Preprint
Review
The Therapeutic Trip of Melatonin Eye Drops: From the Ocular Surface to the Retina
This version is not peer-reviewed.
Submitted:
05 March 2024
Posted:
06 March 2024
You are already at the latest version
A peer-reviewed article of this preprint also exists.
Abstract
Melatonin is a ubiquitous molecule found in living organisms, ranging from bacteria to plants and mammals. It possesses various properties, partly due to its robust antioxidant nature and partly owed to its specific interaction with melatonin receptors present in almost all tissues. Melatonin regulates different physiological functions and contributes to the homeostasis of the entire organism. In the human eye, a small amount of melatonin is also present, produced by cells in the anterior segment and the posterior pole, including the retina. In the eye, melatonin may provide antioxidant protection along with regulating physiological functions of ocular tissues, including intraocular pressure (IOP). Therefore, it is conceivable that the exogenous administration of sufficiently high amounts of melatonin to the eye could be beneficial in several instances: for the treatment of eye pathologies like glaucoma, due to the IOP-lowering and neuroprotection effects of melatonin; for the prevention of other dysfunctions, such as dry eye and refractive defects (cataract and myopia) mainly due to its antioxidant properties; for diabetic retinopathy due to its metabolic influence and neuroprotective effects; for macular degeneration due to the antioxidant and neuroprotective properties; and for uveitis, mostly owing to anti-inflammatory and immunomodulatory properties. This paper reviews the scientific evidence supporting the use of melatonin in different ocular districts and provides data suggesting that the topical administration of melatonin as eye drops is a real possibility. This way, its distribution and concentration in different ocular tissues may support its pleiotropic therapeutic effects.
Keywords:
melatonin
; drug delivery
; eye diseases
1. Introduction
Melatonin (N-acetyl-5-methoxytryptamine) is an indoleamine primarily secreted by the human pineal gland during darkness [1] but also produced in various tissues, including the eyes [2]. Melatonin can be considered the logical natural consequence of what could be described as the paradox of life. Life on Earth over the last 2.5-3.0 billion years has evolved in an oxygen-rich atmosphere, developing an aerobic metabolism that, as a byproduct, generates a substantial amount of free oxygen radicals (reactive oxygen species: ROS) [3]. Known as the oxygen paradox [4], it posits that while oxygen is essential for life, it is also toxic to cells and organisms, ultimately causing aging and death.
Most of the oxidative energetic reactions necessary for metabolism and cell survival occur in mitochondria (chloroplasts in plants), which are the primary producers of these highly reactive free oxygen radicals. Approximately 4% of the oxygen used in aerobic metabolism is estimated to be converted to ROS. To survive, cells had to develop an efficient antioxidant system, and melatonin is likely the product of such an evolutionary effort [5]. Indeed, melatonin is already present in unicellular organisms and has been conserved throughout evolution in plants and animals [6].
The primary site of melatonin biosynthesis is in mitochondria (the major source of ROS generation in living organisms), and its production in other cellular compartments likely derives from this primary site [7,8]. The unique structure of melatonin is responsible for its high efficiency in detoxifying free radicals. Metabolites derived from its antioxidant effect also retain free radical scavenging activity, sometimes even stronger than the original molecule [9]. This potent antioxidant function of melatonin results in the protection of mitochondrial physiology and extends to its other activities, such as anti-inflammatory, neuroprotective, anti-apoptotic, and anti-aging effects [5].
With time, the functions of melatonin have expanded through the development of specific cell receptors. MT1 and MT2 are the main cell membrane receptors, belonging to the class of G-protein–coupled receptors and are widely distributed in many tissues [10,11]. MT3 is the low-affinity cytoplasmic receptor for melatonin, identified as quinone reductase 2 [12], belonging to a group of detoxifying enzymes involved in preventing oxidative stress through the inhibition of electron transfer reactions [13].
Melatonin-producing cells and their related receptors are present throughout the body and in almost all eye tissues, where they play a role in the regulation of eye growth and its dioptric potential, intraocular pressure (IOP), ocular surface physiology, and phototransduction processes [13] (Figure 1). Indeed, the presence of receptor-dependent and -independent functions within the same molecule makes melatonin a very versatile natural drug. This versatility could help rebalance deranged situations leading to ophthalmic pathologies. Here, we aim to explore the possibility of formulating melatonin as eye drops for topical application, to treat or even prevent the development of the most common eye diseases [14,15], as supported by the research data presented subsequently.
2. Topical Formulations of Melatonin
Formulating melatonin as eye drops presents two primary challenges that must be addressed. The first pertains to its stability in aqueous solutions, and the second involves facilitating its transport across the epithelial barrier of the ocular surface.
Melatonin is a small, non-polar molecule with poor solubility in an aqueous solution (0.1 mg/ml) and is typically administered orally in immediate and modified-release formulations. Through HPLC analytical methods, it has been demonstrated that melatonin remains stable in aqueous solution for at least 6 months when stored at 4°C or below, in sterile vacuum-packed vials [16]. Further studies on the stability of aqueous melatonin solutions were conducted at 20°C and 37°C at different pH values (1.2-12) for 21 days [17]. No degradation of melatonin was observed during the initial 48 hours. However, from day 3 to day 21, a gradual decrease in melatonin occurred at all pH values, though not exceeding 30% of the initial value. Due to its poor stability in aqueous solution, various formulations are being investigated for alternative routes of administration, such as sublingual, transbuccal, ocular, intranasal, or injectable methods [18].
Achieving full solubility of higher concentrations of melatonin would require the use of organic solvents, which are incompatible with ocular application. Therefore, the quest for more tolerable formulations containing higher concentrations of melatonin than achievable in water, and devoid of organic solvents, poses a challenging task in developing a commercial product suitable for topical ocular treatments. Another challenge lies in developing a formulation capable of delivering melatonin beyond the ocular surface. To address this, nanotech ophthalmic systems could be considered to traverse the ocular surface epithelial barrier and reach tissues within the eye globe. Several nanocarriers have been described for ocular drug delivery to different eye segments [19], and some of them have shown promising results in improving ocular bioavailability, reducing the frequency of administration, and minimizing toxic effects on healthy tissues.
2.1. Formulations with Liposomes
Liposomes are spherical, closed vesicles composed of double lipid layers that enclose an aqueous compartment. The size of a liposome varies from 10 nm to several micrometers. The main constituents of liposomes are phospholipids, which are amphiphilic molecules. A significant advantage of liposomes is their ability to incorporate hydrophilic molecules into the aqueous compartment, hydrophobic molecules within the lipid membrane, and amphiphilic molecules at the lipid-aqueous interface [20]. This property gives liposomes unique characteristics, making them an ideal transport system in the ophthalmic field.
In a recent study, nano-liposomes (150–200 nm, 6-10 mV zeta potential) were loaded with the melatonin analog 5-methoxycarbonylamino-N-acetyltryptamine (5-MCA-NAT) (100 μM). The formulation also contained a mucoadhesive molecule such as sodium hyaluronate 0.2% (SH) or carboxymethylcellulose 0.5% (CMC) or a thermosensitive amphiphilic poloxamer (PX407 and PX 188; 12/8, w/w). The aim was to prolong the residence time of the eye drop on the ocular surface and enhance its hypotensive efficacy, as evaluated in normotensive rabbits’ eyes [21]. The results showed that the formulation of 5-MCA-MAT with 0.2% sodium hyaluronate was well-tolerated and the most effective in reducing intraocular pressure (IOP) by 39.13%, compared to all other tested formulations (achieving at most a 29% IOP reduction). The effect lasted more than 8 hours. Interestingly, the hypotensive effects of melatonin or 5-MCA-NAT may complement those achieved by the beta-blocker timolol or the alpha-agonist brimonidine. This suggests a mechanism of action different from that exerted by the two classical anti-glaucoma drugs [22]. Since 5-MCA-NAT is a selective MT3 receptor agonist, this suggests the involvement of this receptor in the observed hypotonizing effect [23]. Most recently, melatonin was loaded into liposomes modified with a cell-penetrating-peptide, the trans-activator of transcription (TAT) fragment, one of the CPPs derived from human immunodeficiency virus [24].
2.2. Lipid Nanoparticles
Lipid nanoparticles (LNPs) are spherical vesicles with an average size ranging between 40 and 100 nm, composed of a dispersed phase consisting of a solid lipid stabilized with a biocompatible surfactant used as an emulsifier. These lipid nanoparticles are conventionally classified into SLNs (Solid Lipid Nanoparticles) and NLCs (Nanostructured Lipid Carriers) [25]. SLNs are systems comprising a solid lipid matrix, while NLCs are modified SLNs in which a mixture of solid and liquid lipids is present to enhance their drug-loading capacity and stability. Both have been demonstrated as suitable carriers for ocular drug delivery [26].
The ocular hypotensive effect and tolerability of melatonin encapsulated in cationic solid lipid nanoparticles were evaluated in normotensive rabbits. Melatonin-loaded SLNs (0.05% w/v) were prepared using the QESD method with Softisan100 as the main lipid matrix, and stearic (SA) or palmitic (PA) acid as lipid modifiers, enhancing the physical stability of the nanoparticles. The positive surface charge was achieved by adding a cationic lipid (dodecyl-dimethylammonium bromide) to ensure mucoadhesion and a longer retention time [27]. The effects on intraocular pressure (IOP) after topical administration in albino rabbits’ eyes were compared to an aqueous solution of melatonin over 24 hours of observation. The formulation with SA-SLN loaded with melatonin was the most effective in terms of IOP reduction (maximum IOP reduction: 7 mmHg), and its effect lasted approximately 24 hours with a significant difference (p < 0.01) compared to an aqueous solution of the drug. The ocular tolerability test was evaluated according to a modified Draize test and showed good ocular tolerance of this formulation [27].
2.3. Polymeric Nanoparticles
Polymeric nanoparticles (NPs) are colloidal particles (10-1000nm) composed of biocompatible and biodegradable polymers of synthetic, semisynthetic, or natural origin. The active molecule can be dispersed, encapsulated, or adsorbed on the surface of the nanoparticles. Polylactic-co-glycolic acid (PLGA)—a copolymer of polylactic acid (PLA) and polyglycolic acid (PGA)—is an FDA-approved, highly biocompatible, biodegradable material with mechanical properties modifiable by varying the PLA / PGA ratio and its final molecular weight. It is an excellent drug carrier for ocular administration due to its high hydrophilicity and tolerability. Furthermore, NPs of PLGAs have a high encapsulation efficiency for hydrophilic and hydrophobic drugs, including macromolecules, proteins, peptides, and nucleic acids [28].
PLGA and PLGA-polyethylenglycol (PEG) NPs were used to prepare melatonin-loaded nanocarriers, in which melatonin loading ranged between 44% and 80% [29]. These NPs have been shown to possess adequate characteristics for application to the eye. The hypotensive effect of these NPs was evaluated by measuring IOP during 24 h after instillation in the rabbit eye, as compared to a melatonin aqueous solution at the same concentration (0.08%, w/v). The maximum IOP-lowering effect (5 mmHg) was obtained using melatonin-loaded PLGA-PEG NPs. The tolerability of these formulations was evaluated following a modified Draize test protocol. NPs did not cause any signs of eye inflammation or tissue changes in the rabbit’s eye [29].
Polymeric nanoparticles can be classified into nanospheres and nanocapsules, both serving as effective drug delivery systems. Nanocapsules function as reservoirs, comprising an oily core where the drug is typically dissolved, surrounded by a polymeric shell that controls the drug’s release profile [30,31]. In a rabbit retinal degeneration model, topical formulations of melatonin-loaded ethylcellulose nanocapsules (NCECMEs) demonstrated enhanced transcorneal permeability and neuroprotective effects [32]. These nanocapsules, containing melatonin with an ethylcellulose external cover over an internal oily phase, were prepared using interfacial deposition of preformed polymers. The formulations exhibited favorable properties such as a pH around 5.3, osmolality close to tear fluid, a mean particle size ranging from 150 nm to 180 nm with a narrow size distribution, zeta potential between −25 mV and −30 mV, and encapsulation efficiency around 70%. In vitro release studies showed slower melatonin release from NCECMEs compared to a free melatonin solution. Despite the slower release, NCECMEs facilitated greater penetration without causing irritation during transcorneal application. Stability assessments over 30 days indicated no significant modifications in particle size, polydispersity index, drug content, encapsulation efficiency, zeta potential, and pH. In vivo toxicity and irritation tests after topical application revealed no adverse responses or histopathological changes. Notably, the topical application of melatonin loaded in nanoparticle systems proved more efficient than a melatonin solution, attributed to the prolonged corneal residence time allowing for a more effective and concentrated drug penetration across the cornea after 9 days of treatment. The increased permeation capacity of melatonin observed in NCECMEs correlated with greater neuroprotective and anti-apoptotic effects on retinal ganglion cells, demonstrated by a significant reduction in the apoptosis index and maintained retinal integrity compared to the melatonin solution. Indeed, melatonin has been shown to suppress apoptotic pathways in retinal cells, thereby preventing programmed cell death and promoting cell survival. This anti-apoptotic effect contributes to the preservation of retinal function and structure [33].
In a different study, Carbone et al. [34] compared hybrid nanocapsules and polymeric nanocarriers for delivering melatonin to the eye (0.03 w/w). They used FDA-approved poly-lactic acid (PLA) to prepare nanocapsules without organic solvents. The hybrid nanocapsules, formed by depositing PLA at the interface of a water-in-oil nanoemulsion, included a coating layer with dimethyldioctadecylammonium bromide (DDAB) or cetyltrimethylammonium bromide (CTAB) cationic surfactants. All formulations had sizes <250 nm and a polydispersity index <0.2. Cryo-TEM revealed well-defined surfaces in DDAB hybrid nanocapsules. Stability studies showed reversible instability in samples with sediment, dispersible by agitation. CTAB presence led to significant instability. DDAB was chosen for stable nanocapsules with sustained melatonin release (encapsulation efficiency of 87.69%).
2.4. Nanomicelles
Surfactants, characterized by hydrophilic ‘heads’ and hydrophobic ‘tails,’ spontaneously form micelles in aqueous solutions when concentrations surpass the critical micellar concentration (CMC) [35]. Micelles, aggregates resulting from surfactant self-assembly, play a vital role in drug delivery. The CMC is the minimal concentration needed for amphiphilic molecules to initiate micellization, and it varies for each monomer. Polymer micelle structures, dependent on polymer chemistry, include spherical or cylindrical forms from di-block, tri-block, or graft copolymers [34]. Nanomicelles offer advantages such as ease of preparation, small final size (<200 nm), and high drug encapsulation [36].
In a study by Massimo Dal Monte et al. [37], Soluplus® nanomicelles were used to deliver melatonin and its analog agomelatine, demonstrating a hypotensive effect in both normotensive and hypertensive eyes. Soluplus®, a graft copolymer comprising polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol, forms micelles in aqueous solutions above the CMC (7.6 mg/l). The nanomicelles, prepared with a direct dissolution method, exhibited dimensions suitable for ocular instillation, with a size below 200 nm and high homogeneity. The combination of melatonin and agomelatine, used as a systemic antidepressant, showed a hypotensive activity. The addition of lipoic acid (0.1% w/v) as an antioxidant increased the duration of the lowering effect of melatonin and agomelatine by approximately 50%. Moreover, the hypotensive efficacy of topical nanomicellar formulations of melatonin (0.4% w/v) and agomelatine (0.4% w/v) in rat eyes was demonstrated using the MCE model [38]. The nanomicellar formulation, prepared with 11.5% Soluplus® in Tris buffer, included lipoic acid (0.1% w/v) as a stabilizing excipient and antioxidant. Compared to conventional glaucoma medications, melatonin/agomelatine drastically reduced IOP elevation in the MCE model, indicating higher efficacy. Neuroprotective efficacy was evaluated through electroretinography, inflammatory and apoptotic markers, and retinal ganglion cell density. In a different study [39], the ocular hypotensive effect of melatonin incorporated into lecithin/chitosan nanoparticles and Pluronic® F127/chitosan micelles was evaluated. Lecithin/chitosan nanoparticles (241.8 nm, -35.9 mV ± 4.2 mV) were formed through electrostatic interactions, and Pluronic® F127/chitosan micelles (22.7± 0.7 mV) were prepared using the direct dissolution method. The addition of chitosan in the micelles shifted the surface charge to strongly positive zeta potential values. Although nanoparticles were not cytotoxic, the permeation effect of F127 was reduced in the presence of chitosan, suggesting that the addition of chitosan in the formulation of Pluronic® F127 micelles was not advantageous.
2.5. Self-Nanoemulsifying Drug Delivery Systems
Self-nanoemulsifying drug delivery systems (SNEDDS) were developed and tested for efficient ocular delivery of Sirtuin-1 (SIRT-1) agonists, resveratrol (RSV), and melatonin, for potential applications in diabetic retinopathy treatment. Four types of SNEDDS were made, with different surfactants. Tween® 80-based SNEDDS emerged as the most stable formulation. The optimized formulation, comprising Capryol® PGMC, Tween® 80, and Transcutol® P, loaded with RSV or melatonin, exhibited favorable physico-chemical parameters, including small size, homogeneity, quick emulsion time, transparency, high drug content, mucoadhesion strength, sustained drug release, and cytocompatibility with rabbit corneal epithelial cells. These findings suggest the potential of SNEDDS as effective nanocarriers for ocular drug delivery, addressing challenges associated with stability and bioavailability [40].
2.6. Biomechanical Delivery Systems
In recent studies addressing the challenges of enhancing melatonin delivery for ocular applications, three different approaches have been explored. Romeo et al. investigated the use of nanofiber-based inserts for prolonged ocular surface contact time and improved melatonin delivery. They employed electrospinning to prepare poly (vinyl alcohol) (PVA) and poly (lactic acid) (PLA) nanofiber inserts, demonstrating submicron-sized structures with amorphous melatonin, and observed varying release rates based on the polymer type [41]. Serramito et al. synthesized melatonin-eluting contact lenses (CLs) and assessed their impact on tear volume and intraocular pressure. In vitro, both non-functionalized (HEMA) and functionalized (HEMA/APMA) monomers exhibited similar melatonin loading and release. In vivo, these CLs released melatonin over the ocular surface for at least 2 hours, increasing tear volume without affecting intraocular pressure [42]. Navarro-Gil et al. explored commercially available hydrogel CLs as a delivery system for melatonin analogs, finding that silicone CLs were more effective in releasing agomelatine and 5-MCA-NAT compared to conventional materials. Preloaded CLs triggered higher tear secretion in rabbits than corresponding eye drops, suggesting their potential as an effective strategy for managing dry eye disease. These studies highlight the diverse approaches to optimize melatonin delivery for ocular therapeutic applications [43] (Figure 2).
3. Melatonin as a Potential Therapeutic Approach for Dry Eye Disease
Dry eye disease (DED), a prevalent ocular condition affecting approximately 30% of the global population, is characterized by compromised tear film stability, leading to ocular discomfort, visual disturbances, and potential damage to the ocular surface [45]. Recent research has highlighted inflammation as a significant risk factor for DED development [46]. The presence of ROS contributes to inflammation, suggesting that antioxidants could play a crucial role in DED management by mitigating ROS-induced inflammation. In this context, melatonin emerges as a promising therapeutic agent due to its potent antioxidant properties. Melatonin has been demonstrated as an efficient ROS scavenger in SIRC cells and the entire rabbit cornea. It exhibits the capability to inhibit the formation of superoxide anions induced by UV-B exposure or through incubation with fMLP-stimulated autologous macrophages [47]. Further investigations elucidated the molecular mechanisms underlying melatonin’s anti-inflammatory effects. It has been demonstrated that melatonin administration reduced ROS production in HCE cells via the upregulation of heme oxygenase-1 (HO-1), an antioxidant enzyme [48]. This anti-inflammatory effect was further validated in vivo, as melatonin treatment significantly attenuated the mRNA expression levels of NLRP3 and IL-1β in the mouse cornea [48]. The potential therapeutic benefits of melatonin as an anti-inflammatory agent extended to corneal transplantation. In a mouse model, melatonin administration after corneal allografts effectively inhibited NLRP3 inflammasome activation, thereby reducing the expression of proinflammatory cytokines such as IL-1β, MCP-1, MIP-1, NLRP3, ASC, tumor necrosis factor-alpha (TNF-α), and endothelial growth factor (VEGF)-A. This effect may be mediated by macrophage suppression and T-cell regulation [49]. Interestingly, melatonin has been detected in human tears, with concentrations varying throughout the day in accordance with the circadian rhythm [50]. Melatonin has also been identified in New Zealand white rabbit tears, but unlike humans, its production does not follow a circadian pattern [51]. While melatonin itself failed to induce tear secretion in New Zealand rabbits, melatonin analogs such as 5-MCA-NAT, IIK7, and agomelatine have demonstrated significant tear production-enhancing effects [52]. This suggests that the higher affinity of melatonin analogs for melatonin receptors may contribute to their enhanced tear secretion-inducing capacity [53]. Crooke and collaborators assessed the role of melatonin and its analogs in corneal wound healing. Topical application of melatonin accelerated corneal wound healing in New Zealand rabbits compared to a control condition, mediated by MT2 receptors [51]. Melatonin receptors were also involved in tear secretion mechanisms. It was described that melatonin combined with diadenosine tetraphosphate (Ap4A) synergistically promoted tear secretion when applied topically to the cornea of New Zealand white rabbits. This effect was abolished by luzindole, a melatonin receptor antagonist, supporting the involvement of melatonin receptors in tear secretion [54]. A more recent study aimed to investigate the role and mechanism of melatonin-loaded polymer polyvinyl caprolactam-polyvinyl acetate-polyethylenglycol graft copolymer micelles (Mel-Mic) in dry eye disease [55]. In vitro, Mel-Mic improved the solubility and biological activities of melatonin, reducing apoptosis and ROS in human corneal epithelial cells (HCECs) exposed to hyperosmotic conditions. Mel-Mic also enhanced mitophagy markers and restored impaired mitophagic flux in HCECs. In vivo, Mel-Mic-treated mice exhibited improved clinical parameters, increased tear production, and reduced goblet cell loss. The protective effects involved PINK1-mediated mitophagy and possibly acted through the MT1 receptor, as demonstrated by antagonist studies. The findings suggest Mel-Mic as a potentially effective treatment for DED. A different model system addressed the efficacy of topical administration of TAT-modified melatonin liposomes (TAT-MT-LIPs) (obtained by chemical grafting of Trans-Activator of Transcription (TAT) onto liposomes) on the ocular surface of rats with Benzalkonium-Chloride (BAC)-induced DED [24]. TAT-MT-LIPs significantly alleviated clinical symptoms by inhibiting tissue inflammation and preventing corneal epithelium and conjunctival goblet cell loss. The study revealed that BAC induces NLRP3/Caspase-1/GSDMD-mediated corneal epithelium pyroptosis, a novel finding. TAT-MT-LIPs efficiently suppressed BAC-induced pyroptosis and inflammation by inhibiting mitochondrial DNA oxidation and subsequent signal transmission. This suggests a potential new target for protecting the corneal epithelium when BAC is used as an eye drop preservative, with TAT-MT-LIPs holding promise as a DED treatment.
In summary, melatonin and its analogs hold promise as therapeutic agents for DED. Their potential benefits include modulating the corneal hydration state, increasing tear secretion, and protecting against ROS-induced inflammation [56]. Further studies employing a multi-level strategy utilizing a combination of melatonin and its analogs acting on distinct receptors are warranted to optimize DED management.
4. Melatonin and Cataract
Cataract, a prevalent age-related ocular ailment, involves clouding of the eye lens, a biconvex structure situated between the iris and the vitreous body, responsible for focusing light onto the retina [57]. Comprising water and proteins, the crystalline lens is transparent, enabling unimpeded light transmission to the retina. Structurally, it features a monolayer of cuboidal epithelial cells, a cortex of fibrous cells formed through epithelial cell differentiation, and a nucleus of fibers present from birth [57]. Over the lifespan, fiber cells accumulate high protein concentrations, forming aggregates that scatter light, hindering its focus on the retina [58]. Cataract results primarily from crystallin protein aggregation (α-, β-, and ɣ-crystallins), overexpressed during lens cell differentiation into fiber cells. Despite the necessity of their high protein concentration for maintaining the lens’s refractive gradient and optical performance, aging diminishes the protein biosynthetic capacity of lens fiber cells, limiting damaged crystallin function restoration [59]. Age-related changes in crystallins involve posttranslational structural alterations, including homo- and hetero-oligomeric protein complexes cross-linked through disulfide bridges, a consequence of oxidative stress and glutathione redox imbalance [60]. These changes reduce crystalline protein stability, leading to aggregate formation, uneven protein distribution, light scattering, and subsequent cataract characteristics like reduced transparency, light sensitivity, and visual acuity [60,61]. The role of autophagy and mitophagy in lens organelle degradation is crucial. During maturation, differentiated fiber cells lose internal organelles, including mitochondria, nuclei, Golgi apparatus, and endoplasmic reticulum [62,63]. Mitochondrial degradation triggers the maturation phase, and maintaining mitochondrial homeostasis in lens epithelium is vital for cell viability. Incomplete removal of damaged mitochondria leads to undesirable light scattering in cortical fibers [64].
Melatonin plays a pivotal role in inhibiting cataract formation through its diverse properties. Extensive research has highlighted its presence in crucial ocular components such as the retina, iris, ciliary body, crystalline lens, and lacrimal gland [65,66,67,68,69]. A notable study published in 1994 provided early insights into melatonin’s antioxidative role, demonstrating its capacity to inhibit cataract formation in newborn rats with glutathione deprivation-induced cataract. The findings indicated a significant reduction in cataract incidence, emphasizing melatonin’s protective effects against oxidative stress, potentially attributed to its role in scavenging free radicals and stimulating glutathione production [70]. Building on these observations, subsequent research explored melatonin’s impact on selenite-induced cataracts in rat eyes. The study revealed a significant decrease in cataract incidence following melatonin treatment, accompanied by favorable alterations in oxidative stress markers and enhanced antioxidant enzyme activities [71]. Further elucidating the protective mechanisms of melatonin, Bai et al. in 2013 investigated its role in human lens epithelial cells. The study demonstrated melatonin’s superior efficacy in preventing H2O2-induced damage compared to vitamin E. Melatonin exhibited its protective effects through the activation of antioxidant enzymes and the PI3K/Akt signaling pathway, ensuring cellular survival [72]. More insights into melatonin’s involvement in ocular lens organelle degradation were provided by addressing the role of autophagy. The presence of autophagic vesicles containing mitochondria in various lens compartments was demonstrated, underscoring the importance of mitophagy in maintaining lens homeostasis and preventing cataract formation [73]. Jenwitheesuk et al. in 2014 expanded the understanding of melatonin’s multifaceted role, linking it to aging, neurodegeneration, energy metabolism, epigenetics, autophagy, and circadian rhythm pathways. Their study suggested that melatonin’s impact on autophagy depended on cellular conditions, either inducing or inhibiting it, accordingly [74]. In a more recent investigation, melatonin has been found to inhibit ferroptosis and delay age-related cataract by regulating specific pathways. This inhibition of ferroptosis by melatonin is achieved through the activation of SIRT6/p-Nrf2/GPX4 and SIRT6/NCOA4/FTH1 pathways. It was observed that melatonin rescued the survival of cells exposed to UVB-induced ferroptotic stress, and this effect was attributed to the inhibition of ferroptosis. Melatonin’s mechanism of action involved neutralizing lipid peroxidation toxicity, thereby protecting cells against ferroptotic stress in vitro and delaying cataract formation caused by UVB exposure in rats. These findings suggest that melatonin plays a significant role in modulating ferroptosis, highlighting its potential therapeutic implications for conditions associated with ferroptosis, such as age-related cataracts [75]. Examining melatonin’s ability to counteract oxidative damage, Lledo et al. [76] shed light on its regulatory role in Nrf2 and NLRP3 inflammasome activity. Melatonin’s interventions included preventing ROS generation, promoting antioxidant capacity, and attenuating inflammatory and cytotoxic effects induced by oxidative stressors. These findings underscored melatonin’s potential as a therapeutic agent for cataract prevention [76]. In conclusion, the collective body of research portrays melatonin as a promising candidate for addressing age-related cataracts and stress-related eye diseases. Its diverse properties, encompassing antioxidative, mitochondrial protective, autophagy-modulating, anti-inflammatory, and anti-angiogenic functions, underscore its potential as a valuable component in the treatment and prevention of cataracts [71,77].
5. Melatonin, a Versatile Molecule in the Retina
Melatonin, a hormone primarily synthesized by the pineal gland and additionally produced in the retina, originates from the amino acid tryptophan found in the bloodstream [78]. Its production and release follow a daily rhythm, peaking at night and dipping during the day [79]. Enzymes in the liver, kidneys, and central nervous system then break down circulating melatonin [79]. Vertebrate retinas express melatonin receptors known as MT1 and MT2 [80]. These receptors, when activated by melatonin, function as neuro-hormones or neuro-modulators in the retinal pigment epithelium (RPE). MT1 and MT2 inhibit the adenylate cyclase pathway through the Gi protein (G protein inhibitor) [81]. These receptors can exist as pairs (homodimers) or mixed pairs (heterodimers). In photoreceptor cells, melatonin’s effect is primarily mediated by heterodimeric MT1/MT2 receptors, which activate a different signaling pathway involving other molecules like PLC and PKC [82]. The MT1 melatonin receptor couples with pertussis toxin–sensitive Gi and –insensitive Gq/11 G proteins, inhibiting forskolin-stimulated cAMP, protein kinase A signaling, and CREB phosphorylation. Additionally, the MT1 receptor enhances phosphorylation of mitogen-activated protein kinase 1/2 and extracellular signal–regulated kinase 1/2, while also increasing potassium conductance through Kir inwardly rectifying channels. Activation of the MT2 melatonin receptor inhibits forskolin-stimulated cAMP production and cGMP formation, activates protein kinase C (PKC) in the suprachiasmatic nucleus (SCN), and reduces calcium-dependent dopamine release in the retina [83]. The initial insights into melatonin’s role in countering retinal pathologies emerged from a study on frog photoreceptors. Exposing these photoreceptors to a brief light source resulted in the generation of ROS, a phenomenon effectively neutralized by low doses of melatonin [84].
5.1. Melatonin’s Effects on Retinal Function:
Studies in mice suggest that melatonin, through a pathway involving PKC, can boost the amplitude of the a- and b-waves in the scotopic electroretinogram (ERG), a measure of retinal function in low light [85]. Conversely, in a double-blind placebo-controlled study involving 13 healthy volunteers who ingested 10 mg of pineal melatonin at 4 pm, the b-wave amplitude exhibited a significant reduction, whether measured in dark or light conditions. This suggests that melatonin may have complex and context-dependent effects on retinal signaling, and possesses the ability to transmit signals not only in the pineal gland but also in the retina [86]. Another study explored the influence of melatonin timing on chick ERG. It was found that the circadian rhythm of the chick electroretinogram (ERG) is under the influence of the indoleamine hormone melatonin [87]. To explore whether the melatonin concentration or the timing of administration induces distinct effects on ERG parameters, experiments were conducted with melatonin administered at different times of the day. Circadian rhythms of a- and b-wave implicit times and amplitudes were evident under both light:dark (LD) and continuous darkness (DD) conditions. Intramuscular administration of melatonin at doses of 1 mg/kg and 100 ng/kg resulted in decreased a- and b-wave amplitudes and increased a- and b-wave implicit times. This effect was more pronounced than that observed with 1 ng/kg melatonin, which had minimal impact compared to saline controls. Furthermore, the influence of 1 mg/kg and 100 ng/kg melatonin on a- and b-wave amplitude in LD, and on b-wave amplitude in DD, was more prominent during the night (ZT/CT 17) compared to the day (ZT/CT 5). The fold change in b-wave implicit time over controls was higher during the day (ZT/CT 5) than at night (ZT/CT 17). These findings suggest that melatonin may contribute to regulating a diurnal and nocturnal functional transition in the retina, potentially through the modulation of a retinal clock [87]. Overall, these studies raise intriguing questions about how melatonin interacts with the retina. While promoting PKC activity in mice might enhance low-light vision, other studies show reduced activity in humans and dose-dependent effects in chicks. Further research is needed to understand the nuanced role of melatonin in retinal function and the potential for individual differences.
6. Melatonin and Age-Related Macular Degeneration (AMD)
Melatonin exhibits significant implications for AMD, a condition intricately linked to oxidative stress in the retina, stemming from elevated oxygen consumption rates and exposure to natural or artificial light sources [88]. AMD, characterized by clinical hallmarks like drusen and extracellular depositions indicative of inefficient retinal metabolism, is strongly associated with oxidative stress [89]. Notably, AMD patients present lower circulating melatonin levels compared to their healthy counterparts [90]. Melatonin, renowned for its role as a potent ROS scavenger, emerges as a crucial player in retinal pathophysiology, effectively counteracting oxidative damage to retinal cells. A study administering melatonin to AMD patients demonstrated promising outcomes, with 55 out of 100 patients experiencing stabilized visual acuity at the six-month follow-up [91].
In an in vitro model of RPE, melatonin showcased its antioxidative protective effects by directly or indirectly activating its receptors [92]. The study revealed a concentration-dependent modulation of this protective effect, with luzindole, a melatonin receptor antagonist, blocking the effect at lower concentrations (up to 10−8 M) but proving ineffective at higher concentrations (above 10−6 M) [92]. Cellular aging, often associated with telomere shortening triggered by oxidative environmental factors, is a contributing factor in AMD due to RPE senescence [93]. Melatonin, an antioxidant molecule, has the potential to indirectly modulate telomere shortening [94]. In the retina, melatonin has been shown to down-regulate hTERT expression (telomerase catalytic subunit) and stimulate telomerase activity, thereby preventing telomere shortening [95].
The breakdown of the blood-retinal barrier (BRB) is a hallmark of AMD, and melatonin has been found to counteract structural and functional changes in the BRB associated with AMD development [96]. In a murine model mimicking Nonexudative AMD (NE-AMD), melatonin prevented oxidative stress-induced alterations in Bruch’s membrane structure and mitigated the decrease in RPE melanin and melanosome content, essential for light absorption. Moreover, melatonin significantly impeded the increase in temporal RPE mitochondria superoxide content induced by the model [97].
Mitochondrial impairment in human RPE cells affected by AMD is linked to oxidative/nitrosative stress, contributing to AMD development. Melatonin’s effect on mitochondrial function plays a crucial role in reducing oxidative stress, inflammation, and apoptosis in the retina. These findings underscore melatonin’s potential as a preventive and therapeutic agent for AMD [98].
Furthermore, recent research suggests that melatonin plays a role in regulating immune homeostasis in the retina through regulatory T cells (Tregs) [99]. Studies have shown that melatonin can restore retinal integrity and ameliorate retinal degeneration in mouse models of retinopathy induced by NaIO3. This effect may be attributed to the conversion of pro-inflammatory M1 macrophages to anti-inflammatory M2 macrophages, promoting tissue repair and immune regulation. Melatonin treatment also upregulates TET2 gene expression and demethylates NT5E, potentially enhancing Treg recruitment in the retinal microenvironment. These findings suggest that melatonin could modulate immune responses and provide a promising therapeutic strategy for AMD.
However, while these studies paint a promising picture, it’s crucial to remember that research on melatonin’s role in AMD is still ongoing. More extensive clinical trials are needed to fully understand its efficacy and safety as a potential therapeutic intervention. In conclusion, melatonin’s multifaceted approach to combating oxidative stress, cellular aging, and mitochondrial dysfunction positions it as a potential contender in the fight against AMD. While further research is required, its preliminary promise encourages exploration of its potential to protect our vision from this debilitating disease.
7. Melatonin Effects on Metabolic Syndrome and Diabetic Retinopathy
Melatonin has pleiotropic effects on metabolic dysfunctions and gut microbiota. Aging-induced changes in gut microbiota composition and bile acid patterns are known to contribute to hepatic lipid dysmetabolism [100]. However, oral melatonin treatment may reverse these alterations by inhibiting gut microbiota-mediated deconjugation of bile acids, thereby reducing hepatic lipid dysmetabolism [100]. Additionally, melatonin supplementation has been shown to improve lipid dysmetabolism in the ileum and white adipose tissue by modulating gut microbiota and angiopoietin-like 4 expression, offering potential therapeutic benefits for metabolic syndrome [101]. Furthermore, melatonin’s regulation of circadian rhythms and metabolic processes is evident in studies demonstrating its ability to restore diurnal rhythmicity of clock genes, lipid profiles, and gut microbiota composition in mice fed a high-fat diet [102]. This suggests that melatonin may play a crucial role in regulating metabolic homeostasis, particularly in the context of obesity. Moreover, dietary melatonin supplementation has been shown to mitigate the adverse effects of a high-fat/high-sugar diet on body weight, lipid metabolism, and organ function in mice, indicating its potential as a therapeutic agent for managing metabolic syndrome [103]. Additionally, melatonin prevents lipid accumulation and gut microbiota dysbiosis induced by a high-fat diet in mice, suggesting its protective effects against obesity-related metabolic disorders [104]. These findings underscore the importance of melatonin in modulating gut microbiota composition and metabolic processes to prevent or mitigate the development of metabolic syndrome. Furthermore, dysregulation of circadian rhythms has been implicated in diabetes mellitus, highlighting the potential role of melatonin in regulating carbohydrate metabolism and glycemic control [105]. Studies suggest that melatonin supplementation or analogues may hold promise in diabetes management and prevention by restoring circadian rhythmicity and regulating carbohydrate homeostasis [105]. Overall, melatonin emerges as a multifaceted regulator of metabolic processes and gut microbiota composition, offering potential therapeutic avenues for managing metabolic syndromes and related conditions.
Among the metabolic dysfunctions, diabetes plays a prominent role, and diabetic retinopathy is a feared consequence of poorly controlled diabetes. Diabetic retinas are associated with abnormal vascular changes such as dilatation and deformation. HIF-1a, VEGF-A, and PEDF were all increased because of diabetic injury. Melatonin decreased retinal nitrotyrosine and malondialdehyde levels, showing an antioxidative support. The vasculo-modulator cytokines are decreased accordingly by melatonin therapy. Melatonin normalized retinal vascular changes as well [106]. In a model of early T2DM in adult rats, animals were subcutaneously implanted with a pellet of melatonin. At 12 weeks of treatment, melatonin, which did not affect glucose metabolism in control or diabetic rats, prevented the decrease in the electroretinogram a-wave, b-wave, and oscillatory potential amplitude, and the increase in retinal lipid peroxidation, NOS activity, TNF-α, Muller cells glial fibrillary acidic protein, and vascular endothelial growth factor levels. In addition, melatonin prevented the decrease in retinal catalase activity. These results indicate that melatonin protected the retina from the alterations observed in an experimental model of DR associated with type 2 diabetes [107]. In diabetic Sprague-Dawley rats, melatonin (10 mg/kg daily, i.p.) was administered from the induction of diabetes and continued for up to 12 weeks and retinal samples were collected. The retina of diabetic rats showed depletion of glutathione and downregulation of glutamate cysteine ligase (GCL). Melatonin significantly upregulated GCL by retaining the nuclear factor erythroid 2–related factor 2 (Nrf2) in the nucleus and stimulating Akt phosphorylation. The production of proinflammatory cytokines and proteins, including interleukin-1 β (IL-1β), TNF-α, and inducible nitric oxide (NO) synthase (iNOS), was inhibited by melatonin through the NF-𝜅B pathway. At 12 weeks, melatonin prevented the significant decrease in the ERG a- and b-wave amplitudes under the diabetic condition. These results suggest potent protective functions of melatonin in diabetic retinopathy [108]. In diabetic rats, melatonin (20 mg/kg) was given orally for 7 weeks in diabetic rats starting 1 week after induction of diabetes. Diabetes significantly increased the mean scores of fluorescein leakage, and MDA and ROS levels compared to control group. Treatment of the diabetic rats with melatonin for 7 weeks prevented the alterations induced by diabetes in comparison with the diabetic control group. Based on these findings, it can be concluded that melatonin might have beneficial effects in prevention of diabetic retinopathy [109]. Under starvation, mitochondria can fuse with each other to maintain bioenergetic efficiency. When there is a nutrient overload, fragmenting mitochondria is a way to store nutrients to avoid energy waste. in cultured 661W cells, a photoreceptor-derived cell line, hyperglycemic conditions triggered an increase of the expression of dynamin- related protein 1 (DRP1), a protein marker of mitochondrial fission, and a decrease of mitofusin 2 (MFN2), a protein for mitochondrial fusion. Further, these hyperglycemic cells also had decreased mitochondrial Ca2+ but increased cytosolic Ca2+. Treating these hyperglycemic cells with melatonin, averted hyperglycemia-altered mitochondrial fission-and-fusion dynamics and mitochondrial Ca2+ levels. We gave melatonin to streptozotocin- (STZ-) induced type 1 diabetic mice by daily oral gavage and determined the effects of melatonin on diabetic eyes. We found that melatonin was not able to reverse the STZ-induced systemic hyperglycemic condition, but it prevented STZ-induced damage to the neural retina and retinal microvasculature. The beneficial effects of melatonin in the neural retina in part were through alleviating STZ-caused changes in mitochondrial dynamics and Ca2+ buffering [110]. The following study demonstrated decreased serum melatonin in pre-diabetic rats, as well as, increased concentration of retinal lipid peroxidation TBARS (thiobarbituric acid reactive substances), protein oxidation (advanced oxidation protein products, AOPP). Oral supplementation with melatonin (85 μg/animal/day) caused melatonin and HDL cholesterol levels to rise in treated rats and reduced levels of fasting serum glucose and fructosamine. Finally, supplementation with melatonin reduced concentrations of TBARS, AOPP, iNOS, VEGF, and MMP9 in significant level. Thereby exerting an overall positive effect on oxidative stress and pro-angiogenic signaling in the pre-diabetic retina. Thus, oral melatonin might be considered in an early treatment or in the prevention of retinal changes associated with pre-diabetes [111]. Reactive gliosis and pro-inflammatory cytokine production by Müller cells contribute to the progression of DR. In this study, melatonin inhibited the gliosis activation and inflammatory cytokine production of Müller cells in both in vitro and in vivo models of DR. Furthermore, melatonin inhibited Müller cell activation and pro-inflammatory cytokine production by upregulating the long noncoding RNA maternally expressed gene 3/miR-204/sirtuin 1 axis [112]. In the following study, melatonin inhibited oxidative stress and inflammation by enhancing the expression and activity of silent information regulator factor 2-related enzyme 1 (Sirt1) both in in vitro and in vivo models of DR, and the Sirt1 inhibitor EX-527 counteracted melatonin-mediated antioxidant and anti-inflammatory effects on Müller cells. Moreover, melatonin enhanced Sirt1 activity through the maternally expressed gene 3 (MEG3)/miR-204 axis, leading to the deacetylation of the Sirt1 target genes fork- head box o1 (Foxo1) and nuclear factor kappa B (NF-κB) subunit p65, eventually contribute to the alleviation of oxidative stress and inflammation. The study revealed that melatonin promotes the Sirt1 pathway, thereby protecting the retina from DM-induced damage [113]. In this other study, we characterized the protective effects of melatonin on the inner blood–retinal barrier (iBRB), as well as the possible mechanisms in experimental DR. Results showed that in diabetic rat retinas, the leakage of iBRB and the expression of inflammatory factors (VEGF, TNF-α, IL-1β, ICAM-1, and MMP9) increased dramatically, while the expression of tight junction proteins (ZO-1, occludin, JAM-A, and claudin-5) decreased significantly. The above changes were largely ameliorated by melatonin. Melatonin could maintain the iBRB integrity by upregulating the expression of tight junction proteins [114]. To evaluate the effects of melatonin on DR, we first investigated the role of melatonin in retinal angiogenesis and inner blood-retina barrier (iBRB) under high glucose conditions in vitro and in vivo. Melatonin administration ameliorated high glucose-induced iBRB disruption, cell proliferation, cell migration, invasion, and tube formation, and decreased the expression levels of VEGF, MMP-2, and MMP-9. Furthermore, melatonin treatment increased the level of autophagy but decreased the expression levels of inflammation-related factors under high glucose conditions. We found that melatonin inhibited the activation of the Wnt/β-catenin pathway following DR. Melatonin exerts protective effects on experimental DR via inhibiting the Wnt/β-catenin pathway by, at least partially, alleviating autophagic dysfunction and inflammatory activation [115].
8. Melatonin and Retinal Ischemia
Melatonin, the sleep hormone, shows promise in protecting the retina from damage caused by insufficient oxygen and blood flow. In fact, melatonin’s antioxidant, anti-inflammatory, anti-angiogenic, neuroprotective, and vasculo-protective properties work together to provide a comprehensive approach to protecting the retina from damage caused by ischemia [116]. Several experimental model systems corroborate the efficacy of melatonin in mitigating the pathological consequences of retinal ischemia.
In an experiment with C57BL/6 mice, a sudden increase in pressure inside the eye was induced to create a temporary lack of blood flow in the retina. Prior to, during, and after this induced ischemia, mice were injected with either melatonin or a neutral substance. Twenty-four hours after the ischemia, the levels of HIF-1α and GFAP, important proteins related to tissue response, peaked in the affected retina. When the ischemic retina was treated with melatonin, the overexpression of HIF-1α and GFAP was inhibited. Additionally, two weeks after the ischemia, a greater number of retinal ganglion cells (RGCs) survived in the retinas treated with melatonin compared to those treated with the neutral substance. Melatonin treatment led to increased survival of RGCs in the ischemic mouse retina. This protective effect of melatonin appears to be achieved by preventing the stabilization of HIF-1α and reducing the activity of glial cells in the ischemic retina [117].
Another study aimed to explore the effects of melatonin on retina neovascularization (RNV) and neuroglia in a mouse model of oxygen-induced retinopathy (OIR), a condition mimicking retinal hypoxia in premature infants. The findings indicated a decrease in leakage from retinal blood vessels in OIR mice following melatonin treatment. In response to oxygen-induced injury, there was a reduction in the density of astrocytes along with changes in their structure and function. The activation of the HIF-1α-VEGF pathway observed in the retinas of OIR mice was suppressed in those treated with melatonin. Melatonin not only prevented abnormal blood vessel growth but also protected neuroglial cells and exhibited anti-inflammatory effects by inhibiting the HIF-1α-VEGF pathway in OIR retinas. This suggests that melatonin could be a promising treatment for retinopathy of prematurity (ROP) [118].
In another particular investigation, researchers utilized a mice model of oxygen-induced retinopathy (OIR) to simulate conditions akin to retinal hypoxia and ischemia. Their aim was to delve deeper into the protective impact of melatonin on neonatal retinal nerve cells. Melatonin played a role in maintaining the typical structure and thickness of the inner retina, and it safeguarded populations of inner retinal neurons in areas deprived of blood flow by preventing their programmed cell death. Furthermore, melatonin ameliorated visual impairment. Following melatonin treatment, the heightened levels of cleaved caspase-3 and Bax proteins, indicative of cellular apoptosis in response to hypoxia and ischemia, decreased, while the reduced levels of Bcl-2 protein increased. Moreover, melatonin promotes the expression of neurotrophic factors in the retina, fostering the growth, survival, and function of retinal neurons. This neurotrophic support enhances the resilience of retinal cells against various insults [119]. More specifically, melatonin boosted levels of BDNF (brain-derived neurotrophic factor) and activated downstream pathways involving phospho-TrkB/Akt/ERK/CREB. These findings suggest that melatonin’s anti-apoptotic and neuroprotective effects on inner retinal neurons following hypoxia and ischemia are, at least partially, attributed to its modulation of the BDNF-TrkB pathway [119].
In tandem with these experimental findings, animal model studies accentuated the significance of melatonin in counteracting retinal hypoxia. By attenuating vascular VEGF and NO levels (both of which were elevated in hypoxic animals), melatonin demonstrated its efficacy in reducing retinal permeability and mitigating associated edema. These findings bolster the premise that melatonin supplementation could hold therapeutic promise in managing retinal complications stemming from hypoxia [120,121].
Collectively, these insights reinforce the notion that melatonin serves as a formidable ally in shielding the retina against ischemic insults, offering a multifaceted strategy encompassing neuroprotection, anti-inflammatory action, and vascular stabilization. By targeting various facets of retinal pathology, melatonin emerges as a promising therapeutic intervention for mitigating the detrimental effects of retinal ischemia.
9. Melatonin and Glaucoma
Glaucoma, the second leading cause of visual impairment and blindness globally, manifests as a multifactorial disease marked by the progressive degeneration of retinal ganglion cells (RGCs) and subsequent optic nerve damage, culminating in blindness [122,123]. Central to its pathology is the elevation of intraocular pressure (IOP), predominantly caused by increased resistance to aqueous drainage through the trabecular meshwork (primary open-angle glaucoma) and obstruction of the drainage pathway by the iris (primary closed-angle glaucoma) [124,125]. Notably, high IOP correlates with RGC death, underscoring the significance of IOP management in glaucoma treatment [126,127]. Consequently, current therapeutic strategies predominantly revolve around IOP control through pharmacological and surgical interventions [127,128,129]. However, the side effects associated with glaucoma medications prompt continual exploration for alternative therapies.
Melatonin emerges as a promising avenue for glaucoma management due to its involvement in IOP regulation [12,130]. Dysregulation of ocular melatonin levels and signaling correlates with elevated nocturnal IOP and RGC loss [131]. Melatonin’s actions primarily occur through specific receptors (MT1, MT2, and putative MT3), abundantly expressed in retinal cells and the ciliary epithelium [80,132]. Stimulation of these receptors, particularly in the ciliary body, leads to reduced aqueous humor secretion, thereby lowering IOP [133].
In animal models of glaucoma, such as DBA/2J mice, decreased expression of melatonin receptors, particularly MT2, is associated with disease progression, while aqueous humor melatonin levels remain unchanged [134]. Furthermore, suppression of the MT1 receptor correlates with photoreceptor and RGC loss in mice [135,136]. Human studies also corroborate the role of melatonin in glaucoma, with high IOP patients exhibiting elevated aqueous humor melatonin levels compared to normotensive individuals [137,138]. Moreover, urinary levels of melatonin metabolites correlate with glaucoma severity, indicating its potential as a biomarker [139,140]. Notably, disruptions in circadian rhythms, including sleep patterns, are associated with RGC loss in glaucoma patients [141].
Experimental evidence suggests that exogenous melatonin and its analogs effectively lower IOP in animal models and human patients with glaucoma [142,143,144]. These effects are mediated, in part, by modulating adrenergic receptors involved in aqueous humor production [145]. Notably, nanomicellar formulations of melatonin and its analogs demonstrate prolonged hypotensive effects and neuroprotection in glaucoma models [37,38]. Additionally, combined melatonin formulations exhibit enhanced neuroprotective effects against glutamate-induced cytotoxicity, oxidative stress, and nitrosative stress, all implicated in glaucoma pathology [146].
Human studies further support the therapeutic potential of melatonin in glaucoma, with oral melatonin administration reducing IOP and enhancing surgical outcomes during cataract surgery [147]. Agomelatine, an analog of melatonin, also demonstrates IOP-lowering effects and neuroprotection in humans [148] and animal models [149]. Moreover, melatonin’s antioxidant properties protect against retinal damage induced by glutamate excitotoxicity and oxidative stress, both implicated in glaucoma progression [150,151,152].
Given the neurodegenerative nature of glaucoma, therapeutics with neuroprotective properties, like melatonin, hold significant promise [153,154]. Melatonin’s multifaceted actions, ranging from IOP regulation to antioxidant and neuroprotective effects, position it as a valuable candidate for glaucoma management, warranting further exploration in clinical settings.
10. Melatonin and Uveitis
Uveitis, a sight-threatening ocular inflammatory condition, presents significant challenges in its management. Uveitis encompasses a diverse group of intraocular inflammatory disorders characterized by inflammation of the uvea, retina, and vitreous. The pathogenesis of uveitis involves a complex interplay of immune-mediated processes, cytokine dysregulation, and oxidative stress, resulting in inflammation, tissue damage and vision impairment [155]. In recent years, melatonin, also endowed with potent anti-inflammatory and immunomodulatory properties [156], has emerged as a potential therapeutic candidate for uveitis. Melatonin exerts pleiotropic effects through its interactions with melatonin receptors MT1 and MT2, and its modulation of NF-κB signaling. These actions contribute to the downregulation of pro-inflammatory cytokines, such as TNF-α, interleukin-6 (IL-6), and interleukin-1 beta, thereby attenuating ocular inflammation. Additionally, melatonin’s antioxidative properties play a crucial role in mitigating oxidative stress, a prominent feature in uveitis pathophysiology [157]. Interestingly, topical melatonin instillation also exerted a detectable effect. In a study using rabbits, acute immunogenic uveitis was induced by injections of normal horse serum. Researchers investigated the effects of applying a 0.1% melatonin solution on uveitis symptoms and biochemical markers in tear fluid and aqueous humor. They found that melatonin reduced uveitis symptoms and increased antioxidant activity in tears while lowering levels of α(2)-macroglobulin. A similar trend was observed in aqueous humor, with higher antioxidant activity and lower levels of protein and α(2)-macroglobulin in treated rabbits. These findings suggest that melatonin instillations enhance local antioxidant activity and reduce inflammation severity and permeability of the blood-ocular barrier in uveitis [158].
While the existing evidence is promising, human clinical trials are necessary to determine efficacy, optimal dosing regimens, treatment durations, and potential combination therapies involving melatonin for uveitis. Moreover, research focusing on the safety and efficacy of topical and intravitreal administration of melatonin is crucial for its translation into clinical practice.
In conclusion, the anti-inflammatory, immunomodulatory, and antioxidative properties of melatonin position it as a promising candidate for the adjunctive or alternative management of uveitis. The available literature supports the potential therapeutic role of melatonin in uveitis, underscoring the need for additional research to elucidate its clinical utility and optimize treatment strategies.
11. Melatonin and Retinitis Pigmentosa
The investigation into melatonin’s role in retinal health spans various studies, shedding light on its potential therapeutic benefits for retinal degenerative diseases like retinitis pigmentosa (RP). In a study on patients with night blindness, Banas et al. [159] found that damage to photoreceptors influenced melatonin secretion and its circadian rhythm. This observation underscores the link between retinal health and melatonin regulation. Preclinical studies using animal models further elucidate melatonin’s protective effects on retinal degeneration. Xu et al. [160] demonstrated that daily melatonin injections in rd10 mice, a model of autosomal recessive RP, significantly delayed photoreceptor loss and reduced inflammation-related gene expression. Similarly, Liang et al. [161] found that melatonin treatment in rds/rds mice slowed photoreceptor degeneration and reduced apoptotic cell death, suggesting its potential as a therapeutic intervention for RP. Recognizing the significant impact of RP on patients’ quality of life, Pastor-Idoate et al. [162] proposed a clinical trial to assess the efficacy of oral melatonin (OM) administration, alone or combined with short-wavelength light (SWL)-blocking filters, in alleviating sleep disorders and psychological stress in RP patients. This study aims to evaluate changes in hormone release, sleep quality, retinal function, and patient-reported variables, offering insights into the potential benefits of melatonin supplementation as a low-cost therapeutic approach for RP patients.
Overall, these studies highlight the multifaceted role of melatonin in retinal health and its potential as a therapeutic agent for mitigating retinal degeneration in conditions like RP. Further research and clinical trials are warranted to validate these findings and explore melatonin’s broader applications in preserving retinal function and improving patients’ quality of life.
12. Melatonin and Myopia
Myopia, a prevalent refractive error, poses significant risks of adverse outcomes. Most creatures, including humans, are initially inclined towards hyperopia. As the eye develops, it undergoes axial elongation, a process called emmetropization, influenced by visual stimuli. The surge in myopia rates and its correlation with education levels underscore substantial environmental influences on its development, despite the familial inheritance patterns that suggest a genetic component. Over 150 genetic syndromes include familial high myopia, indicating a genetic predisposition. Therefore, both genetic and environmental factors contribute to myopia’s etiology. Various pathways regulating growth, such as dopamine, ZENK-glucagon, retinoic acid, crystallin, serotonin, melatonin, vasoactive intestinal peptide, enkephalins, nitric oxide, and growth factors, are known to be involved in the control of eye growth and axial elongation [163].
The complex relationship between the sleep hormone melatonin and myopia has intrigued researchers for some time. While experimental models hint at a potential link between disrupted melatonin rhythms and myopia development [164,165], the true picture in humans remains shrouded in uncertainty.
Several studies have addressed the assessment of melatonin levels and their daily fluctuations in diverse body fluids like saliva, blood serum, and urine among both myopic and non-myopic individuals. However, the findings presented a rather discordant symphony. While some studies reported higher, even significantly higher, systemic melatonin concentrations in myopes compared to individuals with normal vision (emmetropes) [166,167], others found no or even negative associations [168,169]. This inconsistency played out across different measuring methods and sampling times. Morning blood serum levels varied greatly between studies, with maximum and minimum values showcasing a wide range. Likewise, salivary melatonin patterns measured every 4 hours over 24 hours revealed no significant differences between groups, while hourly evening measurements hinted at potentially higher levels in myopes. Even the timing of melatonin release after dim-light exposure, known as dim-light melatonin onset, lacked a consistent connection to the refractive group across studies. A lone study offered a different perspective, reporting significantly lower levels of a melatonin metabolite in overnight urine samples from myopes compared to emmetropes [170].
In summary, while the exact role of melatonin in myopia remains unclear, the current evidence suggests a fascinating, yet complex, relationship. Further research with rigorous methodologies and controlled variables is crucial to harmonize the discordant notes and understand the true melody of melatonin’s influence on myopia.
13. Conclusions
Despite the promising findings here reported, the existing clinical trials and research studies are still insufficient, particularly for various ocular diseases and alternative drug administration methods. Further large-scale clinical trials are needed to determine the appropriate dosages, administration routes, and treatment time windows for melatonin in the context of ocular diseases [15].
In summary, the scientific literature suggests that melatonin holds promise as a potential therapeutic agent for a range of ocular diseases, especially in the context of its antioxidant, anti-inflammatory, and neuroprotective properties. However, further extensive research and clinical trials are necessary to fully understand its efficacy, safety, and optimal administration methods for different ocular conditions. The topic administration of melatonin as eye drops remains a challenging, however affordable objective, due to the instability of the molecule in aqueous solution, and the low efficiency of delivery to the posterior pole.
Author Contributions
Conceptualization, DR; data curation, DR and CR; writing—original draft preparation, DR; writing—review and editing, DR and CR. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J Biol Chem 1960, 235, 1992–7. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.; Sengupta, A.; Maitra, S.K. Melatonin: fifty years of scientific journey from the discovery in bovine pineal gland to delineation of functions in human. Indian J Biochem Biophys 2008, 45, 289–304. [Google Scholar] [PubMed]
- Taverne, Y.J.; Merkus, D.; Bogers, A.J.; Halliwell, B.; Duncker, D.J.; Lyons, T.W. Reactive Oxygen Species: Radical Factors in the Evolution of Animal Life: A molecular timescale from Earth’s earliest history to the rise of complex life. Bioessays 2018, 40(3).
- Davies, K.J.A.; Ursini, F. The Oxygen Paradox. Cleup University Press 1995, p. 1-811.
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front Endocrinol 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Zheng, X.; Kong, J.; Manchester, L.C.; Hardeland, R.; Kim, S.J.; Xu, X.; Reiter, R.J. Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: relation to their biological functions. Int J Mol Sci 2014, 15, 15858–90. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J Pineal Res 2013, 54, 127–38. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Qin, L.; Reiter, R.J. Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Int J Mol Sci 2016, 17, 2124. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J Pineal Res 2013, 54, 245–57. [Google Scholar] [CrossRef]
- Singh, S.S.; Deb, A.; Sutradhar, S. Dexamethasone Modulates Melatonin MT2 Receptor Expression in Splenic Tissue and Humoral Immune Response in Mice Biol. Rhythm Res 2017, 48, 425–435. [Google Scholar] [CrossRef]
- Legros, C.; Dupré, C.; Brasseur, C.; Bonnaud, A.; Bruno, O.; Valour, D.; Shabajee, P.; Giganti, A.; Nosjean, O.; Kenakin, T.P.; Boutin, J.A. Characterization of the various functional pathways elicited by synthetic agonists or antagonists at the melatonin MT1 and MT2 receptors. Pharmacol Res Perspect 2019, 8, e00539. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors 2009, 35, 183–92. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol 2008, 85, 335–53. [Google Scholar] [CrossRef]
- Scuderi, L.; Davinelli, S.; Iodice, C.M.; Bartollino, S.; Scapagnini, G.; Costagliola, C.; Scuderi, G. Melatonin: Implications for Ocular Disease and Therapeutic Potential. Curr Pharm Des 2019, 25, 4185–4191. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Q.; Wu, W.; Zeng, W.; Feng, Y. Therapeutic Effects of Melatonin on Ocular Diseases: Knowledge Map and Perspective. Front Pharmacol 2021, 12, 721869. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, A.; Hassan, M. Stability of melatonin in aqueous solution. J Pineal Res 1995, 18, 90–2. [Google Scholar] [CrossRef] [PubMed]
- Daya, S.; Walker, R.B.; Glass, B.D.; Anoopkumar-Dukie, S. The effect of variations in pH and temperature on stability of melatonin in aqueous solution. J Pineal Res 2001, 31, 155–8. [Google Scholar] [CrossRef] [PubMed]
- Vlachou, M.; Siamidi, A. Melatonin Modified Release Formulations Designed for Sleep Disorders [Internet]. Melatonin - Molecular Biology, Clinical and Pharmaceutical Approaches. IntechOpen 2018.
- Souto, E.B.; Dias-Ferreira, J.; López-Machado, A.; Ettcheto, M.; Cano, A.; Camins Espuny, A.; Espina, M.; Garcia, M.L.; Sánchez-López, E. Advanced Formulation Approaches for Ocular Drug Delivery: State-Of-The-Art and Recent Patents. Pharmaceutics 2019, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Rui, M. Liposomal Delivery System for Small Molecular Therapeutic Drugs. In The World Scientific Encyclopedia of Nanomedicine and Bioengineering II: Bioimplants, Regenerative Medicine, and Nano-Cancer Diagnosis and Phototherapy Volume 2, Advanced Nanomaterials for Bioimaging and Cancer Therapy 2017 235-253.
- Quinteros, D.; Vicario-de-la-Torre, M.; Andrés-Guerrero, V.; Palma, S.; Allemandi, D.; Herrero-Vanrell, R.; Molina-Martínez, I.T. Hybrid formulations of liposomes and bioadhesive polymers improve the hypotensive effect of the melatonin analogue 5-MCA-NAT in rabbit eyes. PLoS One 2014, 9, e110344. [Google Scholar] [CrossRef]
- Crooke, A.; Huete-Toral, F.; Martínez-Águila, A.; Martín-Gil, A.; Pintor, J. Melatonin and its analog 5-methoxycarbonylamino-N-acetyltryptamine potentiate adrenergic receptor-mediated ocular hypotensive effects in rabbits: significance for combination therapy in glaucoma. J Pharmacol Exp Ther 2013, 346, 138–45. [Google Scholar] [CrossRef]
- Alkozi, H.A.; Navarro, G.; Franco, R.; Pintor, J. Melatonin and the control of intraocular pressure. Prog Retin Eye Res 2020, 75, 100798. [Google Scholar] [CrossRef]
- Lou, Q.; Pan, L.; Xiang, S.; Li, Y.; Jin, J.; Tan, J.; Huang, B.; Nan, K.; Lin, S. Suppression of NLRP3/Caspase-1/GSDMD Mediated Corneal Epithelium Pyroptosis Using Melatonin-Loaded Liposomes to Inhibit Benzalkonium Chloride-Induced Dry Eye Disease. Int J Nanomedicine 2023, 18, 2447–2463. [Google Scholar] [CrossRef]
- Mrudula, B.; Sanjay, K.; Husain, M.; Kailas, M.; Vaibhav, I.; Kiran, S.; Vishal, S. Nanostructured Lipid Carriers Based Drug Delivery System: A Review. Indo American Journal of Pharmaceutical Research 2017, 7. [Google Scholar]
- Omerović, N.; Vranić, E. Application of nanoparticles in ocular drug delivery systems. Health and Technology 2020, 1. [Google Scholar] [CrossRef]
- Leonardi, A.; Bucolo, C.; Drago, F.; Salomone, S.; Pignatello, R. Cationic solid lipid nanoparticles enhance ocular hypotensive effect of melatonin in rabbit. Int J Pharm 2015, 478, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, L.; Wang, J.; Zhang, H.; Zhang, Z.; Xing, G.; Wang, X.; Liu, M. Drug-loaded PEG-PLGA nanoparticles for cancer treatment. Front Pharmacol 2022, 13, 990505. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, T.; Bucolo, C.; Carbone, C.; Pignatello, R.; Drago, F.; Puglisi, G. Polymeric nanoparticles augment the ocular hypotensive effect of melatonin in rabbits. Int J Pharm 2013, 440, 135–40. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; Santini, A.; Souto, E.B. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. , Oliveira, A.M., Neves, A., Pires, B., Venkatesh, D.N., Durazzo, A., Lucarini, M., Eder, P., Silva, A.M., Santini, A., Souto, E.B. Polymeric Nanoparticles: Production, Characterization, Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.V.R.; Silveira, P.L.; Spingolon, G.; Alves, G.A.L.; Peña, F.P.; Aguirre, T.A.S. Polymeric nanoparticles containing babassu oil: A proposed drug delivery system for controlled release of hydrophilic compounds. Chem Phys Lipids 2023, 253, 105304. [Google Scholar] [CrossRef]
- Bessone, C.D.V.; Martinez, S.M.; Luna, J.D.; Marquez, M.A.; Ramírez, M.L.; Allemandi, D.A.; Carpentieri, Á.R.; Quinteros, D.A. Neuroprotective effect of melatonin loaded in ethylcellulose nanoparticles applied topically in a retinal degeneration model in rabbits. Exp Eye Res 2020, 200, 108222. [Google Scholar] [CrossRef]
- Ye, D.; Xu, Y.; Shi, Y.; Fan, M.; Lu, P.; Bai, X.; Feng, Y.; Hu, C.; Cui, K.; Tang, X.; Liao, J.; Huang, W.; Xu, F.; Liang, X.; Huang, J. Anti-PANoptosis is involved in neuroprotective effects of melatonin in acute ocular hypertension model. J Pineal Res 2022, 73, e12828. [Google Scholar] [CrossRef]
- Carbone, C.; Manno, D.; Serra, A.; Musumeci, T.; Pepe, V.; Tisserand, C.; Puglisi, G. Innovative hybrid vs polymeric nanocapsules: The influence of the cationic lipid coating on the “4S”. Colloids Surf B Biointerfaces 2016, 141, 450–457. [Google Scholar] [CrossRef]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J Control Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, R.; Corsaro, R.; Bonaccorso, A.; Zingale, E.; Carbone, C.; Musumeci, T. Soluplus® polymeric nanomicelles improve solubility of BCS-class II drugs. Drug Deliv Transl Res 2022, 12, 1991–2006. [Google Scholar] [CrossRef] [PubMed]
- Dal Monte, M.; Cammalleri, M.; Amato, R.; Pezzino, S.; Corsaro, R.; Bagnoli, P.; Rusciano, D. A Topical Formulation of Melatoninergic Compounds Exerts Strong Hypotensive and Neuroprotective Effects in a Rat Model of Hypertensive Glaucoma. Int J Mol Sci 2020, 21, 9267. [Google Scholar] [CrossRef] [PubMed]
- Dal Monte, M.; Cammalleri, M.; Pezzino, S.; Corsaro, R.; Pescosolido, N.; Bagnoli, P.; Rusciano, D. Hypotensive Effect of Nanomicellar Formulation of Melatonin and Agomelatine in a Rat Model: Significance for Glaucoma Therapy. Diagnostics (Basel) 2020, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Hafner, A.; Lovrić, J.; Romić, M.D.; Juretić, M.; Pepić, I.; Cetina-Čižmek, B.; Filipović-Grčić, J. Evaluation of cationic nanosystems with melatonin using an eye-related bioavailability prediction model. Eur J Pharm Sci 2015, 75, 142–50. [Google Scholar] [CrossRef] [PubMed]
- Zingale, E.; Bonaccorso, A.; D’Amico, A.G.; Lombardo, R.; D’Agata, V.; Rautio, J.; Pignatello, R. Formulating Resveratrol and Melatonin Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Ocular Administration Using Design of Experiments. Pharmaceutics 2024, 16, 125. [Google Scholar] [CrossRef] [PubMed]
- Romeo, A.; Kazsoki, A.; Omer, S.; Pinke, B.; Mészáros, L.; Musumeci, T.; Zelkó, R. Formulation and Characterization of Electrospun Nanofibers for Melatonin Ocular Delivery. Pharmaceutics 2023, 15, 1296. [Google Scholar] [CrossRef]
- Serramito, M.; Pereira-da-Mota, A.F.; Carpena-Torres, C.; Huete-Toral, F.; Alvarez-Lorenzo, C.; Carracedo, G. Melatonin-Eluting Contact Lenses Effect on Tear Volume: In Vitro and In Vivo Experiments. Pharmaceutics 2022, 14, 1019. [Google Scholar] [CrossRef]
- Navarro-Gil, F.J.; Huete-Toral, F.; Domínguez-Godínez, C.O.; Carracedo, G.; Crooke, A. Contact Lenses Loaded with Melatonin Analogs: A Promising Therapeutic Tool against Dry Eye Disease. J Clin Med 2022, 11, 3483. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv Transl Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Lemp, M.A.; Baudouin, C.; Baum, J.; Dogru, M.; Foulks, G.N.; Kinoshita, S.; Laibson, P.; McCulley, J.; Murube, J.; Pflugfelder, S.C.; et al. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul. Surf 2007, 5, 75–92. [Google Scholar]
- Tsubota, K., Yokoi, N., Shimazaki, J., Watanabe, H., Dogru, M., Yamada, M., Kinoshita, S., Kim, H. M., Tchah, H. W., Hyon, J. Y., Yoon, K. C., Seo, K. Y., Sun, X., Chen, W., Liang, L., Li, M., Liu, Z., ùAsia Dry Eye Society. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. The ocular surface. 2017, 15, 65–76.
- Ciuffi, M.; Pisanello, M.; Pagliai, G.; Raimondi, L.; Franchi-Micheli, S.; Cantore, M.; Mazzetti, L.; Failli, P. Antioxidant protection in cultured corneal cells and whole corneas submitted to UV-B exposure. Journal of photochemistry and photobiology. J Photochem Photobiol B 2003, 71, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zuo, X.; Peng, L.; Wang, X.; Zeng, H.; Zhong, J.; Li, S.; Xiao, Y.; Wang, L.; Ouyang, H.; Yuan, J. Melatonin ameliorates oxidative stress-mediated injuries through induction of HO-1 and restores autophagic flux in dry eye. Exp Eye Res 2021, 205, 108491. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Peng, R.; Shen, H.; Zhong, L.; Song, S.; Wang, T.; Ling, S. Treatment With Melatonin After Corneal Graft Attenuates Rejection. Front Pharmacol 2021, 12, 778892. [Google Scholar] [CrossRef]
- Carracedo, G., Carpena, C., Concepción, P., Díaz, V., García-García, M., Jemni, N., Lledó, V. E., Martín, M., Pastrana, C., Pelissier, R., Veselinova, A., Wang, X., Pintor, J. Presence of melatonin in human tears. J Optom. 2017, 10, 3–4.
- Crooke, A.; Guzman-Aranguez, A.; Mediero, A.; Alarma-Estrany, P.; Carracedo, G.; Pelaez, T.; Peral, A.; Pintor, J. Effect of melatonin and analogues on corneal wound healing: involvement of Mt2 melatonin receptor. Curr Eye Res 2015, 40, 56–65. [Google Scholar] [CrossRef]
- Navarro Gil, F.J.; Huete-Toral, F.; Crooke, A.; Dominguez Godinez, C.O.; Carracedo, G.; Pintor, J. Effect of Melatonin and Its Analogs on Tear Secretion. J Pharmacol Exp Ther 2019, 371, 186–190. [Google Scholar] [CrossRef]
- Alkozi, H.A.; Sánchez Montero, J.M.; Doadrio, A.L.; Pintor, J. Docking studies for melatonin receptors. Expert Opin Drug Discov 2018, 13, 241–248. [Google Scholar] [CrossRef]
- Hoyle, C.H.; Peral, A.; Pintor, J. Melatonin potentiates tear secretion induced by diadenosine tetraphosphate in the rabbit. Eur J Pharmacol 2006, 552, 159–61. [Google Scholar] [CrossRef]
- Xu, J.; Chen, P.; Zhao, G.; Wei, S.; Li, Q.; Guo, C.; Cao, Q.; Wu, X.; Di, G. Copolymer Micelle-administered Melatonin Ameliorates Hyperosmolarity-induced Ocular Surface Damage through Regulating PINK1-mediated Mitophagy. Curr Eye Res 2022, 47, 688–703. [Google Scholar] [CrossRef] [PubMed]
- Wahl, C.; Li, T.; Takagi, Y.; Howland, H. The effects of light regimes and hormones on corneal growth in vivo and in organ culture. J Anat 2011, 219, 766–75. [Google Scholar] [CrossRef]
- Dubbelman, M.; Van der Heijde, G.L.; Weeber, H.A.; Vrensen, G.F. Changes in the internal structure of the human crystalline lens with age and accommodation. Vision Res 2003, 43, 2363–75. [Google Scholar] [CrossRef]
- Petrash, J.M. Aging and age-related diseases of the ocular lens and vitreous body. Invest Ophthalmol Vis Sci 2013, 54, ORSF54–9. [Google Scholar] [CrossRef] [PubMed]
- Bassnett, S. Lens organelle degradation. Exp Eye Res 2002, 74, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.L.; King, J.A. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol Med 2012, 18, 273–82. [Google Scholar] [CrossRef] [PubMed]
- Benedek, G.B. Cataract as a protein condensation disease: the Proctor Lecture. Invest Ophthalmol Vis Sci 1997, 38, 1911–21. [Google Scholar] [PubMed]
- Bassnett, S. Mitochondrial dynamics in differentiating fiber cells of the mammalian lens. Curr Eye Res 1992, 11, 1227–32. [Google Scholar] [CrossRef]
- Bassnett, S. The fate of the Golgi apparatus and the endoplasmic reticulum during lens fiber cell differentiation. Invest Ophthalmol Vis Sci 1995, 36, 1793–803. [Google Scholar]
- Pendergrass, W.; Penn, P.; Possin, D.; Wolf, N. Accumulation of DNA, nuclear and mitochondrial debris, and ROS at sites of age-related cortical cataract in mice. Invest Ophthalmol Vis Sci 2005, 46, 4661–70. [Google Scholar] [CrossRef]
- Bubenik, G.A.; Brown, G.M.; Uhlir, I.; Grota, L.J. Immunohistological localization of N-acetylindolealkylamines in pineal gland, retina and cerebellum. Brain Res 1974, 81, 233–42. [Google Scholar] [CrossRef] [PubMed]
- Aimoto, T., Rohde, B.H., Chiou, G.C., Lauber, J.K. N-acetyltransferase activity and melatonin level in the eyes of glaucomatous chickens. J Ocul Pharmacol. 1985, 1, 149–60.
- Quay, W.B. Increases in volume, fluid content, and lens weight of eyes following systemic administration of melatonin. J Pineal Res 1984, 1, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Itoh, M.T.; Miyata, M.; Shimizu, K.; Sumi, Y. Circadian rhythm of serotonin N -acetyltransferase activity in rat lens. Exp Eye Res 2000, 70, 805–8. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, M.C.; van Jaarsveld, A.S.; Reiter, R.J. Melatonin in the lacrimal gland: first demonstration and experimental manipulation. Biochem Biophys Res Commun 1988, 153, 1186–92. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Reiter, R.J.; Orhii, P.B.; Hara, M.; Poeggeler, B. Inhibitory effect of melatonin on cataract formation in newborn rats: evidence for an antioxidative role for melatonin. J Pineal Res 1994, 17, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Yağci, R., Aydin, B., Erdurmuş, M., Karadağ, R., Gürel, A., Durmuş, M., Yiğitoğlu, R. Use of melatonin to prevent selenite-induced cataract formation in rat eyes. Curr Eye Res 2006, 31, 845-50.
- Bai, J.; Dong, L.; Song, Z.; Ge, H.; Cai, X.; Wang, G.; Liu, P. The role of melatonin as an antioxidant in human lens epithelial cells. Free Radic Res 2013, 47, 635–42. [Google Scholar] [CrossRef]
- Costello, M.J.; Brennan, L.A.; Basu, S.; Chauss, D.; Mohamed, A.; Gilliland, K.O.; Johnsen, S.; Menko, S.; Kantorow, M. Autophagy and mitophagy participate in ocular lens organelle degradation. Exp Eye Res 2013, 116, 141–50. [Google Scholar] [CrossRef]
- Jenwitheesuk, A.; Nopparat, C.; Mukda, S.; Wongchitrat, P.; Govitrapong, P. Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. Int J Mol Sci 2014, 15, 16848–84. [Google Scholar] [CrossRef]
- Mi, Y., Wei, C., Sun, L., Liu, H., Zhang, J., Luo, J., Yu, X., He, J., Ge, H., Liu, P. (2023). Melatonin inhibits ferroptosis and delays age-related cataract by regulating SIRT6/p-Nrf2/GPX4 and SIRT6/NCOA4/FTH1 pathways. Biomed Pharmacother 2023, 157, 114048. 114048.
- Lledó, V. E., Alkozi, H. A., Sánchez-Naves, J., Fernandez-Torres, M. A., Guzman-Aranguez, A. Melatonin counteracts oxidative damage in lens by regulation of Nrf2 and NLRP3 inflammasome activity. Exp Eye Res 2022, 215, 108912.
- Ohannes, A.K.; Hussain, S.A.R.; Rasool, A.A.H.; Mohammed, H.M.; Al Essa, A.; Abdulrazzaq, M.H. Protective effects of melatonin as an eye drops against selenite-induced cataract in rat pups. Saudi Pharmaceutical Journal 2009, 17, 2. [Google Scholar]
- Tosini, G.; Baba, K.; Hwang, C.K.; Iuvone, P.M. Melatonin: an underappreciated player in retinal physiology and pathophysiology. Exp Eye Res 2012, 103, 82–9. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Pozdeyev, N.; Sakamoto, K.; Iuvone, P.M. The circadian clock system in the mammalian retina. Bioessays 2008, 30, 624–33. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.; Wankiewicz, E.; Brown, G.M.; Fujieda, H. MT(1) melatonin receptor in the human retina: expression and localization. Invest Ophthalmol Vis Sc. 2002, 43, 889–97. [Google Scholar]
- Jockers, R.; Maurice, P.; Boutin, J.A.; Delagrange, P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what’s new? Br J Pharmacol 2008, 154, 1182–95. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Pozdeyev, N.; Mazzoni, F.; Contreras-Alcantara, S.; Liu, C.; Kasamatsu, M.; Martinez-Merlos, T.; Strettoi, E.; Iuvone, P.M.; Tosini, G. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci U S A 2009, 106, 15043–8. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev 2010, 62, 343–80. [Google Scholar]
- Marchiafava, P.L.; Longoni, B. Melatonin as an antioxidant in retinal photoreceptors. J Pineal Res 1999, 26, 184–9. [Google Scholar] [CrossRef]
- Piano, I., Baba, K., C Gargini, Tosini, G. Heteromeric MT1/MT2 melatonin receptors modulate the scotopic electroretinogram via PKCζ in mice. Exp Eye Res 2018, 177, 50-54.
- Emser, W.; Dechoux, R.; Weiland, M.; Wirz-Justice, A. Melatonin decreases the amplitude of the b-wave of the human electroretinogram. Experientia 1993, 49, 686–7. [Google Scholar] [CrossRef]
- Peters, J.L.; Cassone, V.M. Melatonin regulates circadian electroretinogram rhythms in a dose- and time-dependent fashion. J Pineal Res 2005, 38, 209–15. [Google Scholar] [CrossRef]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 2000, 45, 115–34. [Google Scholar] [CrossRef]
- Bellezza, I. Oxidative Stress in Age-Related Macular Degeneration: Nrf2 as Therapeutic Target. Front Pharmacol 2018, 9, 1280. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.; Hu, D.N.; Perez, V.; Tai, K.; Yu, G.P.; Chen, M.; Tone, P.; McCormick, S.A.; Walsh, J. Urinary 6-sulfatoxymelatonin level in age-related macular degeneration patients. Mol Vis 2009, 15, 1673–9. [Google Scholar]
- Yi, C.; Pan, X.; Yan, H.; Guo, M.; Pierpaoli, W. Effects of melatonin in age-related macular degeneration. Ann N Y Acad Sci 2005, 1057, 384–92. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.B.; Hu, D.N.; Chen, M.; McCormick, S.A.; Walsh, J.; Roberts, J.E. Effects of melatonin and its receptor antagonist on retinal pigment epithelial cells against hydrogen peroxide damage. Mol Vis 2012, 18, 1640–8. [Google Scholar] [PubMed]
- Lin, J.; Epel, E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res Rev 2022, 73, 101507. [Google Scholar] [CrossRef] [PubMed]
- Rowe-Rendleman, C.; Glickman, R.D. Possible therapy for age-related macular degeneration using human telomerase. Brain Res Bull 2004, 62, 549–53. [Google Scholar] [CrossRef]
- Rastmanesh, R. Potential of melatonin to treat or prevent age-related macular degeneration through stimulation of telomerase activity. Med Hypotheses 2011, 76, 79–85. [Google Scholar] [CrossRef]
- Tisi, A.; Feligioni, M.; Passacantando, M.; Ciancaglini, M.; Maccarone, R. The Impact of Oxidative Stress on Blood-Retinal Barrier Physiology in Age-Related Macular Degeneration. Cells 2021, 10, 64. [Google Scholar] [CrossRef]
- Diéguez, H.H.; González Fleitas, M.F.; Aranda, M.L.; Calanni, J.S.; Keller Sarmiento, M.I.; Chianelli, M.S.; Alaimo, A.; Sande, P.H.; Romeo, H.E.; Rosenstein, R.E.; Dorfman, D. Melatonin protects the retina from experimental nonexudative age-related macular degeneration in mice. J Pineal Res 2020, 68, e12643. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Hemati, K.; Reiter, R.J.; Hosseinzadeh, A. Mitochondrial dysfunction in age-related macular degeneration: melatonin as a potential treatment. Expert Opin Ther Targets 2020, 24, 359–378. [Google Scholar] [CrossRef]
- Ku, L.C.; Sheu, M.L.; Cheng, H.H.; Lee, C.Y.; Tsai, Y.C.; Tsai, C.Y.; Lin, K.H.; Lai, L.C.; Lai, D.W. Melatonin protects retinal integrity through mediated immune homeostasis in the sodium iodate-induced mouse model of age-related macular degeneration. Biomed Pharmacother 2023, 161, 114476. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Li, Y.; Che, M.; Li, C.; Wu, Q.; Sun, C. Melatonin relieves hepatic lipid dysmetabolism caused by aging via modifying the secondary bile acid pattern of gut microbes. Cell Mol Life Sci 2022, 79, 527. [Google Scholar] [CrossRef] [PubMed]
- Rong, B.; Wu, Q.; Reiter, R.J.; Sun, C. The Mechanism of Oral Melatonin Ameliorates Intestinal and Adipose Lipid Dysmetabolism Through Reducing Escherichia Coli-Derived Lipopolysaccharide. Cell Mol Gastroenterol Hepatol 2021, 12, 1643–1667. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Han, H.; Ma, J.; Liu, G.; Wu, X.; Huang, X.; Fang, R.; Baba, K.; Bin, P.; Zhu, G.; Ren, W.; Tan, B.; Tosini, G.; He, X.; Li, T.; Yin, Y. Administration of Exogenous Melatonin Improves the Diurnal Rhythms of the Gut Microbiota in Mice Fed a High-Fat Diet. mSystems 2020, 5, e00002–20. [Google Scholar] [CrossRef] [PubMed]
- Onaolapo, A.Y.; Adebisi, E.O.; Adeleye, A.E.; Olofinnade, A.T.; Onaolapo, O.J. Dietary Melatonin Protects Against Behavioural, Metabolic, Oxidative, and Organ Morphological Changes in Mice that are Fed High-Fat, High- Sugar Diet. Endocr Metab Immune Disord Drug Targets 2020, 20, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Han, H.; Chen, S.; Gao, J.; Liu, G.; Wu, X.; Deng, J.; Yu, Q.; Huang, X.; Fang, R.; Li, T.; Reiter, R.J.; Zhang, D.; Zhu, C.; Zhu, G.; Ren, W.; Yin, Y. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J Pineal Res 2018, 6, e12524. [Google Scholar] [CrossRef] [PubMed]
- Onaolapo, A.Y.; Onaolapo, O.J. Circadian dysrhythmia-linked diabetes mellitus: Examining melatonin’s roles in prophylaxis and management. World J Diabetes 2018, 9, 99–114. [Google Scholar] [CrossRef]
- Ozdemir, G.; Ergün, Y.; Bakariş, S.; Kılınç, M.; Durdu, H.; Ganiyusufoğlu, E. Melatonin prevents retinal oxidative stress and vascular changes in diabetic rats. Eye (Lond) 2014, 28, 1020–7. [Google Scholar] [CrossRef]
- Salido, E.M.; Bordone, M.; De Laurentiis, A.; Chianelli, M.; Keller Sarmiento, M.I.; Dorfman, D.; Rosenstein, R.E. Therapeutic efficacy of melatonin in reducing retinal damage in an experimental model of early type 2 diabetes in rats. J Pineal Res 2013, 54, 179–89. [Google Scholar] [CrossRef]
- Jiang, T.; Chang, Q.; Cai, J.; Fan, J.; Zhang, X.; Xu, G. Protective Effects of Melatonin on Retinal Inflammation and Oxidative Stress in Experimental Diabetic Retinopathy. Oxid Med Cell Longev 2016, 2016, 3528274. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Motevalian, M.; Rezaei Kanavi, M.; Fatemi, I.; Ghaznavi, H.; Shahriari, M. Protective effect of melatonin in the diabetic rat retina. Fundam Clin Pharmacol 2018, 32, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Yu, F.; Shi, L.; Ko, M.L.; Ko, G.Y. Melatonin Affects Mitochondrial Fission/Fusion Dynamics in the Diabetic Retina. J Diabetes Res 2019, 2019, 8463125. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, B., Cvetkovic, T., Stoimenov, T. J., Despotovic, M., Zivanovic, S., Basic, J., Veljkovic, A., Velickov, A., Kocic, G., Pavlovic, D., Sokolovic, D.Oral supplementation with melatonin reduces oxidative damage and concentrations of inducible nitric oxide synthase, VEGF and matrix metalloproteinase 9 in the retina of rats with streptozotocin/nicotinamide induced pre-diabetes. Eur J Pharmacol 2018, 833, 290-297.
- Tu, Y.; Zhu, M.; Wang, Z.; Wang, K.; Chen, L.; Liu, W.; Shi, Q.; Zhao, Q.; Sun, Y.; Wang, X.; Song, E.; Liu, X. Melatonin inhibits Müller cell activation and pro-inflammatory cytokine production via upregulating the MEG3/miR-204/Sirt1 axis in experimental diabetic retinopathy. J Cell Physiol 2020, 235, 8724–8735. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Song, E.; Wang, Z.; Ji, N.; Zhu, L.; Wang, K.; Sun, H.; Zhang, Y.; Zhu, Q.; Liu, X.; Zhu, M. Melatonin attenuates oxidative stress and inflammation of Müller cells in diabetic retinopathy via activating the Sirt1 pathway. Biomed Pharmacother 2021, 137, 111274. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, C.; Yang, Q.; Xie, H.; Liu, D.; Tian, H.; Lu, L.; Xu, J.Y.; Li, W.; Xu, G.; Qiu, Q.; Liu, K.; Luo, D.; Xu, G.T.; Zhang, J. Melatonin maintains inner blood-retinal barrier via inhibition of p38/TXNIP/NF-κB pathway in diabetic retinopathy. J Cell Physiol 2021, 236, 5848–5864. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Wang, H.; Gu, Y.; Li, X.; Tao, L.; Lu, P. Melatonin exerts protective effects on diabetic retinopathy via inhibition of Wnt/β-catenin pathway as revealed by quantitative proteomics. Exp Eye Res 2021, 205, 108521. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Rathnasamy, G.; Foulds, W.S.; Ling, E.A. Cellular and Molecular Mechanisms of Retinal Ganglion Cell Death in Hypoxic-Ischemic Injuries. J Neurol Exp Neurosci 2015, 1, 10–19. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, H.S.; Sung, M.S.; Kim, S.J. The effect of melatonin on retinal ganglion cell survival in ischemic retina. Chonnam Med J 2012, 48, 116–22. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, X.; Hu, Y.; Yang, B.; Tsui, C.K.; Yu, S.; Lu, L.; Liang, X. Melatonin attenuated retinal neovascularization and neuroglial dysfunction by inhibition of HIF-1α-VEGF pathway in oxygen-induced retinopathy mice. J Pineal Res 2018, 64, e12473. [Google Scholar] [CrossRef]
- Miranda-Riestra, A.; Estrada-Reyes, R.; Torres-Sanchez, E.D.; Carreño-García, S.; Ortiz, G.G.; Benítez-King, G. Melatonin: A Neurotrophic Factor? Molecules 2022, 27, 7742. [Google Scholar] [CrossRef]
- Huang, R.; Xu, Y.; Lu, X.; Tang, X.; Lin, J.; Cui, K.; Yu, S.; Shi, Y.; Ye, D.; Liu, Y.; Liang, X. Melatonin protects inner retinal neurons of newborn mice after hypoxia-ischemia. J Pineal Res 2021, 71, e12716. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Sivakumar, V.; Yong, Z.; Lu, J.; Foulds, W.S.; Ling, E.A. Blood-retinal barrier disruption and ultrastructural changes in the hypoxic retina in adult rats: the beneficial effect of melatonin administration. J Pathol 2007, 212, 429–39. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A. Glaucoma. The Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br J Ophthalmol 2012, 96, 614–618. [Google Scholar] [CrossRef]
- Nucci, C.; Tartaglione, R.; Rombolà, L.; Morrone, L. A.; Fazzi, E.; Bagetta, G. Neurochemical Evidence to Implicate Elevated Glutamate in the Mechanisms of High Intraocular Pressure (IOP)-induced Retinal Ganglion Cell Death in Rat. NeuroToxicology 2005, 26, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: a review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Investigators, T.A. The advanced glaucoma intervention study (AGIS) : 7. the relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 2000, 130, 429–440. [Google Scholar]
- Jin, S.W.; Noh, S.Y. Long-Term Clinical Course of Normal-Tension Glaucoma: 20 Years of Experience. Journal of Ophthalmology 2017, e2651645. [Google Scholar] [CrossRef]
- Marquis, R.E.; Whitson, J.T. Management of Glaucoma: Focus on Pharmacological Therapy. Drugs Aging 2005, 22, 1–21. [Google Scholar] [CrossRef]
- Agorastos, A.; Huber, C.G. The role of melatonin in glaucoma: implications concerning pathophysiological relevance and therapeutic potential. J Pineal Res 2011, 50, 1–7. [Google Scholar] [CrossRef]
- Alkozi, H.; Sánchez-Naves, J.; de Lara, M.J.; Carracedo, G.; Fonseca, B.; Martinez-Aguila, A.; Pintor, J. Elevated intraocular pressure increases melatonin levels in the aqueous humour. Acta Ophthalmologica 2017, 95, e185–e189. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Águila, A.; Martín-Gil, A.; Carpena-Torres, C.; Pastrana, C.; Carracedo, G. Influence of Circadian Rhythm in the Eye: Significance of Melatonin in Glaucoma. Biomolecules 2021, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Molecular and Cellular Endocrinology 2012, 351, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Crooke, A.; Huete-Toral, F.; Colligris, B.; Pintor, J. The role and therapeutic potential of melatonin in age-related ocular diseases. Journal of Pineal Research 2017, 63, e12430. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Águila, A.; Fonseca, B.; Pérez de Lara, M.J.; Miras-Portugal, M.T.; Gómez-Villafuertes, R.; Carracedo, G.; Pintor, J. Changes in melatonin receptor expression in a murine model of glaucoma. Mol Vis 2020 26, 530–539.
- Alcantara-Contreras, S.; Baba, K.; Tosini, G. Removal of Melatonin Receptor Type 1 Increases Intraocular Pressure and Retinal Ganglion Cells Death in the Mouse. Neurosci Lett 2011, 494, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Iuvone, M.; Boatright, J.H. Is the melatonin receptor type 1 involved in the pathogenesis of glaucoma? J Glaucoma 2013, 22 Suppl 5, S49–50. [Google Scholar] [CrossRef]
- Alkozi, H.; Sánchez-Naves, J.; de Lara, M.J.; Carracedo, G.; Fonseca, B.; Martinez-Aguila, A.; Pintor, J. Elevated intraocular pressure increases melatonin levels in the aqueous humour. Acta ophthalmologica 2017, 95, e185–e189. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-P.; Shen, M.-Y.; Shen, G.-L.; Qi, Q.-R.; Sun, X.-H. Melatonin concentrations in serum of primary glaucoma patients. International Journal of Ophthalmology 2018, 11, 1337–1341. [Google Scholar]
- Kim, J.Y.; Jeong, A.R.; Chin, H.S.; Kim, N.R. Melatonin Levels in Patients With Primary Open-angle Glaucoma With High or Low Intraocular Pressure. Journal of Glaucoma 2019, 28, 154–160. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Obayashi, K.; Miyata, K.; Saeki, K.; Ogata, N. Decreased melatonin secretion in patients with glaucoma: Quantitative association with glaucoma severity in the LIGHT study. J Pineal Res 2020, 69, e12662. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.G.; Malishevskaya Т, N.; Astakhov, Y.S.; Astakhov, S.Y.; Cornelissen, G.; Kuznetsov, V.A.; Weinert, D. Progressive retinal ganglion cell loss in primary open-angle glaucoma is associated with temperature circadian rhythm phase delay and compromised sleep. Chronobiol Int 2019, 36, 564–577. [Google Scholar] [CrossRef]
- Alarma-Estrany, P., Guzman-Aranguez, A., Huete, F., Peral, A., Plourde, R., Jr, Pelaez, T., Yerxa, B., Pintor, J. (2011). Design of Novel Melatonin Analogs for the Reduction of Intraocular Pressure in Normotensive Rabbits. J Pharmacol Exp Ther 2011, 337, 703–709.
- Crooke, A.; Huete-Toral, F.; Martínez-Águila, A.; Martín-Gil, A.; Pintor, J. Melatonin and Its Analog 5-Methoxycarbonylamino-N-Acetyltryptamine Potentiate Adrenergic Receptor-Mediated Ocular Hypotensive Effects in Rabbits: Significance for Combination Therapy in Glaucoma. J Pharmacol Exp Ther 2013, 346, 138–145. [Google Scholar] [CrossRef]
- Martinez-Aguila, A.; Fonseca, B.; Perez de Lara, M.J.; Pintor, J. Effect of Melatonin and 5-Methoxycarbonylamino-N-Acetyltryptamine on the Intraocular Pressure of Normal and Glaucomatous Mice. Journal of Pharmacology and Experimental Therapeutics 2016, 357, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, D.S.; Liebmann, J.M.; Ritch, R. Brimonidine: a new alpha2-adrenoreceptor agonist for glaucoma treatment. J Glaucoma 1997, 6, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Arranz-Romera, A.; Davis, B.M.; Bravo-Osuna, I.; Esteban-Pérez, S.; Molina-Martínez, I.T.; Shamsher, E.; Ravindran, N.; Guo, L.; Cordeiro, M.F.; Herrero-Vanrell, R. Simultaneous co-delivery of neuroprotective drugs from multi-loaded PLGA microspheres for the treatment of glaucoma. Journal of controlled release: official journal of the Controlled Release Society 2019, 297, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.A.; Mowafi, H.A. Melatonin Provides Anxiolysis, Enhances Analgesia, Decreases Intraocular Pressure, and Promotes Better Operating Conditions During Cataract Surgery Under Topical Anesthesia. Anesthesia & Analgesia 209, 108, 1146–1151.
- Pescosolido, N.; Gatto, V.; Stefanucci, A.; Rusciano, D. Oral treatment with the melatonin agonist agomelatine lowers the intraocular pressure of glaucoma patients. Ophthalmic and Physiological Optics 2015, 35, 201–205. [Google Scholar] [CrossRef]
- Martínez-Águila, A.; Fonseca, B.; Bergua, A.; Pintor, J. Melatonin analogue agomelatine reduces rabbit’s intraocular pressure in normotensive and hypertensive conditions. European Journal of Pharmacology 2013, 701, 213–217. [Google Scholar] [CrossRef]
- Southgate, G.; Schubert, M.; Daya, S. Melatonin Offers Protection against Glutamate Receptor Agonists in Neuronal Cultures. Annals of Neurosciences 2010, 15, 1–5. [Google Scholar] [CrossRef]
- Espinar, A.; García-Oliva, A.; Isorna, E.M.; Quesada, A.; Prada, F.A.; Guerrero, J.M. Neuroprotection by melatonin from glutamate-induced excitotoxicity during development of the cerebellum in the chick embryo. J Pineal Res 2000, 28, 81–88. [Google Scholar] [CrossRef]
- del Valle Bessone, C., Fajreldines, H. D., de Barboza, G. E. D., Tolosa de Talamoni, N. G., Allemandi, D. A., Carpentieri, A. R., et al. Protective role of melatonin on retinal ganglionar cell: In vitro an in vivo evidences. Life Sciences 2019, 218, 233–240.
- Gupta, N.; Yücel, Y.H. Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol 2007, 18, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Rusciano, D.; Pezzino, S.; Mutolo, M.G.; Giannotti, R.; Librando, A.; Pescosolido, N. Neuroprotection in Glaucoma: Old and New Promising Treatments. Adv Pharmacol Sci 2017, 4320408–4320408. [Google Scholar] [CrossRef] [PubMed]
- Egwuagu, C.E.; Alhakeem, S.A.; Mbanefo, E.C. Uveitis: Molecular Pathogenesis and Emerging Therapies. Front Immunol 2021, 12, 623725. [Google Scholar] [CrossRef]
- Aranda, M.L.; Fleitas, M.F.G.; Dieguez, H.; Iaquinandi, A.; Sande, P.H.; Dorfman, D.; Rosenstein, R.E. Melatonin as a Therapeutic Resource for Inflammatory Visual Diseases. Curr Neuropharmacol 2017, 15, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Sande, P.H.; Dorfman, D.; Fernandez, D.C.; Chianelli, M.; Domínguez Rubio, A.P.; Franchi, A.M.; Silberman, D.M.; Rosenstein, R.E.; Sáenz, D.A. Treatment with melatonin after onset of experimental uveitis attenuates ocular inflammation. Br J Pharmacol 2014, 171, 5696–707. [Google Scholar] [CrossRef] [PubMed]
- Chesnokova, N.B.; Beznos, O.V.; Lozinskaya, N.A.; Beyshenova, G.A.; Nesterova, T.V. Effect of melatonin instillations on the clinical course of experimental uveitis and biochemical processes in tears and aqueous humor. Biomed Khim 2016, 62, 164–8. [Google Scholar] [CrossRef] [PubMed]
- Banaś, I., Buntner, B., Niebrój, T., Ostrowska, Z. Stezenie melatoniny w surowicy chorych ze zwyrodnieniem barwnikowym siatkówki [Levels of melatonin in serum of patients with retinitis pigmentosa]. Klin Oczna 1995, 97, 321-3.
- Xu, X.J.; Wang, S.M.; Jin, Y.; Hu, Y.T.; Feng, K.; Ma, Z.Z. Melatonin delays photoreceptor degeneration in a mouse model of autosomal recessive retinitis pigmentosa. J Pineal Res 2017, 63. [Google Scholar] [CrossRef] [PubMed]
- Liang, F. Q., Aleman, T. S., ZaixinYang, Cideciyan, A. V., Jacobson, S. G., Bennett, J. Melatonin delays photoreceptor degeneration in the rds/rds mouse. Neuroreport 2001, 12, 1011-4.
- Pastor-Idoate, S.; Mateos-Olivares, M.; Sobas, E.M.; Marcos, M.; Toribio, A.; Pastor, J.C.; Usategui Martín, R. Short-Wavelength Light-Blocking Filters and Oral Melatonin Administration in Patients With Retinitis Pigmentosa: Protocol for a Randomized Controlled Trial. JMIR Res Protoc 2023, 12, e49196. [Google Scholar] [CrossRef]
- Morgan, I.G. The biological basis of myopic refractive error. Clin Exp Optom 2003, 86, 276–88. [Google Scholar] [CrossRef]
- Hoffmann, M.; Schaeffel, F. Melatonin and deprivation myopia in chickens. Neurochem Int 1996, 28, 95–107. [Google Scholar] [CrossRef]
- Morgan, I.G.; Rose, K.A.; Ashby, R.S. Animal Models of Experimental Myopia: Limitations and Synergies with Studies on Human Myopia. In: Spaide, R., Ohno-Matsui, K., Yannuzzi, L. (eds) Pathologic Myopia. Springer, New York, NY 2014.
- Kearney, S.; O’Donoghue, L.; Pourshahidi, L.K.; Cobice, D.; Saunders, K.J. Myopes have significantly higher serum melatonin concentrations than non-myopes. Ophthalmic Physiol Opt 2017, 37, 557–567. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, N.; Velpandian, T.; Gupta, V.; Vanathi, M.; Vashist, P.; Gowtham, L.; Saxena, R.; Tandon, R. Myopia, Melatonin and Conjunctival Ultraviolet Autofluorescence: A Comparative Cross-sectional Study in Indian Myopes. Curr Eye Res 2021, 46, 1474–1481. [Google Scholar] [CrossRef]
- Fulton, J.M.; Flanagan, S.C.; Sittlington, J.J.; Cobice, D.; Dobbin, S.; McCullough, S.J.; Orr, G.; Richardson, P.; Saunders, K.J. A Cross-Sectional Study of Myopia and Morning Melatonin Status in Northern Irish Adolescent Children. J Ophthalmol 2023, 2023, 7961623. [Google Scholar] [CrossRef]
- Chakraborty, R.; Seby, C.; Scott, H.; Tang, V.; Kemps, E.; Anstice, N.; Juers, E.; Lovato, N.; Taranath, D.A.; Mills, R.A.; Lack, L.C. Delayed melatonin circadian timing, lower melatonin output, and sleep disruptions in myopic, or short-sighted, children. Sleep 2024, 47, zsad265. [Google Scholar] [CrossRef]
- Hussain, A.; Gopalakrishnan, A.; Scott, H.; Seby, C.; Tang, V.; Ostrin, L.; Chakraborty, R. Associations between systemic melatonin and human myopia: A systematic review. Ophthalmic Physiol Opt 2023, 43, 1478–1490. [Google Scholar] [CrossRef]
Figure 1.
Properties of melatonin and their supposed effects on the eye.
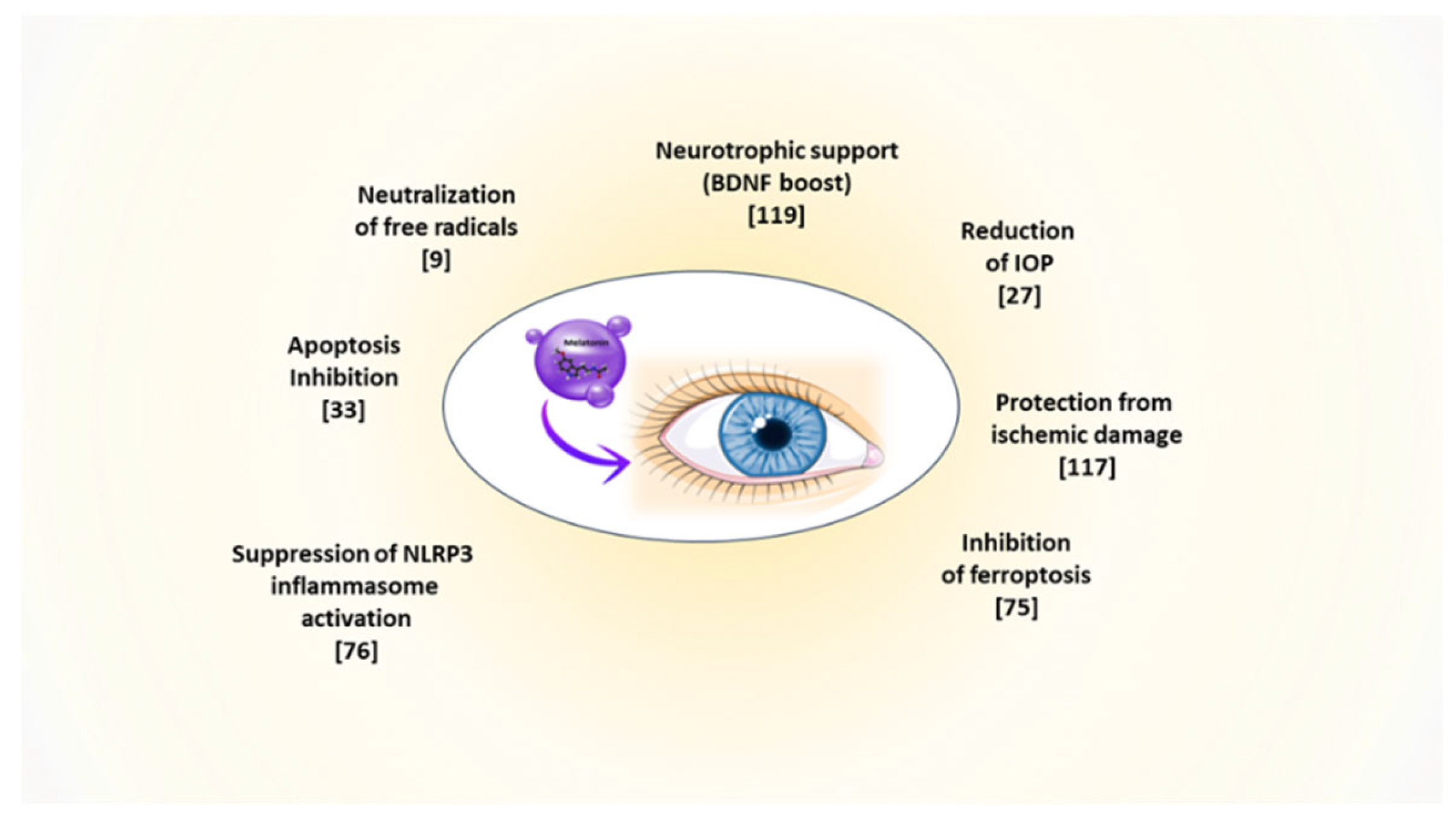
Figure 2.
Hypothetical diffusion of melatonin in nanomicelles from the ocular surface to different eye tissues. The figure illustrates a possible ‘vertical’ diffusion, going from the ocular surface through the aqueous humor, the lens, the vitreous body to the retina; and a ‘lateral’ diffusion through the anterior uvea and the local blood circulation [44].
Figure 2.
Hypothetical diffusion of melatonin in nanomicelles from the ocular surface to different eye tissues. The figure illustrates a possible ‘vertical’ diffusion, going from the ocular surface through the aqueous humor, the lens, the vitreous body to the retina; and a ‘lateral’ diffusion through the anterior uvea and the local blood circulation [44].
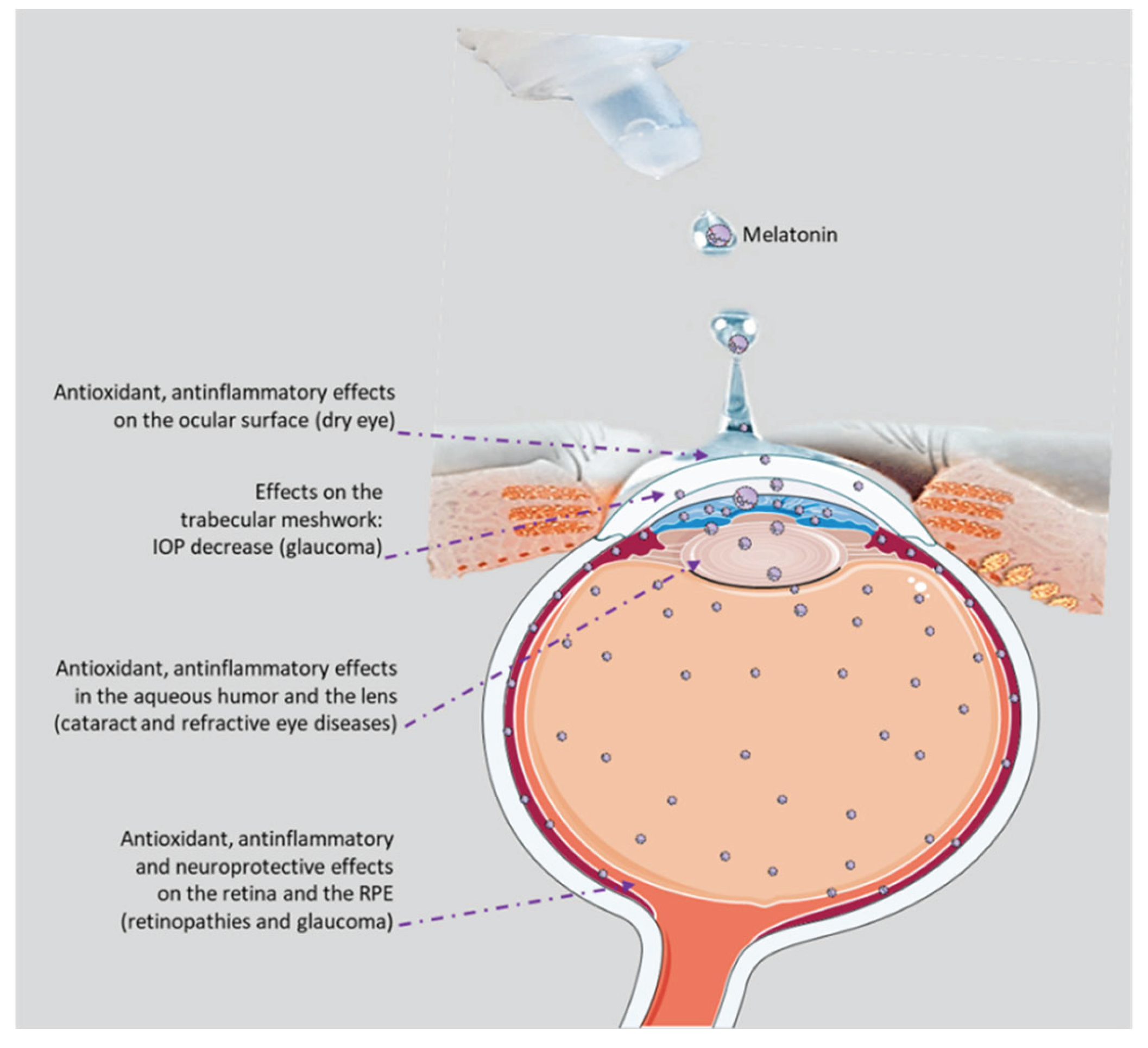
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Downloads
156
Views
122
Comments
0
Subscription
Notify me about updates to this article or when a peer-reviewed version is published.
MDPI Initiatives
Important Links
© 2025 MDPI (Basel, Switzerland) unless otherwise stated







