1. Introduction
Extracellular Vesicles (EVs) are key in intercellular communication, facilitating the exchange of biological information among cells. Their intrinsic ability to transfer proteins, lipids and nucleic acids in small fragments between cells, influences several physiological and pathological functions inside of the body. In 2015, the European Cooperation in Science and Technology (COST) action initiative “
Microvesicles and Exosomes in Disease and Health” (ME-HaD) confirmed that the current literature and knowledge about EVs is still poor and need to be improved [
1]. In our research, we are dedicated to utilizing EVs to selectively target pathological cells while preserving healthy ones, thereby modulating the intercellular communication between them. Numerous studies on the composition and subtypes of EVs have culminated in the creation of public online databases such as EVpedia, Vesiclepedia, and Exocarta. These resources are instrumental in classifying different EVs, facilitating a deeper understanding of their potential applications for various therapeutic needs [
2,
3,
4].
Between the major EVs’ subtypes such as microvesicles/ectosomes and apoptotic bodies, exosomes have gained particular importance for their unique properties, clinical and therapeutical applications. Exosomes, are small vesicles (30-150nm) that originate from the endosomal pathway and are released into the extracellular space upon the fusion of multivesicular bodies with the plasma membrane. By engineering exosomes, research is searching valuable therapeutic options to control many diseases, aid in disease diagnosis or direct the delivery of a specific molecule to a desired target. For example, Ebrahimian et al. [
5] showed how the local delivery of
Thymoquinone–loaded mesenchymal stem cell–derived (MSCs) exosomes is an efficient delivery system to bring the chemotherapy inside of the breast cancer core. In another study, Shojaei et al. [
6] showed how
miR–218–enriched MSCs exosomes reduce the epithelial–to–mesenchymal transition (EMT) and angiogenesis which are both key in the most malignant breast cancer, the Triple–Negative Breast Cancer (TNBC).
TNBC represents the most aggressive and challenging breast cancer subtype worldwide. Growth and local invasion of the tumor core occurs at the expense of the surrounding tissues in a well-coordinated interplay [
7]. The tumor microenvironment (TME) is key in cancer progression and represents a fundamental target for new therapeutical systems, particularly for TNBC therapy. While surgery, chemotherapy, and radiotherapy remain the cornerstone treatments, research is actively pursuing novel therapeutic options in order to target the tumor and its tumor microenvironment (TME) while simultaneously preserving the integrity of the surrounding healthy tissues. The possibility to impact cancer at the core of the tumor, at its TME and surrounding tissues, including metastasis, is challenging.
In this review, the authors delve into the world of extracellular vesicles exploring, in particular, the biological properties and potential applications of Adipose-derived Stem Cells Exosomes (ASCs Exos). Upon detailing the biological attributes and functions of ASCs exos, we succinctly underscore their existing applications across various clinical scenarios, as well as their prospective efficacy against the most aggressive form of breast cancer TNBC. Finally, we highlight the development of a novel Triple ASCs–Exos [T.A.E.] Technology able to act as therapeutical trifecta towards the TNBC at three different levels: the tumor core, its local microenvironment and the surrounding tissues.
2. Extracellular Vesicles, Exosomes and Clinical Applications of Ascs Exosomes
2.1. Extracellular Vesicles: Composition and Subtypes
EVs are membrane–contained vesicles comprising different subtypes based on their content, size (from 10μm to 30nm) and membrane composition (e.g. cytoskeletal-, cytosolic-, heat shock– and surface proteins). In addition to the traditional juxtacrine, autocrine and paracrine communication, EVs influence and communicate with the surrounding environment by transferring proteins, lipids and nucleic acids to the other cells
1. The characterization of EVs is largely determined by their cytoplasmic content and membrane composition. However, the markers of EVs are shared with a multitude of vesicle types and there is no unique surface protein that can definitively distinguish EVs, pinpoint their cellular origin, or identify their distinctive characteristics [
8,
9,
10].
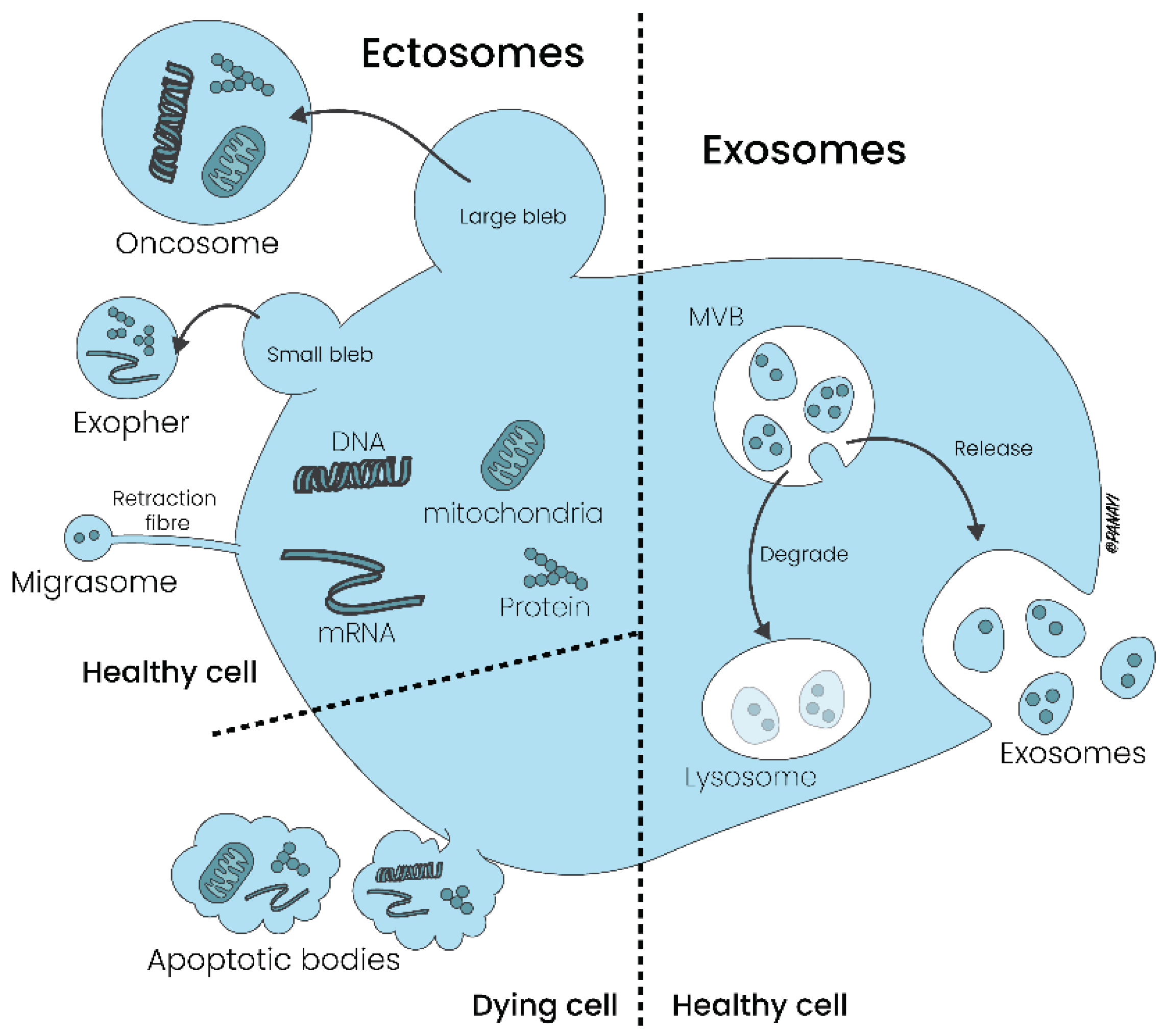
The main EVs subgroups are identified because of their size and are represented by large Oncosomes (1-10μm) and Exophers (3500-4000nm), Apoptotic bodies (1000-5000nm) and Migrasomes (500-3000nm), Cellular Microvesicles/Ectosomes (100-1000nm), Extracellular Particles (30-50nm) and intraluminal vesicles named as Exosomes (30-150nm) [
Table 1].
Whereas microvesicles are formed by outward budding of the plasma membrane, apoptotic bodies are released during programmed cell death, contain organelles and nuclear fragments and are involved in the clearance of dying cells by phagocytosis. Due to these characteristics, these vesicles are also named as Ectosomes. In contrast, exosomes are derived from the endosomal pathway and are released into the extracellular milieu following the fusion of multivesicular bodies (MVBs) with the plasma membrane [
Figure 1].
There are several isolation and characterization methods such as ultracentrifugation, filtration, size–exclusion– or affinity–chromatography, reactive ligands or antibody technology that aim to differentiate EVs’ subgroups. The paucity of exclusive markers for EVs currently complicates the definitive distinction of the aforementioned subpopulations [
1]. The current main proteins which mark EVs’ subpopulations include tetraspanins (CD9, CD63, CD81 and CD82), major histocompatibility complex (MHC), 14–3–3 proteins, Tumor susceptibility gene 101 (Tsg101), heat–shock proteins and the Endosomal Sorting Complex Required for Transport 3 (ESCRT–3) [
11,
12,
13]. Protein glycosylation patterns, such as N-glycans and N-acetyl lactosamine, along with lectin-binding profiles, for instance, galectin-3 binding, play significant roles in cellular sorting and homeostasis. However, these markers are not currently sufficient for identifying specific subgroups EVs.
EVs can rearrange the distribution of Tetraspanins, ESCRT complexes and the BAR (Bin/Amphiphysin/Rvs) domain–containing proteins, thus minimizing the cellular surface free energy, alter their structural curvature and promote their release via cellular “budding” [
14,
15,
16]. However, due to the small differences even in this last characteristic, the discrimination between different EVs’ subgroups during their release remains challenging.
2.2. Exosomes: Composition, Biological Properties and ASCs Exosomes
As shown in
Table 1, EVs can be classified into two main categories: large EVs, which are released through outward budding of the plasma membrane, and small EVs, with endosomal origin. The exosomal surface pattern composed by the Ras–related GTP binding protein B, tetraspanins, the Soluble N–ethylamide–sensitive factor Activating protein Receptor (SNARE)–Complex proteins, Sytenin–1, Tsg101, the Apoptosis–Linked gene 2–Interacting protein X (ALIX), Syndecan–1, and ESCRT are correlated to their biogenesis and functional impact [
17,
18]. On the other side, even the recipient cells influence exosomes’ content in a well-coordinated interplay. After being released, exosomes transport their bioactive content to the other cells through ligand/receptor interactions, membrane fusion and cargo internalization [
11,
19]. Current research efforts are aimed at engineering exosomes to manage a variety of diseases, enhance diagnostic capabilities, and ensure targeted delivery to specific sites within the body.
Exosomes can be isolated from several tissues and biofluids including blood serum, plasma, saliva, urine, breast milk, amniotic and cerebrospinal fluid [
20]. Among the other tissues, the Adipose Tissue (AT) is a valuable source of high–quality exosomes, particularly those present inside the Stromal Vascular Fraction (SVF) [
21]. In addition to immune cells, erythrocytes and endothelial cells, the SVF contains a particular subset of mesenchymal stem cells: the Adipose–derived Stem Cells (ASCs) [
22,
23]. Their particular mesenchymal–like morphology, the expression profile of CD34
+, CD44
+, CD31
- and CD45
- and their ability to produce osteoblasts, chondrocytes, myocytes, epithelial and neuronal cells allows ASCs to act as chemoattractant, angiogenic and prosurvival molecules [
24]. The presence of ASCs gives to the AT immunomodulatory, wound healing and regeneration properties [
25,
26]. All these functions are supported by additional mediators that live within the SVF and are released by ASCs paracrine named as ASCs Exosomes. Even if both ASCs and ASCs Exos co-exist within the SVF, they perform their functions via different mechanisms. In contrast to ASCs, ASCs Exos orchestrate tissue regeneration, immunological functions, tissue and organ homeostasis avoiding cell therapy–associated problems such as cell survival, unfavorable differentiation, senescence–induced instability and immune rejection [
21].
Because of these intrinsic properties and the possibility to collect them in a very simple way, for example through a lipoaspiration session, ASCs Exos are gaining particular attention. By inducing cell migration and proliferation, ASCs Exos enhance tissue regeneration impacting skin–aging, wound healing, scar quality, flap vitality and fat grafting [
21,
27,
28]. Because of their immunomodulatory features, ASCs Exos have potential anti–inflammatory properties against osteoarthritis [
29]. In another study, Fang et al. treated CCl4–induced acute liver injury with ASCs exosomes loaded with vitamin A and quercetin [
30]. In another paper, the group of Qian et al. demonstrated how hypoxic ASCs exosomes can attenuate colitis by regulating macrophage polarization [
31]. Even in the neurological field, ASCs exosomes showed promising in–vitro results by ameliorating phenotype of Huntington’s disease [
32]. Despite the promising therapeutic and clinical applications of ASCs-derived exos, there is a significant need for more clinical trials to establish their efficacy and, crucially, their safety for clinical use.
3. Breast Cancer as Triple Entity: Classification System and General Therapeutical Options
According to WHO Data and Globocan 2020, breast cancer represents the most commonly diagnosed cancer worldwide with an estimated 2.3 million new cases in 2020 [
33]. Breast cancer has evolved in our understanding from a solitary oncological mass to a dynamic constellation involving a central core, a responsive local microenvironment, and the interplay with adjacent healthy tissues. The TME, once relegated to a non-active role, is now recognized as a critical influencer within the oncological landscape, facilitating tumor genesis and promoting the progression of cancer [
7]. Exactly as cancer invasion is dictated by mutual interaction between the core, its microenvironment and the surrounding tissues, the most innovative therapeutic strategies should act on these three entities.
The interplay between a cancerous mass and its local TME is orchestrated through a symphony of direct cell–to–cell interactions, paracrine signaling, and the exchange of vesicles. Among the myriad of signaling entities, tumor–derived exosomes are prominent players in this intricate dialogue, exerting a profound influence on the processes of cancer invasion, metastasis, and the development of chemoresistance [
34]. The possibility of taking advantage from the pro–oncogenic role of tumor exosomes and develop an ASCs exosome–based therapy against breast cancer is real. However, novel ideas and technologies have to be tested firstly in–vitro and then in–vivo.
3.1. Breast Cancer Classification Systems: Tumor Size, Local Receptors and Immunohistochemistry
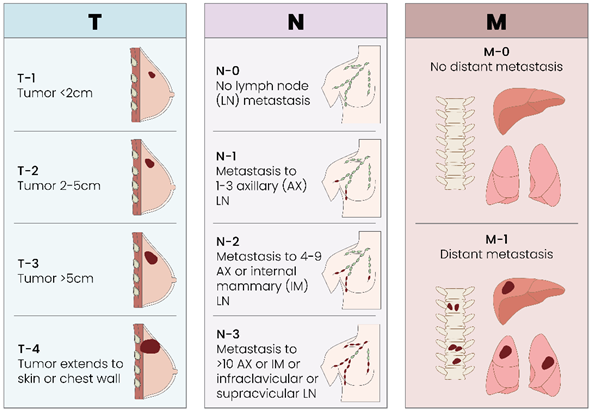
As with all solid tumors, the traditional TMN classification for breast cancer is current, valid and effective for staging a tumor [
Table 2].
From the perspective of anatomical pathology, breast cancer is categorized according to its histological type, immunohistochemical characteristics, or receptor profile. The predominant histological types are ductal and lobular carcinoma. These are further classified as either in situ or invasive, contingent upon whether the tumor breaches the basement membrane. Among the less common varieties are mucinous carcinoma, primary breast lymphoma, neuroendocrine, medullary, and inflammatory carcinoma [
35]. From the immunohistochemical side, the Luminal–A like tumor (
ER+, PR+, Her–2 –, Ki67<20%) has estrogen and progesterone receptors, has no Her2–Neu receptors and a growth rate (Ki67) of less than 20%. Luminal-B like tumors (
ER+, PR+, Her-2+, Ki67>20%) also have a high percentage of estrogen and progesterone receptors but with the Her2-Neu receptor on their surface and a growth rate (Ki67) that exceeds 20%. A third category is represented by the Her2 positive tumors (
ER–, PR–, Her-2+, Ki67>20%) with a very low percentage of estrogen and progesterone receptors, a large quantity of Her2–Neu receptors and a growth speed (Ki67) that is usually high. Finally, the TNBC (
ER–, PR–, Her-2–, Ki67>20%) does not possess receptors for estrogen, progesterone and Her2–Neu and also has a usually high growth rate (Ki67) [
Figure 2].
Despite receiving standard frontline therapy, TNBC often exhibits recurrence or metastasis within the first 3 years post-surgery [
36]. In addition, Goh et al. [
37] recently reported that the 5–year survival rate for TNBC patients is 77% compared to 93% for other breast cancer subtypes. Despite it was traditionally considered a “basal–like” breast cancer, the TNBC is currently divided into 6 different subtypes: (a; b) basal–like 1 and 2, (c) immunomodulatory, (d) luminal androgen receptor, (e; f) mesenchymal and mesenchymal–stem–like. All these categories present different response to standard chemotherapy [
38].
3.2. Basic Knowledge and Novel Therapeutical Options for Breast Cancer Therapy
As we widely highlighted by Brett et al., traditional surgery, chemotherapy and radiotherapy represent the main traditional options for breast cancer. The therapeutical strategy is based on the severity, the clinical condition of the patient and his response to treatment. Whereas surgery is indicated for breast tumors in stages I, II or III, radiotherapy is perfomed for the higher stages and shrinks the tumor core and the surrounding tissues in a non-specific way [
7].
The standard chemotherapy regimen for breast cancer includes Anthracyclines (Doxorubicin, Epirubicin) and Taxanes (Docetaxel and Paclitaxel). Tamoxifen, a Selective Estrogen Receptor Modulator (SERM), blocks estrogen effects in estrogen–sensitive women, contributing to the reduction of tumor development. Conversely, postmenopausal women may benefit from the androgen and anabolic steroid Fluoxymesterone, as well as the aromatase inhibitors Exemestane, Anastrozole, and Letrozole. Other valuable options are represented by the monoclonal antibodies Trastuzumab, Lapatinib and Bevacizumab, most of the time in combination with Paclitaxel [
7]. In addition to these consolidated therapeutical options, blocking or knocking down the CCL5/CCR5 Axis is also a potential way to control breast cancer progression and invasion, even metastasis formation. The possibility of targeting the CCL5/CCR5 axis and induce an antitumor environment is real and will be discussed in the last section of this paper. However, despite these innovative tools, prognosis is strongly influenced by the clinical stage at which the cancer is diagnosed.
4. Tnbc and Tumor Exosomes: Mutual Friends or Potential Enemies?
4.1. TNBC and Tumor Exosomes: Mutual Friends…?
Exosomes allow the communication between tumor cells and the TME supporting proliferation, EMT transition, immunological switch, neo–angiogenesis and metastasis. The connection between the tumor core and tumor-derived exosomes has long been a subject of debate. Initially, it appeared that exosomes primarily fostered tumor growth. However, recent research has unveiled a transformation in the role of exosomes, as they can shift from being allies to becoming formidable adversaries of the tumor mass. Even Goh et al. [
37] widely discussed this topic, wondering if exosomes were humble “garbage disposal” or brave “Trojan horses” in TNBC.
First of all, breast cancer patients present a higher concentration of exosomes in their plasma compared to healthy age controls. The oncological upregulation of the p–53 gene product “TSAP 6” in breast cancer women, for example, enhances exosomes concentrations in their blood plasma [
39,
40,
41]. The senescence-associated secretory phenotype (SASP), known for conferring specific chemoresistance to certain cancer cells, is intricately linked to an increased intracellular concentration of EVs. About this, Kavanagh et al. [
42] showed that treatment with Paclitaxel results in the development of chemoresistant therapeutic induced senescent (TIS Cal51) cells which produce more exosomes than non–senescent control cells. Secondly, tumor exosomes are modulated by the TME but also modulate recipient cells based on their cells of origin. O’Brien et al. [
43] demonstrated that TNBCs Exos transfer phenotypic traits to secondary cells representing their cells of origin. By comparing the effects of tumor exosomes derived from the TNBC cell line Hs578T and its more invasive Hs578T
(i)8 variant, authors showed that Hs5787T
(i)8–Exos can increase proliferation, migration and invasiveness of the following recipient cells lines: SKBR3, MDA-MB-231 and HCC1954. In addition, Hs5787T
(i)8–Exos were able to increase sensitivity to anoikis on recipient cells, reflecting the enhanced sensitivity of Hs578Ts
(i)8 to anoikis. Finally, Hs5787T
(i)8–Exos stimulated significantly more endothelial tubules formation, influencing the vascular profile of the recipient site.
Not only the exosomes themselves, but also their intravesicular components play an important oncogenic role. Bobrie et al. [
44] and Yang et al. [
45], for example, showed how the exosomal miR–223 or the protein Rab27a facilitate exosomes secretion and promote TNBC progression and invasiveness. Last but not least, tumor exosomes play a role in drug resistance. There is brilliant literature from Boelens et al. [
46] and Diluvio et al. [
47] that explains how stromal cells exosomes can mediate drug resistance in TNBC cells by modulating the NOTCH3 pathway. In aggregate, a significant portion of the scientific literature perceives exosomes as genuine allies of the tumor, capable of fostering a pro-tumor microenvironment that facilitates cancer progression, drug resistance, and both local and distant metastasis.
4.2. TNBC and Tumor Exosomes: … or Potential Enemies?
Considering their pivotal role in tumor progression, exosomes emerge as potential therapeutic targets. The exosomal pathway presents opportunities for intervention at multiple biological stages, encompassing biogenesis, release, and uptake. Moreover, exosomes can be transformed into delivery systems to carry chemotherapeutic drugs or nucleic acids [
37]. The ESCRT protein machinery (ESCRT 0–III) facilitate receptor sorting into the lumen of multivesicular endosomes and can be inhibited using sphingolipid ceramid–generating sphingomyelinase to prevent exosomes formation
48. Even Rab27a was silenced in mouse mammary tumor 4T1 cells in order to reduce local growth and metastasis [
44]. In the above–mentioned study of O’Brien, authors showed how exosomes–mediated transfection of miR–134 into Hs578Ts(i)8 cells was associate with lower expression of STAT5B, Bcl–2 and HSP90 with consequent reduced cellular proliferation [
43]. In another paper of Li et al. [
49], authors modified the TME of TNBC by engineering exosomes with miR–770. TNBC cells became more sensitive to Doxorubicin because of a synergistic action of miR–770 and Doxorubicin in therapeutic–induced apoptosis. Doxorubicin can also be loaded inside of exosomes in order to transport chemotherapeutic drugs with low toxicity and immunogenicity. Gomari et al. [
50] reported how Doxorubicin–loaded exosomes are able to target Her2
+ cells with reduction of tumor growth rate and reduced adverse effects in a murine breast cancer model.
The prospect of surface modification of exosomes using specific peptides or tissue-specific antibodies to fine-tune their homing capabilities is also a highly intriguing avenue of research. In the aforementioned study by Gomari, it was reported that exosomes could be engineered to target Her2+ tumor cells by incorporating pLEX-LAMP DARPin, thereby enabling DARPin expression on the exosome’s surface.
As the research article of Goh et al. reported and gave us the inspiration of starting this journey, there is the need to face TNBC research with exosomes further and, in particular, with ASCs–Exosomes. The possibility to use them as Trojan horses for TNBC therapy is real and is the focus of our research. The concept and the T.A.E. preconditioning model is presented in the next section of the paper.
5. The Triple Ascs Exosomes [t.a.e.] Technology as Preconditioning Tool for Tnbc Patients
In the perpetual fight against breast cancer, novel technologies try to offer hope for effective treatments and improved patient outcomes. The lack of ER, PR and Her2 receptors means neo–adjuvant chemotherapy and/or radiotherapy first and traditional surgery after against TNBC. Because of our clinical duty as plastic surgeons, the possibility of harvesting ASCs exosomes from a breast cancer patient through a liposuction session is real and possible. Hence, we are developing a novel Triple ASCs–Exosomes [T.A.E.] Technology able to impact breast cancer immediately after diagnosis by preconditioning the tumor core, its TME and surrounding tissues, including metastasis. This to act as integrated and supportive tool with traditional breast cancer therapies. In this process, ASCs exosomes are taken from the patient during a liposuction session, engineered and mixed at different concentrations and in three different ways to precondition cancer at its three different levels.
Due to their intrinsic low toxicity, low immunogenicity, high–flexibility engineering, inherent targeting and interplay with recipient cells, ASCs Exos are ideal drug carriers for breast cancer therapy. About this, Jung et al. [
51] developed an exosome platform to target regions of tumor hypoxia. After isolating four types of exosomes from MDA–MB–231 human breast cancer cells, the authors engineered them to carry supermagnetic iron oxide (SPIO) nanoparticles and the anti–tumoral PARP inhibitor Olaparib, used in the treatment of ovarian and breast cancer. In this way, the research group obtained increased apoptosis and slower tumor growth in–vivo in hypoxic tumors after exosomes treatment.
#1: Pembrolizumab–ASCs Exosomes
At the heart of the T.A.E. Technology lies the transformative power of ASCs and their related exosomes. Central to T.A.E. Technology is its ability to locally target TNBC cells within the tumor core, bypassing systemic barriers and minimizing off–target effects. Engineered exosomes can deliver anti–cancer drugs, RNA interference molecules or immune–modulating molecules, specifically to TNBC cells, inducing apoptosis or inhibiting proliferation. This to minimize off–target effects and enhance therapeutic efficacy. Tumor Exos bestow several advantages upon the tumor core, including the activation of stromal components, initiation of the angiogenic switch, enhancement of vascular permeability, facilitation of the epithelial-to-mesenchymal transition, and the establishment of a pre-metastatic niche [
52].
As example of one of the possibilities of targeting the tumor core, we are thinking about the possibility of developing the first of the three T.A.E. components by engineering ASCs Exosomes with Pembrolizumab to deliver it within the tumor core. In a phase 3 trial of Cortes et al.53, the addition of Pembrolizumab to chemotherapy resulted in longer overall survival than chemotherapy along among TNBC patients whose tumors expressed programmed death ligand–1 (PD–L1) with a combined positive score (CPS) of 10 or more.
#2: FAP–ASCs Exosomes
Beyond the tumor cells themselves, the TME plays is key in TNBC progression and treatment resistance. The second T.A.E. component should therefore impact this intricate milieu, leveraging ASCs exosomes to modulate the TME in favor of anti–tumor immune responses and inhibit pro–tumorigenic signals. By promoting the recruitment and activation of cytotoxic T cells and Natural Killer (NK) cells, the T.A.E. Technology aims to unleashes the immune system’s full potential against TNBC, fostering an inhospitable environment for cancer growth and metastasis. Moreover, exosomes loaded with immunomodulatory factors (e.g. cytokines, chemokines, or immune checkpoint inhibitors) can stimulate immune cells to recognize and eliminate TNBC cells more effectively. The T.A.E. Technology may also target cancer–associated fibroblasts and endothelial cells within the TME, impeding angiogenesis and reducing supply of nutrients essential for tumor growth. Before the beginning of our study, even Hu et al. [
54] demonstrated that engineered exosomes can be used as vaccines to reprogram the immune response of the TME. By producing Fibroblast Activation Protein–alpha (FAP) gene–engineered tumor cell–derived exosome–like nanovesicles (eNVs–FAP) as tumor vaccine, the authors were able to inhibit tumor growth inducing cytotoxic T lymphocyte (CTL) immunity against colon, melanoma, lung and breast cancer models and reprogrammed the TME. Because of its activity, a eNVs–FAP–engineered ASCs Exos represent a special candidate as tumor vaccine to be used for our second T.A.E. component to target locally the tumor parenchyma and the surrounding stroma.
#3: MVC–ASCs Exosomes
The third T.A.E. component should be developed for the surrounding tissues or breast cancer metastasis. As exosomes derived from metastatic breast cancers have natural organotropism to lung and brain, therapeutic drugs can be encapsulated with exosomes as third component to address the different metastatic foci. Huang et al. [
55], for example, loaded a nanomaterial for photothermal therapy named as gold nanorods into lung metastatic cancer–cells derived exosomes exhibiting better therapeutic effects on lung metastasis. Another therapeutical option could be represented by Maraviroc (MVC), the non–peptidic antiretroviral CCR5 blocker which is used for HIV patients. Pervaiz et al. [
56] is analyzing the relationship between MCV and breast cancer. In one of their preclinical studies, the group demonstrated that blocking CCR5 reduces Breast cancer proliferation, colony formation and metastasis. Furthermore, MVC inhibits bone metastasis in implanted MDA–MB–231 breast cancer cells in rats. Even Jiao et al. [
56] induced TNBC cell proliferation and migration via IL–6 and CCL5 by exploiting MVC and Tocilizumab. Hence, a MVC–ASCs–Exos complementary therapy inhibiting these mechanisms may therefore be a valuable tool for TNBC patients.
At present, T.A.E. Technology is undergoing laboratory and preclinical development with the potential to address and support traditional chemotherapeutical treatments against TNBC. This with the final prospect of a future where TNBC can be controlled and effectively treated with a combination treatment. As research progresses and clinical trials advance, the transformative impact of “T.A.E. preconditioning” on TNBC treatment is poised to be realized, ushering in a new era of hope and healing in the fight against cancer.
6. Conclusions
TNBC remains one of the most common and dangerous malignancies worldwide. Due to the lack of ER, PR and Her2 expression, TNBC defies conventional therapies, necessitating novel therapeutical approaches, in particular for the pre–surgical treatment of the tumor mass. The possibility of harvesting ASCs exosomes from breast cancer patients with liposuction, engineering them after and support traditional breast cancer therapies at three different levels is challenging. By targeting not only the tumor core but also its TME and surrounding tissues including metastasis, the authors work to develop a supportive T.A.E. Technology for breast cancer in order to performed a selective preconditioning of the cancer before or while the traditional treatments are running. The possibility to exploit the transformative impact of ASCs exosomes within the T.A.E. technology against TNBC is real, highlighting a new era for supportive measures against breast cancer.
List of abbreviations: ADSC: Adipose-Derived Stem Cell; AT: Adipose Tissue; CPS: Combined Positive Score; CTL: Cytotoxic T Lymphocyte; EMT: Epithelial–to– Mesenchymal Transition; eNVs–FAP: Fibroblast Activation Protein–alpha gene–engineered tumor cell–derived exosome–like nanovesicles; ESCRT–3: Endosomal Sorting Complex Required for Transport 3; EV: Extracellular vesicle; FAP: Fibroblast Activation Protein–alpha; FGF: Fibroblast Growth Factor; GF: Growth Factor; IGF–II: Insulin Growth Factor II; MHC: Major Histocompatibility Complex; MSC: Mesenchymal Stem Cell; NK: Natural Killer; MVC: Maraviroc PD–L1: Programmed Death Ligand–1; SERM: Selective Estrogen Receptor Modulator; SVF: Stromal Vascular Fraction; TGF-Beta: Transforming Growth Factor–Beta; TME: Tumor Microenvironment; TNBC: Triple–Negative Breast Cancer; Tsg101: Tumor Susceptibility Gene 101; VEGF: Vascular Endothelial Growth Factor
Author Contributions
Conceptualization, A. Pag. and D.D.; methodology, A. Pag and L.K.; formal analysis, D.O. and S.K.; resources, O.F. and L.P.; data curation, A. Pan.; Awriting—original draft preparation, A. Pag., S.K., D.D.; writing—review and editing, L.K., A. Pan and D.O.; visualization, O.F.; supervision, L.P.; project administration, L.P., S.G. and S.K.; funding acquisition, L.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yáñez-Mó M, Siljander PRM, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [CrossRef]
- Kalra H, Simpson RJ, Ji H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10(12):e1001450. [CrossRef]
- Kim DK, Kang B, Kim OY, et al. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J Extracell Vesicles. 2013;2. [CrossRef]
- Simpson RJ, Kalra H, Mathivanan S. ExoCarta as a resource for exosomal research. J Extracell Vesicles. 2012;1. [CrossRef]
- Ebrahimian M, Hashemi M, Etemad L, Salmasi Z. Thymoquinone-loaded mesenchymal stem cell-derived exosome as an efficient nano-system against breast cancer cells. Iran J Basic Med Sci. 2022;25(6). [CrossRef]
- Shojaei S, Moradi-Chaleshtori M, Paryan M, Koochaki A, Sharifi K, Mohammadi-Yeganeh S. Mesenchymal stem cell-derived exosomes enriched with miR-218 reduce the epithelial–mesenchymal transition and angiogenesis in triple-negative breast cancer cells. Eur J Med Res. 2023;28(1):516. [CrossRef]
- Brett E, Duscher D, Pagani A, Daigeler A, Kolbenschlag J, Hahn M. Naming the Barriers between Anti-CCR5 Therapy, Breast Cancer and Its Microenvironment. Int J Mol Sci. 2022;23(22):14159. [CrossRef]
- Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11:108. [CrossRef]
- Gonzales PA, Pisitkun T, Hoffert JD, et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol JASN. 2009;20(2):363-379. [CrossRef]
- Jeppesen DK, Nawrocki A, Jensen SG, et al. Quantitative proteomics of fractionated membrane and lumen exosome proteins from isogenic metastatic and nonmetastatic bladder cancer cells reveal differential expression of EMT factors. Proteomics. 2014;14(6):699-712. [CrossRef]
- Van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213-228. [CrossRef]
- Aatonen MT, Ohman T, Nyman TA, Laitinen S, Grönholm M, Siljander PRM. Isolation and characterization of platelet-derived extracellular vesicles. J Extracell Vesicles. 2014;3. [CrossRef]
- Colombo M, Moita C, van Niel G, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553-5565. [CrossRef]
- Huang KC, Ramamurthi KS. Macromolecules that prefer their membranes curvy. Mol Microbiol. 2010;76(4):822-832. [CrossRef]
- Van Meer G, Vaz WLC. Membrane curvature sorts lipids: Stabilized lipid rafts in membrane transport. EMBO Rep. 2005;6(5):418-419. [CrossRef]
- Abels ER, Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol Neurobiol. 2016;36(3):301-312. [CrossRef]
- Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9-17. [CrossRef]
- Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;188:1-11. [CrossRef]
- Xia L, Zhang C, Lv N, et al. AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages. Theranostics. 2022;12(6):2928-2947. [CrossRef]
- Duan P, Tan J, Miao Y, Zhang Q. Potential role of exosomes in the pathophysiology, diagnosis, and treatment of hypoxic diseases. Am J Transl Res. 2019;11(3):1184-1201.
- Xiong M, Zhang Q, Hu W, et al. Exosomes From Adipose-Derived Stem Cells: The Emerging Roles and Applications in Tissue Regeneration of Plastic and Cosmetic Surgery. Front Cell Dev Biol. 2020;8:574223. [CrossRef]
- Al-Ghadban S, Bunnell BA. Adipose Tissue-Derived Stem Cells: Immunomodulatory Effects and Therapeutic Potential. Physiology. 2020;35(2):125-133. [CrossRef]
- Suh A, Pham A, Cress MJ, et al. Adipose-derived cellular and cell-derived regenerative therapies in dermatology and aesthetic rejuvenation. Ageing Res Rev. 2019;54:100933. [CrossRef]
- Bajek A, Gurtowska N, Olkowska J, Kazmierski L, Maj M, Drewa T. Adipose-Derived Stem Cells as a Tool in Cell-Based Therapies. Arch Immunol Ther Exp (Warsz). 2016;64(6):443-454. [CrossRef]
- Zocchi ML, Vindigni V, Pagani A, et al. Regulatory, ethical, and technical considerations on regenerative technologies and adipose-derived mesenchymal stem cells. Eur J Plast Surg. 2019;42(6):531-548. [CrossRef]
- Zocchi ML, Facchin F, Pagani A, et al. New perspectives in regenerative medicine and surgery: the bioactive composite therapies (BACTs). Eur J Plast Surg. 2022;45(1):1-25. [CrossRef]
- Liu YX, Sun JM, Ho CK, et al. Advancements in adipose-derived stem cell therapy for skin fibrosis. World J Stem Cells. 2023;15(5):342-353. [CrossRef]
- Lee JH, Won YJ, Kim H, et al. Adipose Tissue-Derived Mesenchymal Stem Cell-Derived Exosomes Promote Wound Healing and Tissue Regeneration. Int J Mol Sci. 2023;24(13):10434. [CrossRef]
- Li C, Li W, Pu G, Wu J, Qin F. Exosomes derived from miR-338-3p-modified adipose stem cells inhibited inflammation injury of chondrocytes via targeting RUNX2 in osteoarthritis. J Orthop Surg. 2022;17(1):567. [CrossRef]
- Fang J, Liang W. ASCs -derived exosomes loaded with vitamin A and quercetin inhibit rapid senescence-like response after acute liver injury. Biochem Biophys Res Commun. 2021;572:125-130. [CrossRef]
- Qian W, Huang L, Xu Y, et al. Hypoxic ASCs-derived Exosomes Attenuate Colitis by Regulating Macrophage Polarization via miR-216a-5p/HMGB1 Axis. Inflamm Bowel Dis. 2023;29(4):602-619. [CrossRef]
- Lee M, Liu T, Im W, Kim M. Exosomes from adipose-derived stem cells ameliorate phenotype of Huntington’s disease in vitro model. Eur J Neurosci. 2016;44(4):2114-2119. [CrossRef]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. [CrossRef]
- Bantug GR, Hess C. The immunometabolic ecosystem in cancer. Nat Immunol. 2023;24(12):2008-2020. [CrossRef]
- Watkins, EJ. Watkins EJ. Overview of breast cancer. JAAPA Off J Am Acad Physician Assist. 2019;32(10):13-17. [CrossRef]
- Steward L, Conant L, Gao F, Margenthaler JA. Predictive Factors and Patterns of Recurrence in Patients with Triple Negative Breast Cancer. Ann Surg Oncol. 2014;21(7):2165-2171. [CrossRef]
- Goh CY, Wyse C, Ho M, et al. Exosomes in triple negative breast cancer: Garbage disposals or Trojan horses? Cancer Lett. 2020;473:90-97. [CrossRef]
- Colwell AS, Taylor EM. Recent Advances in Implant-Based Breast Reconstruction. Plast Reconstr Surg. 2020;145(2):421e-432e. [CrossRef]
- Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66(9):4795-4801. [CrossRef]
- Stevic I, Müller V, Weber K, et al. Specific microRNA signatures in exosomes of triple-negative and HER2-positive breast cancer patients undergoing neoadjuvant therapy within the GeparSixto trial. BMC Med. 2018;16(1):179. [CrossRef]
- Galindo-Hernandez O, Villegas-Comonfort S, Candanedo F, et al. Elevated concentration of microvesicles isolated from peripheral blood in breast cancer patients. Arch Med Res. 2013;44(3):208-214. [CrossRef]
- Kavanagh EL, Lindsay S, Halasz M, et al. Protein and chemotherapy profiling of extracellular vesicles harvested from therapeutic induced senescent triple negative breast cancer cells. Oncogenesis. 2017;6(10):e388. [CrossRef]
- O’Brien K, Rani S, Corcoran C, et al. Exosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cells. Eur J Cancer. 2013;49(8):1845-1859. [CrossRef]
- Bobrie A, Krumeich S, Reyal F, et al. Rab27a Supports Exosome-Dependent and -Independent Mechanisms That Modify the Tumor Microenvironment and Can Promote Tumor Progression. Cancer Res. 2012;72(19):4920-4930. [CrossRef]
- Yang M, Chen J, Su F, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. [CrossRef]
- Boelens MC, Wu TJ, Nabet BY, et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159(3):499-513. [CrossRef]
- Diluvio G, Del Gaudio F, Giuli MV, et al. NOTCH3 inactivation increases triple negative breast cancer sensitivity to gefitinib by promoting EGFR tyrosine dephosphorylation and its intracellular arrest. Oncogenesis. 2018;7(5):42. [CrossRef]
- Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic Cph Den. 2009;10(7):925-937. [CrossRef]
- Li Y, Liang Y, Sang Y, et al. MiR-770 suppresses the chemo-resistance and metastasis of triple negative breast cancer via direct targeting of STMN1. Cell Death Dis. 2018;9(1):14. [CrossRef]
- Gomari H, Forouzandeh Moghadam M, Soleimani M, Ghavami M, Khodashenas S. Targeted delivery of doxorubicin to HER2 positive tumor models. Int J Nanomedicine. 2019;14:5679-5690. [CrossRef]
- Jung KO, Jo H, Yu JH, Gambhir SS, Pratx G. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials. 2018;177:139-148. [CrossRef]
- Brinton LT, Sloane HS, Kester M, Kelly KA. Formation and role of exosomes in cancer. Cell Mol Life Sci CMLS. 2015;72(4):659-671. [CrossRef]
- Cortes J, Rugo HS, Cescon DW, et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2022;387(3):217-226. [CrossRef]
- Hu S, Ma J, Su C, et al. Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis. Acta Biomater. 2021;135:567-581. [CrossRef]
- Huang H, Shao L, Chen Y, et al. Synergistic strategy with hyperthermia therapy based immunotherapy and engineered exosomes-liposomes targeted chemotherapy prevents tumor recurrence and metastasis in advanced breast cancer. Bioeng Transl Med. 2022;7(2):e10284. [CrossRef]
- Pervaiz A, Zepp M, Georges R, et al. Antineoplastic effects of targeting CCR5 and its therapeutic potential for colorectal cancer liver metastasis. J Cancer Res Clin Oncol. 2021;147(1):73-91. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).