Submitted:
05 March 2024
Posted:
06 March 2024
You are already at the latest version
Abstract
Keywords:
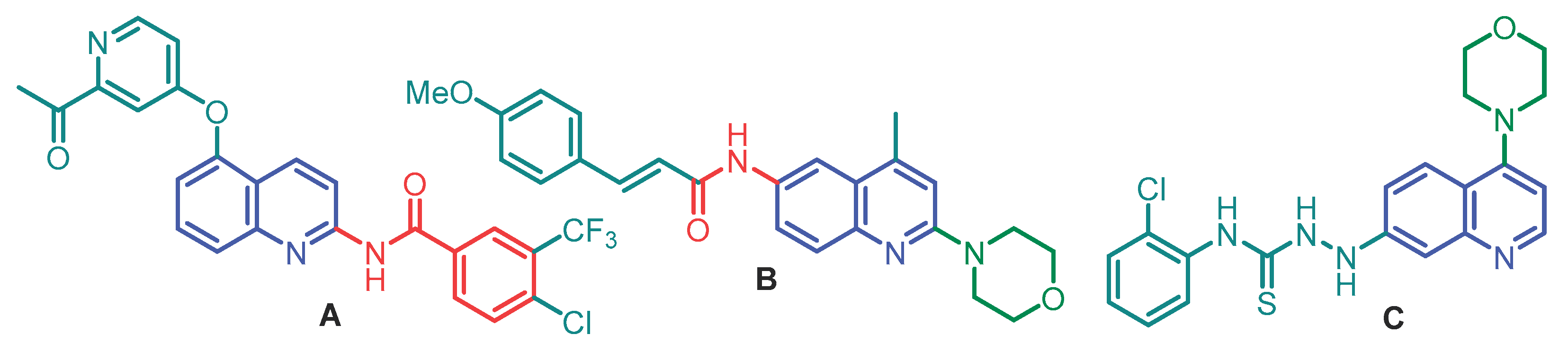
1. Introduction
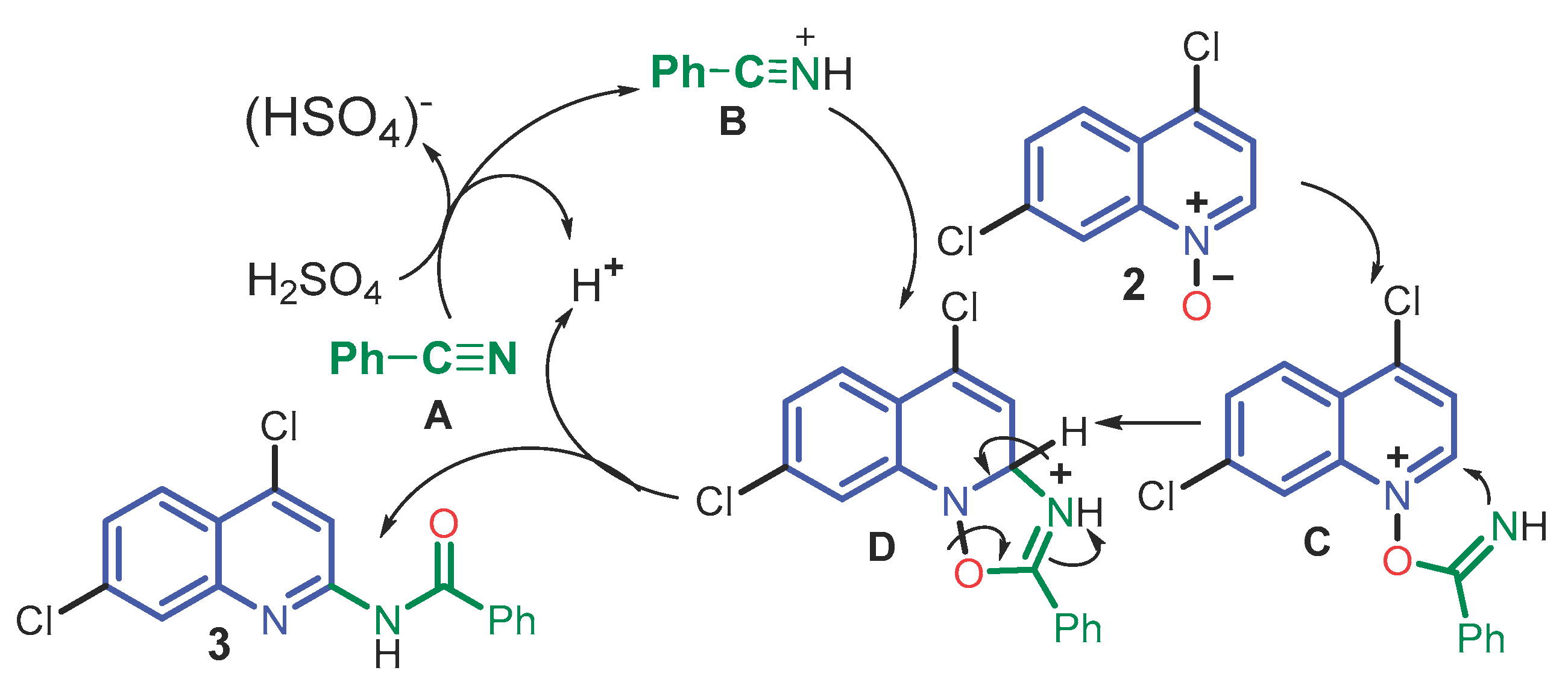
2. Results and Discussion
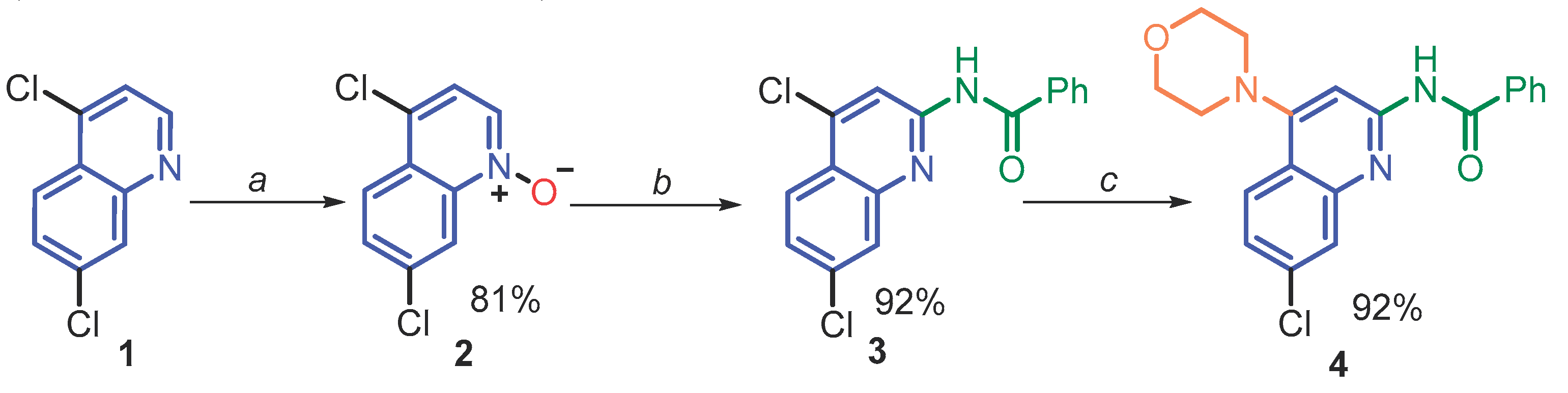
3. Materials and Methods
3.1. Chemical Analysis
3.2. Step 1: Preparation of 4,7-dichloroquinoline 1-oxide (2)
3.3. Step 2: Synthesis of N-(4,7-dichloroquinolin-2-yl) benzamide (3)
3.4. Step 3: Synthesis of N-(7-chloro-4-morpholinoquinolin-2-yl) benzamide (4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef] [PubMed]
- Ajani, O.O.; Iyaye, K.T.; Ademosun, O.T. Recent advances in chemistry and therapeutic potential of functionalized quinoline motifs–a review. RSC Adv. 2022, 12, 18594–18614. [Google Scholar] [CrossRef]
- Basavarajaiah, S.M. The Versatile Quinoline and Its Derivatives as anti-Cancer Agents: An Overview. Polycycl. Aromat. Compd. 2022, 43, 4333–4345. [Google Scholar]
- Kumari, A.; Singh, R.K. Morpholine as Ubiquitous Pharmacophore in Medicinal Chemistry: Deep Insight into the Structure-Activity Relationship (SAR). Bioorg. Chem. 2020, 96, 103578. [Google Scholar] [CrossRef]
- Kourounakis, A.P.; Xanthopoulos, D.; Tzara, A. Morpholine as a Privileged Structure: A Review on the Medicinal Chemistry and Pharmacological Activity of Morpholine Containing Bioactive Molecules. Med. Res. Rev. 2020, 40, 709–752. [Google Scholar] [CrossRef]
- Tzara, A.; Xanthopoulos, D.; Kourounakis, A.P. Morpholine as a Scaffold in Medicinal Chemistry: An Update on Synthetic Strategies. Chem. Med. Chem. 2020, 15, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Daddario, N.; Minassi, A.; Moriello, A.S.; De Petrocellis, L.; Di Marzo, V. The taming of capsaicin. Reversal of the vanilloid activity of N-acylvanillamines by aromatic iodination. J. Med. Chem. 2005, 48, 4663–4669. [Google Scholar] [CrossRef]
- Jetter, M.C.; McNally, J.J.; Youngman, M.A.; McDonnell, M.E.; Dubin, A.E.; Nasser, N.; et al. N-pyridin-3-yl-and N-quinolin-3-yl-benzamides: modulators of human vanilloid receptor 1 (TRPV1). Bioorg. Med. Chem. Lett. 2008, 18, 2730–2734. [Google Scholar] [CrossRef]
- Ulven, T.; Frimurer, T.M.; Receveur, J.M.; Little, P.B.; Rist, Ø.; Nørregaard, P.K.; Högberg, T. 6-Acylamino-2-aminoquinolines as potent melanin-concentrating hormone 1 receptor antagonists. Identification, structure− activity relationship, and investigation of binding mode. J. Med. Chem. 2005, 48, 5684–5697. [Google Scholar] [CrossRef]
- Delattin, N.; Bardiot, D.; Marchand, A.; Chaltin, P.; De Brucker, K.; Cammue, B.P.; Thevissen, K. Identification of fungicidal 2,6-disubstituted quinolines with activity against Candida biofilms. Molecules 2012, 17, 12243–12251. [Google Scholar] [CrossRef]
- Basak, A.; Abouelhassan, Y.; Kim, Y.S.; Norwood, V.M.; Jin, S.; Huigens, R.W. 3rd Halogenated quinolines bearing polar functionality at the 2-position: identification of new antibacterial agents with enhanced activity against Staphylococcus epidermidis. Eur. J. Med. Chem. 2018, 155, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Collins, J.; Nyamwihura, R.; Ware, S.; Kaiser, M.; Ogungbe, I.V. Discovery of a quinoline-based phenyl sulfone derivative as an antitrypanosomal agent. Bioorg. Med. Chem. Lett. 2018, 28, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Nefertiti, A.S.G.; Batista, M.M.; Da Silva, P.B.; Batista, D.G.J.; Da Silva, C.F.; Peres, R.B.; et al. In vitro and in vivo studies of the trypanocidal effect of novel quinolines. Antimicrob. Agents Chemother. 2018, 62, 10.1128/aac.01936-17. [Google Scholar] [CrossRef] [PubMed]
- El-Damasy, A.K.; Seo, S.H.; Cho, N.C.; Kang, S.B.; Pae, A.N.; Kim, K.S.; Keum, G. Design, synthesis, in-vitro antiproliferative activity and kinase profile of new picolinamide based 2-amido and ureido quinoline derivatives. Eur. J. Med. Chem. 2015, 101, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.J.; Patel, A.P.; Chikhalia, K.H. Design and synthesis of some novel 7-substituted thiosemicarbazinyl-quinolines via Ullmann coupling reaction and examination of their antimicrobial activities. Res. Chem. Intermed. 2018, 44, 813–828. [Google Scholar] [CrossRef]
- Pandiri, H.; Soni, V.; Gonnade, R.G.; Punji, B. Development of (quinolinyl) amido-based pincer palladium complexes: a robust and phosphine-free catalyst system for C–H arylation of benzothiazoles. New J. Chem. 2017, 41, 3543–3554. [Google Scholar] [CrossRef]
- O'Neill, P.M.; Bray, P.G.; Hawley, S.R.; Ward, S.A.; Park, B.K. 4-Aminoquinolines—Past, present, and future; A chemical perspective. Pharmacol. Therapeut. 1998, 77, 29–58. [Google Scholar] [CrossRef]
- Solomon, V.R.; Pundir, S.; Lee, H. Examination of novel 4-aminoquinoline derivatives designed and synthesized by a hybrid pharmacophore approach to enhance their anticancer activities. Sc. Rep. 2019, 9, 6315. [Google Scholar] [CrossRef]
- Nandi, S.; Chauhan, B.; Tarannum, H.; Khede, M.K. Multi-target polypharmacology of 4-aminoquinoline compounds against malaria, tuberculosis and cancer. Curr. Top. Med. Chem. 2023, 23, 403–414. [Google Scholar] [CrossRef]
- Ravindar, L.; Hasbullah, S.A.; Rakesh, K.P.; Raheem, S.; Agustar, H.K.; Ismail, N.; Ling, L.Y.; Hassan, N. I. Exploring diverse frontiers: Advancements of bioactive 4-aminoquinoline-based molecular hybrids in targeted therapeutics and beyond. Eur. J. Med. Chem. 2023, 264, 116043. [Google Scholar] [CrossRef]
- Corio, A.; Gravier-Pelletier, C.; Busca, P. Regioselective Functionalization of Quinolines through CH Activation: A Comprehensive Review. Molecules 2021, 26, 5467. [Google Scholar] [CrossRef] [PubMed]
- Singha, K.; Habib, I.; Hossain, M. Quinoline N-Oxide: A Versatile Precursor in Organic Transformations. ChemistrySelect 2022, 7, e202203537. [Google Scholar] [CrossRef]
- Yan, G.; Borah, A.J.; Yang, M. Recent Advances in Catalytic Functionalization of N-Oxide Compounds via C-H Bond Activation. Adv. Synth. Catal. 2014, 356, 2375–2394. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Vargas Méndez, L.Y.; Puerto Galvis, C.E.; Ortiz Villamizar, M.C. The direct C–H alkenylation of quinoline N-oxides as a suitable strategy for the synthesis of promising antiparasitic drugs. New J. Chem. 2020, 44, 12–19. [Google Scholar] [CrossRef]
- Dong, D.; Sun, Y.; Li, G.; Yang, H.; Wang, Z.; Xu, X. Recent Progress in the Functionalization of Quinoline N-Oxide. Chin. J. Org. Chem. 2020, 40, 4071–4086. [Google Scholar] [CrossRef]
- Wang, D.; Désaubry, L.; Li, G.; Huang, M.; Zheng, S. Recent Advances in the Synthesis of C2-Functionalized Pyridines and Quinolines Using N-Oxide Chemistry. Adv. Synth. Catal. 2021, 363, 2–39. [Google Scholar] [CrossRef]
- Couturier, M.; Caron, L.; Tumidajski, S.; Jones, K.; White, T.D. Mild and direct conversion of quinoline N-oxides to 2-amidoquinolines with primary amides. Org. Lett. 2006, 8, 1929–1932. [Google Scholar] [CrossRef]
- Medley, J.W.; Movassaghi, M. Direct dehydrative N-pyridinylation of amides. J. Org. Chem. 2009, 74, 1341–1344. [Google Scholar] [CrossRef]
- Chen, X.; Peng, M.; Huang, H.; Zheng, Y.; Tao, X.; He, C.; Xiao, Y. TsOH· H2O-mediated N-amidation of quinoline N-oxides: facile and regioselective synthesis of N-(quinolin-2-yl) amides. Org. Biomol. Chem. 2018, 16, 6202–6205. [Google Scholar] [CrossRef]
- Xie, L.Y.; Peng, S.; Liu, F.; Yi, J.Y.; Wang, M.; Tang, Z.; Xu, X.; He, W.M. Metal-free deoxygenative 2-amidation of quinoline N-oxides with nitriles via a radical activation pathway. Adv. Synth. Catal. 2018, 360, 4259–4264. [Google Scholar] [CrossRef]
- Xie, L.Y.; Peng, S.; Lu, L.H.; Hu, J.; Bao, W.H.; Zeng, F.; Tang, Z.; He, W.M. Brønsted acidic ionic Liquid-Promoted amidation of quinoline N-Oxides with nitriles. ACS Sustain. Chem. Engin. 2018, 6, 7989–7994. [Google Scholar] [CrossRef]
- Josephitis, C.M.; Nguyen, H.M.; McNally, A. Late-Stage C–H Functionalization of Azines. Chem. Rev. 2023, 123, 7655–7691. [Google Scholar] [CrossRef]
- Rojas Ruiz, F.A.; Kouznetsov, V.V. Property-based Design and Synthesis of New Chloroquine Hybrids via Simple Incorporation of 2-Imino-thiazolidin-4-one or 1H-Pyrrol-2,5-dione Fragments on the 4-Amino-7-chloroquinolines Side Chain. J. Braz. Chem. Soc. 2011, 22, 1774–1781. [Google Scholar] [CrossRef]
- Fonseca-Berzal, C.; Rojas Ruiz, F.A.; Escario, J.A.; Kouznetsov, V.V.; Gómez-Barrio, A. In vitro phenotypic screening of 7-chloro-4-amino(oxy)quinoline derivatives as putative anti-Trypanosoma cruzi agents. Bioorg. Med. Chem. Lett. 2014, 24, 1209–1213. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Sojo, F.; Rojas Ruiz, F.A.; Merchán-Arenas, D.R.; Arvelo, F. Synthesis and cytotoxic evaluation of 7-chloro-4-phenoxyquinolines with formyl, oxime and thiosemicarbazone scaffolds. Med. Chem. Res. 2016, 25, 2718–2727. [Google Scholar] [CrossRef]
- Luna-Parada, L.K.; Kouznetsov, V.V. 5-Chloro-8-{[1-(2-chlorobenzyl)-1H-1,2,3-triazol-4-yl] methoxy}quinoline. Molbank 2019, 2019, M1038. [Google Scholar] [CrossRef]
- Rosado-Solano, D.N.; Barón-Rodríguez, M.A.; Sanabria-Florez, P.L.; Luna-Parada, L.K.; Puerto-Galvis, C.E.; Zorro-González, A.F.; Kouznetsov, V.V.; Vargas-Méndez, L.Y. Synthesis, Biological Evaluation and in silico Computational Studies of 7-Chloro-4-(1H-1,2,3-triazol-1-yl)quinoline Derivatives. Search for new controlling agents against Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. J. Agric. Food Chem. 2019, 67, 9210–9219. [Google Scholar] [CrossRef]
- Rodríguez Enciso, D.A.; Puerto Galvis, C.E.; Kouznetsov, V.V. Microwave-assisted synthesis of pharmacologically active 4-phenoxyquinolines and their benzazole-quinoline hybrids through SNAr reaction of 4,7-dichloroquinoline and phenols using [bmim][PF6] as a green solvent. Curr. Org. Synth. 2023, 20, 546–559. [Google Scholar]
- Elslager, E.F.; Gold, E.H.; Tendick, F.H.; Werbel, L.M.; Worth, D.F. Amodiaquine N-oxides and other 7-chloro-4-aminoquinoline N-oxides. J. Heterocyclic Chem. 1964, 1, 6–12. [Google Scholar] [CrossRef]
- Solomon, V.R.; Haq, W.; Srivastava, K.; Puri, S.K.; Katti, S.B. Design and synthesis of 3-[(7-chloro-1-oxidoquinolin-4-ylamino) alkyl]-1,3-thiazolidin-4-ones as antimalarial agents. J. Enzyme Inhib. Med. Chem. 2013, 28, 1048–1053. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, F.; Deng, G.J.; Gong, H. Amination of aromatic halides and exploration of the reactivity sequence of aromatic halides. J. Org. Chem. 2018, 84, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Vamos, M.; Cosford, N.D. 2-Aminopyridines via reaction of pyridine N-oxides and activated isocyanides. J. Org. Chem. 2014, 79, 2274–2280. [Google Scholar] [CrossRef] [PubMed]
- Ryzhakov, A.V.; Rodina, L.L. Aromatic N-oxides as 1,3-dipoles and π-donors in reactions with unsaturated compounds. Review. Chem. Heterocycl. Compd. 1992, 28, 483–493. [Google Scholar] [CrossRef]
- Swiss Institute of Bioinformatics. Available online: http://www.swissadme.ch/ (accessed on 26 February 2024).
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Ortiz Villamizar, M.C.; Puerto Galvis, C.E.; Pedraza Rodríguez, S.A.; Zubkov, F.I.; Kouznetsov, V.V. Synthesis, In Silico and In Vivo Toxicity Assessment of Functionalized Pyridophenanthridinones via Sequential MW-Assisted Intramolecular Friedel-Crafts Alkylation and Direct C–H Arylation. Molecules 2022, 27, 8112. [Google Scholar] [CrossRef]
- Swiss Institute of Bioinformatics. Available online: http://www.swisstargetprediction.ch/ (accessed on 26 February 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




