the soil microbiome in long-term field experiments as revealed by network analysis. Soil Biol Biochem 2016, 99, 137–49. [CrossRef]
- Mendes, L.W.; Tsai, S.M.; Navarrete, A.A.; de Hollander, M.; van Veen, J.A.; Kuramae, E.E. Soil-borne microbiome: Linking diversity to function. Microb Ecol 2015, 70, 255–65. [CrossRef]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P.; Burmølle, M.; Herschend, J.; Bakker, P.A.H.M.; Pieterse, C.M.J. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J 2018, 12, 1496–507. [CrossRef]
- Stringlis, I.A.; Proietti, S.; Hickman, R.; Van Verk, M.C.; Zamioudis, C.; Pieterse, C.M.J. Root transcriptional dynamics induced by beneficial rhizobacteria and microbial immune elicitors reveal signatures of adaptation to mutualists. Plant J 2018, 93, 166–80. [CrossRef]
- Tsolakidou, M-D.; Stringlis, I.A.; Fanega-Sleziak, N.; Papageorgiou, S.; Tsalakou, A.; Pantelides, I.S. Rhizosphere-enriched microbes as a pool to design synthetic communities for reproducible beneficial outputs, FEMS Microbiology Ecology 2019, 95(10), 138. [CrossRef]
- Oliveira, D.F.; Santos Júnior, H.M.; Nunes, A.S.; Campos, V.P.; Pinho, R.S.; Gajo, G.C. Purification and identification of metabolites produced by Bacillus cereus and B. subtilis active against Meloidogyne exigua, and their in silico interaction with a putative phosphoribosyltransferase from M. incognita. An Acad Bras Cienc 2014, 86(2), 525-538. [CrossRef]
- Cao, G.T.; Dai, B.; Wang, K.L.; Yan, Y.; Xu, Y.L.; Wang, Y.X.; Yang, C.M. Bacillus licheniformis, a potential probiotic, inhibits obesity by modulating colonic microflora in C57BL/6J mice model. J Appl Microbiol 2019, 127(3), 880-888. [CrossRef]
- Engelbrecht, G.; Claassens, S.; Mienie, C.M.S.; Fourie, H. Filtrates of mixed Bacillus spp. inhibit second-stage juvenile motility of root-knot nematodes Rhizosphere 2022, 22, 10052. [CrossRef]
- Moghaddam, R.M.; Moghaddam, M.E.; Ravari, B.S.; Rouhani, H. The first report of Bacillus pumilus influence against Meloidogyne javanica in Iran. JCP 2014, 3(1), 105-112. [CrossRef]
- Sansinenea, E.; Ortiz, A. Secondary metabolites of soil Bacillus spp. Biotechnol Lett 2011, 33(8), 1523-38. [CrossRef]
- El-Nagdi, W.M.A.; Abd-El-Khair, H. Biological control of Meloidogyne incognita and Fusarium solani in dry common bean in the field Arch. Phytopath Plant Prot 2014, 47(4), 388-397. [CrossRef]
- Munawar, M.; Khan, S.A.; Javed, N.; Ul Haq, I.; Gondal, A.S. Bio-management of tomato wilt complex caused by Meloidogyne incognita and Fusarium oxysporum f. sp. lycopersici. Nematology 2015, 17(4),479-485. [CrossRef]
- Patil, J.A.; Yadav, S.; Kumar, A. Management of root-knot nematode, Meloidogyne incognita and soil borne fungus, Fusarium oxysporum in cucumber using three bioagents under polyhouse conditions, Saudi J Biol Sci 2021, 28(12), 7006-7011. [CrossRef]
- Shi, X .; Qiao, K.; Li, B.; Zhang, S. Integrated management of Meloidogyne incognita and Fusarium oxysporum in cucumber by combined application of abamectin and fludioxonil Crop Protection 2019, 126, 104922. [CrossRef]
- Chialva, M.; Zouari, I.; Salvioli, A.; Novero, M.; Vrebalov, J.; Giovannoni, J.J.; Bonfante, P. Gr and hp-1 tomato mutants unveil unprecedented interactions between arbuscularmycorrhizal symbiosis and fruit ripening. Planta 2016, 244, 155–65. [CrossRef]
- Antoniou, A.; Tsolakidou, M-D.; Stringlis, I.A.; Pantelides, I.S. Rhizosphere microbiome recruited from a suppressive compost improves plant fitness and increases protection against vascular wilt pathogens of tomato. Front Plant Sci 2017, 8, 2022. [CrossRef]
- Baermann, G. Eine einfache Methode zur Auffindung von Anklostomum (Nematoden) Larven in Erdproben. – Tijdschr Diergeneeskd 1917, 57, 131–137.
- Abd-Elgawad, M.; Askary, T. Impact of Phytonematodes on Agriculture Ecology. In Biocontrol Agents of Phytonematodes. Askary T.H., Martinelli P.R.P., editors. CAB International Wallingford; Wallingford, UK 2015, 3–49.
- Phani, V.; Khan, M.R.; Dutta, T.K. Plant-parasitic nematodes as a potential threat to protected agriculture: Current status and management options. Crop Prot 2021, 144, 105573. [CrossRef]
- Sikora, R.A.; Fernandez, E. Nematode parasites of vegetables. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture. CABI Publishing; Wallingford, UK 2005, 319–392.
- Abd-Elgawad, M.M. Optimizing safe approaches to manage plant-parasitic nematodes. Plants 2021, 10,1911. [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front Microbiol 2020, 11,992. [CrossRef]
- Zhou, D.; Feng, H.; Schuelke, T.; De Santiago, A.; Zhang, Q.; Zhang, J.; Luo, C.; Wei, L. Rhizosphere Microbiomes from Root Knot Nematode Non-infested Plants Suppress Nematode Infection. Microb Ecol 2019, 78, 470–481. [CrossRef]
- Gamalero, E.; Glick, B.R. The Use of Plant Growth-Promoting Bacteria to Prevent Nematode Damage to Plants. Biology (Basel) 2020, 9(11), 381. [CrossRef]
- Kamalanathan, V.; Sevugapperumal, N.; Nallusamy, S. Antagonistic Bacteria Bacillus velezensis VB7 Possess Nematicidal Action and Induce an Immune Response to Suppress the Infection of Root-Knot Nematode (RKN) in Tomato. Genes (Basel) 2023, 25;14(7), 1335. [CrossRef]
- Mhatre, P.H.; Karthik, C.; Kadirvelu, K.; Divya, K.; Venkatasalam, E.; Srinivasan, S.; Ramkumar, G.; Saranya, C.; Shanmuganathan, R. Plant growth promoting rhizobacteria (PGPR): A potential alternative tool for nematodes bio-control. Biocat Agricul Biotechnol 2019, 17, 119–128. [CrossRef]
- Sidhu, H.S. Potential of plant growth-promoting rhizobacteria in the management of nematodes: a review. J Entomol Zool Stud 2018, 6(3), 1536-1545.
- Wharton, D. Nematode eggshells. Parasitology 1980, 81, 447–463. [CrossRef]
- Ray, S.; Reddigarim, S.R.; Jansma, P.L.; Allen, R.; Hussey, R.S. Immunocytochemical analysis of the stage-specific distribution of collagen in the cuticle of Meloidogyne incognita. Fund Appl Nematol 1996, 19, 71–78.
- Khanna, K.; Kohli, S.K.; Ohri, P.; Bhardwaj, R. Plants-nematodes-microbes crosstalk within soil: A trade-off among friends or foes, Microbiological Research 2021, 248, 126755, ISSN 0944-5013. [CrossRef]
- Krechel, A.; Faupel, A.; Hallmann, J.; Ulrich, A.; Berg, G. Potato-associated bacteria and their antagonistic potential towards plant-pathogenic fungi and the plant-parasitic nematode Meloidogyne incognita (Kofoid & White) Chitwood Can J Microbiol 2002, 48(9), 772-786. [CrossRef]
- El-Hadad, M.E.; Mustafa, M.I.; Selim, S.M.; El-Tayeb, T.S.; Mahgoob, A.E.A.; Aziz, N.H.A. The nematicidal effect of some bacterial biofertilizers on Meloidogyne incognita in sandy soil. Braz J Microbiol 2011, 42(1), 105-113. [CrossRef]
- Siddiqui, I.A.; Shaukat, S.S. Rhizobacteria-mediated induction of systemic resistance (ISR) in tomato against Meloidogyne javanica. J Phytopathol 2002, 150, 469–473. [CrossRef]
- Sikora, R.A. Interrelationship between plant health promoting rhizobacteria, plant parasitic nematodes and soil microorganisms. Med Fac Landbouww Rijksuniv Gent 1988, 53/2b, 867-878.
- Kloepper, J.W.; Ryu, C.-M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259-1266. [CrossRef]
- Siddiqui, Z.A.; Mahmood I. Role of bacteria in the management of plant parasitic nematodes: a review Bioresour Technol 1999, 69, 167-179. [CrossRef]
- Ramamoorthy, V.; Viswanathan, R.; Raguchander, T.; Prakasam, V.; Samiyappan R. Induction by systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and diseases Crop Prot 2001, 20, 1-11. [CrossRef]
- Van Loon, L.C.; Geraats, B.P.J.; Linthorst, H.J.M. Ethylene as a modulator of disease resistance in plants. Trends Plant Sci 2006, 11, 184–191. [CrossRef]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 2010, 34, 1037–62. [CrossRef]
- Falardeau, J.; Wise, C.; Novitsky, L.; Avis, T.J. Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J Chem Ecol 2013, 39, 869–78. [CrossRef]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Gowda, C.L.L.; Krishnamurthy, L. Plant growth promoting rhizobia: challenges and opportunities 3 Biotech, 2015, 355-377. [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 2003, 118, 10–5. [CrossRef]
- Zamioudis, C.; Mastranesti, P.; Dhonukshe, P.; Blilou, I.; Pieterse, C.M.J. Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria. Plant Physiol 2013, 162, 304–18. [CrossRef]
- Asari, S.; Tarkowská, D.; Rolčík, J.; Novák, O.; Palmero, D.V.; Bejai, S.; Meijer, J. Analysis of plant growth-promoting properties of Bacillus amyloliquefaciens UCMB5113 using Arabidopsis thaliana as host plant. Planta 2017, 245(1), 15-30. [CrossRef]
- Arora, N.K.; Tewari, S.; Singh R. Multifaceted plant-associated microbes and their mechanisms diminish the concept of direct and indirect PGPRs. In N.K. Arora (Ed.), Plant Microbe Symbiosis: Fundamentals and Advances Springer 2013, 411-449.
- Shaikh, S.; Saraf M. Biofortification of Triticum aestivum through the inoculation of zinc solubilizing plant growth promoting rhizobacteria in field experiment. Biocatal Agric Biotechnol 2016, 9, 120-126. [CrossRef]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 2010, 367-374. [CrossRef]
- Khan, M.R.; Khan, M.M.; Anwer, M.A.; Haque Z. Laboratory and field performance of some soil bacteria used as seed treatments on Meloidogyne incognita in chickpea. Nematol Mediterr 2012, 40, 143-151.
- Rashad, F.M.; Fathy, H.M.; El-Zayat, A.S.; Elghonaimy A.M. Isolation and characterization of multifunctional Streptomyces species with antimicrobial, nematicidal and phytohormone activities from marine environments. Egypt Microbiol Res 2015, 175, 34-47. [CrossRef]
- Ruanpanun, P.; Tangchitsomkid, N.; Hyde, K.D.; Lumyong S. Actinomycetes and fungi isolated from plant-parasitic nematode infested soils: screening of the effective biocontrol potential, indole-3-acetic acid and siderophore production World J Microbiol Biotechnol 2010, 1569-1578. [CrossRef]
- Van Loon, L.C.; Bakker, P.A.H.M.; Pieterse C.M.J. Systemic resistance induced by rhizosphere bacteria Annu Rev Phytopathol 1988, 453-483.
- Nascimento, F.X.; Vicente, C.S.; Barbosa, P.; Espada, M.; Glick, B.R.; Mota, M.; Oliveira, S. Evidence for the involvement of ACC deaminase from Pseudomonas putida UW4 in the biocontrol of pine wilt disease caused by Bursaphelenchus xylophilus. Biol Control 2013, 58, 427–433. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




