1. Introduction
Long QT syndrome (LQTS) is characterized by a disruption in the repolarization of the ventricular myocardium, which is evident in the elongation of the electrocardiographic QT interval. This condition primarily results from mutations related to myocyte ion channels, presenting as a monogenic disorder with an autosomal-dominant inheritance pattern [
1,
2]. Ventricular arrhythmias in LQTS can result in syncope, seizures, or sudden cardiac death.
In the management of LQTS, β-adrenergic blocking agents, left cardiac sympathetic denervation, and implantable cardioverter-defibrillator (ICD) placement are commonly utilized. In cases of documented cardiac arrest, the general consensus is to promptly implant an ICD, regardless of any ongoing treatments [
3]. ICD placement in children with inherited arrhythmia syndromes is intended to prolong life, but the ICD implantation procedure carries risks, including potential infection, malfunction, pocket complications, hematoma, and pneumothorax [
4]. Children have smaller thoracic cavities than adults, along with thinner soft tissue in the chest wall. Therefore, when children receive large thoracic implants, they are at increased risk of implant exposure. Olde Nordkamp et al. [
4] found that 22% of 4916 pediatric patients experienced ICD-related complications, with 1.6% requiring reintervention because of pocket complications.
Since the report of the initial study on breast reconstruction using acellular dermal matrix (ADM) as an implant was published in 2001, numerous subsequent reports have suggested that ADM can help reduce the risk of capsular contracture and maintain the shape of the reconstructed breast [
5]. Additionally, a case series reported by Baxter in 2003 indicated that the use of ADM in breast surgery can augment atrophied tissue and reinforce the capsule [
6]. As of November 24, 2023, no study found on PubMed or Google Scholar using the terms “implantable cardioverter defibrillator,” “skin erosion,” and “acellular dermal matrix” (without any restrictions on date or language) described the strengthening of subcutaneous sacs following ICD placement in children.
In this report, we present the case of an 8-year-old boy diagnosed with LQTS. The boy experienced skin erosion over his ICD site. This issue was successfully resolved by reinserting the ICD using ADM.
2. Case Presentation
As part of LQTS (type 8, CACNA1C gene mutation) management by our institution’s cardiology department, an 8-year-old boy had an ICD implanted in a subcutaneous pocket of his left anterolateral chest wall (
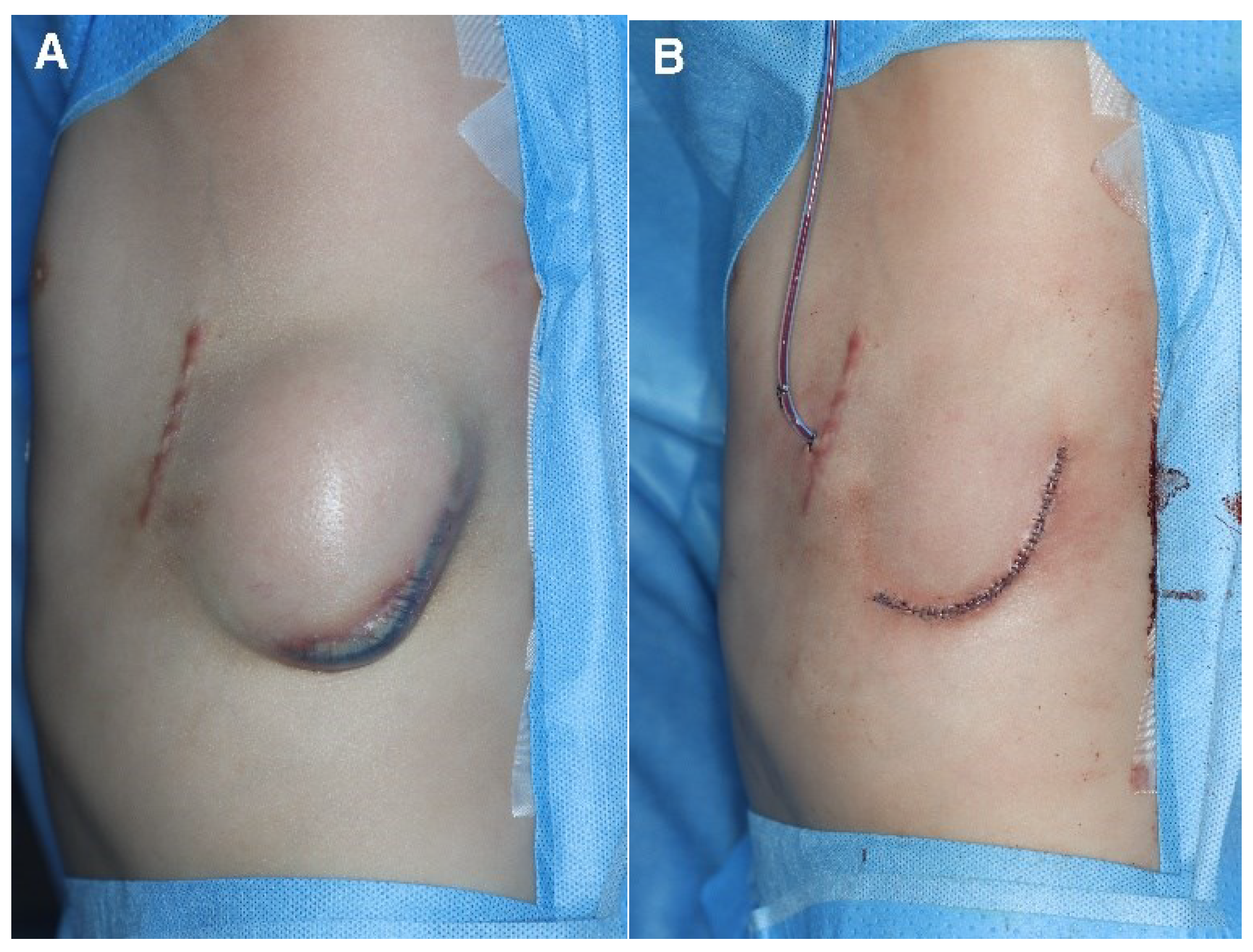
Figure 1). The cardiology department later requested an ICD replacement based on concerns about the risk of ICD exposure caused by thinning of the distal and lateral aspects of the ICD pocket. A rupture of the pocket and subsequent exposure to the external environment would significantly increase the risk of infection; therefore, the ICD was removed and the pocket was primarily closed (
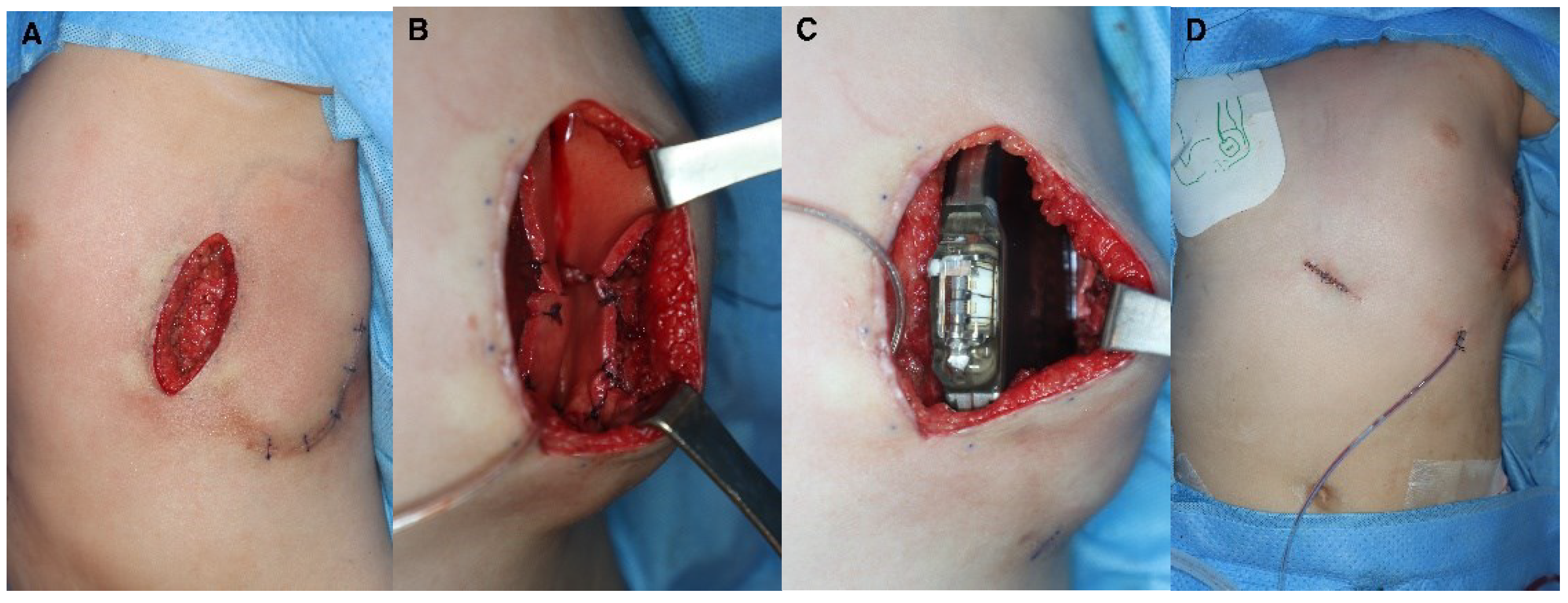
Figure 2). The scar from the initial ICD insertion was preserved, while debridement was performed on the area where the skin had thinned due to the descending ICD. This facilitated ICD reinsertion. Two weeks later, with the absence of inflammation confirmed, a new pocket was created in the suprafascial layer at the same location as the original implantation. ADM was attached to the inner wall of the pocket to prevent ICD protrusion, and a new ICD was inserted (
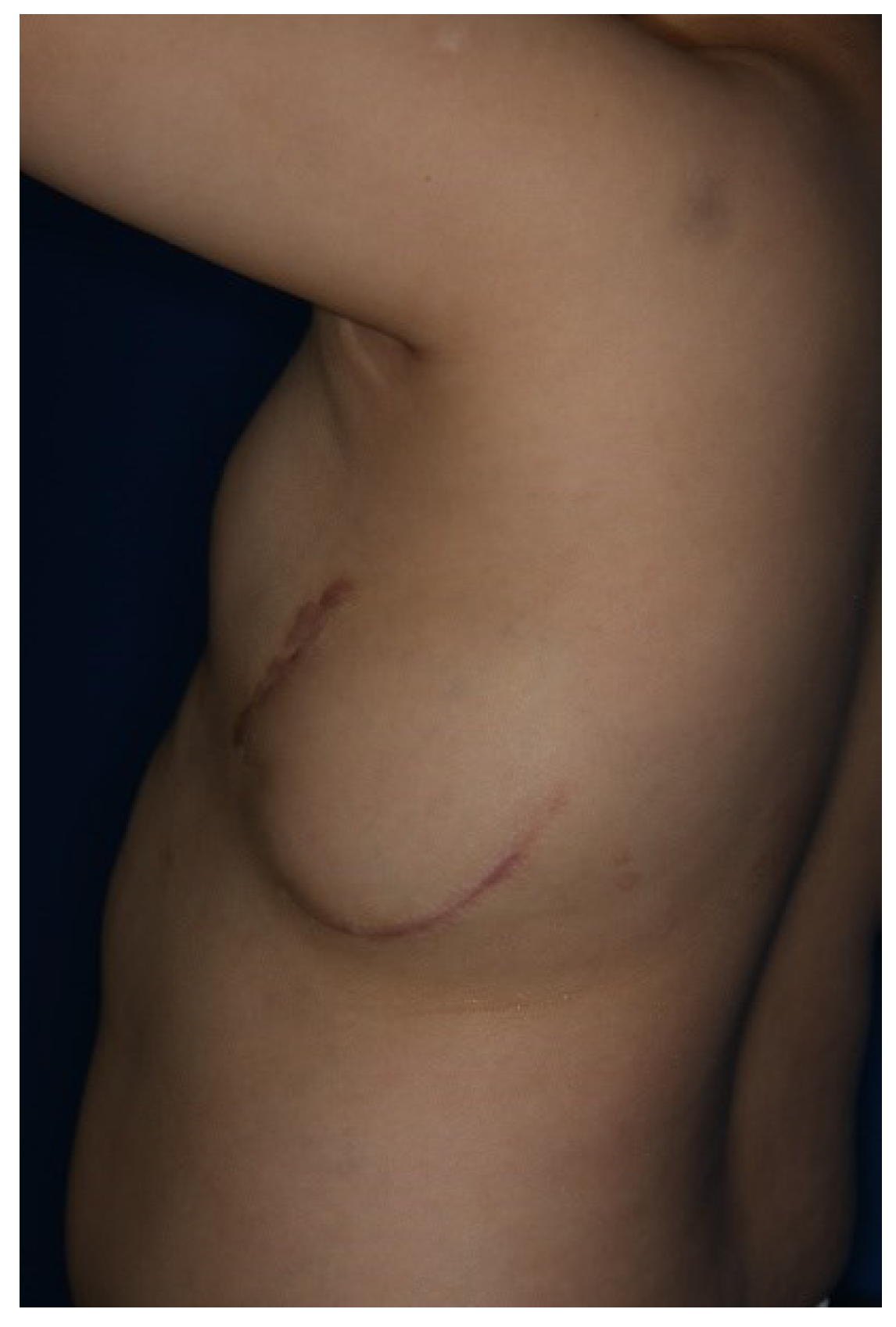
Figure 3). The ICD (EMBLEM A219, Boston Scientific Corp., Marlborough, MA) measures 83.1 × 69.1 × 12.7 mm, weighs 130 g, and has a volume of 59.5 cm³. The ADM (AlloDerm RTU, Allergan Inc., Madison, NJ) used for this procedure measured 20 × 10 × 0.24 cm. Postoperatively, it was necessary for the patient to consistently wear specialized supportive clothing to counteract gravitational forces and prevent skin erosion. One year after the operation, the ADM had successfully engrafted, and no complications had arisen, such as seroma, inflammation, or pocket rupture were observed (
Figure 4).
3. Discussion
The recommended position for the pulse generator of an ICD’s subcutaneous pocket is between the anterior axillary line of the fifth intercostal space and the midaxillary line. The subcutaneous lead is situated parallel to the sternum on the left side and is anchored at the xiphoid process level. The subcutaneous ICD employs a modified surface electrocardiogram, creating three vectors between the two sensing electrodes and the pulse generator to detect ventricular arrhythmias. When a ventricular arrhythmia is detected, the device charges 80 J of energy and delivers a biphasic waveform defibrillating shock [
7]. In this case, a patient who had previously been successfully resuscitated from sudden cardiac arrest was diagnosed with LQTS. In accordance with the prescribed treatment algorithm, a subcutaneous ICD was successfully inserted.
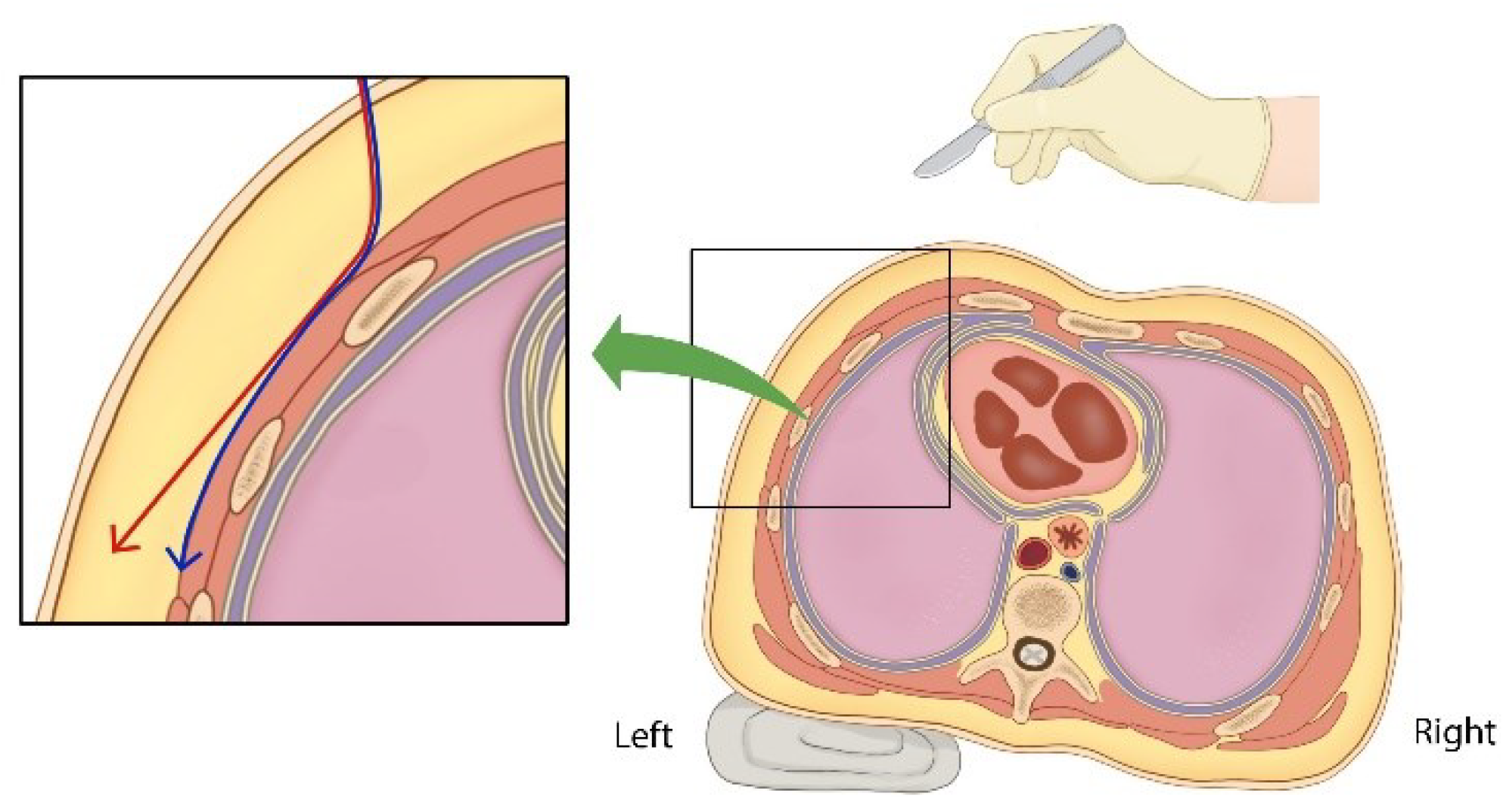
However, there are several precautions to consider when inserting a subcutaneous ICD in thin people. When making an incision in the anterior chest and performing subcutaneous dissection laterally at the suprafascial level, it is important to note that there is a risk of thinning the pocket’s deepest subcutaneous layer (
Figure 5). In our case, when skin erosion occurred after the initial ICD insertion, the subcutaneous pocket was thinnest at its most inferior aspect owing to the influence of gravity. Inadequate dissection also resulted in thinning of the tissue in the superficial portion of the pocket’s deepest area (
Figure 2). Children’s thoraxes have smaller anteroposterior-to-transverse diameter ratios and are rounder than adult thoraxes [
8]. Therefore, caution is required when performing pocket dissection for ICD insertion, as the angle of dissection gradually steepens laterally. When operating on thin people, especially children, collaborating with plastic surgeons proficient in handling soft tissues could lower the complication rates.
Furthermore, research using ultrasound and X-ray imaging has demonstrated that the thickness of the skin and subcutaneous fat in the chest wall increases with age. Children, therefore, typically have thin layers of skin and fat [
9,
10]. Patients who lack sufficient subcutaneous tissue coverage for ICD devices may be at a higher risk of experiencing pocket-related complications, such as infection and skin erosion [
11]. There are various surgical techniques available to reinforce soft tissue structures, including fat grafts and dermofat grafts. However, given the potential for donor site morbidity among children (who are still growing), using autologous tissues may not be the optimal choice [
12]. In our case, when considering the ICD reimplantation, we contemplated dissecting the serratus anterior or latissimus dorsi muscles to transition the subcutaneous pocket to an intramuscular level. However, this was not a viable option for effective chest wall reinforcement because of the thinness of pediatric muscles. Furthermore, selecting an intramuscular pocket was not considered as a solution because continuous pressure from the ICD directly above the bone could lead to deformation of the bone. For thin people, even adults, this may not be an effective option for the same reasons.
ADM and synthetic mesh are frequently used to reinforce soft tissue structures [
13]. ADM is a biological graft material obtained from decellularized human cadaveric tissue or animal dermis. The process of cellular component removal minimizes the risk of eliciting an immune response in the recipient. However, ADM retains the structural and functional properties of the dermis, including the basement membrane, cellular matrix, and collagen fibers. As a biological scaffold, ADM encourages angiogenesis and speeds up tissue ingrowth and cellular repopulation, leading to tissue regeneration [
14,
15]. Furthermore, ADM acts as a blueprint for the formation of neodermis tissue, offering an advantage in minimizing scar contracture postoperatively [
16]. Despite the high costs associated with ADM, particularly considering the limited insurance coverage for its use in conjunction with ICD implantation, this case report is significant because it marks the first documented instance of ADM being used to reinforce the pocket for ICD insertion. This highlights the potential role of ADM in addressing skin erosion resulting from the ICD implantation process.
To enhance the successful maintenance of an ICD, several external factors must be considered: first, designing the ICD device to be thinner, with a reduced surface area and lighter weight, would likely decrease the likelihood of skin erosion. Second, crafting the ICD to have an appropriate concave shape (rather than flat) to conform to the chest wall’s curvatures, could reduce the chance of the device’s ends protruding from the pocket and causing skin thinning. Third, when creating a subcutaneous pocket on the left side of the patient’s chest, a task typically performed by a right-handed surgeon from the patient’s right side, tilting the patient approximately 30 degrees to the right during surgery may allow for dissection at the suprafascial level. This positioning helps the surgeon maintain a consistent skin envelope thickness and provides adequate visibility when dissecting areas distant from the pocket opening (
Figure 5). These measures can prevent incorrect dissection angles and ensure sufficient visibility for the surgeon when dissecting areas far from the pocket opening.
4. Conclusions
When inserting a subcutaneous ICD in thin people, reinforcing the subcutaneous pocket with ADM may help minimize complications, such as skin erosion.
Author Contributions
Conceptualization, K.S.K..; Data curation: J.H.C. and H.J.L.; Formal analysis: H.W.P, I.C. and J.H.H.; Investigation: J.H.C. and H.J.L.; Methodology: H.W.P., I.C. and S.Y.L.; Project administration: K.S.K.; Writing - original draft: J.H.C., H.J.L, K.S.K., H.W.P., I.C., J.H.H. and S.Y.L.; Writing - review & editing: K.S.K.
Funding
The authors received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Chonnam National University Hospital (IRB No., CNUH-EXP-2023-333) and conducted in accordance with the principles of the Declaration of Helsinki.
Informed Consent Statement
Written informed consent for publication of the clinical details and images was obtained from the patient.
Data Availability Statement
All data created or analyzed in this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ADM = acellular dermal matrix, ICD = implantable cardioverter-defibrillator, LQTS = long QT syndrome.
References
- Goldenberg, I.; Zareba, W.; Moss, A.J. Long QT Syndrome. Curr. Probl. Cardiol. 2008, 33, 629–694. [Google Scholar] [CrossRef] [PubMed]
- Wilde, A.A.M.; Amin, A.S.; Postema, P.G. Diagnosis, management and therapeutic strategies for congenital long QT syndrome. Heart 2022, 108, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Ackerman, M.J. The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. Eur. Heart J. 2013, 34, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Olde Nordkamp, L.R.; Postema, P.G.; Knops, R.E.; van Dijk, N.; Limpens, J.; Wilde, A.A.M.; de Groot, J.R. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: a systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm 2016, 13, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Macadam, S.A.; Lennox, P.A. Acellular dermal matrices: economic considerations in reconstructive and aesthetic breast surgery. Clin. Plast. Surg. 2012, 39, 187–216. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.A. Intracapsular allogenic dermal grafts for breast implant-related problems. Plast. Reconstr. Surg. 2003, 112, 1692–1696; discussion 1697–1698. [Google Scholar] [CrossRef] [PubMed]
- Bordachar, P.; Marquié, C.; Pospiech, T.; Pasquié, J.L.; Jalal, Z.; Haissaguerre, M.; Thambo, J.B. Subcutaneous implantable cardioverter defibrillators in children, young adults and patients with congenital heart disease. Int. J. Cardiol. 2016, 203, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Casal-Beloy, I.; Míguez Fortes, L.; Gómez Tellado, M.A.; Gonzalez-Rivas, D. Thinking uniportal in pediatric thoracic surgery. Pediatr. Med. 2019, 13, 1–7. [Google Scholar] [CrossRef]
- Tafeit, E.; Möller, R.; Jurimae, T.; Sudi, K.; Wallner, S.J. Subcutaneous adipose tissue topography (SAT-Top) development in children and young adults. Coll. Antropol. 2007, 31, 395–402. [Google Scholar]
- Seidenari, S.; Giusti, G.; Bertoni, L.; Magnoni, C.; Pellacani, G. Thickness and echogenicity of the skin in children as assessed by 20-MHz ultrasound. Dermatology 2000, 201, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.; Giofrè, F.; De Filippo, P. Intermuscular pocket for subcutaneous implantable cardioverter defibrillator: Single-center experience. J. Arrhythm. 2016, 32, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Kim, S.H.; Nam, S.B.; Lee, J.W.; Jeong, D.K.; Kim, Y.H. Reconstruction of temporal hollowing deformities using silicone implants made using a toy-clay model: a report of three cases. Arch. Craniofac. Surg. 2022, 23, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.; O’Donnell, J.P.; Ryan, É.J.; O’Neill, B.L.; Boland, M.R.; Lowery, A.J.; Kerin, M.J.; McInerney, N.M. Immediate breast cancer reconstruction with or without dermal matrix or synthetic mesh support: a review and network meta-analysis. Plast. Reconstr. Surg. 2023, 151, 563e–574e. [Google Scholar] [CrossRef] [PubMed]
- Gravina, P.R.; Pettit, R.W.; Davis, M.J.; Winocour, S.J.; Selber, J.C. Evidence for the use of acellular dermal matrix in implant-based breast reconstruction. Semin. Plast. Surg. 2019, 33, 229–235. [Google Scholar] [CrossRef]
- Macadam, S.A.; Lennox, P.A. Acellular dermal matrices: use in reconstructive and aesthetic breast surgery. Can. J. Plast. Surg. 2012, 20, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Ki, S.H.; Park, T.J.; Yoon, J.M. Surgical outcomes of suprafascial and subfascial radial forearm free flaps in head and neck reconstruction. Arch. Craniofac. Surg. 2023, 24, 105–110. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).