1. Introduction
Heart failure is still a current major healthcare concern worldwide, especially in the well developed countries, such as US and European countries, where heart failure is the third cause of cardiovascular mortality, with about 1 million hospitalizations annually [
1,
2].
Several circulating biomarkers have been studied for the use in appreciating the prognostic risk and the response to therapy in heart failure patients. Among these the natriuretic peptides are the most used and studied biomarkers of heart failure [
2,
3,
4].
Cancer Antigen 125 (CA 125) has recently emerged as a potential prognostic indicator and biomarker for guiding decongestive therapy in heart failure patients [
4,
5,
6].
Carbohydrate antigen 125 (CA 125) is a high molecular weight transmembrane glycoprotein belonging to the mucin family, also known as MUC1644. It was first detected in ovarian cancer cells, but several studies showed that it is normally expressed on different cell surfaces present in various organs( lung, prostate, pleura, pericardium, peritoneum), with the role of hydrating and lubricating epithelial surfaces, thus protecting them from mechanical stress [
7,
8,
9,
10].
Clinically it has been used as a marker of ovarian cancer, in monitoring, risk stratification and prognostication. CA 125 levels also rises in other malignancies such as lung cancer, mediastinal teratoma and non-Hodgkin lymphoma [
11,
12].
Although CA 125 is a well-known marker of ovarian cancer, i’s serum levels are also upregulated in multiple nonmalignant pathological states but also physiologic conditions: pregnancy, menstruation, liver cirrhosis, pelvic inflammatory disease, peritoneal trauma, ascites, lung cancer and congestion due to heart failure [
13,
14]. The exact mechanism is not known but it is probably related to several mechanisms.
One proposed pathophysiological mechanism is the increased mechanical stress produced by excessive fluid accumulation. For example CA 125 levels are positively correlated with pleural effusion volume in patients with chronic obstructive pulmonary disease and also with serosal fluid accumulation seen in patients with benign and malignant diseases such as ovarian cancer or liver cirrhosis [
15].
Inflammation seems to be another factor involved in the elevation of CA 125 levels in heart failure. We know that heart failure generates a systemic inflammatory state and that several proinflammatory cytokines (IL-6, IL-10, TNF alpha), are released in the blood circulation, and those cytokines can enhance the secretion of CA125 [
16]. Venous congestion produces changes in expressions patterns of the endothelium and perivascular tissue, leading to upregulation of pro oxidant, proinflammatory and vasoconstrictive factors. Bulska-Bedkowska et al reported that CA 125 levels positively correlated with high sensitivity C reactive protein and IL – 6 [
8,
17].
There is also data to suggest that CA 125 is implicated in the process of cardiac remodelling by modifying the intracellular matrix [
18,
19,
20,
21,
22]. In patients hospitalized for acute heart failure there was a positive correlation between galectin-3 and signs of inflammation only in patients with elevated CA 125 levels [
18,
22,
23,
24].
It appears that CA 125 is also implicated in the processes of fluid and cell transport, inflammation, tissue repair and tumor dissemination. CA 125 associated N-glycans are involved in modulating immune responses; for example CA 125 can suppress natural killer activity by the interaction with several proteins.
The exact mechanisms of CA 125 increased secretion in heart failure is not completely known. Nagele at al firstly reported the findings of several relevant tumor markers, especially CA 125, in heart failure patients before and after heart transplantation [
19,
21].
Duman et al and Kouris et al reported, in small studies, a weak association between CA 125 levels and pulmonary artery pressure, with no correlation to left side cardiac dysfunction. They also show that CA 125 was associated with left atrial volume as an index of diastolic dysfunction [
25,
26].
Larger scale studies began to show the link between CA 125 and heart failure. D’ Aloia et al [
27] demonstrated a correlation between CA 125 levels and right heart dysfunction and left ventricular diastolic dysfunction. D’Aloia’s study had a larger total patient population and a wider range of heart failure severity, and thus more conclusive results. They also showed that lower levels of CA 125 at follow up correlated with clinical improvement. In another large scale study, Vizzardi et al [
28] reported that systolic and diastolic indices and cardiac diameter correlated with CA 125 levels.
More recent and also larger scale studies performed by Nunez et al, demonstrated the predictive value of CA 125 for all-cause mortality at 6 months after acute heart failure discharge [
29,
30].
There is also a moderate correlation between CA 125 and natriuretic peptides, and the combination of the two seems to improve risk stratification of heart failure.
Production of CA 125 in patients with heart failure is supposed to happen by increased mechanical stress in mesothelial cells, in response to hemodynamic and inflammation stimuli. Fluid overload and consecutive high venous pressure in heart failure may increase pressure in mesothelium, which could induce the release of several inflammatory markers (IL-6, IL-10, tumor necrosis factors). This inflammation process along with the increased mechanical stress can stimulate mesothelial cells to secrete CA 125. Congestion in heart failure can involve pulmonary congestion, pleural effusion, ascites, which are interrelated with systemic inflammation in a vicious cycle [
31,
32].
The capacity of the mesothelium to secrete CA 125 was investigated and demonstrated by Zeillemaker et al, using a mesothelial cell monolayer model in vitro and utilizing inflammatory cytokines such as interleukin 1, tumor necrosis factor alpha and lipopolysaccharides as stimuli [
16]. Peak secretion of CA 125 was observed in 6 hours, and the most effective stimuli was IL 1.
As an additional mechanism, the translocation of bacteria and endotoxin formation during acute heart failure with bowel congestion leading to gastrointestinal functional impairment, may play a role in the secretion of CA 125 [
33].
Seo et al. observed a link between pericardial stimuli and elevation of CA 125 in patients with heart failure [
34].
2. Methodology
Our literature search strategy based on a thorough analysis aiming to identify articles on the selected topic, using the relevant key words, within broadly used databases and aggregators (PubMed, MEDLINE, Google Scholar). We performed this narrative review based on a relevant pool of over 200 articles selected after screening- reviews, meta-analyses and randomized or observational clinical trials results published in the last 20 years.
The population included in these studies had a mean age of 70 years old and were diagnosed with heart failure accord to the current guidelines of medical practice. Serum CA 125 levels were correlated with several clinical and imagistic parameters of heart failure and also with the short and medium term follow up of patients after discharge.
This review is conducted following PRISMA guidelines. Although the search criteria started from a 20-year time span, we focused our work on presenting latest data in order to maximize the clinical impact of the data.
3. Results
3.1. CA125 and Congestion in Heart Failure
Congestion in heart failure is linked to poor outcome, as such we recognize the importance of early detection of congestion. However, the quantification of congestion can be quite challenging, expecially in different settings such as in early phases of acute heart failure or close to discharge for hospitalisation. Fluid overload and retention are the most common reasons for heart failure hospitalisations, and decongestion represents an important therapeutic target in these patients. However complete decongestion in heart failure patients can be challenging , and so the residual congestion may be underappreciated leading to an increased risk of early rehospitalisation and increased mortality [
11,
35].
CA 125 provides additional information regarding signs and symptoms of congestion , peripheral oedema and serosal effusion in patients with heart failure. For example in a studies done by Falcao at al, in patients with heart failure complicating ST elevation myocardial infarction, circulating CA 125 levels correlated with pulmonary congestion and had a similar prognostic power for mortality as N terminal pro B type natriuretic peptide ( NT-pro BNP) [
36,
37,
38]. Minana and al. showed that in cases of systemic congestion and right ventricular dysfunction CA 125 can outperform NT-pro BNP in the prediction of mortality [
31,
39].
Fluid retention and congestion are the primary reasons for hospitalisation of heart failure patients. Therefore congestion is an important therapeutic target and it’s evaluation si very important as it is often difficult.
There are two types of congestion that need to be evaluated in heart failure patients: intravascular congestion and tissue congestion. Underappreciation of congestion at discharge increases the risk of early rehospitalisation and death in heart failure patients [
40].
As such correct evaluation of residual congestion before discharge is very important. For this there is an increasing interest in the establishment of reliable and cost effective biomarkers of fluid overload in heart failure [
40,
41].
CA 125 was studied as a potential biomarker of fluid overload in heart failure.
Studies conducted by Soler [
42] and Minana [
31] suggested that CA 125 may outperform NTpro BNP in predicting mortality in cases of systemic congestion and right ventricular dysfunction. Minana at al conducted a study of 2949 patients hospitalised for acute heart failure, in which they tried to determine the main factors associated with CA 125 and NTpro BNP [
31] . The median value for NTpro BNP was 4840 pg/ml and for CA 125 was 58 U/ml. The main factors associated with NTpro BNP levels were: glomerular filtration rate, left ventricle ejection fraction and age, as opposed to CA 125 leves that were: presence of pleural effusion, tricuspid regurgitation severity and peripheral oedema. In conclusion CA125 was a more useful marker of right heart failure, being less influenced by age and renal function than NTpro BNP [
31] .
In a prospective observational study including 191 patients admitted for acute heart failure, conducted by Pau Llacer et al [
43], CA 125 levels were associated more significantly than NTpro BNP with the state of congestion. CA 125 was positively associated with signs of congestion such as: peripheral oedema, pleural effusion and elevated inferior vena cava diameter. CA 125 was the most important predictor of inferior vena cava dilatation.
In another study, Gonzalo Nunez-Marin et al aimed to determine if CA 125 and NTpro BNP were associated with patterns of congestion as measured by intrarenal venous flow doppler ultrasound, in patients hospitalized with acute heart failure. A number of 70 patients with the mean age of 73 years were enrolled and renal doppler ultrasound was assessed during the first 24 hours of admission. CA 125, with a cut off value of 63.5 U/ml, showed a positive association with congestive renal ultrasound patterns and not NT-pro BNP [
44].
In a meta-analysis performed by Li et al, including sixteen studies, with a total of 8401 patients with acute heart failure, high CA 125 levels were associated with acute heart failure symptoms and with more severe fluid overload [
41].
Two studies have shown the relation between CA 125 and echocardiographic parameters of congestion and heart failure. D’Aloia et al observed that CA 125 levels correlated with pulmonary artery pressure, right atrial pressure and deceleration time as measured by Doppler echocardiography [
27]. Yilmaz et al showed that CA 125 levels were negatively correlated with left ventricle ejection fraction and positively correlated with pulmonary artery pressure as measured by Doppler echocardiography [
45].
3.2. CA125 and Risk Stratification in Heart Failure
Apart from its role in evaluating congestion, CA 125 also seems to correlate with prognosis and mortality in heart failure patients.
In a retrospective trial including 2961 patients discharged after acute heart failure hospitalisation, with mean age of 74 years, Soler and Minana compared NT-pro BNP and CA125 as predictors of poor outcome [
42]. Both biomarkers were analysed in relation to the severity of tricuspid regurgitation (which is a factor of poor prognosis in heart failure). NT-pro BNP was found to be linearly linked to mortality in non severe tricuspid regurgitation, but not in severe tricuspid regurgitation. High CA 125 levels were associated with increased mortality especially in patients with more severe tricuspid regurgitation. In conclusion CA 125 may outperform NTpro BNP in predicting mortality in heart failure patients with more involvement of the right ventricle and with more severe tricuspid regurgitation [
42,
46,
47].
In the CHANCE-HF trial, conducted by Nunez et al, CA 125 emerged as a marker of prognosis in heart failure patients, by reducing the risk of acute heart failure readmissions when used to guide therapy after acute heart failure discharge [
33].
Hung et al conducted a trial including 158 female patients with acute heart failure and preserved ejection fraction. During follow-up those with CA 125 levels > 17.29 U/ml had a greater incidence of heart failure rehospitalisation, and also CA 125 levels correlated with maximum left atrial volume [
48].
A study performed by Nunez et al sought to determine the CA 125 cutpoint for identifying patients at low risk of 1 month death and composite death/heart failure readmissions. In patients with acute heart failure a serum level of CA 125 < 23 U/ml was corellated with low risk of adverse events after discharge. This cutoff value remained significant up to 6 months after discharge [
33].
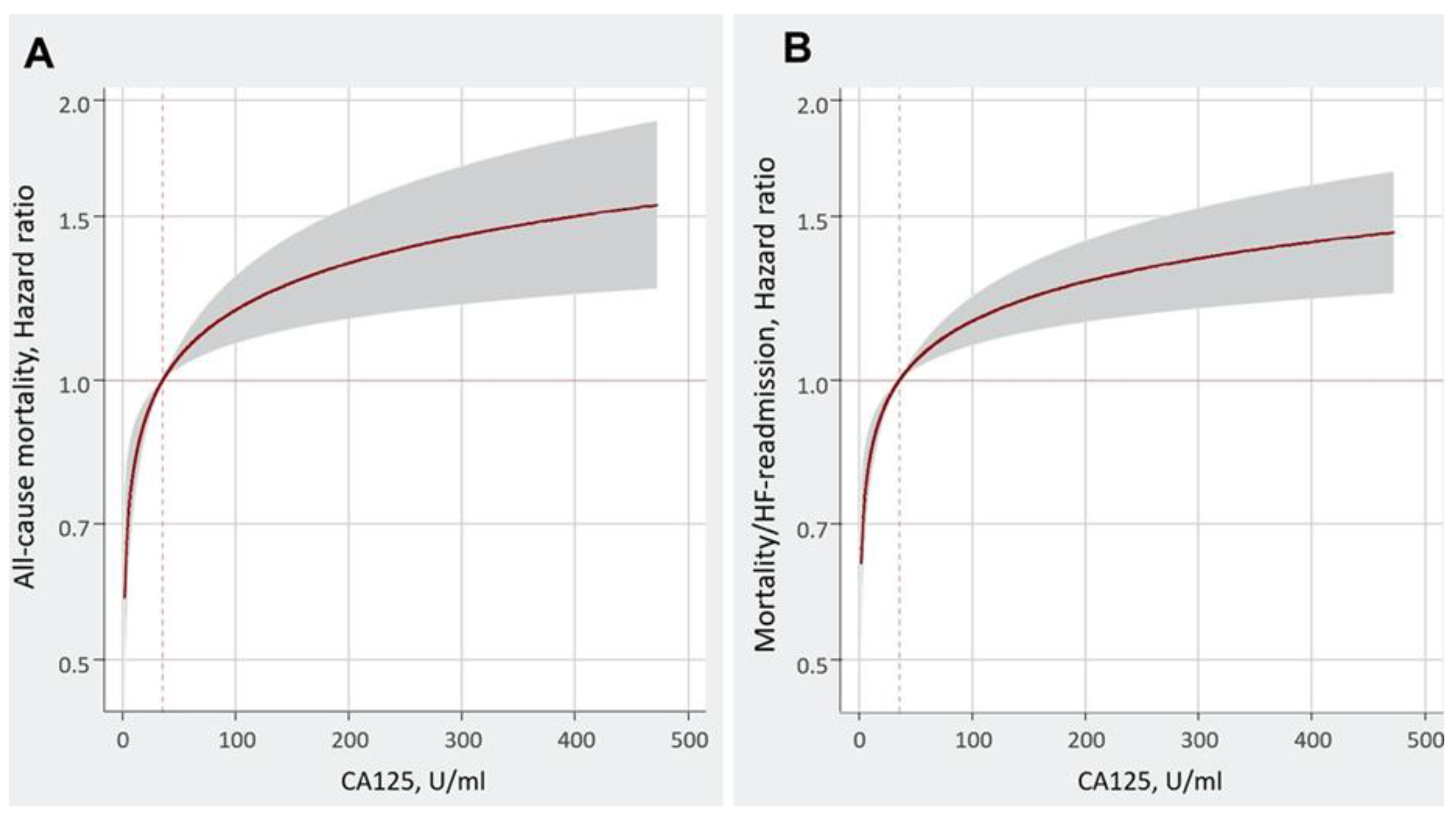
In a subanalysis of BIOSTAT – CHF Study [
30], there was a evaluation of the association between CA 125 and the risk of one year clinical outcomes in patients with heart failure. Higher levels of CA 125 were associated with an increased risk of death and composite of death/heart failure readmissions (
Figure 1).
As show by Yoon el al, patients hospitalized for acute heart failure, with high levels of CA 125 and low levels of NTpro BNP, had worse mid term prognosis then patients with both CA 125 and NTpro BNP low levels and patients with both CA 125 and NTpro BNP high levels had the worse prognosis [
49].
The most relevant publications presenting CA125 involvement in the heart failure risk stratification are presented in
Table 1.
3.3. CA125 Use in Guiding Heart Failure Therapy
One of the cornerstones of heart failure management represents decongestion therapy. The treatment of fluid overload in heart failure is based on diuretics, although no trials have shown a mortality benefit from diuretic therapy in heart failure patients. Also there isn’t a clear strategy of diuretic therapy titration in these patients. The main interest is to escalate treatment intensity in patients with more volume overload and also to reduce diuretic doses in patients who would not benefit from more intense diuretic therapy, thus reducing adverse reactions.
Because of the link between CA 125 and the levels of fluid overload (congestion) in heart failure, CA 125 has emerged as a potential marker of guiding treatment in acute heart failure.
As shown by Nunez et al in a trial of 946 patients with acute heart failure, where NTpro BNP and CA 125 were measured repeatedly at each doctor-patient visit, the levels of CA 125 decreased in the subset of patients with lower risk, and in patients in whom the CA 125 levels remained high, the mortality risk was also high. The association between CA 125 and mortality risk in heart failure patients, would argue for a role of CA 125 in tailoring decongestion therapy in these patients [
40].
In the CHANCE – HF trial, the authors compared CA 125 guided therapy versus standard of care therapy in terms of composite of one year death/ heart failure readmissions, in patients discharged after acute heart failure hospitalisation. CA 125 guided therapy resulted in a reduction of the composite endpoint at one year follow-up, mainly by a significant reduction of acute heart failure readmissions but no effect on mortality [
50].
Nunez et al proposed an algorithm for the use of CA 125 in heart failure therapy guidance. Using data from the CHANCE – HF trial [
50], a cutoff value for CA 125 was proposed at 35 U/ml. A number of 380 patients, discharged after acute heart failure hospitalization, and a high level of CA 125 > 35 U/ml, were assigned to CA 125 guided therapy versus standard of care therapy16. In patients enrolled in CA 125 guided therapy the diuretic treatment was intensified when the CA 125 levels remained high or increased. Also when CA 125 levels decreased below 35 U/ml, the diuretics were down titrated. These patients allocated to CA 125 guided therapy had 50% morre diuretic dose modification that patients with standard of care treatment. The CA 125 guided therapy was superior to standard of care therapy in terms of reducing the composite of mortality/ acute heart failure readmissions at one year. Patients who had CA 125 levels above 35 U/ml at the first outpatient visit, had a threefols increased risk of six months heart failure readmission [
50,
51,
52].
In another study by Nunez et al [
53], 160 patients with mean age of 78 years, acute heart failure and renal dysfunction, were randomized into two groups: one with loop diuretic guided therapy using CA 125 levels and the second group with standard care therapy. After 72 hours from admission, patients with with CA 125 guided therapy, with CA 125 levels over 35 U/ml, received the highest doses of loop diuretics and had the highest volume depletion, with significant improvement of glomerular filtration rate as opposed to patiens with usual loop diuretic therapy. CA 125 guided diuretic therapy improved glomerular filtration rate at 72 hours in patients admitted for acute heart failure and renal dysfunction [
53].
4. Discussion
There is sufficient data to suggest that CA 125 has a potential role in the clinical workup of patients with acute heart failure, as a prognostic tool, a biomarker of congestion and a guide for decongestion therapy.
The role and benefits of CA 125 in acute heart failure patients are supported by several facts:
There is no other biomarker that correlates with the congestive status of patients with acute heart failure.
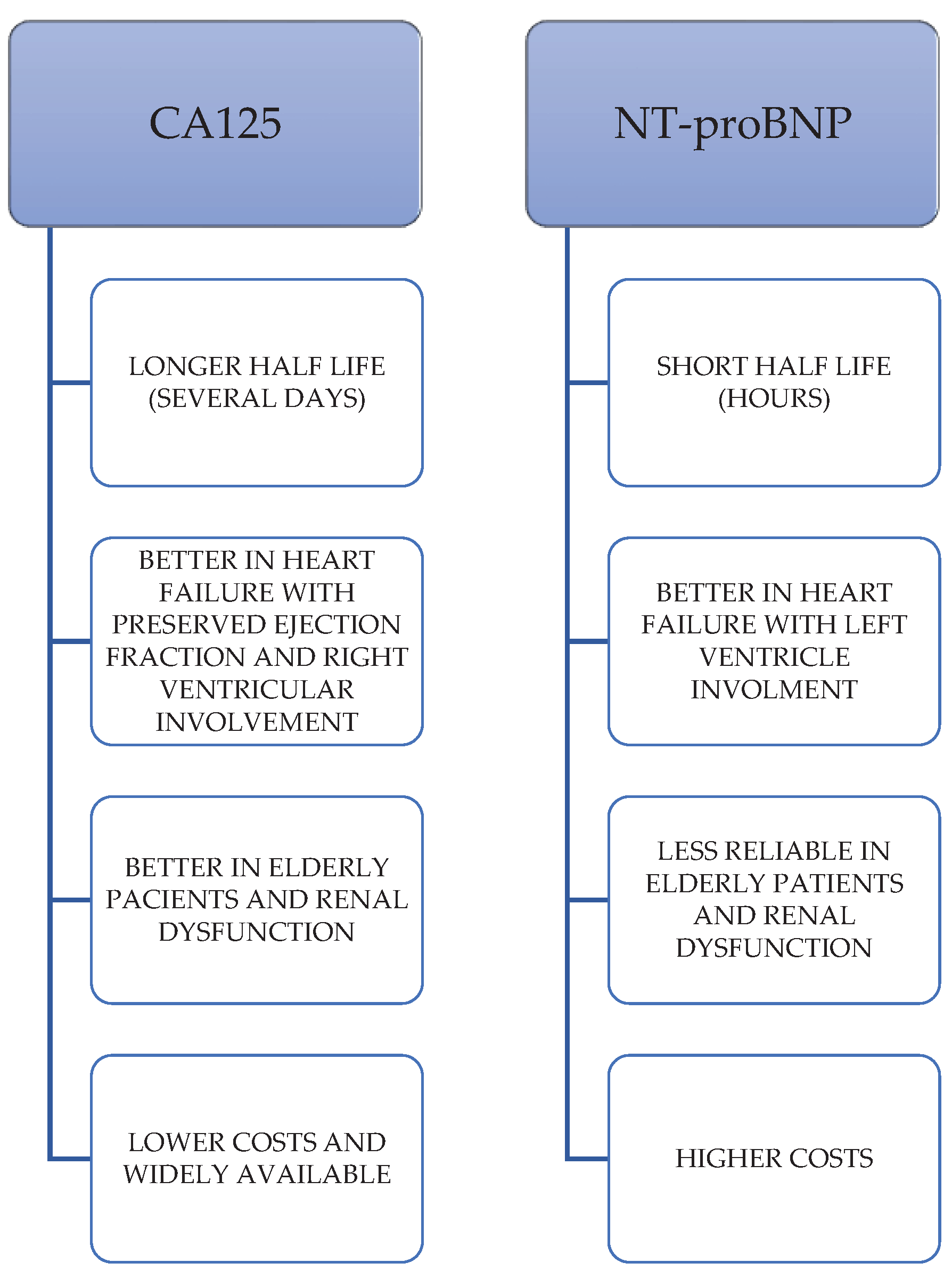
CA 125 has additional prognostic information beyond classical biomarkers of heart failure (BNP, NT-proBNP); the addition of CA 125 to NT-proNP may be a superior tool of risk estimation than NTpro BNP alone.
CA 125 levels are not significantly modified by factors such as age and renal dysfunction as opposed to NT-pro BNP.
CA 125 has a longer half-life that NTpro BNP, which makes it more stable and more reliable as a prognostic marker.
CA 125 levels correlate better with right heart side involvement
CA 125 levels correlate with echocardiographic parameters of heart failure.
CA 125 can have some advantages over NT-proBNP (a larger scale used biomarker of heart failure) in circumstances such as: heart failure with predominant involvement of right ventricle (especially heart failure with preserved ejection fraction), renal dysfunction and elderly patients [
40,
53,
54]. Other advantages of CA 125 over NT-proBNP can be of logistical nature, such as: CA 125 is widely available as it has already been used as a cancer marker for several decades and the cost of assessment is lower than for natriuretic peptides (
Figure 2).
Cancer Antigen 125 (CA 125) should be measured at admission of patients with acute decompensated heart failure. As CA 125 in not a cardiac specific biomarker, and its upregulation may be in relation with other diseases, in the absence of heart failure diagnosis, CA 125 levels should be interpreted accordingly.
There is also a difference between patients with acute heart failure and stable heart failure. Up to two thirds of patients with acute decompensated heart failure have elevated CA 125 levels (above 35 U/ml) but only a few patients with stable heart failure have CA 125 levels above this cutoff value. As such it is important to correlate CA 125 levels with signs and symptoms, echocardiographic parameters and other biomarkers such as natriuretic peptides [
1,
13,
19,
20].
A cutoff value of 35 U/ml was proposed for CA 125 levels that can identify low risk of adverse events following acute heart failure admission. High CA 125 levels (above 35 U/ml) can identify high risk patients and may guide a more intensive decongestion therapy with higher doses of loop diuretics.
It appears that during the first months after acute heart failure hospitalization, CA 125 kinetics are correlated with the patients’ clinical outcome. Because CA 125 has a long half-life (several days as opposed to NT-proBNP which has a half-life of hours), it is reasonable to measure CA 125 levels at admission and at least seven days after the initial measurement, for obtaining information about the response to therapy.
Although there are many studies that show the role of CA 125 in acute heart failure patients, there is less data about CA 125 in chronic ambulatory heart failure. One study performed by Kieran F. Docherty et al, and published in Journal of Am College of Cardiology in 2023, addressed this issue [
55]. They examined the association between baseline CA 125 levels and outcome in patients from the DAPA – HF trial (Dapaglifozin and Prevention of Adverse Outcomes in Heart Failure) and also the relationship with the effect of dapaglifozin. The DAPA – HF was a prospective, randomized trial that examined the efficacy and safety of dapaglifozin 10 mg per day compared with placebo, in patients with heart failure with reduced ejection fraction. The patients included in this trial had stable, non-decompensated heart failure with reduced ejection fraction (LVEF < 40 %). CA 125 was measured at baseline and after 12 months after randomization. CA 125 was analyzed according to the upper normal limit of 35 U/ml. Patients with higher concentrations of CA 125 were: older, had worse NYHA functional class, higher heart rate and also higher NT-proBNP and hs Troponin T levels. They also exhibited lower left ventricular ejection fraction and more renal impairment. Higher CA 125 levels also correlated with more peripheral congestion. A history of atrial fibrillation was associated with more elevated CA 125 levels. Using the cutoff value of 35 U/ml, patients with elevated CA 125 concentrations had higher risks of primary and secondary morbidity/mortality outcomes. The risk of death caused by worsening heart failure was also higher in patients with CA 125 levels above 35 U/ml. Renal function also declined at a greater rate over time in patients with elevated CA 125. CA 125 levels in the DAPA – HF trial were lower than in patients with acute heart failure, with only 12% of patients having levels above the cutoff value of 35 U/ml [
55,
56,
57,
58]. Elevated levels of CA 125 at baseline and also an increase of CA 125 levels from baseline to 12 months, were independent predictors of the risk of worsening heart failure and mortality. The effects of dapaglifozin were not influenced by the levels of CA 125 [
55,
56,
57,
59,
60].
5. Conclusions
CA 125 is a promising biomarker of congestion in the setting of acute heart failure and there is evidence to support its role in risk stratification, monitoring and guiding therapy in acute heart failure. More studies are necessary for the implementation of CA 125 in the clinical practice of heart failure management, to establish reference ranges for heart failure and appropriate algorithms for diagnosis and treatment monitoring.
While it is commonly available, for a better clinical effectiveness in screening and early detection CA 125 serum levels should be used by physicians in combination with both clinical manifestations as well as with other biomarkers or ultrasound and other multimodal methods.
Author Contributions
Conceptualization, M.C.M. and L.C.N.; methodology, V.D.O. and A.R.; formal analysis, M.C.M.; resources, A.N.; data curation, S.N.M. and D.T.; writing—original draft preparation, M.C.M.; writing—review and editing, V.D.O. and A.R.; visualization, L.C.N.; supervision, A.R.; project administration, M.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by “Dunarea de Jos” University of Galati, Romania, as academic support with no involvement in conducting the research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hollenberg, S.M.; et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: a report of the American College of Cardiology; J Am Coll Cardiology 2019, 74. [CrossRef]
- Rogers, J.K.; Pocock, S.J.; McMurray, J.J.; Granger, C.B.; Michelson, E.L.; Östergren, J.; Pfeffer, M.A.; Solomon, S.D.; Swedberg, K.; Yusuf, S. Analysing recurrent hospitalizations in heart failure: a review of statistical methodology, with application to CHARM-Preserved. Eur J Heart Fail. 2014, 16, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhang, P.; Hu, H. Serum Levels of Hcy, sST2 and CA-125 in CHF Patients and Their Correlation with Cardiac Function Classification. Heart Surg Forum. 2023, 26, E449–E454. [Google Scholar] [CrossRef] [PubMed]
- Pandhi, P.; Ter Maaten, J.M.; Anker, S.D.; Ng, L.L.; Metra, M.; Samani, N.J.; Lang, C.C.; Dickstein, K.; de Boer, R.A.; van Veldhuisen, D.J.; Voors, A.A.; Sama, I.E. Pathophysiologic Processes and Novel Biomarkers Associated With Congestion in Heart Failure. JACC Heart Fail. 2022, 10, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Santas, E.; Palau, P.; Bayés-Ge, A.; Núñez, J. The emerging role of carbohydrate antigen 125 in heart failure. Biomark Med. 2020, 14, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Zhang, Z.; Fan, Q. Carbohydrate antigen 125 in congestive heart failure: ready for clinical application? Front Oncol. 2023, 13, 1161723. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; et al. New mechanism of elevated CA 125 in heart failure: the mechanical stress and inflammatory stimuli initiate CA 125 synthesis; Med Hypotheses 2012, 79. [CrossRef]
- Ley, I.; et al. ERO1L promotes IL6/sIL6R signaling and regulates MUC16 expresion to promote CA 125 secretion and the metastasis of lung cancer cell; Cell Death Dis 2020, 11. [CrossRef]
- Scholler, N.; et al. CA 125 in ovarian cancer; Biomark Med 2017, 1. [CrossRef]
- Lloyd, K.O.; et al; Synthesis and secretion of the ovarian cancer antigen CA 125 by the human cell line NIH:OVCAR-3; Tumour Biol 2001; 22. [CrossRef]
- Bottoni, P. et al; The role of CA 125 as tumor marker: biochemical and clinical aspects; Adv Exp Med Biol, 2015, 867. [CrossRef]
- Miralles, C. et al; Cancer antigen 125 associated with multiple benign and malignant pathologies; Ann Surg Oncol 2003, 10. [CrossRef]
- Nunez, J. et al; Clinical utility of antigen carbohydrate 125 in heart failure; Heart Fail Rev 2014, 19. [CrossRef]
- Llacer, P. et al; Carbohydrate antigen 125 in heart failure. New era in the monitoring and control of treatment; Med Clin 2019, 152. [CrossRef]
- Topalak, O. et al; Serum, pleural effusion and ascites CA 125 levels in ovarian cancer and nonovarian benign and malignant diseases: a comparative study; Gynecol Oncol 2002, 85. [CrossRef]
- Zeillemaker, A.M. et al; CA 125 secretion by peritoneal mesothelial cells; J Clin Pathology 1994, 47. [CrossRef]
- Bulska-Będkowska, W.; Chełmecka, E.; Owczarek, A.J.; Mizia-Stec, K.; Witek, A.; Szybalska, A.; Grodzicki, T.; Olszanecka-Glinianowicz, M.; Chudek, J. CA125 as a Marker of Heart Failure in the Older Women: Population-Based Analysis. J Clin Med. 2019, 8, 607. [Google Scholar] [CrossRef] [PubMed]
- Nunez, J. et al; Prognostic value of the interaction between galectin-3 and antigen carbohydrate 125 in acute heart failure; PLoS One 2015, 10. [CrossRef]
- Nagele, H. et al; CA 125 and its relation to cardiac function; Am Heart J 1999, 137. [CrossRef]
- Nunez, J. et al; Carbohydrate antigen 125: an emerging prognostic risk factor in acute heart failure? Heart 2007, 93. [CrossRef]
- Nunez, J. et al; Antigen carbohydrate 125 in heart failure: not just a surrogate for serosal effusions?; Int J Cardiology 2011, 146. [CrossRef]
- Minana, G. et al; CA 125 and immunoinflammatory activity in acute heart failure; Int J Cardiology 2010, 145. [CrossRef]
- Colombo, P.C. et al; Peripheral venous congestion causes inflammation, neurohormonal and endothelial cell activation; European Heart J 2014, 35. [CrossRef]
- Murphy, S.P. et al; Inflammation in heart failure: JACC state of the art review; J Am Coll Cardiologu 2020, 75. [CrossRef]
- Duman, D.; Palit, F.; Simsek, E.; Bilgehan, K. Serum carbohydrate antigen 125 levels in advanced heart failure: relation to B-type natriuretic peptide and left atrial volume. Eur J Heart Fail. 2008, 10, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Kouris, N.T.; Zacharos, I.D.; Kontogianni, D.D.; Goranitou, G.S.; Sifaki, M.D.; Grassos, H.E.; Kalkandi, E.M.; Babalis, D.K. The significance of CA125 levels in patients with chronic congestive heart failure. Correlation with clinical and echocardiographic parameters. Eur J Heart Fail. 2005, 7, 199–203. [Google Scholar] [CrossRef] [PubMed]
- D’Aloia, A.; et al, Serum levels of carbohydrate antigen 125 in patients with chronic heart failure: relation to clinical severity, hemodynamic and Doppler echocardiographic abnormalities, and short-term prognosis; Am Coll Cardiology 2003, 41, 1805–1811. [CrossRef]
- Vizzardi, E. et al; Carbohydrate antigen 125: a new biomarker in heart failure; Cardiol Rev 2013, 21. [CrossRef]
- Nunez, J. et al, CA 125 guided diuretic treatment versus usual of care in patients with acute heart failure and renal dysfunction; The American Journal of Medicine 2020, 133. [CrossRef]
- Nunez, J. et al; Clinical role of CA 125 in worsening heart failure: a BIOSTAT-CHF study subanalysis; JACC Heart Failure 2020, 8, 386–397. [CrossRef]
- Minana, G. et al; Factors associated with plasma antigen cardohydrate 125 and amino-terminal pro B type natriuretic peptide concentrations in acute heart failure; European Heart J Acute Cardiovasc Care 2020, 9, 437–447. [CrossRef]
- Nunez, J. et al; Improvement in risk stratification with the combination of the tumor marker antigen carbohydrate 125 and brain natriuretic peptide in patients with acute heart failure; European Heart J 2010, 31, 1752–1763. [CrossRef]
- Nunez, J. Nunez, J. et al; Optimal carbohydrate antigen 125 cutpoint for identifying low risk patients after acute heart failure admission; Rev Esp Cardiol, march 2021. [CrossRef]
- Seo, T.; Ikeda, Y.; Onaka, H.; Hayashi, T.; Kawaguchi, K.; Kotake, C.; et al. Usefulness of serum CA 125 measurement for monitoring pericardial effusion. Jpn Circ J. 1993, 57, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Kumric, M. et al; Carbohydrate Antigen 125: A biomarker at the crossroads of congestion and inflammation in heart failure; Radcliffe Cardiology, 2021. [CrossRef]
- Falcao, F. et al; Carbohydrate antigen 125 predicts pulmonary congestion in patients with ST segment elevation myocardial infarction; Braz J Med Biol Res 2019, 52. [CrossRef]
- Falcao, F. et al; Carbohydrate antigen 125 for mortality risk prediction following acute myocardial infarction; Sci Rep 2020, 10. [CrossRef]
- Bobeica, C.; Niculet, E.; Tatu, A.L.; Craescu, M.; Vata, D.; Statescu, L.; Iancu, A.V.; Musat, C.L.; Draganescu, M.L.; Onisor, C.; Lungu, M.; Fotea, S.; Nechita, A.; Stefanescu, B.I.; Gheuca-Solovastru, L. Old and new therapeutic strategies in systemic sclerosis (Review). Exp Ther Med. 2022, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Minana, G.; Nunez, J. et al; Carbohydrate antigen 125 serial measurements after an admission for acute heart failure and risk of early readmission; Med Clin 2012, 139. [CrossRef]
- Nunez, J. et al; Long term serial kinetics of N terminal pro B tyoe natriuretic peptide and carbohydrate antigen 125 for moratlity risk prediction following acute heart failure; Eur Heart J Acute Cardiovasc Care 2017, 6, 685–696. [CrossRef]
- Li, K.H.C.; et al; Cancer antigen 125 and outcomes in acute heart failure: a systematic review and meta-analysis; Heart Asia 2018, 10. [CrossRef]
- Soler, M. et al; CA 125 outperforms NTproBNP in acute heart failure with severe tricuspid regurgitation; Int J Cardiology 2020, 308, 54–59. [CrossRef]
- Llacer, P. et al; Comparison between CA 125 and NTpro BNP for evaluating congestion in acute heart failure; Med Clin 2021, 156. 2021. [CrossRef]
- Nunez, G. et al; CA 125 but not NTpro BNP predicts the presence of a congestive intrarenal venous flow in patients with acute heart failure; Eur Heart J Acute Cardiovascular Care 2021, 10. [CrossRef]
- Yilmaz, M.B.; et al, Plasma CA 125 level is related to both sides of the heart: a retrospective analysis; Int J Cardiology 2011, 149, 80–82. [CrossRef]
- Oprea, V.D.; Marinescu, M.; Rișcă Popazu, C.; Sârbu, F.; Onose, G.; Romila, A. Cardiovascular Comorbidities in Relation to the Functional Status and Vitamin D Levels in Elderly Patients with Dementia. Diagnostics. 2022, 12, 2994. [Google Scholar] [CrossRef] [PubMed]
- Menghoum, N.; Badii, M.C.; Deltombe, M.; Lejeune, S.; Roy, C.; Vancraeynest, D.; Pasquet, A.; Gerber, B.L.; Horman, S.; Gruson, D.; Beauloye, C.; Pouleur, A.C. Carbohydrate antigen 125: a useful marker of congestion, fibrosis, and prognosis in heart failure with preserved ejection fraction. ESC Heart Fail. 2024. [CrossRef] [PubMed]
- Hung, C.L. et al; Beyond malignancy: the role of carbohydrate antigen 125 in heart failure; Biomark Res 2013, 1. [CrossRef]
- Yong, Y.J. et al; Serum levels of carbohydrate antigen 125 in combination with N-terminal pro-brain natriuretic peptide in patients with acute decompensated heart failure; Korean J Int Medicine 2019, 34. [CrossRef]
- Nunez, J. et al; Carbohydrate antigen 125 guided therapy in acute heart failure: CHANCE-HF a randomized study; JACC Heart Failure 2016, 4, 833–843. [CrossRef]
- Nunez, J. et al; Antigen carbohydrate 125 and creatinine on admission for prediction of renal function response following loop diuretic administration in acute heart failure; Int J Cardiology 2014, 174. [CrossRef]
- Nunez, J. et al; Differential mortality association of loop diuretic dosage according to blood urea nitrogen and carbohdrate antigen 125 following a hospitalization for acute heart failure; Eur J Heart Failure 2012, 14, 974–984. [CrossRef]
- Nunez et al; Ca 125 guided diuretic treatment versus usual care in patients with acute heart failure and renal dysfuntion; Am J Medicine 2020; 133. [CrossRef]
- Ordu, S.; et al. Carbohydrate antigen 125 and N terminal pro brain natriuretic peptide levels: compared in heart failure prognostication. Tex Heart Inst J 2012, 9. [Google Scholar]
- Kieran, F. Docherty et al; Association of Carbohydrate Antigen 125 on the Response to Dapaglifozin in Patients with Heart Failure; J of Am Coll of Card; 2023, 82. [CrossRef]
- De la Espriella Juan, R. et al; Carbohydrate antigen 125 in heart failure: an overlooked biomarker of congestion; JACC Heart Failure 2018, 6. [CrossRef]
- Nunez, J. et al, Antigene carbohydrate 125 as a biomarker in heart failure: a narrative review; European Journal of Heart Failure 2021, 23. [CrossRef]
- Sikaris, K.A. et al; CA 125: a test with a change of heart; Heart Lung Circ 2011, 20. [CrossRef]
- Minana, G. et al; Carbohydrate antigen 125 and risk of heart failure readmissions in patients with heart failure and preserved ejection fraction; Scientific Reports 2022, 12, 1344. [CrossRef]
- Xu, K.; Wu, M.; Huang, M.; Zhuo, X.; Weng, Y.; Chen, X. Carbohydrate antigen 125 combined with N-terminal pro-B-type natriuretic peptide in the prediction of acute heart failure following ST-elevation myocardial infarction. Medicine (Baltimore). 2022, 101, e32129. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).