Submitted:
06 March 2024
Posted:
07 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
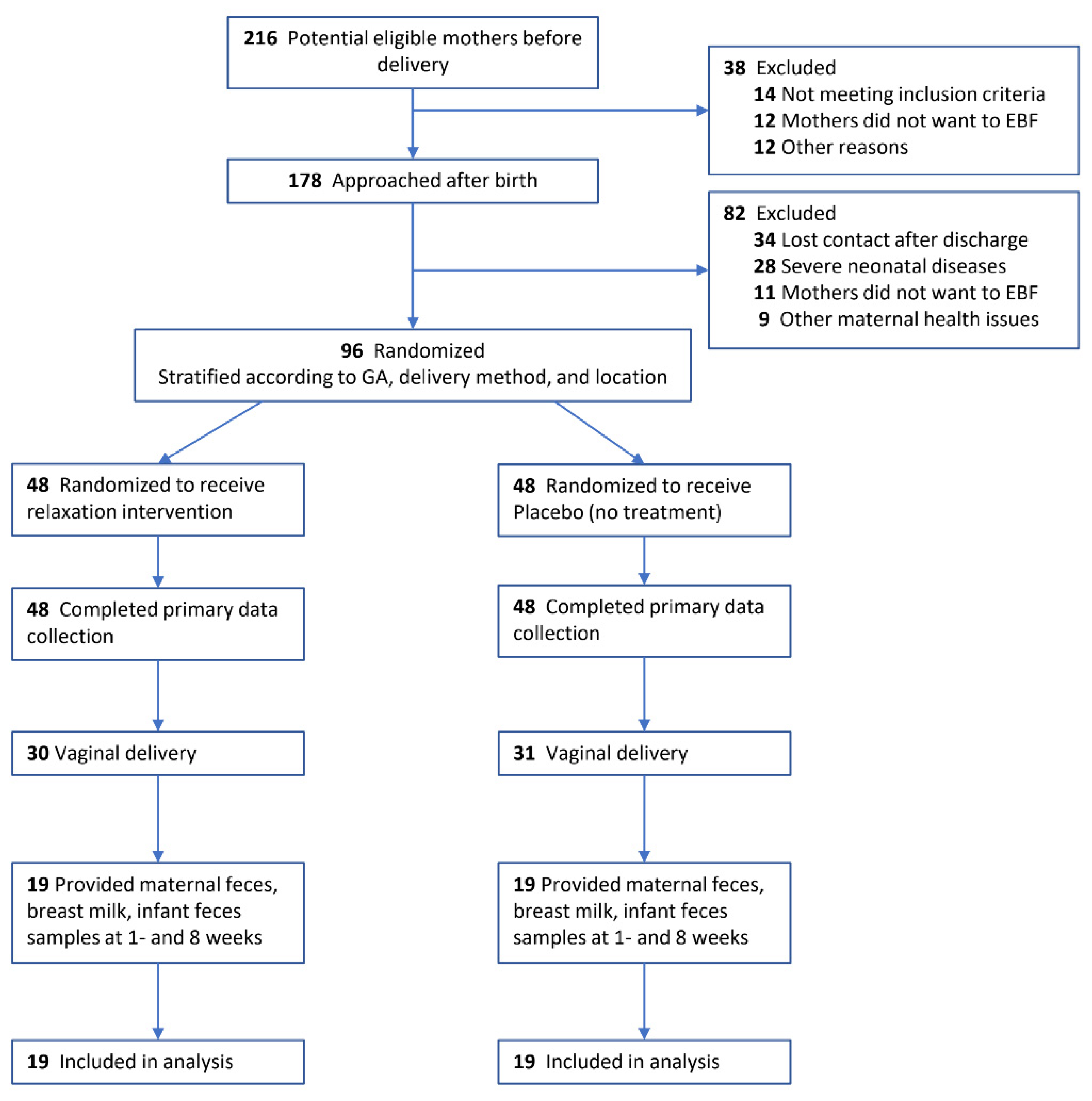
2.1. Study Design and Participants
2.2. Randomization, Procedures, and Intervention
2.3. Outcomes and Measures
2.4. Statistical Analysis
3. Results
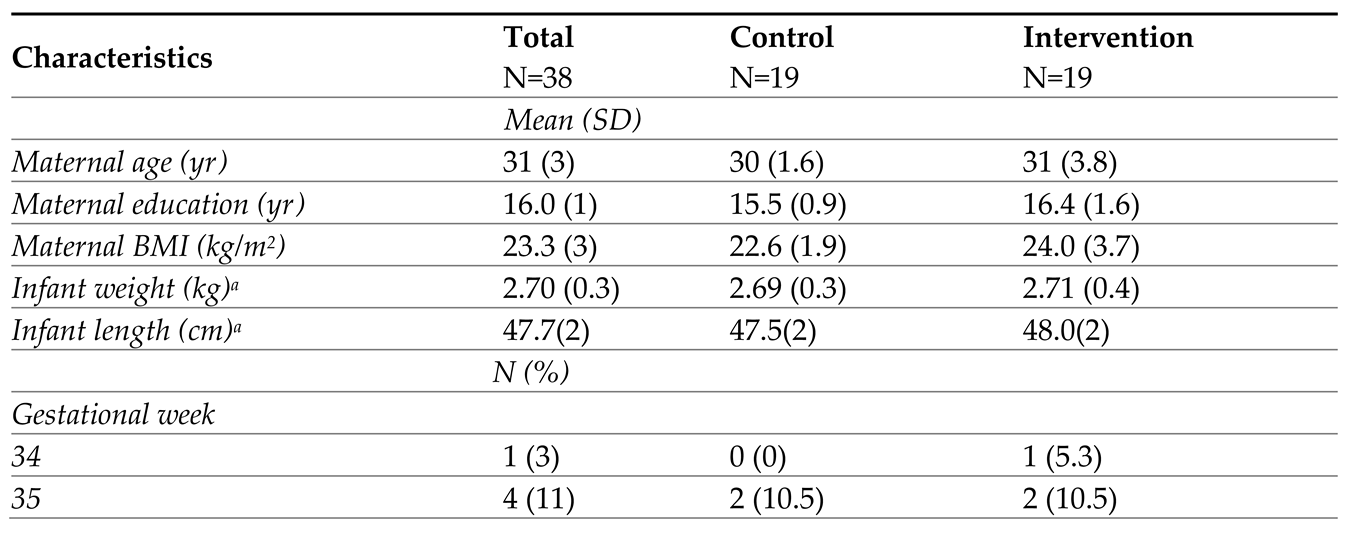
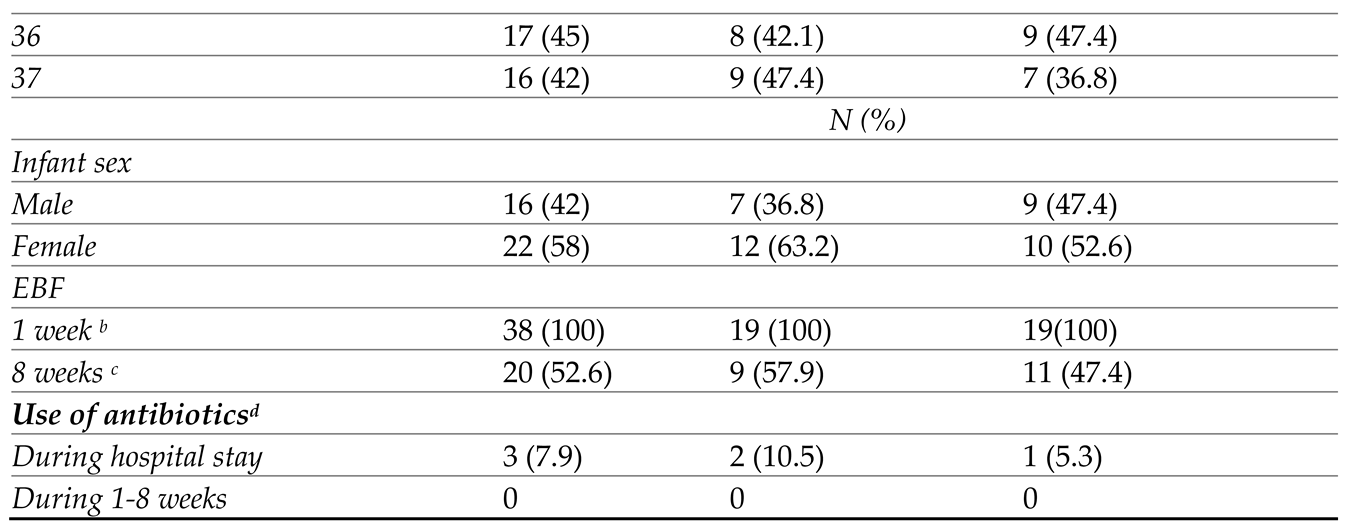
3.1. Study Population and Baseline Characteristics
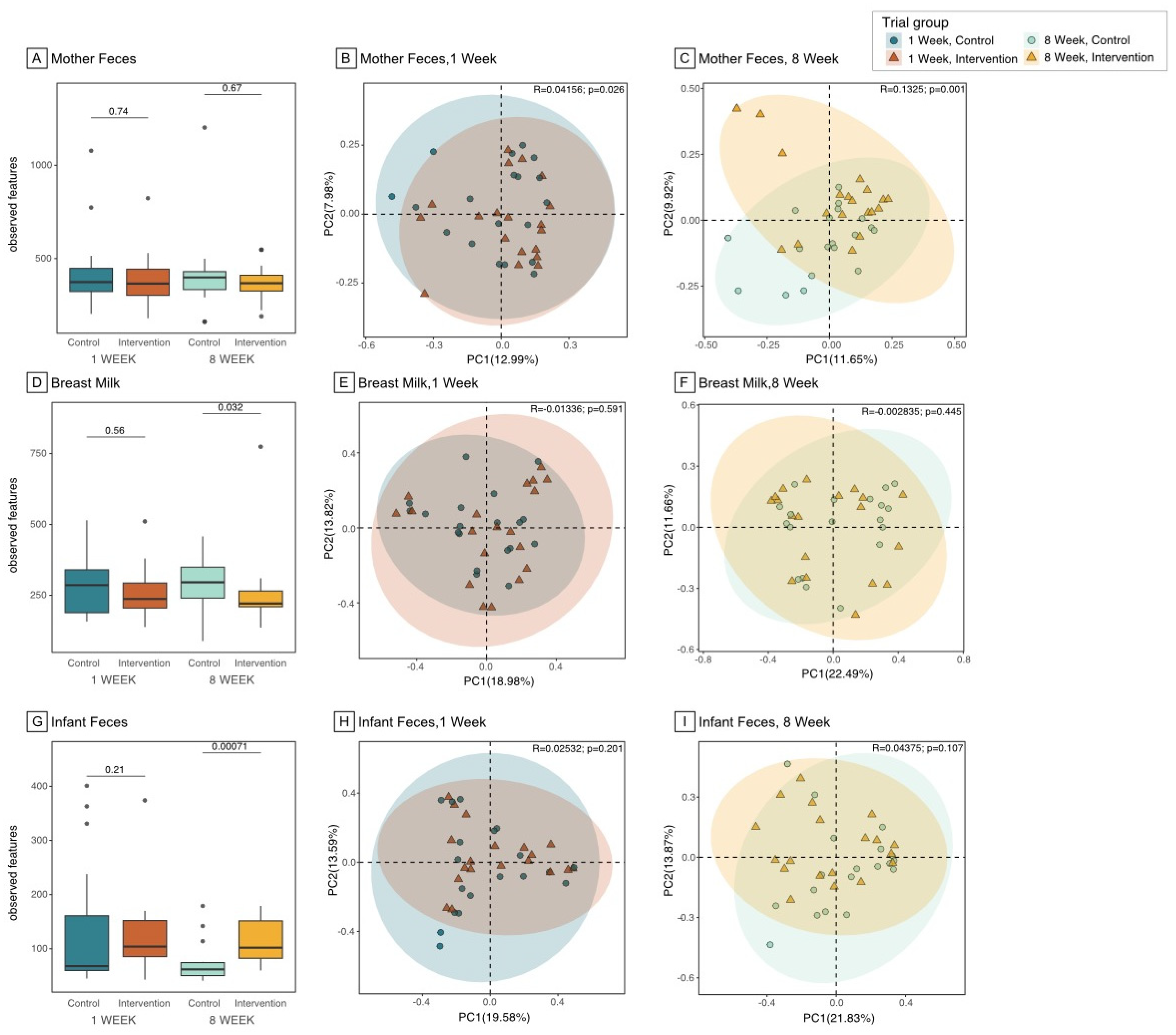
3.2. Microbiome Composition and Diversity in the Maternal Gut
3.3. Microbiome Composition and Diversity in Breast Milk
3.4. Microbiome Composition and Diversity in Infant Gut
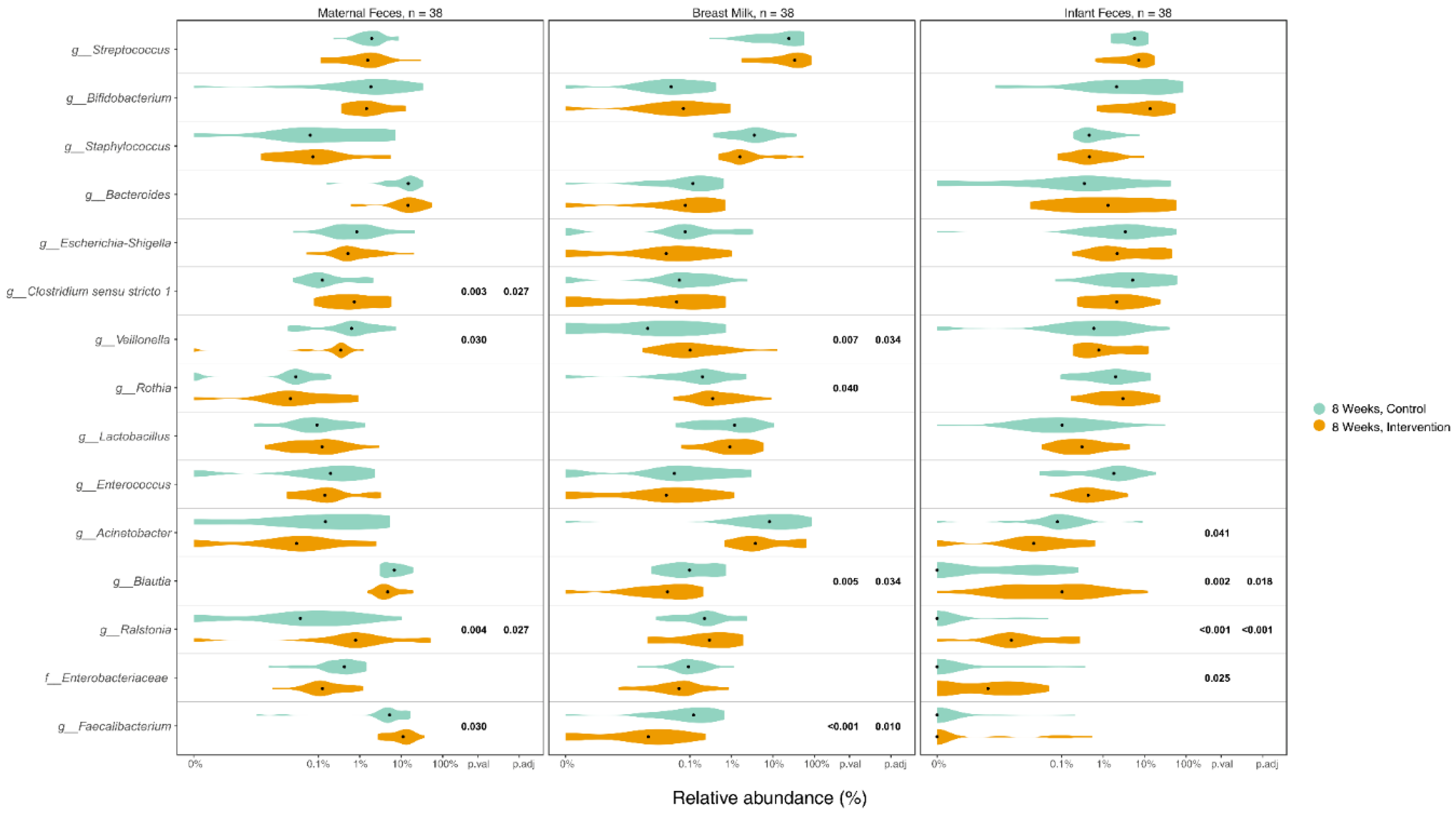
3.5. Differences in Microbiome Composition in Maternal Gut, Breast Milk and Infant Gut between Groups
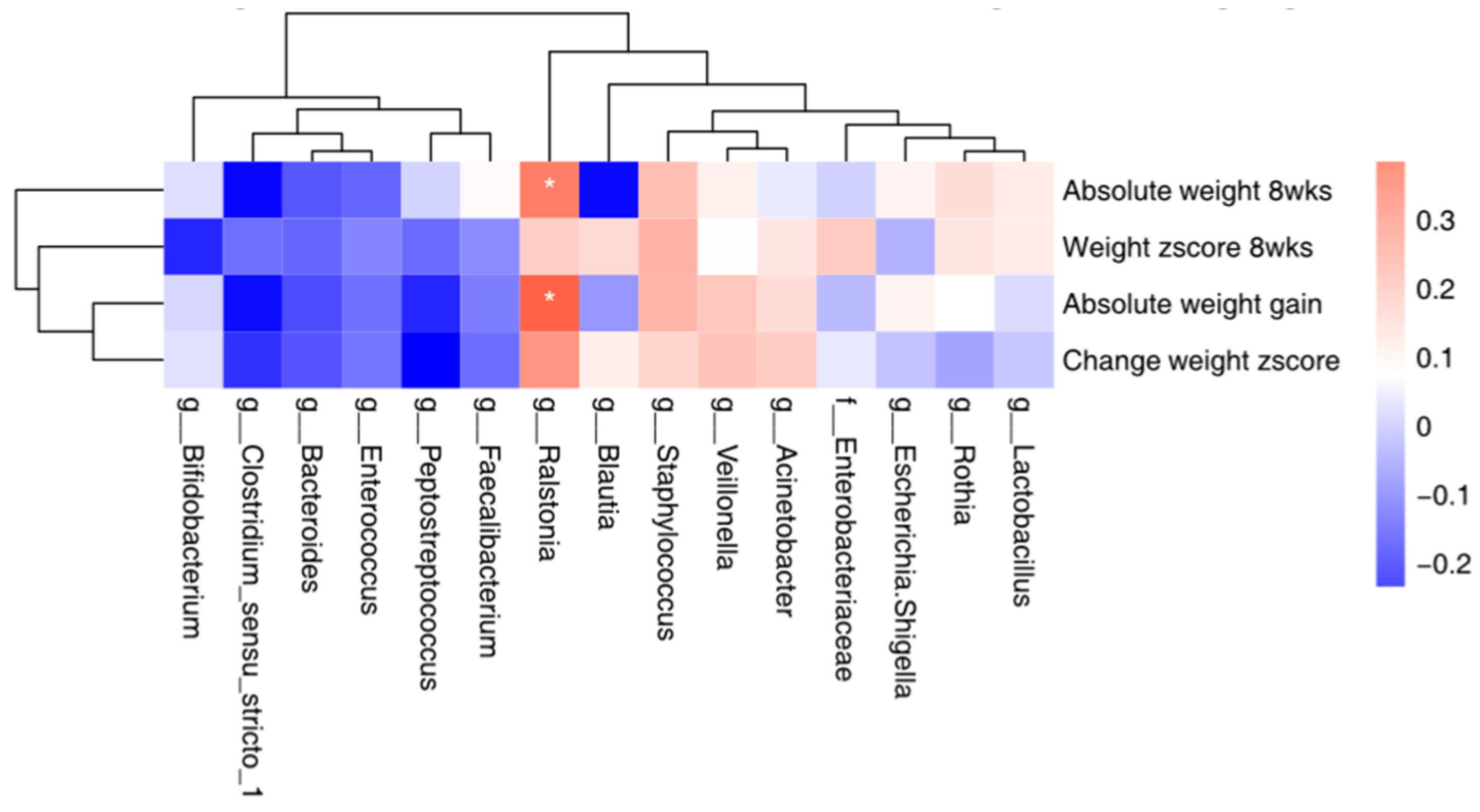
3.6. Correlation between the Top 15 Microbial and Maternal Stress/Infant Weight
3.7. Unintended Effects
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailey, M.T.; Dowd, S.E.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 2011, 25, 397–407. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.M.; Quigley, E.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbe communication in health and disease. Front Physiol 2011, 2, 94. [Google Scholar] [CrossRef]
- Donnet-Hughes, A.; Perez, P.F.; Dore, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J. Potential role of the intestinal microbiota of the mother in neonatal immune education. Proc Nutr Soc 2010, 69, 407–415. [Google Scholar] [CrossRef]
- Fernandez, L.; Langa, S.; Martin, V.; Maldonado, A.; Jimenez, E.; Martin, R.; Rodriguez, J.M. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res 2013, 69, 1–10. [Google Scholar] [CrossRef]
- Perez, P.F.; Dore, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J.; Donnet-Hughes, A. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics 2007, 119, e724–732. [Google Scholar] [CrossRef] [PubMed]
- Kapourchali, F.R.; Cresci, G.A. Early-Life gut microbiome—the importance of maternal and infant factors in its establishment. Nutrition in Clinical Practice 2020, 35, 386–405. [Google Scholar] [CrossRef] [PubMed]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA pediatrics 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Rochat, F.; Chassard, C. Vertical mother–neonate transfer of maternal gut bacteria via breastfeeding. Environmental microbiology 2014, 16, 2891–2904. [Google Scholar] [CrossRef]
- Nagpal, R.; Kurakawa, T.; Tsuji, H.; Takahashi, T.; Kawashima, K.; Nagata, S.; Nomoto, K.; Yamashiro, Y. Evolution of gut Bifidobacterium population in healthy Japanese infants over the first three years of life: a quantitative assessment. Scientific reports 2017, 7, 10097. [Google Scholar] [CrossRef] [PubMed]
- Galley, J.D.; Mashburn-Warren, L.; Blalock, L.C.; Lauber, C.L.; Carroll, J.E.; Ross, K.M.; Hobel, C.; Coussons-Read, M.; Schetter, C.D.; Gur, T.L. Maternal anxiety, depression and stress affects offspring gut microbiome diversity and bifidobacterial abundances. Brain, Behavior, and Immunity 2023, 107, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, J.; Ren, E.; Su, Y.; Zhu, W. Co-occurrence of early gut colonization in neonatal piglets with microbiota in the maternal and surrounding delivery environments. Anaerobe 2018, 49, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Marques, T.M.; Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Ryan, C.A.; Stanton, C. Programming infant gut microbiota: influence of dietary and environmental factors. Current opinion in biotechnology 2010, 21, 149–156. [Google Scholar] [CrossRef]
- Cong, X.; Xu, W.; Romisher, R.; Poveda, S.; Forte, S.; Starkweather, A.; Henderson, W.A. Focus: Microbiome: Gut microbiome and infant health: Brain-gut-microbiota axis and host genetic factors. The Yale journal of biology and medicine 2016, 89, 299. [Google Scholar]
- Tanaka, M.; Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergology International 2017, 66, 515–522. [Google Scholar] [CrossRef]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The human microbiome and child growth–first 1000 days and beyond. Trends in microbiology 2019, 27, 131–147. [Google Scholar] [CrossRef]
- Yu, J.; Wei, Z.; Wells, J.C.; Fewtrell, M. Effects of relaxation therapy on maternal psychological status and infant growth following late preterm and early term delivery: a randomized controlled trial. The American Journal of Clinical Nutrition 2022. [Google Scholar] [CrossRef]
- Dib, S.; Wells, J.C.; Eaton, S.; Fewtrell, M. A Breastfeeding Relaxation Intervention Promotes Growth in Late Preterm and Early Term Infants: Results from a Randomized Controlled Trial. Nutrients 2022, 14, 5041. [Google Scholar] [CrossRef]
- Mohd Shukri, N.H.; Wells, J.; Eaton, S.; Mukhtar, F.; Petelin, A.; Jenko-Pražnikar, Z.; Fewtrell, M. Randomized controlled trial investigating the effects of a breastfeeding relaxation intervention on maternal psychological state, breast milk outcomes, and infant behavior and growth. The American journal of clinical nutrition 2019, 110, 121–130. [Google Scholar] [CrossRef]
- Yu, J.; Wells, J.; Wei, Z.; Fewtrell, M. Effects of relaxation therapy on maternal psychological state, infant growth and gut microbiome: protocol for a randomised controlled trial investigating mother-infant signalling during lactation following late preterm and early term delivery. Int Breastfeed J 2019, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cai, Y.; Dai, W.; Jiang, W.; Tang, W. The difference of gut microbiome in different biliary diseases in infant before operation and the changes after operation. BMC pediatrics 2022, 22, 502. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2′s q2-feature-classifier plugin. Microbiome 2018, 6, 1–17. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell host & microbe 2015, 17, 690–703. [Google Scholar]
- Méndez-Salazar, E.O.; Ortiz-López, M.G.; Granados-Silvestre, M.d.l.Á.; Palacios-González, B.; Menjivar, M. Altered gut microbiota and compositional changes in Firmicutes and Proteobacteria in Mexican undernourished and obese children. Frontiers in microbiology 2018, 2494. [Google Scholar]
- Rajilić-Stojanović, M.; De Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS microbiology reviews 2014, 38, 996–1047. [Google Scholar] [CrossRef]
- LaTuga, M.S.; Stuebe, A.; Seed, P.C. A review of the source and function of microbiota in breast milk. In Proceedings of Seminars in reproductive medicine; pp. 068–073.
- Moloney, R.D.; Desbonnet, L.; Clarke, G.; Dinan, T.G.; Cryan, J.F. The microbiome: stress, health and disease. Mammalian Genome 2014, 25, 49–74. [Google Scholar] [CrossRef]
- Marcobal, A.; Barboza, M.; Froehlich, J.W.; Block, D.E.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Consumption of human milk oligosaccharides by gut-related microbes. J Agric Food Chem 2010, 58, 5334–5340. [Google Scholar] [CrossRef]
- Vazquez, E.; Barranco, A.; Ramirez, M.; Gruart, A.; Delgado-Garcia, J.M.; Martinez-Lara, E.; Blanco, S.; Martin, M.J.; Castanys, E.; Buck, R. , et al. Effects of a human milk oligosaccharide, 2’-fucosyllactose, on hippocampal long-term potentiation and learning capabilities in rodents. J Nutr Biochem 2015, 26, 455–465. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; O’sullivan, A.; Barile, D.; German, J.B.; Lönnerdal, B.; Slupsky, C.M. The human milk metabolome reveals diverse oligosaccharide profiles. The Journal of nutrition 2013, 143, 1709–1718. [Google Scholar] [CrossRef]
- Kortesniemi, M.; Slupsky, C.M.; Aatsinki, A.-K.; Sinkkonen, J.; Karlsson, L.; Linderborg, K.M.; Yang, B.; Karlsson, H.; Kailanto, H.-M. Human milk metabolome is associated with symptoms of maternal psychological distress and milk cortisol. Food Chemistry 2021, 356, 129628. [Google Scholar] [CrossRef]
- Mikami, K.; Kimura, M.; Takahashi, H. Influence of maternal bifidobacteria on the development of gut bifidobacteria in infants. Pharmaceuticals 2012, 5, 629–642. [Google Scholar] [CrossRef]
- Makino, H.; Kushiro, A.; Ishikawa, E.; Kubota, H.; Gawad, A.; Sakai, T.; Oishi, K.; Martin, R.; Ben-Amor, K.; Knol, J. Mother-to-infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant’s microbiota. PloS one 2013, 8, e78331. [Google Scholar] [CrossRef]
- Fernández, L.; Ruiz, L.; Jara, J.; Orgaz, B.; Rodríguez, J.M. Strategies for the preservation, restoration and modulation of the human milk microbiota. Implications for human milk banks and neonatal intensive care units. Frontiers in Microbiology 2018, 9, 2676. [Google Scholar] [CrossRef]
- LeBouder, E.; Rey-Nores, J.E.; Raby, A.-C.; Affolter, M.; Vidal, K.; Thornton, C.A.; Labéta, M.O. Modulation of neonatal microbial recognition: TLR-mediated innate immune responses are specifically and differentially modulated by human milk. The Journal of Immunology 2006, 176, 3742–3752. [Google Scholar] [CrossRef]
- Benítez-Páez, A.; Gómez del Pugar, E.M.; López-Almela, I.; Moya-Pérez, Á.; Codoñer-Franch, P.; Sanz, Y. Depletion of Blautia species in the microbiota of obese children relates to intestinal inflammation and metabolic phenotype worsening. Msystems 2020, 5. [Google Scholar] [CrossRef]
- Salter, S.J.; Cox, M.J.; Turek, E.M.; Calus, S.T.; Cookson, W.O.; Moffatt, M.F.; Turner, P.; Parkhill, J.; Loman, N.J.; Walker, A.W. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC biology 2014, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





