Submitted:
04 April 2024
Posted:
09 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment
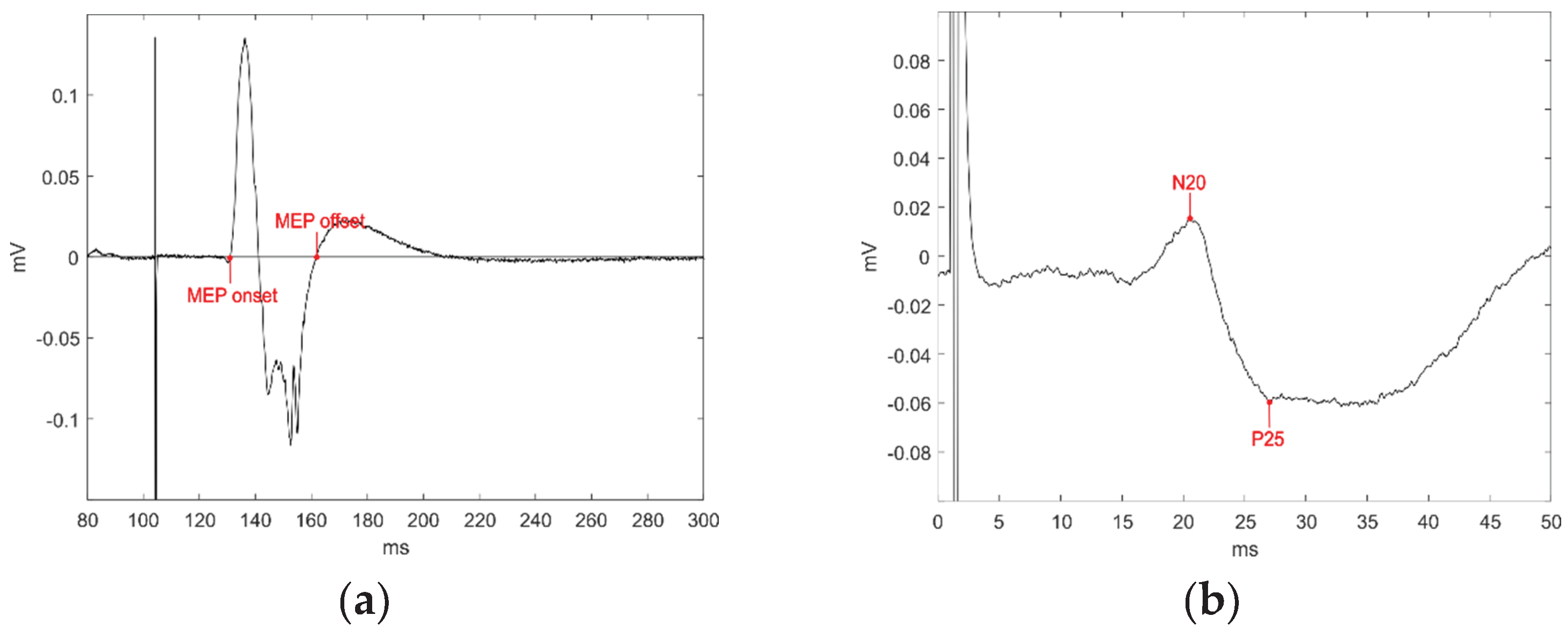
2.3. Experimental Procedures
2.4. Data Analysis
3. Results
3.1. MEPs: AUC
3.2. MEPs: Peak-to-Peak Amplitude
3.3. Relationship between the N20 Component and Corticospinal Excitability (AUC and Peak-to-Peak Amplitude)
4. Clinical Data at Admission and Discharge
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Giacino, J.T.; Fins, J.J.; Laureys, S.; Schiff, N.D. Disorders of consciousness after acquired brain injury: the state of the science. Nat. Rev. Neurol. 2014, 10, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Bodart, O.; Laureys, S.; Gosseries, O. Coma and Disorders of Consciousness: Scientific Advances and Practical Considerations for Clinicians. Semin. Neurol. 2013, 33, 083–090. [Google Scholar] [CrossRef] [PubMed]
- Laureys, S.; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Sazbon, L.; Schmutzhard, E.; von Wild, K.R.; Zeman, A.; et al. Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med. 2010, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Jennett, B.; Plum, F. Persistent vegetative state after brain damage: A Syndrome in Search of a Name. Lancet 1972, 299, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Kelly, J.P.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; et al. The minimally conscious state: Definition and diagnostic criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Ragazzoni, A.; Cincotta, M.; Giovannelli, F.; Cruse, D.; Young, G.B.; Miniussi, C.; Rossi, S. Clinical neurophysiology of prolonged disorders of consciousness: From diagnostic stimulation to therapeutic neuromodulation. Clin. Neurophysiol. 2017, 128, 1629–1646. [Google Scholar] [CrossRef] [PubMed]
- Rossi Sebastiano, D.; Panzica, F.; Visani, E.; Rotondi, F.; Scaioli, V.; Leonardi, M.; Sattin, D.; D’Incerti, L.; Parati, E.; Ferini Strambi, L.; et al. Significance of multiple neurophysiological measures in patients with chronic disorders of consciousness. Clin. Neurophysiol. 2015, 126, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Fleming SM, Frith CD, Goodale M (2023) The integrated information theory of consciousness as pseudoscience. PsyArXiv. Advance online publication. Retrieved October 30, 2023. 10.31234. [CrossRef]
- Seth, A.K.; Izhikevich, E.; Reeke, G.N.; Edelman, G.M. Theories and measures of consciousness: An extended framework. Proc. Natl. Acad. Sci. 2006, 103, 10799–10804. [Google Scholar] [CrossRef]
- Tononi, G.; Edelman, G.M. Consciousness and Complexity. Science (80-. ). 1998, 282, 1846–1851. [Google Scholar] [CrossRef]
- Rosanova, M.; Gosseries, O.; Casarotto, S.; Boly, M.; Casali, A.G.; Bruno, M.-A.; Mariotti, M.; Boveroux, P.; Tononi, G.; Laureys, S.; et al. Recovery of cortical effective connectivity and recovery of consciousness in vegetative patients. Brain 2012, 135, 1308–1320. [Google Scholar] [CrossRef]
- Ragazzoni, A.; Pirulli, C.; Veniero, D.; Feurra, M.; Cincotta, M.; Giovannelli, F.; Chiaramonti, R.; Lino, M.; Rossi, S.; Miniussi, C. Vegetative versus Minimally Conscious States: A Study Using TMS-EEG, Sensory and Event-Related Potentials. PLoS One 2013, 8, e57069. [Google Scholar] [CrossRef] [PubMed]
- Sarasso, S.; Rosanova, M.; Casali, A.G.; Casarotto, S.; Fecchio, M.; Boly, M.; Gosseries, O.; Tononi, G.; Laureys, S.; Massimini, M. Quantifying Cortical EEG Responses to TMS in (Un)consciousness. Clin. EEG Neurosci. 2014, 45, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Berlingeri, M.; Magnani, F.G.; Salvato, G.; Rosanova, M.; Bottini, G. Neuroimaging Studies on Disorders of Consciousness: A Meta-Analytic Evaluation. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.M.; Menon, D.K.; Johnsrude, I.S.; Bor, D.; Scott, S.K.; Manly, T.; Williams, E.J.; Mummery, C.; Pickard, J.D. Detecting residual cognitive function in persistent vegetative state. Neurocase 2002, 8, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Crone, J.S.; Ladurner, G.; Höller, Y.; Golaszewski, S.; Trinka, E.; Kronbichler, M. Deactivation of the Default Mode Network as a Marker of Impaired Consciousness: An fMRI Study. PLoS One 2011, 6, e26373. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Espejo, D.; Junque, C.; Cruse, D.; Bernabeu, M.; Roig-Rovira, T.; Fábregas, N.; Rivas, E.; Mercader, J.M. Combination of diffusion tensor and functional magnetic resonance imaging during recovery from the vegetative state. BMC Neurol. 2010, 10, 77. [Google Scholar] [CrossRef]
- Owen, A.; Coleman, M.; Menon, D.; Johnsrude, I.; Rodd, J.; Davis, M.; Taylor, K.; Pickard, J. Residual auditory function in persistent vegetative state: a combined pet and fmri study. Neuropsychol. Rehabil. 2005, 15, 290–306. [Google Scholar] [CrossRef]
- Di, H.B.; Yu, S.M.; Weng, X.C.; Laureys, S.; Yu, D.; Li, J.Q.; Qin, P.M.; Zhu, Y.H.; Zhang, S.Z.; Chen, Y.Z. Cerebral response to patient’s own name in the vegetative and minimally conscious states. Neurology 2007, 68, 895–9. [Google Scholar] [CrossRef]
- Qin, P.; Di, H.; Liu, Y.; Yu, S.; Gong, Q.; Duncan, N.; Weng, X.; Laureys, S.; Northoff, G. Anterior cingulate activity and the self in disorders of consciousness. Hum. Brain Mapp. 2010, 31, 1993–2002. [Google Scholar] [CrossRef]
- Staffen, W.; Kronbichler, M.; Aichhorn, M.; Mair, A.; Ladurner, G. Selective brain activity in response to one’s own name in the persistent vegetative state. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1383–4. [Google Scholar] [CrossRef]
- Kotchoubey, B.; Merz, S.; Lang, S.; Markl, A.; Müller, F.; Yu, T.; Schwarzbauer, C. Global functional connectivity reveals highly significant differences between the vegetative and the minimally conscious state. J. Neurol. 2013, 260, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Sharon, H.; Pasternak, Y.; Ben Simon, E.; Gruberger, M.; Giladi, N.; Krimchanski, B.Z.; Hassin, D.; Hendler, T. Emotional Processing of Personally Familiar Faces in the Vegetative State. PLoS One 2013, 8, e74711. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.M.; Coleman, M.R.; Boly, M.; Davis, M.H.; Laureys, S.; Pickard, J.D. Detecting awareness in the vegetative state. Science 2006, 313, 1402. [Google Scholar] [CrossRef] [PubMed]
- Bekinschtein, T.A.; Manes, F.F.; Villarreal, M.; Owen, A.M.; Della-Maggiore, V. Functional imaging reveals movement preparatory activity in the vegetative state. Front. Hum. Neurosci. 2011, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.M.; Vanhaudenhuyse, A.; Coleman, M.R.; Boly, M.; Pickard, J.D.; Tshibanda, L.; Owen, A.M.; Laureys, S. Willful Modulation of Brain Activity in Disorders of Consciousness. N. Engl. J. Med. 2010, 362, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, E.F.M.; Hijdra, A.; Young, G.B.; Bassetti, C.L.; Wiebe, S. Quality Standards Subcommittee of the American Academy of Neurology Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006, 67, 203–210. [Google Scholar] [PubMed]
- Cruse, D.; Norton, L.; Gofton, T.; Young, G.B.; Owen, A.M. Positive Prognostication from Median-Nerve Somatosensory Evoked Cortical Potentials. Neurocrit. Care 2014, 21, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Guérit, J.-M.; Amantini, A.; Amodio, P.; Andersen, K.V.; Butler, S.; de Weerd, A.; Facco, E.; Fischer, C.; Hantson, P.; Jäntti, V.; et al. Consensus on the use of neurophysiological tests in the intensive care unit (ICU): Electroencephalogram (EEG), evoked potentials (EP), and electroneuromyography (ENMG). Neurophysiol. Clin. Neurophysiol. 2009, 39, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Logi, F.; Fischer, C.; Murri, L.; Mauguière, F. The prognostic value of evoked responses from primary somatosensory and auditory cortex in comatose patients. Clin. Neurophysiol. 2003, 114, 1615–1627. [Google Scholar] [CrossRef]
- Young, G.B.; Doig, G.; Ragazzoni, A. Anoxic–Ischemic Encephalopathy: Clinical and Electrophysiological Associations With Outcome. Neurocrit. Care 2005, 2, 159–164. [Google Scholar] [CrossRef]
- Carter, B.G.; Butt, W. Are somatosensory evoked potentials the best predictor of outcome after severe brain injury? A systematic review. Intensive Care Med. 2005, 31, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Amantini, A.; Grippo, A.; Fossi, S.; Cesaretti, C.; Piccioli, A.; Peris, A.; Ragazzoni, A.; Pinto, F. Prediction of “awakening” and outcome in prolonged acute coma from severe traumatic brain injury: evidence for validity of short latency SEPs. Clin. Neurophysiol. 2005, 116, 229–35. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.G.; Butt, W. Review of the use of somatosensory evoked potentials in the prediction of outcome after severe brain injury. Crit. Care Med. 2001, 29, 178–186. [Google Scholar] [CrossRef]
- Vanhaudenhuyse, A.; Laureys, S.; Perrin, F. Cognitive Event-Related Potentials in Comatose and Post-Comatose States. Neurocrit. Care 2008, 8, 262–270. [Google Scholar] [CrossRef]
- Rothwell, J. C. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J. Neurosci. Meth. 1997, 74, 113–122. [Google Scholar]
- Rizzolatti, G.; Sinigaglia, C. The mirror mechanism: a basic principle of brain function. Nat. Rev. Neurosci. 2016, 17, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Fadiga, L.; Fogassi, L.; Pavesi, G.; Rizzolatti, G. Motor facilitation during action observation: a magnetic stimulation study. J. Neurophysiol. 1995, 73, 2608–11. [Google Scholar]
- Rizzolatti, G.; Craighero, L. The mirror neuron system. Annu. Rev. Neurosci. 2004, 27, 169–192. [Google Scholar] [PubMed]
- Gallese, V.; Fadiga, L.; Fogassi, L.; Rizzolatti, G. Action recognition in the premotor cortex. Brain 1996, 119, 593–609. [Google Scholar]
- Pistoia, F.; Sacco, S.; Carolei, A.; Sarà, M. Corticomotor facilitation in vegetative state: results of a pilot study. Arch. Phys. Med. Rehabil. 2013, 94, 1599–606. [Google Scholar]
- Furubayashi, T.; Ugawa, Y.; Terao, Y.; Hanajima, R.; Sakai, K.; Machii, K.; Mochizuki, H.; Shiio, Y.; Uesugi, H.; Enomoto, H.; et al. The human hand motor area is transiently suppressed by an unexpected auditory stimulus. Clin. Neurophysiol. 2000, 111, 178–83. [Google Scholar] [CrossRef] [PubMed]
- Davis, M. The Mammalian Startle Response. In Neural Mechanisms of Startle Behavior; Springer US: Boston, MA, 1984; pp. 287–351. [Google Scholar]
- Kelly, C.P.; Pothoulakis, C.; LaMont, J.T. Medical aspects of the persistent vegetative state (1). The Multi-Society Task Force on PVS. N. Engl. J. Med. 1994, 330, 1499–508. [Google Scholar]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Avanzini, G.; Bestmann, S.; Berardelli, A.; Brewer, C.; Canli, T.; Cantello, R.; et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [PubMed]
- Mancuso, M.; Abbruzzese, L.; Canova, S.; Landi, G.; Rossi, S.; Santarnecchi, E. Transcranial random noise stimulation does not improve behavioral and neurophysiological measures in patients with subacute Vegetative-Unresponsive Wakefulness State (VS-UWS). Front. Hum. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Hagen C, Malkmus D, Durham P. Levels of cognitive functioning. Downey (CA): Rancho Los Amigos Hospital, 1972.
- JASPER, H. The ten twenty electrode system of the international federation. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 371–375. [Google Scholar]
- Guide for the Uniform Data Set for Medical Rehabilitation (Adult FIM), version 4.0. Buffalo: State University of New York at Buffalo, 1993.
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.B.; George, M.S.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application: An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [PubMed]
- Gangitano, M.; Mottaghy, F.M.; Pascual-Leone, A. Phase-specific modulation of cortical motor output during movement observation. Neuroreport 2001, 12, 1489–1492. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.; Devi, B.I.; Agrawal, A. Outcome measures for traumatic brain injury. Clinical neurology and neurosurgery 2011, 113, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Rossato, E.; Verzini, E.; Scandola, M.; Ferrari, F.; Bonadiman, S. Role of LCF scale as an outcome prognostic index in patients with traumatic brain injury. Neurological Sciences 2021, 42, 2747–2752. [Google Scholar]
- Blair, R.C.; Karniski, W. An alternative method for significance testing of waveform difference potentials. Psychophysiology 1993, 30, 518–24. [Google Scholar]
- Leitner, D.S.; Powers, A.S.; Hoffman, H.S. The neural substrate of the startle response. Physiol. Behav. 1980, 25, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Naro, A.; Pignolo, L.; Lucca, L. F.; Calabrò, R. S. An action-observation/motor-imagery based approach to differentiate disorders of consciousness: what is beneath the tip of the iceberg? Rest. Neurol. Neurosci. 2021, 39, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Keysers, C.; Fadiga, L. The mirror neuron system: New frontiers. Soc. Neurosci. 2008, 3, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Dayan, E.; Sella, I.; Mukovskiy, A.; Douek, Y.; Giese, M.A.; Malach, R.; Flash, T. The Default Mode Network Differentiates Biological From Non-Biological Motion. Cereb. Cortex 2016, 26, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, E.; Maffongelli, L.; Jacono, M.; D’Ausilio, A. Representing tools as hand movements: Early and somatotopic visuomotor transformations. Neuropsychologia 2014, 61, 335–344. [Google Scholar] [CrossRef]
- Franca, M.; Turella, L.; Canto, R.; Brunelli, N.; Allione, L.; Andreasi, N.G.; Desantis, M.; Marzoli, D.; Fadiga, L. Corticospinal Facilitation during Observation of Graspable Objects: A Transcranial Magnetic Stimulation Study. PLoS One 2012, 7, e49025. [Google Scholar] [CrossRef]
| No. | Primitive Pathology | Age (Years) | Diagnosis | CRS-R | Time from Injury (Days) |
|---|---|---|---|---|---|
| 1 | Subarachnoid haemorrhage | 76 | VS/UWS | 1 | 26 |
| 2 | Ischemic stroke | 59 | VS/UWS | 5 | 33 |
| 3 | Intraparenchymal haemorrhage | 72 | VS/UWS | 5.6 | 75 |
| 4 | Subarachnoid haemorrhage | 62 | VS/UWS | 6.4 | 96 |
| 5 | Subarachnoid haemorrhage | 46 | VS/UWS | 6.4 | 60 |
| 6 | Ischemic stroke | 64 | VS/UWS | 6.8 | 80 |
| 7 | Subarachnoid haemorrhage | 81 | VS/UWS | 7.8 | 121 |
| 8 | Ischemic stroke | 74 | MCS | 8.4 | 51 |
| 9 | Subarachnoid haemorrhage | 79 | MCS | 8.4 | 130 |
| 10 | Intraparenchymal haemorrhage | 79 | MCS | 8.4 | 24 |
| 11 | Intraparenchymal haemorrhage | 81 | MCS | 9.2 | 61 |
| No. | Baseline | Action Observation | Acoustic Startle | Pendulum | Diagnosis | CRS-R |
|---|---|---|---|---|---|---|
| 1 | 13.58 (6.63) | 28.62 (18.52) | 25.47 (16.12) | VS/UWS | 1 | |
| 2 | 11.18 (2.30) | 15.09 (8.28) | 13.69 (5.04) | VS/UWS | 5 | |
| 3 | 20.07 (20.83) | 21.54 (18.94) | 20.37 (16.48) | 19.20 (21.67) | VS/UWS | 5.6 |
| 4 | 28.65 (3.29) | 31.32 (1.60) | 31.01 (2.10) | 31.32 (1.86) | VS/UWS | 6.4 |
| 5 | 91.82 (20.86) | 81.01 (18.40) | 95.42 (22.11) | 92.00 (17.28) | VS/UWS | 6.4 |
| 6 | 20.21 (11.23) | 31.45 (2.92) | 28.18 (3.14) | 25.84 (4.31) | VS/UWS | 6.8 |
| 7 | 43.93 (29.64) | 35.66 (23.95) | 41.81 (8.91) | VS/UWS | 7.8 | |
| 8 | 32.63 (12.36) | 47.94 (42.04) | 46.96 (47.29) | 39.80 (26.64) | MCS | 8.4 |
| 9 | 26.60 (18.64) | 20.15 (8.75) | 46.71 (16.90) | 25.23 (18.84) | MCS | 8.4 |
| 10 | 35.90 (24.73) | 38.02 (27.13) | 80.32 (44.35) | 84.18 (46.62) | MCS | 8.4 |
| 11 | 34.16 (24.72) | 59.53 (21.13) | 15.29 (8.67) | 27.36 (19.30) | MCS | 9.2 |
| No. | Baseline | Action Observation | Acoustic Startle | Pendulum | Diagnosis | CRS-R |
|---|---|---|---|---|---|---|
| 1 | 0.38 (0.19) | 0.82 (0.55) | 0.68 (0.44) | VS/UWS | 1 | |
| 2 | 0.50 (0.10) | 0.77 (0.38) | 0.68 (0.23) | VS/UWS | 5 | |
| 3 | 1.18 (0.86) | 1.20 (0.99) | 1.12 (0.80) | 1.15 (1.15) | VS/UWS | 5.6 |
| 4 | 1.03 (0.11) | 1.11 (0.07) | 1.10 (0.11) | 1.13 (0.07) | VS/UWS | 6.4 |
| 5 | 4.15 (0.84) | 3.67 (0.82) | 3.92 (1.02) | 4.51 (0.66) | VS/UWS | 6.4 |
| 6 | 0.72 (0.41) | 1.08 (0.16) | 1.02 (0.10) | 0.93 (0.16) | VS/UWS | 6.8 |
| 7 | 1.11 (0.76) | 0.98 (0.74) | 1.46 (0.63) | VS/UWS | 7.8 | |
| 8 | 1.08 (0.43) | 1.12 (0.40) | 1.56 (1.64) | 1.32 (0.95) | MCS | 8.4 |
| 9 | 0.85 (0.56) | 0.61 (0.26) | 1.37 (0.55) | 0.80 (0.55) | MCS | 8.4 |
| 10 | 1.42 (0.92) | 1.60 (0.91) | 2.83 (1.49) | 3.09 (1.67) | MCS | 8.4 |
| 11 | 1.44 (0.84) | 2.18 (0.81) | 0.80 (0.39) | 1.20 (0.81) | MCS | 9.2 |
| LCF | FIM | Lifespan (months) |
||||
|---|---|---|---|---|---|---|
| No. | CRS-R | Admission | Discharge | Admission | Discharge | |
| 1 | 1 | 2 | - | 18 | - | 1 |
| 2 | 5 | 2 | 3 | 18 | 18 | Alive |
| 3 | 5.6 | 2 | 2 | 18 | 18 | 4 |
| 4 | 6.4 | 2 | 4 | 18 | 23 | 41 |
| 5 | 6.4 | 2 | 2 | 18 | 18 | 8 |
| 6 | 6.8 | 2 | 2 | 18 | 18 | 6 |
| 7 | 7.8 | 1 | 2 | 18 | 18 | 35 |
| 8 | 8.4 | 2 | 2 | 18 | 18 | - |
| 9 | 8.4 | 2 | deceased | 18 | deceased | 4 |
| 10 | 8.4 | 2 | 2 | 18 | 18 | 6 |
| 11 | 9.2 | 3 | 3 | 21 | - | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





