1. Introduction
While antibiotic treatment is available for
Chlamydia trachomatis (
Ct) infections,
Ct is still the most prevalent bacterial sexually transmitted infection (STI), due to difficulties with health care access and the lack of symptoms for most
Ct infections which leads to lack of presentation of persons to the clinic for diagnosis and treatment. These untreated
Ct infections can then cause chronic pelvic pain, ectopic pregnancies, and infertility in women [
1,
2,
3]. Therefore, infection prevention is urgently needed. Currently, there are some
Ct vaccine candidates in pre-clinical testing [
4,
5] and one candidate has completed clinical phase I [
6]. So far however, no vaccine is available to the public and additional research into
Ct vaccine candidates is strongly needed.
Based on high similarities regarding size, physiology, reproductive cycle, immunology, and susceptibility to
Ct, pigs have been used as biomedical animal models [
7] including the study of immunity [
8,
9], STIs [
10], and for
Ct vaccine development [
11,
12,
13,
14,
15]. Previously, we have shown that pigs can be infected genitally with both
Chlamydia suis (
Cs) and
Ct, and that the induced porcine CD4 T-cell response is cross-reactive between
Cs and
Ct [
16]. This finding led us to use outbred
Cs pre-exposed pigs as an animal model for
Ct vaccine development. One advantage of using this pig model is that it closely mimics the clinical trial participant population that will likely be recruited for clinical (phase 3) trials – genetically diverse, high-risk
Ct patients. High-risk
Ct patients will have pre-existing immunity to
Ct. Hence, our outbred pig model has a high chance of translating study outcomes to humans (including clinical trials). In a recent proof-of-principle vaccine study using TriAdj-adjuvanted UV-inactivated
Cs as vaccine, we demonstrated that this proof-of-principle vaccine induced a robust CD4 IFN-γ (=T-helper 1, Th1) response. This Th1 response has been shown to be critical in the anti-
Ct response [
3]. In addition, upon challenge with
Cs, our TriAdj-adjuvanted UV-
Cs vaccine candidate reduced the genital
Cs load compared to unvaccinated pigs demonstrating vaccine efficacy. Thereby, we have demonstrated that this
Cs pre-exposed outbred pig model can be used to accurately assess both vaccine immunogenicity and efficacy.
After this successful establishment of the
Cs pre-exposed outbred pig model, we now tested immunogenicity of our first
Ct vaccine candidate – TriAdj-adjuvanted chlamydial protease-like activity factor (CPAF). Antigen selection is based on a previous study that screened human
Ct patients for
Ct antigens that induce an IFN-γ response: with 16/30 patients eliciting an IFN-γ response in PBMC upon CPAF restimulation, CPAF was the most immunoprevalent antigen in this study [
17]. After selecting the TriAdj adjuvant and the CPAF vaccine antigen, we selected the vaccination regimen. Based on the study of Abrahams et al. [
6], we tested sequential intramuscular (IM) and intranasal (IN) vaccinations: One group (IN/IM) received two weekly IN vaccinations followed by two weekly IM vaccinations (n=6); the administration sites for the other group were reversed (IM/IN, n=6); in the MOCK group, 50% of pigs received IN/IM MOCK vaccinations (no CPAF, n=3) and 50% IM/IN MOCK vaccinations (no CPAF, n=3). Throughout the study, the vaccine-induced cellular and humoral immune responses were analyzed.
While IN/IM administration led to mixed results regarding immunogenicity, this study demonstrates that IM/IN administration of the TriAdj-adjuvanted Ct CPAF vaccine candidate induced a strong, high-avidity serum anti-CPAF IgG response, mucosal anti-CPAF IgG and IgA responses, and a robust IFN-γ response by CCR7- CD4 T cells. The strong induction of relevant humoral and cellular immune responses makes this IM/IN vaccination with the TriAdj-adjuvanted Ct CPAF a highly promising vaccine candidate.
2. Materials and Methods
Animal Trial
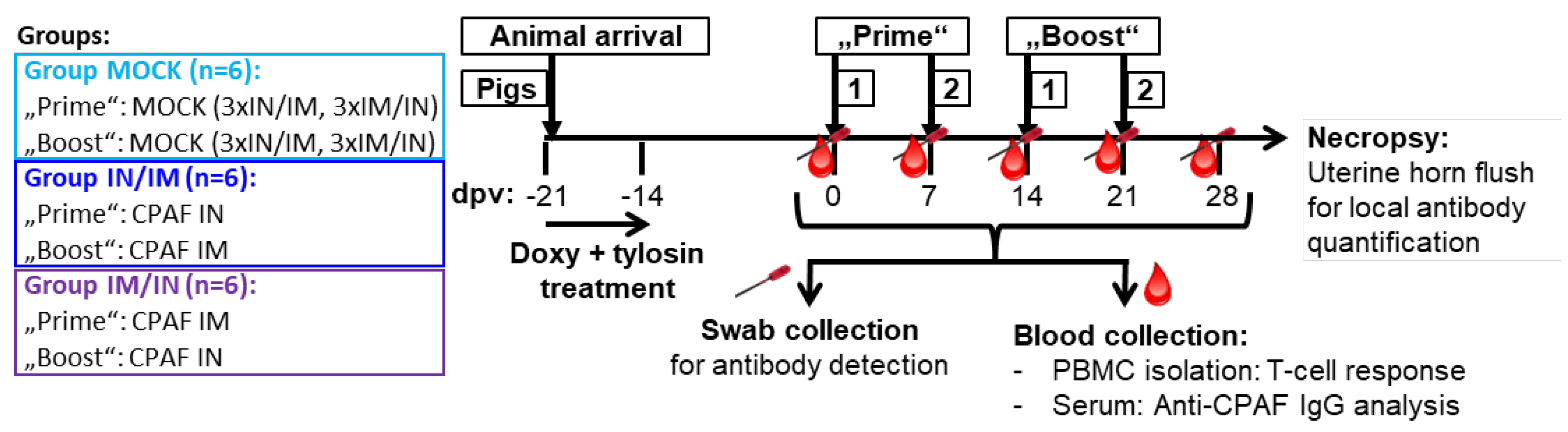
The animal trial was designed as illustrated in
Figure 1. Eighteen 7-week-old pigs from the North Carolina State University (NC State) Swine Educational Unit were brought to the BSL-2 Laboratory Animal Research (LAR) facility at the NC State College of Veterinary Medicine (Raleigh, NC, USA). At this point, pigs had not developed anti-
Cs antibodies (d.n.s.); however, as confirmed by
Cs-specific qPCR [
18], all pigs were qPCR-confirmed to be rectally infected with
Cs (d.n.s.). Pigs were randomly distributed into three groups – MOCK, IN/IM, IM/IN. After a resting period of three days (at -21 days post first vaccination, dpv), pigs were daily administered 1.44 g of doxycycline (Doxycycline Hyclate, West-Ward, Eatontown, NJ, USA) for four days and additionally 3 g of tylosin (Tylan soluble, Elanco
TM, Indianapolis, IN, USA) twice a day for 3.5 days to treat pre-existing
Cs infections. Then, a 14-day resting period was included to provide time for the anti-
Cs immune response to subside. Thereafter, pigs received their first of four vaccinations at 7-day intervals according to their group allocation (0 dpv, see
Figure 1 for group details). For simplicity reasons, the first two vaccinations are called “Prime” and the last two “Boost”. IN/IM and IM/IN groups received TriAdj-adjuvant-formulated CPAF; MOCK groups received the TriAdj adjuvant without CPAF (details: see below). Blood and swabs were taken prior to any vaccination day as well as 6 days after the last vaccination – at -1, 6, 13, 20, and 27 dpv. One week after the last vaccination (28 dpv), pigs were sacrificed to collect uterine horn flushes. Blood was used to collect sera and PBMC to study the systemic humoral and cellular anti-CPAF immune response, respectively. Swabs and uterine horn flushes were used to analyze the mucosal anti-CPAF antibody response.
This animal trial was approved by the Institutional Review Board of the North Carolina State University (ID# 21-199B; approval date: May 13, 2021).
Vaccine Antigen Production and Formulation with TriAdj
CPAF was expressed in E.coli strain BL21 DE3 using a codon optimised open reading frame based on the Ct sequence WP_015506580 cloned into pRSETA. Expressed protein was harvested in inclusion bodies which were washed extensively prior to urea solubilization and IMAC purification on Cobalt agarose (Talon®). Residual LPS was removed by cloud-point detergent extraction and the purified protein was lyophilised for storage. The TriAdj adjuvant was prepared according to the manufacturer’s instructions with final per pig compositions as follows – 150 μg of poly I:C, 300 μg of host defense peptide, and 150 μg of polyphosphazene. Within 1 hour of vaccination, per pig, 30 μg of CPAF were formulated with the TriAdj adjuvant and kept on ice until vaccine administration.
Isolation and Storage of Swabs, Sera, and PBMC
For rectal swab collection, swabs were inserted into the pig’s rectum. For vaginal swab collection, the vulva was cleaned, a speculum inserted into the vagina, and the swab was rotated on the vaginal epithelium surrounding the cervix. Swabs were inserted into 1 mL phosphate buffered saline (PBS) in a 1.5 mL snap-cap tube and stored on ice until further processing in the laboratory. There, swab tubes were vortexed, the swab was taken out of the liquid and rotated against the tube wall before being completely removed from the tube and discarded. The obtained swab samples were then frozen at -20°C for future antibody quantification.
Blood was collected into SST and heparin vacutainers (BD Bioscience, San Jose, CA, USA) for serum and PBMC isolation, respectively. Blood was rested upright for at least 30 min before centrifugation at 2,000 g for 20 min at room temperature (RT). Serum was harvested, aliquoted, and frozen at -20°C for future anti-CPAF IgG analysis. Isolation of PBMC was performed by density centrifugation using Sepmate tubes (StemCell, Vancouver, BC, Canada) and Ficoll-Paque (GE Healthcare, Uppsala, Sweden). After isolation, PBMCs were used fresh for in vitro restimulation to study the anti-CPAF specific T-cell IFN-γ response via ELISpot and flow cytometry intracellular cytokine staining. Additionally, PBMC were frozen in liquid nitrogen for future IL-17A ELISpots.
Interferon-γ and Interleukin-17A ELISpots
ELISpots were performed according to manufacturer’s descriptions. In brief, plates were activated with ethanol and coated overnight at 4°C with either anti-IFN-γ [3130-3, MabTech, Nacka Strand, Sweden] or anti-IL-17A [MT49A7 clone, MAbTech] capture antibodies. For IFN-γ ELISpots, fresh PBMC, for IL-17A ELISpots, thawed PBMC were seeded at 500,000 cells per well. Cells were stimulated for two days with 10 μg/mL CPAF. Media or Concanavalin A (ConA, 5 μg/mL) were used as negative and positive controls, respectively. Afterwards, cells were washed and stained with the respective biotinylated detection antibodies – IFN-γ [3130-6, MabTech] or IL-17A [MTP853-biotin, MabTech]. Streptavidin-alkaline phosphatase combined with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate (100 μL/well, Sigma-Aldrich, Cat # B3679) was used to visualize spots. ELISpot plates were dried and spots counted by a Mabtech ASTORTM ELISpot reader (Mabtech Inc., Cincinnati, OH, USA, for IFN-γ) or an AID ELISpot reader (AID, Straßberg, Germany) (for IL-17A). Data shown are based on three replicates.
Flow Cytometry Including Intracellular Cytokine Staining
Fresh PBMCs were plated in octuplicates at 500,000 cells/well in round-bottom 96-well plates and allowed to rest for 2-4 h. Afterwards, cells were stimulated overnight with 10 μg/mL CPAF. Media or Concanavalin A (ConA, 5 μg/mL) were used as negative and positive controls, respectively. The following day, after 14 h of culture, Monensin (5 mg/mL, Alfa Aesar, Haverhill, MA, USA) was added for an additional 4 h to block Golgi transport. Replicates were pooled and stained for flow cytometry analysis according to
Table 1. Data were acquired on a Cytoflex using the CytExpert software (Beckman Coulter). Data analysis was performed with FlowJo version 10.5.3 (FLOWJO LLC) with gates set based upon relevant FMO controls.
Anti-CPAF IgA, IgG, and IgM ELISAs Including IgG Avidity
For anti-CPAF IgA and IgG ELISAs, sera were diluted 1:5,000 in assay buffer (PBS +0.01% Tween-20 +0.1% bovine serum albumin, BSA); swabs and uterine horn flushes were used undiluted. All assays were run in duplicates. First, polystyrene 96-well plates (NUNC MaxiSorp, Thermo Fisher Scientific, Waltham, MA, USA) were coated overnight at 4°C with 10 μg/mL CPAF. Plates were washed twice with wash buffer (PBS + 0.02% Tween 20) and blocked for at least 1 h at RT using 1% BSA in PBS. After four washes, diluted sera, swabs, and uterine horn flushes were added and incubated overnight at 4°C. After four washes, horse-radish peroxidase-conjugated anti-pig IgA or IgG detection antibodies were added to the wells (Bethyl Laboratories Inc., A100.104P and A100-117P, respectively) at a 1:200,000 dilution for 2 h at RT. After four final washes, substrate (3,3′,5,5′-Tetramethylbenzidine, TMB) was added and incubated for 30 min at RT. Color reaction was quantified by optical density measurement at 450/620 nm using a Tecan Sunrise ELISA reader (Tecan, Männedorf, Switzerland).
For anti-CPAF serum IgG avidity assessment, a control or 6 M urea treatment was added after serum incubation: after washing, wells were incubated for 10 min at RT either in assay buffer (control treatment) or 6 M urea followed by four washes. Avidity index was calculated by dividing the OD value of the 6 M Urea treatment by the OD value of the control treatment [
19,
20].
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 10.2.0 (GraphPad Software, San Diego, CA, USA). Statistical significance was analyzed by either one-way (endpoint measurement) or two-way (longitudinal data) ANOVA each with Dunnett’s multiple comparisons test.
3. Results
The main goal of the study was to determine vaccine immunogenicity of the TriAdj-adjuvanted
Ct CPAF protein vaccine. Hence, prior to, during, and one week after the vaccination series, the humoral and cellular immune response was analyzed: the systemic cellular immunity was studied after in vitro restimulation of PBMC with CPAF via IFN-γ and IL-17A ELISpots (
Figure 2) and via flow cytometric intracellular cytokine staining (
Figure 3). The systemic humoral immune response was analyzed by serum anti-CPAF IgG ELISA including antibody avidity testing (
Figure 4). The mucosal antibody response was analyzed by anti-CPAF IgG and IgA ELISAs in vaginal and rectal swabs as well as uterine horn flushes (
Figure 5).
3.1. The Systemic Cellular Immune Response – IFN-γ and IL-17A ELISpots
CPAF-stimulated PBMC from MOCK vaccinated animals showed IFN-γ or IL-17A production at background levels (
Figure 2, light blue, MOCK). In contrast, PBMC from both CPAF-vaccinated groups increased both IFN-γ and IL-17A production over time (
Figure 2, dark blue and purple groups). However, IN vaccination alone (blue group, 6 and 13 dpv) did not induce systemic IFN-γ production. In contrast, IM vaccination induced significant systemic IFN-γ production (6 and 13 dpv, purple group) which became significant as early as 13 dpv with IFN-γ spots of up to 800 spots per 500,000 PBMC.
It must be noted that in contrast to the IFN-γ ELISpot which used fresh PBMCs, the IL-17A ELISpot was performed using thawed PBMCs that had been shipped from the original site of the study where the animal trial was performed (NCSU) to the current institution of Dr. Käser – the Vetmeduni Vienna. To ensure only fully functional PBMC were evaluated, a threshold of 200 IL-17A spots after ConA stimulation was applied. The consequent exclusion of several data points reduces the power of the IL-17A ELISpot assay. However, while the IL-17A data lack statistical significance, a similar trend is visible as for IFN-γ: mainly the IM vaccination induced a systemic response with the highest values in the IM/IN vaccination group.
In summary, while both anti-CPAF vaccinations induced at least by number a systemic IFN-γ and IL-17A production, the IM/IN vaccination regimen induced a strong and significant IFN-γ production.
3.2. The Systemic Cellular Immune Response – Flow Cytometry
In addition to the IFN-γ and IL-17A ELISpots, the systemic cellular immune response of fresh PBMC to in vitro CPAF restimulation was also analyzed by flow cytometry. The staining panel was chosen not only to quantify IFN-γ production but also to identify the cellular source of IFN-γ as well as the homing pattern via CCR7 expression of IFN-γ producing cells (
Table 1 and
Figure 3A). As in the IFN-γ ELISpot, CPAF-stimulated CD4, CD8, and TCR-γδ T cells from MOCK vaccinated animals showed IFN-γ production at low background levels (
Figure 3B, light blue, MOCK). In this IFN-γ flow assay, PBMC from the IN/IM CPAF-vaccinated groups also did not show increased IFN-γ production in either of the analyzed cell subsets (
Figure 3B, dark blue, IN/IM). In contrast, the IM/IN vaccination regimen induced significant IFN-γ production as early as 13 dpv. This systemic response was not further elevated by IN vaccination. The induced IFN-γ was solely produced by CD4 T cells (
Figure 3B, dark blue, IM/IN).
Expression of CCR7 in T cells corresponds to lymph node homing – e.g., by naïve and central memory T cells. Loss of CCR7 expression is a sign of homing to non-lymphoid tissues – e.g., inflamed mucosal tissue [
21]. Hence CCR7 expression was analyzed in IFN-γ-producing CD4 T cells. Within the IFN-γ
+ CD4 T cells, the IN/IM vaccination group led to only non-significant changes; in contrast, the IM/IN vaccination regimen led to an increased frequency of CCR7
- cells (
Figure 3C).
In summary, IM/IN vaccination with TriAdj-adjuvanted CPAF induced significant IFN-γ production in CD4 T cells and induced their homing to non-lymphoid tissues – such as mucosal sites like the genital or gastrointestinal tract.
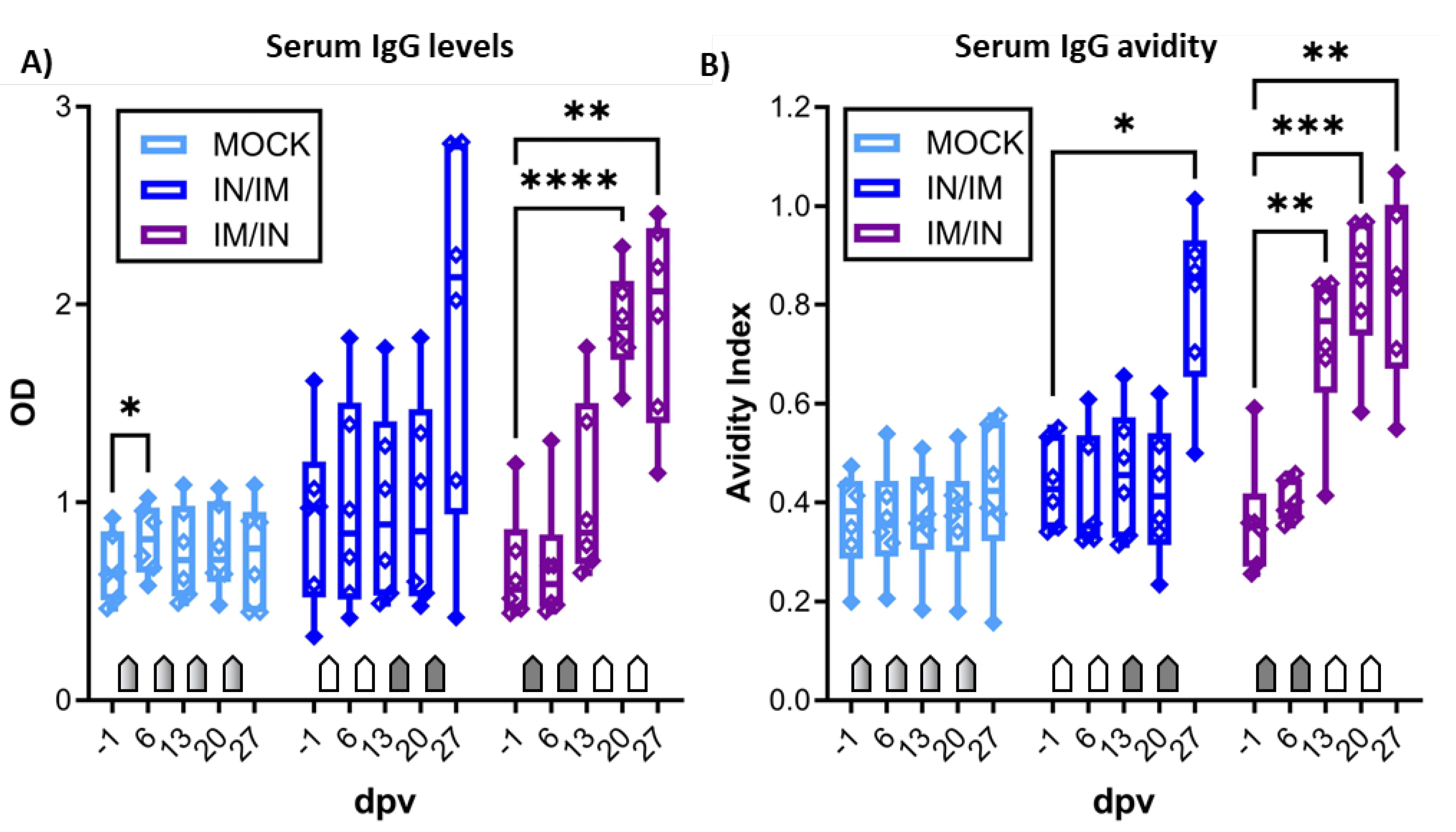
3.3. The Systemic Humoral Immune Response – anti-CPAF IgG Levels and Antibody Avidity
After demonstrating the induction of robust IFN-γ production by blood CD4 T cells, the systemic humoral response was analyzed by serum anti-CPAF IgG ELISA (
Figure 4A) and antibody avidity ELISA (
Figure 4B). While serum IgG levels in MOCK animals stayed at background levels, anti-CPAF serum IgG levels in IN/IM vaccinated pigs showed an increase at 27 dpv but variation in the response among animals prevented a statistically significant result. In IM/IN-vaccinated pigs, vaccination induced robust responses in all vaccinated pigs resulting in a significant increase in serum anti-CPAF IgG levels at 20 and 27 dpv (
Figure 4A). Anti-CPAF IgG avidity was tested by treatment with 6M urea. Anti-CPAF IgG avidity was increased in sera from IN/IM-vaccinated animals at 27 dpv and for IM/IN vaccinated animals as early as 13 dpv. After completion of the vaccination regimen, antibody avidity in both IN/IM and IM/IN vaccinated animals reached very high median avidity indices of >0.8. This shows that in anti-CPAF vaccinated animals, over 80% of antibodies have a strong avidity to the
Ct CPAF vaccine antigen (
Figure 4B).
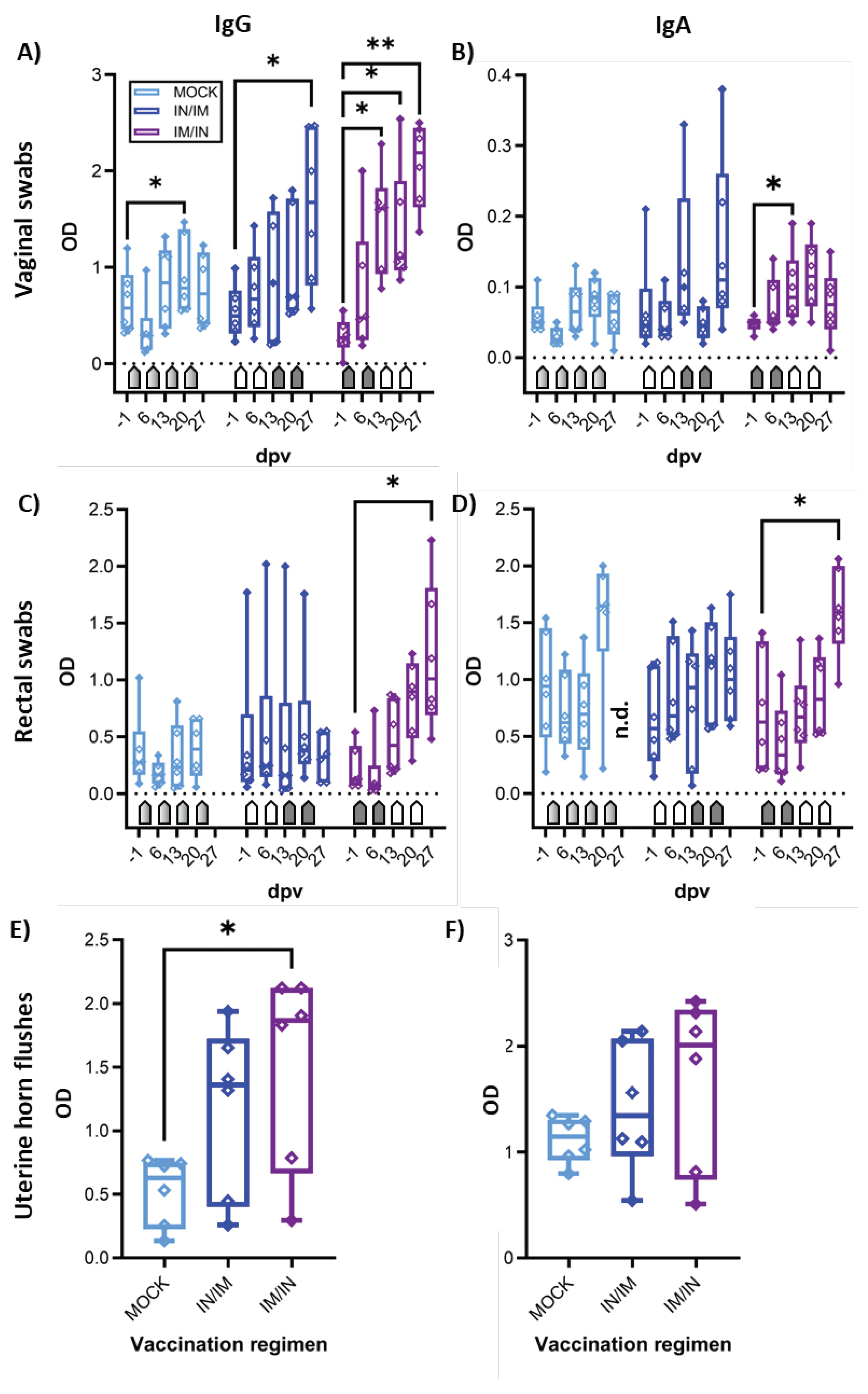
3.4. The Mucosal Humoral Immune Response – anti-CPAF IgG and IgA Levels
The mucosal humoral immune response to CPAF was analyzed via IgG and IgA ELISA in vaginal and rectal swabs, as well as in uterine horn flushes (
Figure 5). In vaginal swabs, both TriAdj-adjuvanted CPAF vaccinations induced significant increases in anti-CPAF IgG levels (
Figure 5A). Vaginal anti-CPAF IgA levels were detected at lower levels and only IM/IN vaccination induced a significant increase at 13 dpv (
Figure 5B). Rectal swabs not only showed increased background levels but also higher data variability, especially for IgA (27 dpv data are not available for MOCK). Nevertheless, also in rectal swabs, IM/IN vaccination once more led to an increased immune response with significantly higher anti-CPAF IgG and IgA levels after completion of the IM/IN vaccination regimen (
Figure 5C,D, 27 dpv). Anti-CPAF IgG and IgA levels were also quantified in uterine horn flushes to determine the humoral immune response in the upper genital tract. Both vaccination regimens led to increased anti-CPAF IgG and IgA levels in three (IgA, IN/IM) or four out of six vaccinated animals. In the IM/IN vaccination group, the increase in uterine horn anti-CPAF IgG levels was significant.
Summarizing, while both vaccination regimens led to increased mucosal IgG and/or IgA levels, at least by number, the IM/IN vaccination regimen produced more consistent responses resulting in significantly increased anti-CPAF IgG and IgA levels at all analyzed mucosal sites – the gut, the lower genital tract, and in most animals also in the upper genital tract.
Taken together, IM/IN vaccination with the TriAdj-adjuvanted Ct CPAF vaccine candidate not only induced strong and significant systemic IFN-γ production by CD4 T cells with increased homing potential to non-lymphoid tissue, but also generated high serum levels of high-avidity anti-CPAF IgG and a robust mucosal anti-CPAF IgG and IgA response in the gut as well as the lower and upper genital tract.
4. Discussion
With a global estimation of 127 million new
Ct cases in 2016 [
22] and the lack of available
Ct vaccines, the search for protective
Ct vaccine candidates becomes more urgent. Over the last ten years, we established
Cs-pre-exposed outbred pigs as an animal model with an arguably high chance of translating data into human clinical trials [
16,
18]. Hence, the main goal of this study was to determine vaccine immunogenicity of a TriAdj-adjuvanted CPAF vaccine candidate.
The TriAdj adjuvant has been selected based on its induction of effective long-term humoral and cellular immunity with various vaccine antigens including chlamydia [
23,
24,
25] and its strong induction of a Th1 response in our proof-of-principle
Cs vaccination study [
18]. As indicated above, CPAF was chosen as the vaccine antigen based on its broad recognition by Th1 cells in human
Ct patients [
17]. While combinations of IM and IN administrations have been successful vaccination strategies including for
Ct vaccines [
6], data are sparse on the optimal order of this combination for
Ct – IN/IM or IM/IN. Hence, we compared vaccine immunogenicity of our TriAdj-adjuvanted CPAF vaccine candidate in both orders.
Immunogenicity of the IN/IM vaccine administration was often non-significant. Especially the IN administration did not induce much of a systemic immune response (both T-cell and antibody responses) at the earlier time points – 6 and 13 dpv. This is in line with our previous data in which IN vaccination using UV-inactivated
Cs adjuvanted by TriAdj failed to induce a systemic IFN-γ response pre-challenge. In this previous study however, vaccination primed for a stronger post-challenge IFN-γ response by CD4 T cells as well as their differentiation into effector-memory T cells. This priming of CD4 T cells can explain a faster and stronger immune response in the genital tract and the observed decrease in
Cs particles in the vaccinated animals [
18]. The most recent data on
Ct vaccination in a clinical phase I trial also lacked a further increase of IM-induced systemic immunity levels by IN boosts [
6]. Nevertheless, one should not underestimate the benefits of IN vaccination without a detailed analysis of the mucosal immune response: Stary et al. also demonstrated that IN vaccination can induce potent protection by elevating mucosal immunity. In that study, the systemic response was also minimal: the only induction of systemic immunity data was a transient and non-significant increase in mostly
Ct-specific NR1 cells. This Stary et al. study further demonstrates that even in the absence of major induction of systemic immune responses, IN vaccination can be highly protective [
26]. Hence, future studies on
Ct vaccination should include a comprehensive analysis of mucosal immunity to optimally demonstrate vaccine immunogenicity – especially for IN vaccination.
In contrast to the minimal effects of IN vaccination on systemic immunity, our results demonstrate that in the IM/IN vaccination regimen, the TriAdj-adjuvanted CPAF vaccine candidate induces a strong systemic Th1 response and differentiation of IFN-γ producing CD4 T-cells into CCR7
- cells. It is well-established that this Th1 response is crucial in the protection against
Ct [
3]. Furthermore, CCR7
- Th1 cells have the ability to home to mucosal tissue; these mucosal tissue-homing cells have been further implicated in protection against
Ct [
26]. Therefore, IM/IN administration of the TriAdj-adjuvanted CPAF vaccine candidate induces the cellular immune response that has been most strongly linked with protection against
Ct.
In addition to the Th1 response, the humoral response contributes to protection against genital tract
Ct reinfections [
27,
28]. The humoral immune response seems to contribute mainly via antibody-induced cellular responses through Fcγ receptors. Moore et al. named enhanced phagocyte-induced
Ct killing through increased opsonophagocytosis, FcR mediated enhancement of antigen presentation, inhibition of
Ct growth and antibody-dependent cellular cytotoxicity (ADCC) as examples; they further suggest that “a future anti-chlamydia vaccine should elicit both humoral and T-cell-mediated immune responses for optimal memory response and vaccine efficacy.” [
27]. However, these benefits of antibody may only apply when the immunogen is on the outer membrane of
Ct. CPAF is a chlamydial protein that is secreted into the host cytosol and released into the extracellular milieu when the host epithelial cell lyses. It paralyzes neutrophils and inhibits their chlamydiacidal activities [
29]. It is possible that antibodies could neutralize this function of CPAF. Besides inducing a strong Th1 response, we show that IM/IN vaccination induced a pronounced humoral immune response: the systemic anti-CPAF IgG response was not only strongly increased but antibody avidity was also significantly elevated. Besides being an indication for successful germinal center formation and antibody maturation, antibody avidity correlates well with cross-neutralization e.g., for SARS-CoV-2 [
20]. In addition, Stanley Plotkin concluded (at least for
Haemophilus influenzae type b), that “antibodies must be of high avidity in order to protect” [
30]. Therefore, the induction of high levels of high-avidity serum anti-CPAF IgG antibodies demonstrates a potent induction of a relevant systemic immune response.
Besides the systemic immune response, anti-CPAF IgG and IgA levels have been analyzed in mucosal samples – vaginal swabs, rectal swabs, and uterine horn flushes. As systemically, IM/IN administration of TriAdj-adjuvanted CPAF induced a strong antibody response with increased IgA levels and particularly high and significantly increased IgG levels at basically all analyzed mucosal sites – rectally and in the lower and upper female genital tract. These high mucosal anti-CPAF antibody levels indicate that IM/IN administration of the TriAdj-adjuvanted CPAF vaccine candidate induces a prominent local humoral immune response. Together with the systemic Th1 response and high-avidity IgG antibodies, these mucosal antibodies have the potential to limit genital and rectal Ct infections.
5. Conclusions
In conclusion, in our Cs pre-exposed outbred pig model, IM/IN administration of the TriAdj-adjuvanted CPAF vaccine candidate not only induces a strong systemic Th1 and IgG response but also high mucosal antibody levels against the broadly recognized Ct CPAF protein. The strong immunogenicity in a relevant large biomedical animal model indicates that TriAdj-adjuvanted CPAF is a viable vaccine candidate against Ct. Future studies should extend the vaccine immunogenicity analysis to determine vaccine efficacy. These vaccine efficacy data will then be able to demonstrate if the TriAdj-adjuvanted CPAF vaccine candidate can limit Ct infections – the most prevalent bacterial STI.
Author Contributions
Conceptualization, J.P., V.G., K.W.B., J.M.H., T.D., T.K. methodology, J.P., M.S., L.M.C., A.I.S., T.I.S., T.K.; validation, J.P., M.S., L.M.C., V.G., K.W.B., J.M.H., T.D., T.K.; formal analysis, J.P., S.B., J.M.H., T.K.; investigation, J.P., M.S., L.M.C., D.B., L.P., V.G., S.B., K.W.B., J.M.H., T.D., T.K.; resources, V.G., A.I.S., T.I.S., K.W.B., J.M.H., T.D.; data curation, J.P., T.K.; writing—original draft preparation, T.K.; writing—review and editing, all authors; visualization, T.K.; supervision and project administration, J.P., T.K.; funding acquisition, V.G., K.W.B., J.M.H., T.D., T.K. All authors have read and agreed to the published version of the manuscript.
Funding
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI162709.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of the North Carolina State University (protocol code 21-199B, approval date: May 13, 2021).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank all students and Laboratory Animal Resources personnel who assisted in the organization of the animal trial, animal handling, and sample processing during the study at NCSU. All flow cytometry was performed in the NCSU CVM Flow Cytometry Core facility managed by Javid Mohammed. We thank Stacy Strom for handling the shipment of the TriAdj adjuvant. We would also like to thank Sandra Groiß, Juliane Müssauer, and Lea Schreitl for their help with the IgG avidity ELISA. As stated in
Table 1, BEI resources provided monoclonal antibodies.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Newman, L.; Rowley, J.; Vander Hoorn, S.; Wijesooriya, N.S.; Unemo, M.; Low, N.; Stevens, G.; Gottlieb, S.; Kiarie, J.; Temmerman, M. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS One 2015, 10, e0143304. [Google Scholar] [CrossRef]
- Witkin, S.S.; Minis, E.; Athanasiou, A.; Leizer, J.; Linhares, I.M. Chlamydia trachomatis: the Persistent Pathogen. Clin. Vaccine Immunol. 2017, 24. [Google Scholar] [CrossRef] [PubMed]
- Darville, T.; Hiltke, T.J. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J. Infect. Dis. 2010, 201 Suppl 2, S114–25. [Google Scholar] [CrossRef]
- Borges, Á.H.; Follmann, F.; Dietrich, J. Chlamydia trachomatis vaccine development - a view on the current challenges and how to move forward. Expert Rev. Vaccines 2022, 21, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Poston, T.B.; Gottlieb, S.L.; Darville, T. Status of vaccine research and development of vaccines for Chlamydia trachomatis infection. Vaccine 2019, 37, 7289–7294. [Google Scholar] [CrossRef]
- Abraham, S.; Juel, H.B.; Bang, P.; Cheeseman, H.M.; Dohn, R.B.; Cole, T.; Kristiansen, M.P.; Korsholm, K.S.; Lewis, D.; Olsen, A.W.; et al. Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: a first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2019, 19, 1091–1100. [Google Scholar] [CrossRef]
- Lunney, J.K.; van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Käser, T. Swine as biomedical animal model for T-cell research-Success and potential for transmittable and non-transmittable human diseases. Mol. Immunol. 2021, 135, 95–115. [Google Scholar] [CrossRef]
- Pabst, R. The pig as a model for immunology research. Cell Tissue Res. 2020, 380, 287–304. [Google Scholar] [CrossRef]
- Käser, T.; Renois, F.; Wilson, H.L.; Cnudde, T.; Gerdts, V.; Dillon, J.-A.R.; Jungersen, G.; Agerholm, J.S.; Meurens, F. Contribution of the swine model in the study of human sexually transmitted infections. Infect. Genet. Evol. 2018, 66, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Bøje, S.; Olsen, A.W.; Erneholm, K.; Agerholm, J.S.; Jungersen, G.; Andersen, P.; Follmann, F. A multi-subunit Chlamydia vaccine inducing neutralizing antibodies and strong IFN-γ⁺ CMI responses protects against a genital infection in minipigs. Immunol. Cell Biol. 2016, 94, 185–195. [Google Scholar] [CrossRef]
- Schautteet, K.; Stuyven, E.; Beeckman, D.S.A.; van Acker, S.; Carlon, M.; Chiers, K.; Cox, E.; Vanrompay, D. Protection of pigs against Chlamydia trachomatis challenge by administration of a MOMP-based DNA vaccine in the vaginal mucosa. Vaccine 2011, 29, 1399–1407. [Google Scholar] [CrossRef]
- Schautteet, K.; de Clercq, E.; Jönsson, Y.; Lagae, S.; Chiers, K.; Cox, E.; Vanrompay, D. Protection of pigs against genital Chlamydia trachomatis challenge by parenteral or mucosal DNA immunization. Vaccine 2012, 30, 2869–2881. [Google Scholar] [CrossRef]
- Schautteet, K.; Stuyven, E.; Cox, E.; Vanrompay, D. Validation of the Chlamydia trachomatis genital challenge pig model for testing recombinant protein vaccines. J. Med. Microbiol. 2011, 60, 117–127. [Google Scholar] [CrossRef]
- Vanrompay, D.; Hoang, T.Q.T.; de Vos, L.; Verminnen, K.; Harkinezhad, T.; Chiers, K.; Morré, S.A.; Cox, E. Specific-pathogen-free pigs as an animal model for studying Chlamydia trachomatis genital infection. Infect. Immun. 2005, 73, 8317–8321. [Google Scholar] [CrossRef]
- Käser, T.; Pasternak, J.A.; Delgado-Ortega, M.; Hamonic, G.; Lai, K.; Erickson, J.; Walker, S.; Dillon, J.R.; Gerdts, V.; Meurens, F. Chlamydia suis and Chlamydia trachomatis induce multifunctional CD4 T cells in pigs. Vaccine 2017, 35, 91–100. [Google Scholar] [CrossRef]
- Li, Y.; Warren, J.; Poston, T.; Shaw, F.; Conrad, S.; Xu, Y.; Zheng, X.; O’Connell, C.M.; Hillier, S.L.; Wiesenfeld, H.C.; et al. Identification of CPAF as the immunoprevalent antigen of Chlamydia trachomatis. The Journal of Immunology 2022, 208, 181–05. [Google Scholar] [CrossRef]
- Amaral, A.F.; Rahman, K.S.; Kick, A.R.; Cortes, L.M.; Robertson, J.; Kaltenboeck, B.; Gerdts, V.; O’Connell, C.M.; Poston, T.B.; Zheng, X.; et al. Mucosal Vaccination with UV-Inactivated Chlamydia suis in Pre-Exposed Outbred Pigs Decreases Pathogen Load and Induces CD4 T-Cell Maturation into IFN-γ+ Effector Memory Cells. Vaccines (Basel) 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G.; Struck, F.; Schreiner, P.; Staschik, E.; Soutschek, E.; Motz, M. The challenge of avidity determination in SARS-CoV-2 serology. J. Med. Virol. 2021, 93, 3092–3104. [Google Scholar] [CrossRef] [PubMed]
- Nakagama, Y.; Candray, K.; Kaku, N.; Komase, Y.; Rodriguez-Funes, M.-V.; Dominguez, R.; Tsuchida, T.; Kunishima, H.; Nagai, E.; Adachi, E.; et al. Antibody Avidity Maturation Following Recovery From Infection or the Booster Vaccination Grants Breadth of SARS-CoV-2 Neutralizing Capacity. J. Infect. Dis. 2023, 227, 780–787. [Google Scholar] [CrossRef]
- Sallusto, F.; Lenig, D.; Förster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Huai, P.; Li, F.; Chu, T.; Liu, D.; Liu, J.; Zhang, F. Prevalence of genital Chlamydia trachomatis infection in the general population: a meta-analysis. BMC Infect. Dis. 2020, 20, 589. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Waugh, C.; Rawlinson, G.; Brumm, J.; Nilsson, K.; Gerdts, V.; Potter, A.; Polkinghorne, A.; Beagley, K.; Timms, P. Vaccination of koalas (Phascolarctos cinereus) with a recombinant chlamydial major outer membrane protein adjuvanted with poly I:C, a host defense peptide and polyphosphazine, elicits strong and long lasting cellular and humoral immune responses. Vaccine 2014, 32, 5781–5786. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Desclozeaux, M.; Waugh, C.; Hanger, J.; Loader, J.; Gerdts, V.; Potter, A.; Polkinghorne, A.; Beagley, K.; Timms, P. Antibody and Cytokine Responses of Koalas (Phascolarctos cinereus) Vaccinated with Recombinant Chlamydial Major Outer Membrane Protein (MOMP) with Two Different Adjuvants. PLoS One 2016, 11, e0156094. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Babiuk, L.; van Drunen Littel-van den Hurk, S.; Gerdts, V. A novel combination adjuvant platform for human and animal vaccines. Vaccine 2017, 35, 4486–4489. [Google Scholar] [CrossRef]
- Stary, G.; Olive, A.; Radovic-Moreno, A.F.; Gondek, D.; Alvarez, D.; Basto, P.A.; Perro, M.; Vrbanac, V.D.; Tager, A.M.; Shi, J.; et al. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 2015, 348, aaa8205. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.; Ananaba, G.A.; Bolier, J.; Bowers, S.; Belay, T.; Eko, F.O.; Igietseme, J.U. Fc receptor regulation of protective immunity against Chlamydia trachomatis. Immunology 2002, 105, 213–221. [Google Scholar] [CrossRef]
- Hafner, L.; Beagley, K.; Timms, P. Chlamydia trachomatis infection: host immune responses and potential vaccines. Mucosal Immunol. 2008, 1, 116–130. [Google Scholar] [CrossRef]
- Rajeeve, K.; Das, S.; Prusty, B.K.; Rudel, T. Chlamydia trachomatis paralyses neutrophils to evade the host innate immune response. Nat. Microbiol. 2018, 3, 824–835. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef]
Figure 1.
TriAdj-adjuvanted Ct CPAF vaccination trial – groups and layout. Eighteen 7-week old female pigs were distributed into three groups—MOCK, intranasal/ intramuscular (IN/IM), and IM/IN vaccination. After a one-week antibiotic treatment and two resting weeks to allow the anti-Cs response to decline, pigs were vaccinated according to their group allocation at 0-, 7-, 14-, and 21-days post (first) vaccination (dpv). As indicated in the timeline, blood and vaginal/ rectal swabs were collected throughout the study the day before each vaccination and at six days after the last vaccination (27 dpv) to assess the humoral and T-cell immune response. The day after the last blood collection (28 dpv), pigs were sacrificed to collect uterine horns for local antibody quantification.
Figure 1.
TriAdj-adjuvanted Ct CPAF vaccination trial – groups and layout. Eighteen 7-week old female pigs were distributed into three groups—MOCK, intranasal/ intramuscular (IN/IM), and IM/IN vaccination. After a one-week antibiotic treatment and two resting weeks to allow the anti-Cs response to decline, pigs were vaccinated according to their group allocation at 0-, 7-, 14-, and 21-days post (first) vaccination (dpv). As indicated in the timeline, blood and vaginal/ rectal swabs were collected throughout the study the day before each vaccination and at six days after the last vaccination (27 dpv) to assess the humoral and T-cell immune response. The day after the last blood collection (28 dpv), pigs were sacrificed to collect uterine horns for local antibody quantification.
Figure 2.
Systemic IFN-γ and IL-17A production to in vitro CPAF restimulation. A) The systemic IFN-γ production was measured by ELISpot after in vitro CPAF restimulation of fresh PBMC. B) IL-17A production was also measured by ELISpot upon in vitro CPAF restimulation using frozen and thawed PBMC. Thawing process reduced viability and responsiveness of some PBMC. Only PBMC with adequate response to control stimulation (ConA) were included in the results. Pentagonal arrows depict vaccinations – gradient: 50%IN/50%IM, white: IN, grey: IM. Statistical analysis was performed via GraphPad via 2-way ANOVA with time and vaccination as the two parameters and Dunnett’s multiple comparison. *p<0.05.
Figure 2.
Systemic IFN-γ and IL-17A production to in vitro CPAF restimulation. A) The systemic IFN-γ production was measured by ELISpot after in vitro CPAF restimulation of fresh PBMC. B) IL-17A production was also measured by ELISpot upon in vitro CPAF restimulation using frozen and thawed PBMC. Thawing process reduced viability and responsiveness of some PBMC. Only PBMC with adequate response to control stimulation (ConA) were included in the results. Pentagonal arrows depict vaccinations – gradient: 50%IN/50%IM, white: IN, grey: IM. Statistical analysis was performed via GraphPad via 2-way ANOVA with time and vaccination as the two parameters and Dunnett’s multiple comparison. *p<0.05.
Figure 3.
IFN-γ production of T-cell subsets and differentiation of IFN-γ producing CD4 T cells. A) shows the gating hierarchy to assess the IFN-γ response of T-cell subsets. A live/dead discrimination dye was included to exclude dead cells. Within live cells, doublets were excluded via a FSC-area (FSC-A)/FSC-width (FSC-W) gate on live singlets. Live singlets were then used to identify live lymphocytes via a FSC/SSC single live lymphocytes (SLLs) gate. These SLLs were used to gate on CD4 T cells (CD4/SSC-A), and CD4- T cells were used to further discriminate TCR-αβ and TCR-γδ T cells (CD3/ TCR-γδ). CD4- TCR-αβ T cells were further used to identify CD8 T cells (CD3/CD8α). Within CD4, CD8, and TCR-γδ T cells, IFN-γ production was analyzed by intracellular cytokine staining (IFN-γ/SSC-A) with gate set based on fluorescence minus one (FMO) control. Within IFN-γ-producing CD4 T cells, expression of the lymph node homing marker CCR7 was analyzed. B) shows the frequency of IFN-γ+ cells within CD4 (left), CD8 (middle), and TCR-γδ (right) T cells after in vitro CPAF restimulation of PBMC from MOCK-vaccinated pigs (light blue), IN/IM vaccinated pigs (dark blue), or IM/IN vaccinated pigs (purple). Panel C) shows the frequency of CCR7- cells within IFN-γ-producing CD4 T cells. Pentagonal arrows depict vaccinations – gradient: 50%IN/50%IM, white: IN, grey: IM. Data were statistically analyzed using a 2-way ANOVA with time and vaccination as the two parameters and Dunnett’s multiple comparison. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05.
Figure 3.
IFN-γ production of T-cell subsets and differentiation of IFN-γ producing CD4 T cells. A) shows the gating hierarchy to assess the IFN-γ response of T-cell subsets. A live/dead discrimination dye was included to exclude dead cells. Within live cells, doublets were excluded via a FSC-area (FSC-A)/FSC-width (FSC-W) gate on live singlets. Live singlets were then used to identify live lymphocytes via a FSC/SSC single live lymphocytes (SLLs) gate. These SLLs were used to gate on CD4 T cells (CD4/SSC-A), and CD4- T cells were used to further discriminate TCR-αβ and TCR-γδ T cells (CD3/ TCR-γδ). CD4- TCR-αβ T cells were further used to identify CD8 T cells (CD3/CD8α). Within CD4, CD8, and TCR-γδ T cells, IFN-γ production was analyzed by intracellular cytokine staining (IFN-γ/SSC-A) with gate set based on fluorescence minus one (FMO) control. Within IFN-γ-producing CD4 T cells, expression of the lymph node homing marker CCR7 was analyzed. B) shows the frequency of IFN-γ+ cells within CD4 (left), CD8 (middle), and TCR-γδ (right) T cells after in vitro CPAF restimulation of PBMC from MOCK-vaccinated pigs (light blue), IN/IM vaccinated pigs (dark blue), or IM/IN vaccinated pigs (purple). Panel C) shows the frequency of CCR7- cells within IFN-γ-producing CD4 T cells. Pentagonal arrows depict vaccinations – gradient: 50%IN/50%IM, white: IN, grey: IM. Data were statistically analyzed using a 2-way ANOVA with time and vaccination as the two parameters and Dunnett’s multiple comparison. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05.
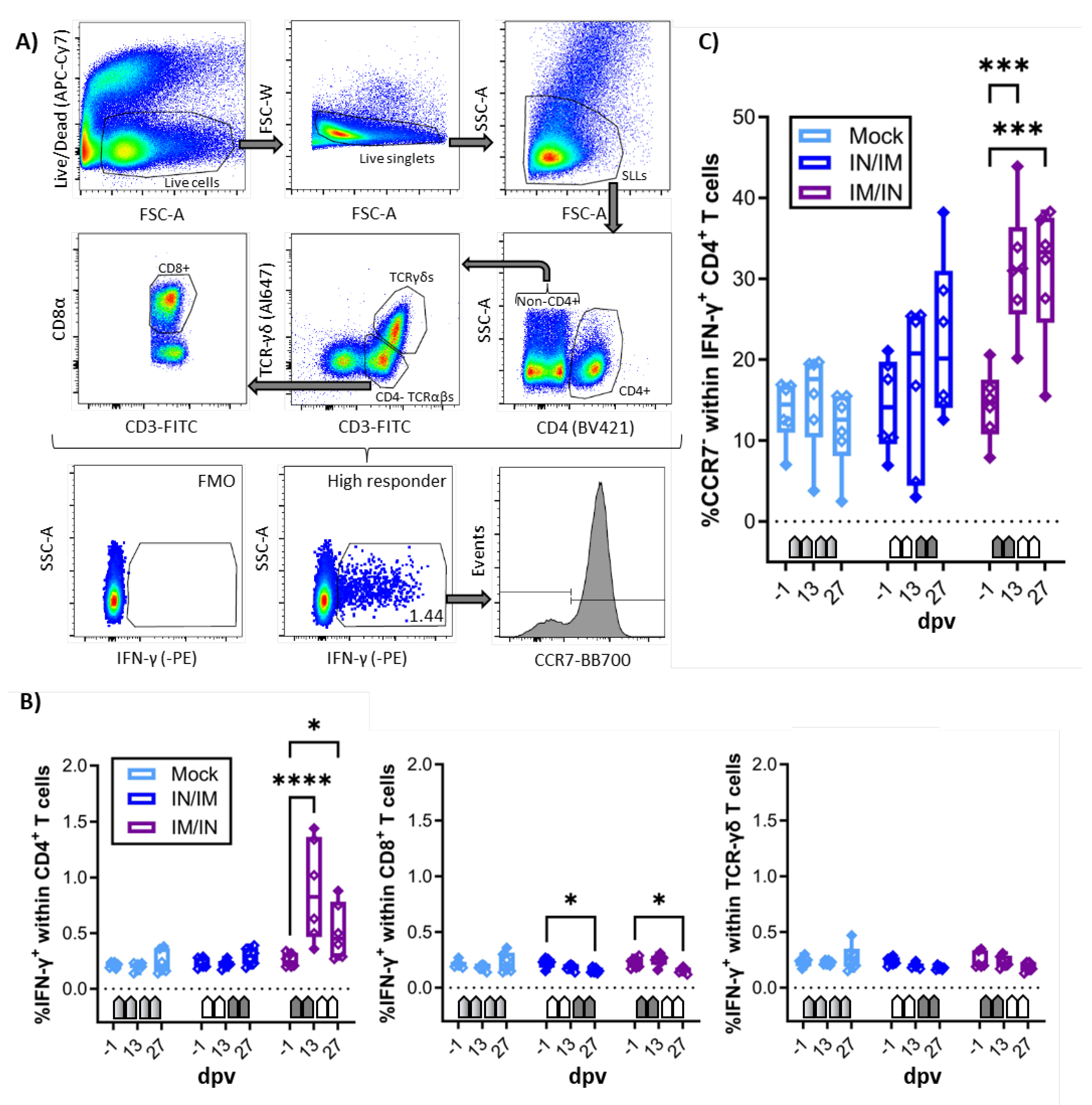
Figure 4.
Anti-CPAF IgG levels and avidity in sera. A) Immunoglobulin G (IgG) levels were quantified by anti-CPAF IgG ELISA in sera from animals receiving MOCK (light blue), IN/IM (dark blue), or IM/IN vaccination. Data are shown in optical density (OD). B) To assess IgG avidity in sera, a control or 6M urea treatment was included in the ELISA assay. Data are shown as avidity index which is calculated by dividing the OD value of the 6M urea treated sample by the OD value of the control treated sample. Pentagonal arrows depict vaccinations – gradient: 50%IN/50%IM, white: IN, grey: IM. Statistical analysis was performed via GraphPad via 2-way ANOVA with time and vaccination as the two parameters and Dunnett’s multiple comparison. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05.
Figure 4.
Anti-CPAF IgG levels and avidity in sera. A) Immunoglobulin G (IgG) levels were quantified by anti-CPAF IgG ELISA in sera from animals receiving MOCK (light blue), IN/IM (dark blue), or IM/IN vaccination. Data are shown in optical density (OD). B) To assess IgG avidity in sera, a control or 6M urea treatment was included in the ELISA assay. Data are shown as avidity index which is calculated by dividing the OD value of the 6M urea treated sample by the OD value of the control treated sample. Pentagonal arrows depict vaccinations – gradient: 50%IN/50%IM, white: IN, grey: IM. Statistical analysis was performed via GraphPad via 2-way ANOVA with time and vaccination as the two parameters and Dunnett’s multiple comparison. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05.
Figure 5.
Anti-CPAF IgG and IgA levels in vaginal and rectal swabs and in uterine horn flushes. Immunoglobulin G levels (IgG, A), C), E)) or IgA levels (B), D), F)) were quantified by isotype-specific anti-CPAF IgG or IgA ELISA in vaginal swabs (A)+B)), rectal swabs (C)+D)), or uterine horn flushes (E)+F)) from pigs receiving MOCK (light blue), IN/IM (dark blue), or IM/IN (purple) vaccination. Data are shown in optical density (OD). Pentagonal arrows depict vaccinations – gradient: 50%IN/50%IM, white: IN, grey: IM. Statistical analysis was performed via GraphPad via 2-way ANOVA with time and vaccination as the two parameters and Dunnett’s multiple comparison. **p < 0.01, *p < 0.05.
Figure 5.
Anti-CPAF IgG and IgA levels in vaginal and rectal swabs and in uterine horn flushes. Immunoglobulin G levels (IgG, A), C), E)) or IgA levels (B), D), F)) were quantified by isotype-specific anti-CPAF IgG or IgA ELISA in vaginal swabs (A)+B)), rectal swabs (C)+D)), or uterine horn flushes (E)+F)) from pigs receiving MOCK (light blue), IN/IM (dark blue), or IM/IN (purple) vaccination. Data are shown in optical density (OD). Pentagonal arrows depict vaccinations – gradient: 50%IN/50%IM, white: IN, grey: IM. Statistical analysis was performed via GraphPad via 2-way ANOVA with time and vaccination as the two parameters and Dunnett’s multiple comparison. **p < 0.01, *p < 0.05.
Table 1.
Flow cytometry staining panel.
Table 1.
Flow cytometry staining panel.
| Antigen |
Clone |
Isotype |
Fluorochrome |
Labeling Strategy |
Primary Ab source |
2nd Ab source |
| CD3 |
PPT3 |
IgG1 |
FITC |
Directly conjugated |
Southern Biotech |
- |
| CD4 |
74-12-4 |
IgG2b |
Brilliant Violet 421 |
Secondary antibody |
BEI Resources |
Jackson Immunoresearch |
| CD8α |
76-2-11 |
IgG2a |
Brilliant Violet 605 |
Biotin-Streptavidin |
Southern Biotech |
Biolegend |
| TCR-γδ |
PGBL22A |
IgG1 |
Alexa Fuor 647 |
In house conjugation1
|
Invitrogen |
- |
| CCR7 |
3D12 |
rIgG2a |
Brilliant Blue 700 |
Directly Conjugated |
BD Biosciences |
- |
| Live/Dead |
- |
- |
Near Infra-red |
- |
Invitrogen |
- |
| IFN-γ |
P2G10 |
IgG1 |
PE |
Directly conjugated |
BD Biosciences |
- |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).