Submitted:
06 March 2024
Posted:
07 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction

2. Materials and Methods
2.1. Material
2.2. Mango fiber pretreatment
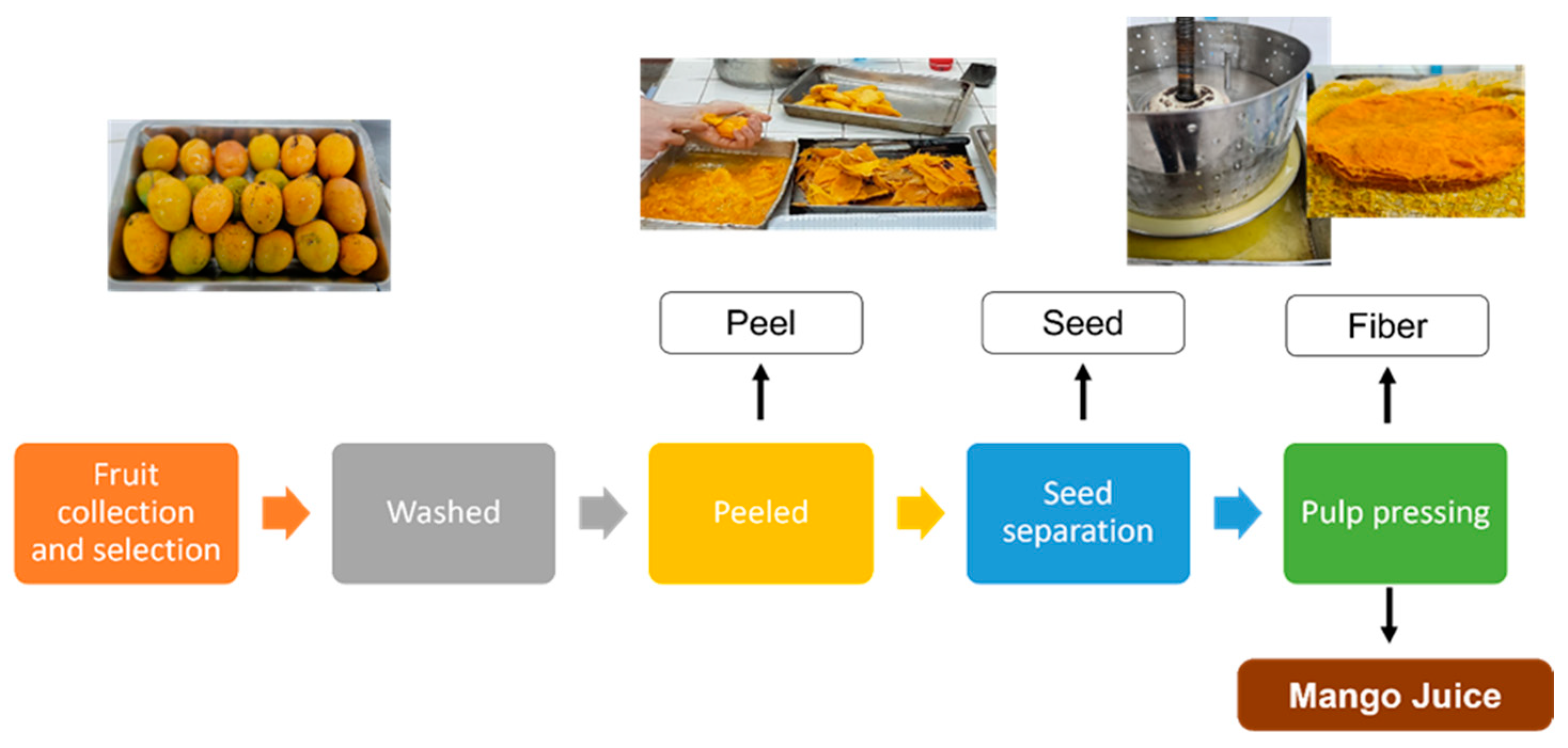
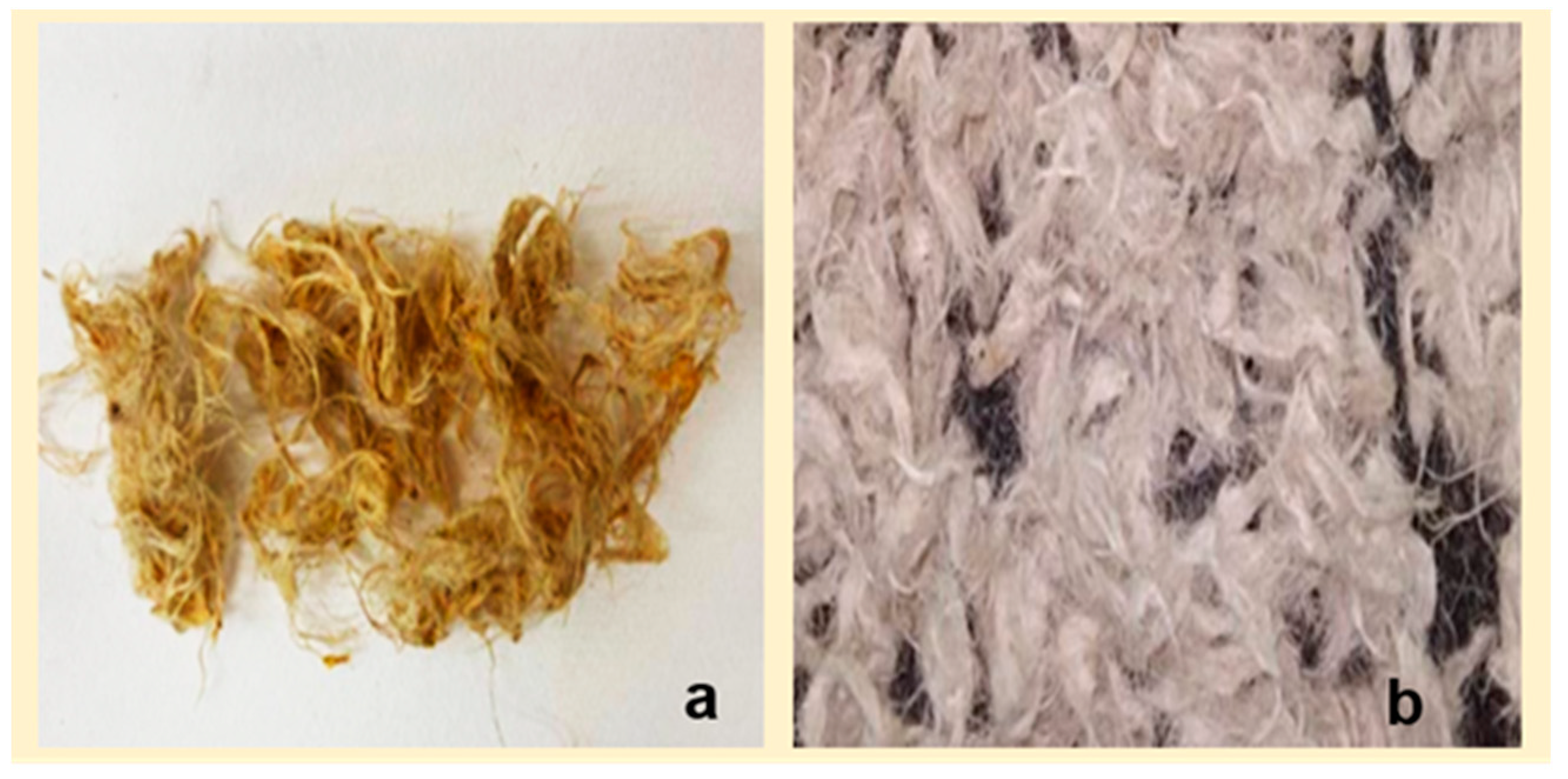
2.3. Mango fiber characterization
2.3.1. Fiber characterization
2.4. Weaving process
3. Results and discussion.
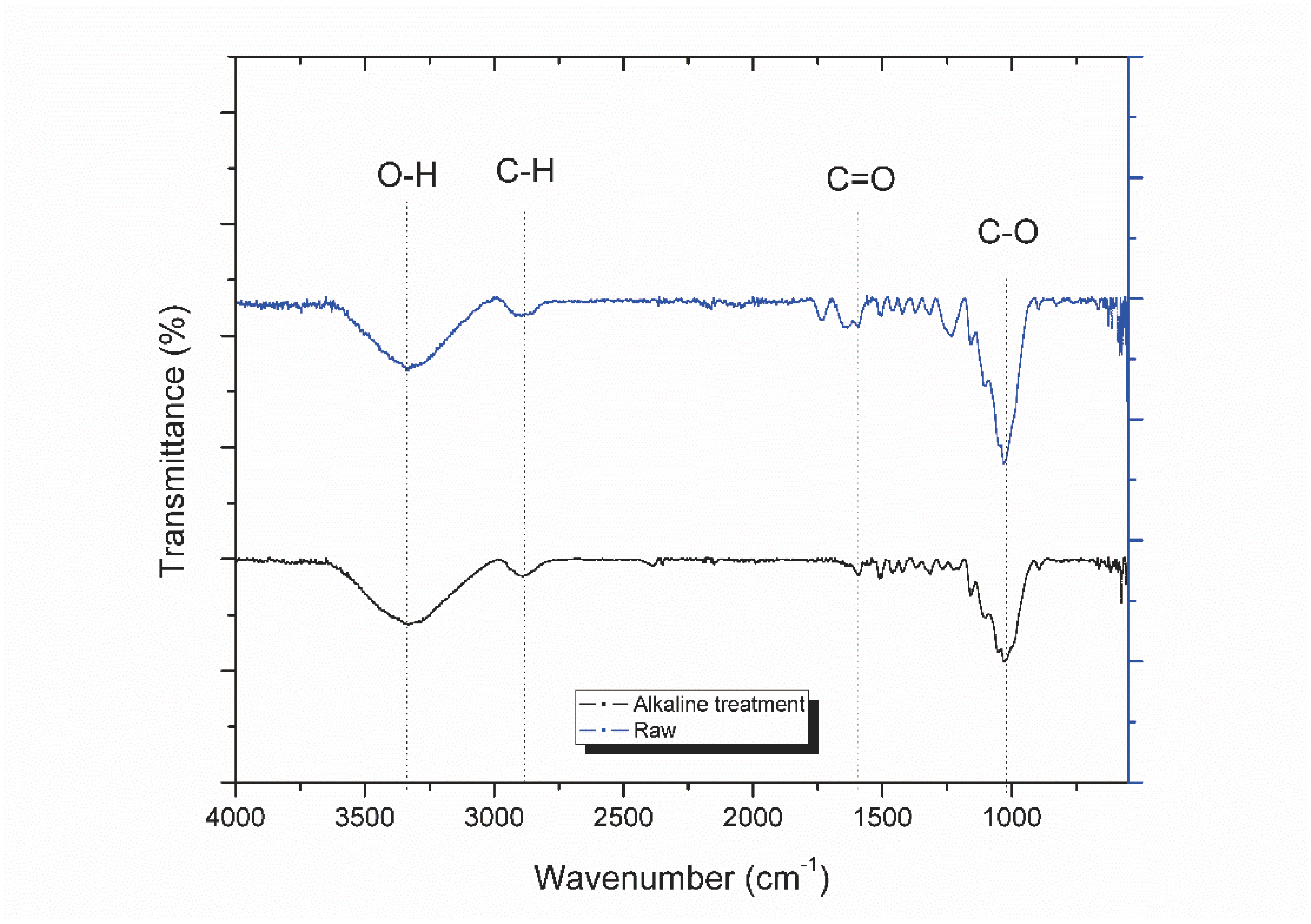
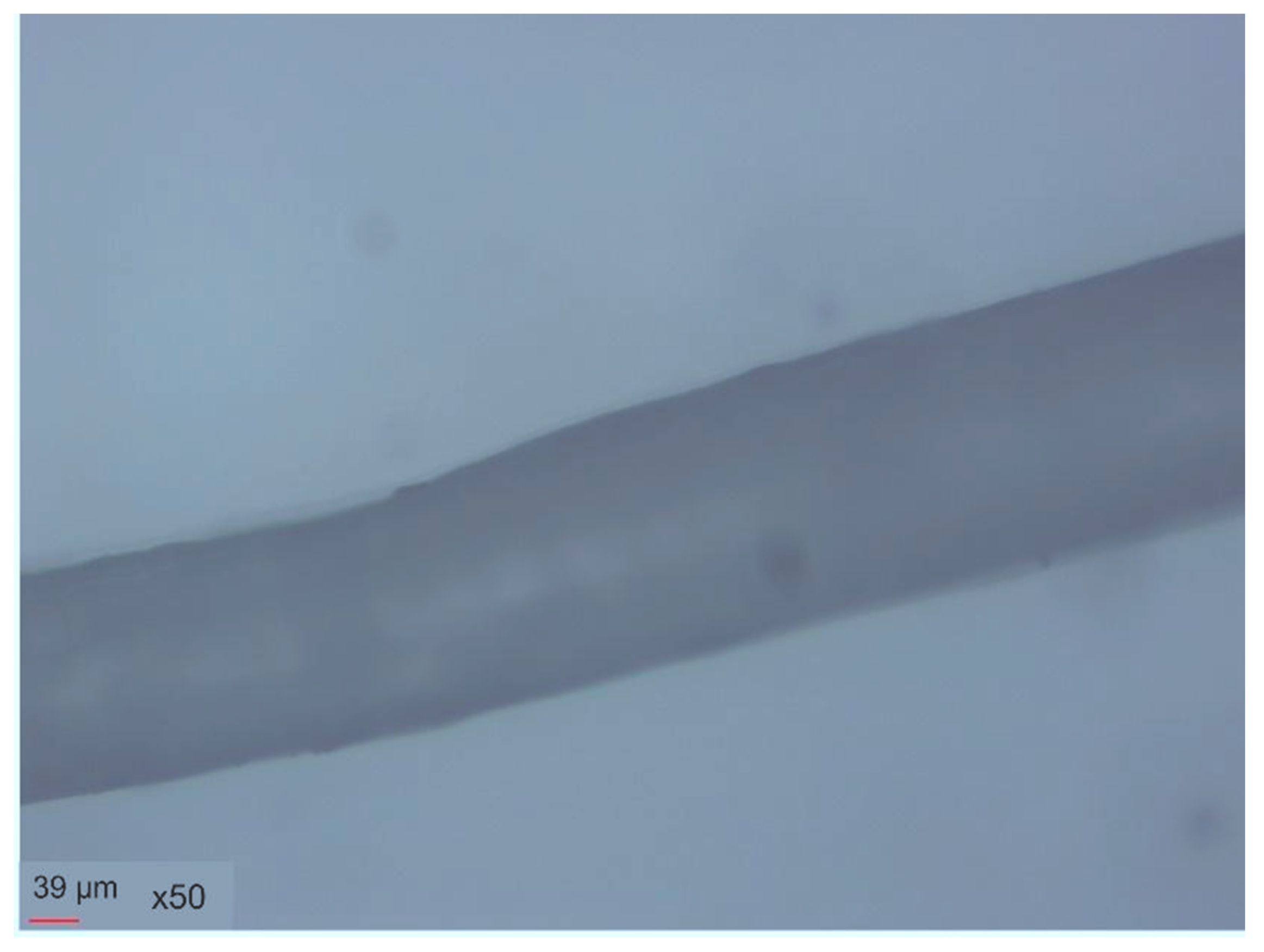
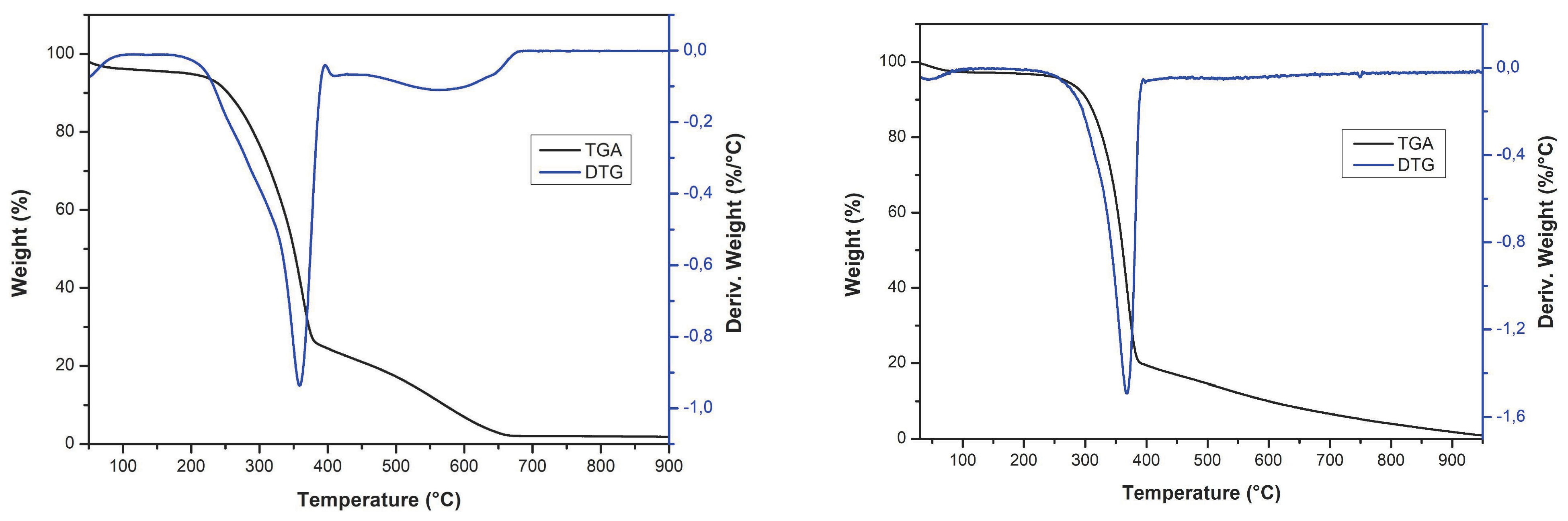
3.1. Characterization
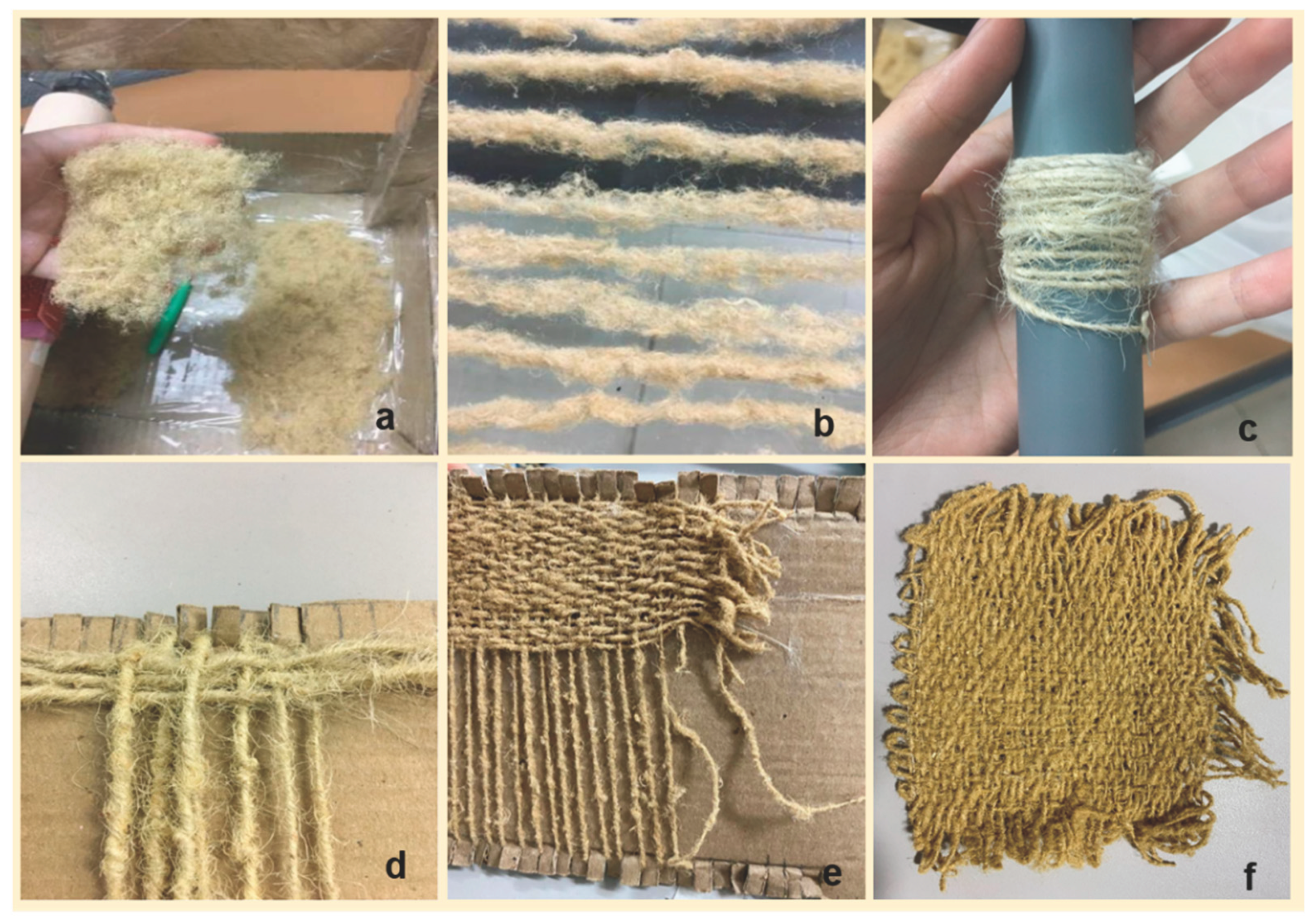
3.2. Weaving process
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Costa, S.M.; Tuesta, E.F.; Costa, S.A. Residuos agro-industriales utilizados como materias-primas en estudios de desarrollo de fibras textiles. Cuad. Cent. Estud. Diseñ. Comun. 2016, 58, 299–309. [Google Scholar] [CrossRef]
- Henrique, M. A.; Silverio, H. A.; Flauzino Neto, W. P.; Pasquini, D. Valorization of an Agro-Industrial Waste, Mango Seed, by the Extraction and Characterization of Its Cellulose Nanocrystals. J. Environ. Manage. 2013, 121, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Stenius, P. Forest Products Chemistry. In Papermaking Science and Technology; Stenius, P., Ed.; 2000. [Google Scholar]
- Uma Maheswari, C.; Obi Reddy, K.; Muzenda, E.; Guduri, B. R.; Varada Rajulu, A. Extraction and Characterization of Cellulose Microfibrils from Agricultural Residue – Cocos nucifera L. Biomass Bioenergy 2012, 46, 555–563. [Google Scholar] [CrossRef]
- Yousef, S.; Tatariants, M.; Tichonovas, M.; Sarwar, Z.; Jonuškienė, I.; Kliucininkas, L. A New Strategy for Using Textile Waste as a Sustainable Source of Recovered Cotton. Resour. Conserv. Recycl. 2019, 145, 359–369. [Google Scholar] [CrossRef]
- Roy, M.; Islam, M.; Khan, M.; Sarker, K.; Mamun, M. Mango Seed Kernel Agronomical Bio-Waste for Ecofriendly Cotton Dyeing: Optimization of Dyeing Period and Temperature. Chem. Mater. Eng. 2018, 6, 36–45. [Google Scholar] [CrossRef]
- Islam, M.; Liman, M.; Roy, M.; Hossain, M.; Repon, M.; Mamun, M. Cotton Dyeing Performance Enhancing Mechanism of Mangiferin Enriched Bio-Waste by Transition Metals Chelation. J. Text. Inst. 2021, 113, 567–579. [Google Scholar] [CrossRef]
- Patti, A.; Cicala, G.; Acierno, D. Eco-Sustainability of the Textile Production: Waste Recovery and Current Recycling in the Composites World. Polymers 2021, 13, 134. [Google Scholar] [CrossRef]
- Shahid-ul-Islam, M.; Mohammad, F. Sustainable Natural Fibres from Animals, Plants and Agroindustrial Wastes—An Overview. In Sustainable Fibres for Fashion Industry: Environmental Footprints and Eco-design of Products and Processes; Subramanian Senthilkannan, M., Miguel Angel, G., Eds.; Springer: Singapore, 2016. [Google Scholar]
- Mercado-Mercado, G.; Montalvo-González, E.; Sánchez-Burgos, J. A.; Velázquez-Estrada, R. M.; Álvarez-Parrilla, E.; González-Aguilar, G. A.; Sáyago-Ayerdi, S. G. Optimization of β-Carotene from ‘Ataulfo’ Mango (Mangifera indica L.) By-Products Using Ultrasound-Assisted Extraction. Rev. Mex. Ing. Quim. 2019, 18, 1051–1061. [Google Scholar] [CrossRef]
- Wongkaew, M.; Chaimongkol, P.; Leksawasdi, N.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Phimolsiripol, Y.; Chaiyaso, T.; Ruksiriwanich, W.; Jantrawut, P.; Sommano, S. R. Mango Peel Pectin: Recovery, Functionality and Sustainable Uses. Polymers 2021, 13, 3898. [Google Scholar] [CrossRef]
- Díaz, F.; Duarte, S.; Ferreiro, O. Clarificación de la pulpa extraída de frutos de mango criollo paraguayo (Mangifera indica). Cienc. Lat. Rev. Cient. Multidiscip. 2020, 4, 1524–1531. [Google Scholar]
- Burgos, N.; Valdés, A.; Jiménez, A. Valorization of Agricultural Wastes for the Production of Protein-Based Biopolymers. J. Renew. Mater. 2016, 4, 165–177. [Google Scholar] [CrossRef]
- Matharu, A. S.; Houghton, J. A.; Lucas-Torres, C.; Moreno, A. Acid-Free Microwave-Assisted Hydrothermal Extraction of Pectin and Porous Cellulose from Mango Peel Waste – Towards a Zero Waste Mango Biorefinery. Green Chem. 2016, 18, 5280–5287. [Google Scholar] [CrossRef]
- Djafari Petroudy, S.R. Physical and Mechanical Properties of Natural Fibers. In Advanced High Strength Natural Fibre Composites in Construction; 2017; pp. 59–83. [Google Scholar]
- Maldonado-Celis, M. E.; Yahia, E. M.; Bedoya, R.; Landazuri, P.; Loango, N.; Aguillon, J.; Guerrero Ospina, J. C. Chemical Composition of Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef] [PubMed]
- Mussig, J. Industrial Applications of Natural Fibres: Structure, Properties and Technical Applications; Wiley: Chichester, West Sussex, U.K, 2010. [Google Scholar]
- Mohl, C.; Weimer, T.; Caliskan, M.; Baz, S.; Bauder, H. J.; Gresser, G. T. Development of Natural Fibre-Reinforced Semi-Finished Products with Bio-Based Matrix for Eco-Friendly Composites. Polymers 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Mustata, F. S. C.; Asandulesa, M.; Varganici, C. D.; Curteza, A. Composites Based on Cotton Fabrics, Acrylic Rubber and Powder from Used Tires: Thermal and Electrical Characterization. J. Therm. Anal. Calorim. 2023, 148, 3325–3339. [Google Scholar] [CrossRef]
- Porras, A.; Maranon, A.; Ashcroft, I.A. Characterization of a Novel Natural Cellulose Fabric from Manicaria Saccifera Palm as Possible Reinforcement of Composite Materials. Compos. Part B-Eng 2015, 74, 66–73. [Google Scholar] [CrossRef]
- Lobregas, M. O. S.; Buniao, E. V. D.; Leaño, J. L. Alkali-Enzymatic Treatment of Bambusa Blumeana Textile Fibers for Natural Fiber-Based Textile Material Production. Ind. Crops Prod. 2023, 194. [Google Scholar] [CrossRef]
- Subash, M. C.; Perumalsamy, M. Identification of Efficient Bioprocessing of Banana Pseudostem Waste Biomass for Sustainable Fibers in the Textile Industry. Waste Biomass Valor. 2022, 14, 631–644. [Google Scholar] [CrossRef]
- Lord, P. R. (Ed.) Handbook of Yarn Production: Technology, Science and Economics; Woodhead Publishing: Cambridge, England, 2003; Elsevier. [Google Scholar]
- Shakil, S.; Ullah, R.; Lutfi, M. Process Flow Chart and Factor Analysis in Production of a Jute Mills. J. Ind. Intell. Inf. 2013, 1, 247–254. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP). NREL/TP-510-42618. 2008. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf.
- National Renewable Energy Laboratory. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples: Laboratory Analytical Procedure (LAP). NREL/TP-510-42621, 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42621.
- Alvarez, A.; Cachero, S.; Gonzalez-Sanchez, C.; Montejo-Bernardo, J.; Pizarro, C.; Bueno, J. L. Novel Method for Holocellulose Analysis of Non-Woody Biomass Wastes. Carbohydr. Polym. 2018, 189, 250–256. [Google Scholar] [CrossRef]
- ASTM International. ASTM D1103-60 (1960) Standard Method for Alpha-Cellulose in Wood and Paper.
- Pastore, C.; Kiekens, P.; Eds. Surface Characteristics of Fibers and Textiles; 1st, *!!! REPLACE !!!* (Eds.) ; CRC Press: Boca Raton, FL, 2000.
- Government, R. M. Significance of Alkaline Treatment on the Composition of Mango Seed Shell Fiber for Polymer Composite Application. Indian J. Sci. Technol. 2020, 13, 2168–2174. [Google Scholar] [CrossRef]
- Mamatha, M.; Ramchandran, M.; Kurinjimalar, R. Influence of Chemical Treatment of Natural Fibres Using the SPSS Method. J. Mater. Its Charact. 2023, 2, 28–39. [Google Scholar] [CrossRef]
- Suwinarti, W.; Wulandari, N.; Haqiqi, M. Potential of Natural Fiber Based on Plant Characteristics. IOP Conf. Ser.: Earth Environ. Sci. 2023, 1282, 012036. [Google Scholar] [CrossRef]
- Sathishkumar, T.; Navaneethakrishnan, P.; Shivaram, S.; Kanna, S.; Rajeshkumar, L.; Rajeshkumar, G. Characterization of New Cellulose Fiber Extracted from Pithecellobium dulce Tree. Appl. Sci. Eng. Prog. 2023, 16, 6845. [Google Scholar] [CrossRef]
- Indran, S.; Raj, R. E. Characterization of New Natural Cellulosic Fiber from Cissus Quadrangularis Stem. Carbohydr. Polym. 2015, 117, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Cecci, R. R. R.; Passos, A. A.; de Aguiar Neto, T. C.; Silva, L. A. Banana Pseudostem Fibers Characterization and Comparison with Reported Data on Jute and Sisal Fibers. SN Appl. Sci. 2019, 2. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M. C.; B, A. K.; H, R. M.; Joy, J.; Moores, A.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Tian, D.; Chandra, R. P.; Lee, J. S.; Lu, C.; Saddler, J. N. A Comparison of Various Lignin-Extraction Methods to Enhance the Accessibility and Ease of Enzymatic Hydrolysis of the Cellulosic Component of Steam-Pretreated Poplar. Biotechnol. Biofuels 2017, 10, 157. [Google Scholar] [CrossRef]
- Wan Azelee, N. I.; Md Jahim, J.; Rabu, A.; Abdul Murad, A. M.; Abu Bakar, F. D.; Md Illias, R. Efficient Removal of Lignin with the Maintenance of Hemicellulose from Kenaf by Two-Stage Pretreatment Process. Carbohydr. Polym. 2014, 99, 447–453. [Google Scholar] [CrossRef]
- Kim, I.; Han, J.-I. Optimization of Alkaline Pretreatment Conditions for Enhancing Glucose Yield of Rice Straw by Response Surface Methodology. Biomass Bioenergy 2012, 46, 210–217. [Google Scholar] [CrossRef]
- Oriez, V.; Peydecastaing, J.; Pontalier, P. Y. Lignocellulosic Biomass Fractionation by Mineral Acids and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ching, Y. C.; Chuah, C. H. Applications of Lignocellulosic Fibers and Lignin in Bioplastics: A Review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Amroune, S.; Bezazi, A.; Belaadi, A.; Zhu, C.; Scarpa, F.; Rahatekar, S.; Imad, A. Tensile Mechanical Properties and Surface Chemical Sensitivity of Technical Fibres from Date Palm Fruit Branches (Phoenix dactylifera L.). Compos. Part A-Appl. S. 2015, 71, 95–106. [Google Scholar] [CrossRef]
- Senthamaraikannan, P.; Kathiresan, M. Characterization of Raw and Alkali Treated New Natural Cellulosic Fiber from Coccinia grandis L. Carbohydr. Polym. 2018, 186, 332–343. [Google Scholar] [CrossRef]
- Singh, J.K. , Rout, A.K. Characterization of raw and alkali-treated cellulosic fibers extracted from Borassus flabellifer L. Biomass Conv. Bioref.
- Azanaw, A.; Haile, A.; Gideon, R. K. Extraction and Characterization of Fibers from Yucca Elephantine Plant. Cellulose 2018, 26, 795–804. [Google Scholar] [CrossRef]
- Elmogahzy, Y.E. Engineering Textiles: Integrating the Design and Manufacture of Textile Products, 2nd ed.; Woodhead Publishing: Sawston, UK, 2020; ISBN 9780081024898. [Google Scholar]
- Asmat-Campos, D.; de Oca-Vásquez, G.M.; Rojas-Jaimes, J.; Delfín-Narciso, D.; Juárez-Cortijo, L.; Nazario-Naveda, R.; Batista Meneses, D.; Pereira, R.; de la Cruz, M.S. Cu2O Nanoparticles Synthesized by Green and Chemical Routes, and Evaluation of Their Antibacterial and Antifungal Effect on Functionalized Textiles. Biotechnol. Rep. 2023, 37, e00785. [Google Scholar] [CrossRef]
| Variables | Level | ||
| -1 | 0 | +1 | |
| A: Alkali concentration (%) | 2 | 6 | 10 |
| B: Liquid to solid ratio (g/mL) | 20 | 25 | 30 |
| C: Reaction time (h) | 2 | 4 | 6 |
| Fiber | Cellulose % | Hemicellulose % | Lignin % | References |
|---|---|---|---|---|
| Mango | 49.3 | 31.9 | 12.2 | This work |
| Bamboo | 61.50 | - | 25.11 | [32] |
| Cotton | 82.7 | 5.7 | - | [34] |
| Jute | 50-70 | 12-20 | 5-21 | [35] |
| Sisal | 60-78 | 10-20 | 8-14 | [34,35] |
| Cissus quadrangularis stem | 82.73 | 7.96 | 11.27 | [34] |
| Cissus quadrangularis root | 77.17 | 11.02 | 10.45 | [34] |
| Banana | 60-70 | 10-30 | 5-12 | [34] |
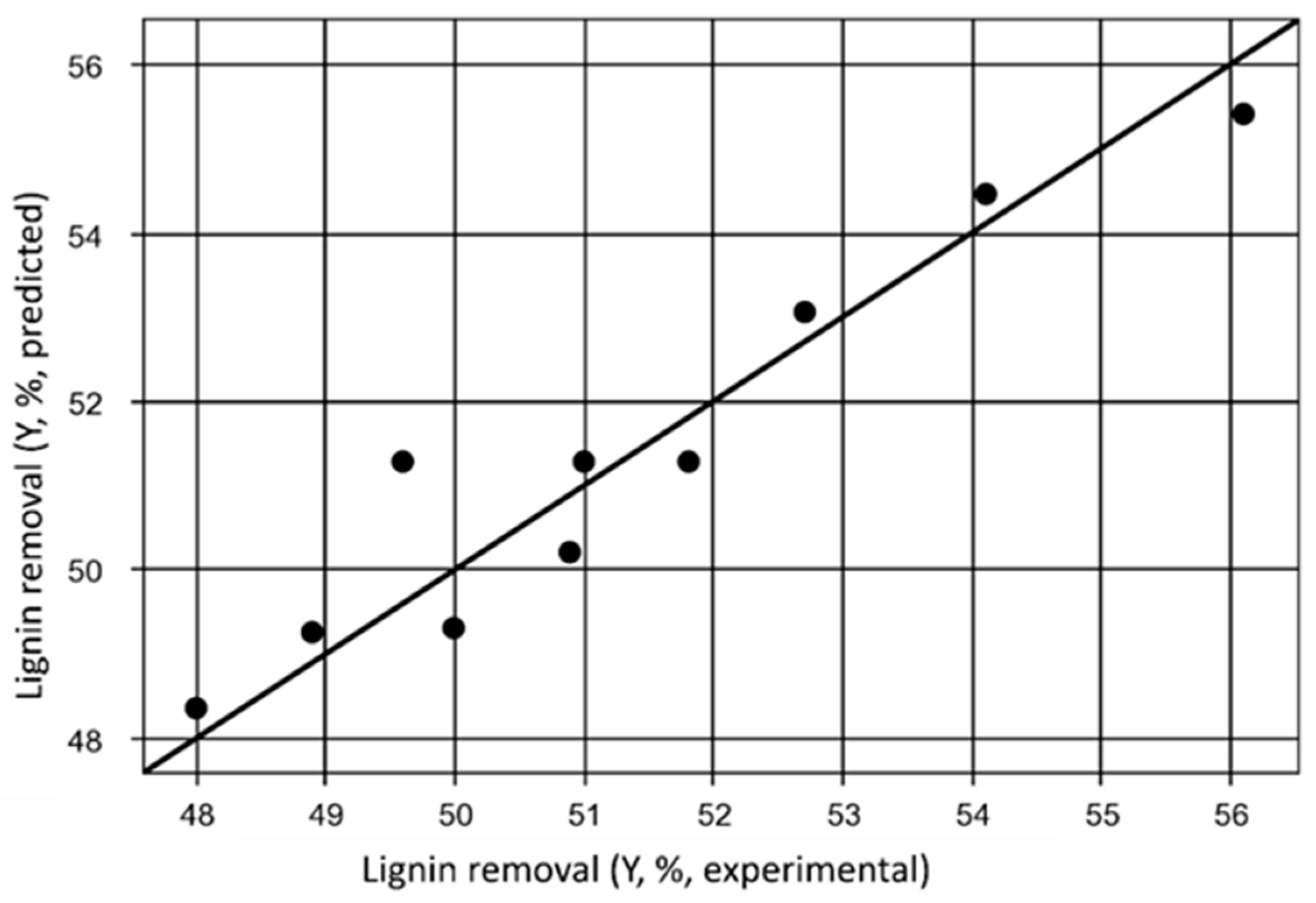
| Experiment | NaOH concentration (%) A | Liquid to solid rate (mL/g) B | Reaction time (h) C | Total ligninremoval(%) |
|---|---|---|---|---|
| 1 | 2 | 20 | 2 | 17.5 |
| 2 | 2 | 20 | 6 | 68.0 |
| 3 | 2 | 30 | 2 | 29.9 |
| 4 | 2 | 30 | 6 | 72.7 |
| 5 | 10 | 20 | 2 | 13.3 |
| 6 | 10 | 20 | 6 | 62.7 |
| 7 | 10 | 30 | 2 | 36.9 |
| 8 | 10 | 30 | 6 | 82.7 |
| 9 | 6 | 25 | 4 | 46.0 |
| 10 | 6 | 25 | 4 | 41.7 |
| 11 | 6 | 25 | 4 | 48.7 |
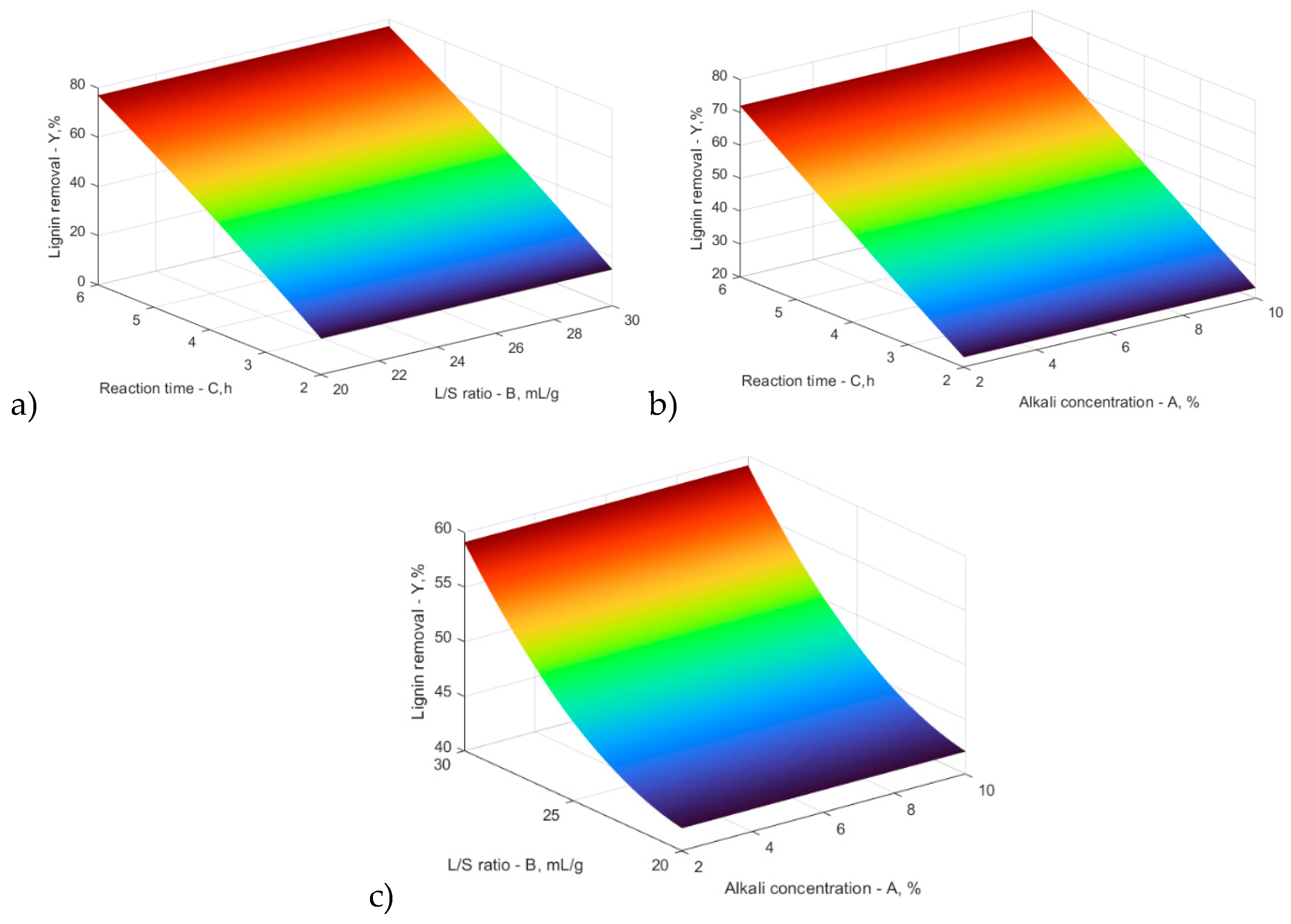
| Variable | Coefficient/Sum of squares | Degree of freedom | Mean sum of squares | F ratio | p | ||
|---|---|---|---|---|---|---|---|
| Model | 47.27 | 0.000 | Significant | ||||
| A – NaOH (%) | 0.93 | 0.4558 | |||||
| B – L/S ratio (mL/g) | 7.58 | 0.0025 | |||||
| C – Time (h) | 23.56 | 0.0000 | |||||
| AB | 3.32 | 0.0416 | |||||
| AC | 0.25 | 0.8339 | |||||
| BC | -1.41 | 0.2787 | |||||
| Regression | 5010.8 | 6 | 835.1 | 82.8 | 0.00038 | ||
| Residual | 40.4 | 4 | 10.1 | ||||
| Lack of fit | 15.6 | 2 | 7.8 | 0.6 | 0.61396 | Non-significant | |
| Pure error | 5051.2 | 10 | |||||
| R2 | 99.20% | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





