Submitted:
06 March 2024
Posted:
07 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Viruses and Cells
2.2. Animals and Experimental Design
2.3. Sampling, Clinical and Post-Mortem Analysis
2.4. Samples Processing
2.5. Assessment of the Presence of Virus in Blood and Tissues
2.6. Assessment of ASFV-Specific Antibodies and Cytokines in Serum
2.7. Assessment of ASFV-Specific T Cell Cytokine Responses in Blood
2.7.1. IFNγ ELISpot Assay
2.7.2. Flow Cytometric Analysis
2.8. Statistical Data Analysis
3. Results
3.1. Evaluation of Immunization Efficacy
3.1.1. Clinical Signs and Viraemia
3.1.2. Pathological Finding and Presence of Virus Genome in Tissues
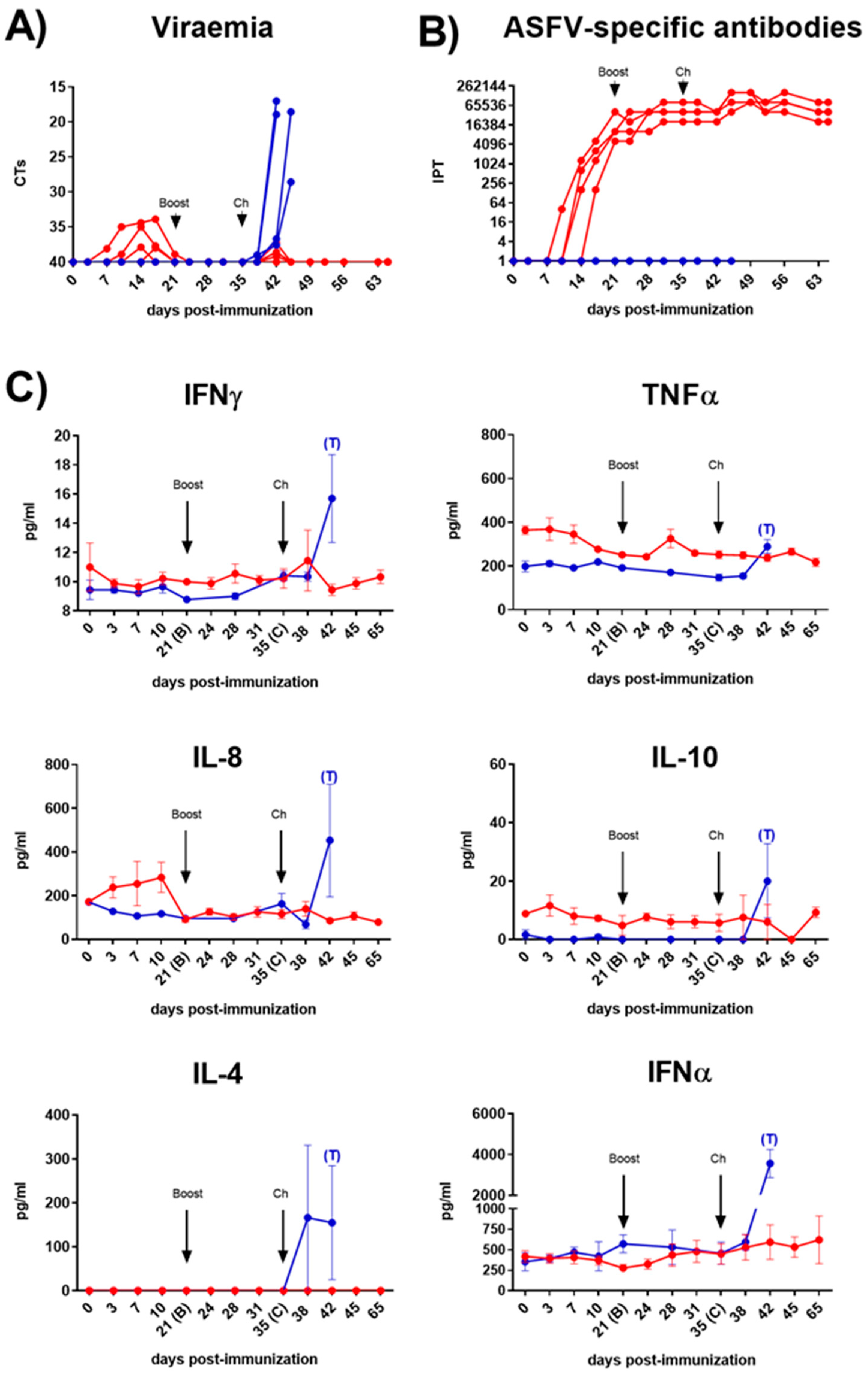
3.2. Evaluation of ASFV-Specific Antibodies and Cytokine Profiles in Serum
3.3. Evaluation of ASFV-Induced Protective Cellular Responses after Immunization with the Lv17/WB/Rie1 Strain and Homologous Virulent Challenge with Armenia/07
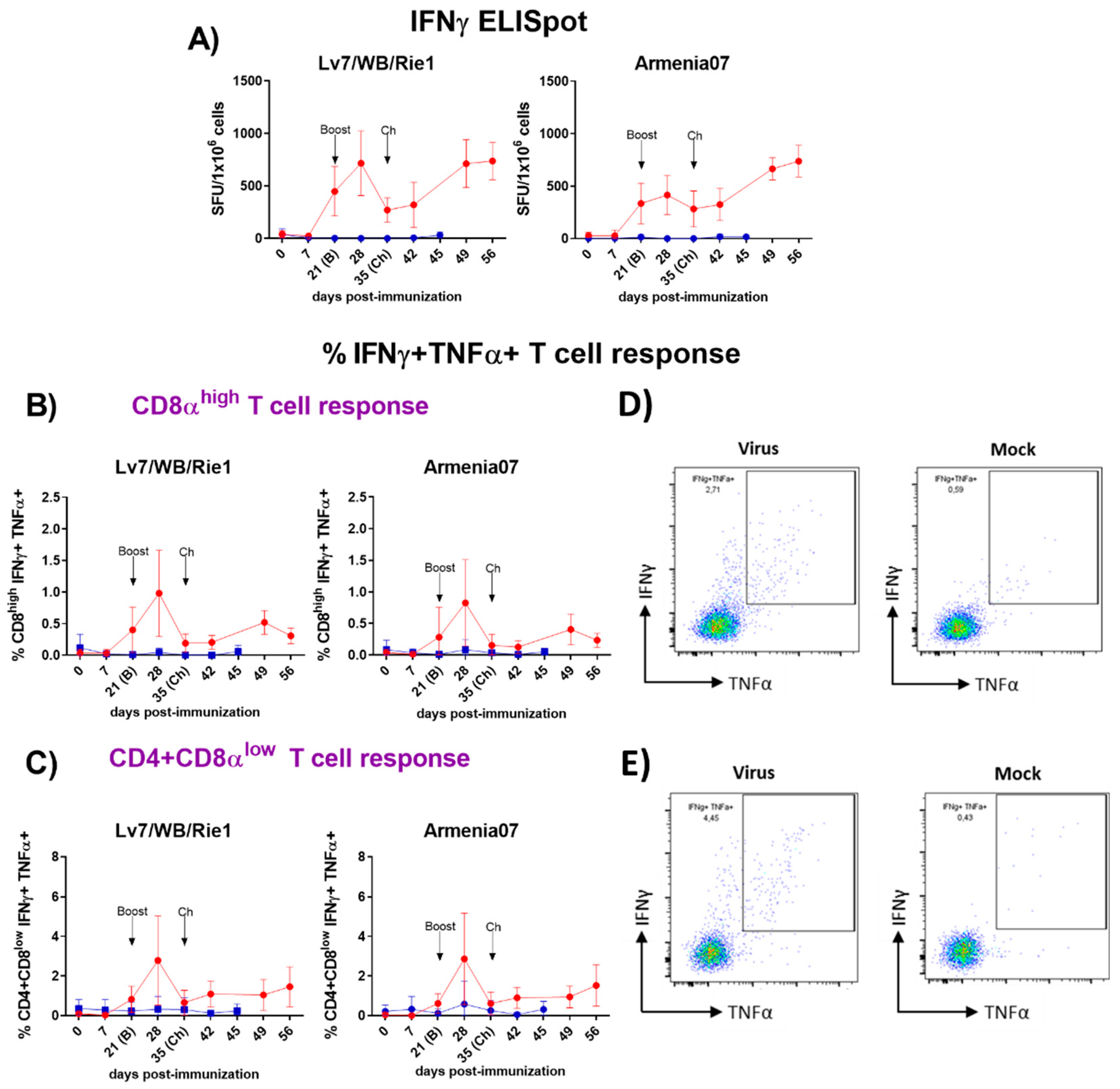
3.3.1. Analysis of Virus-Specific IFNγ Responses by IFNγ T Cell ELISpot
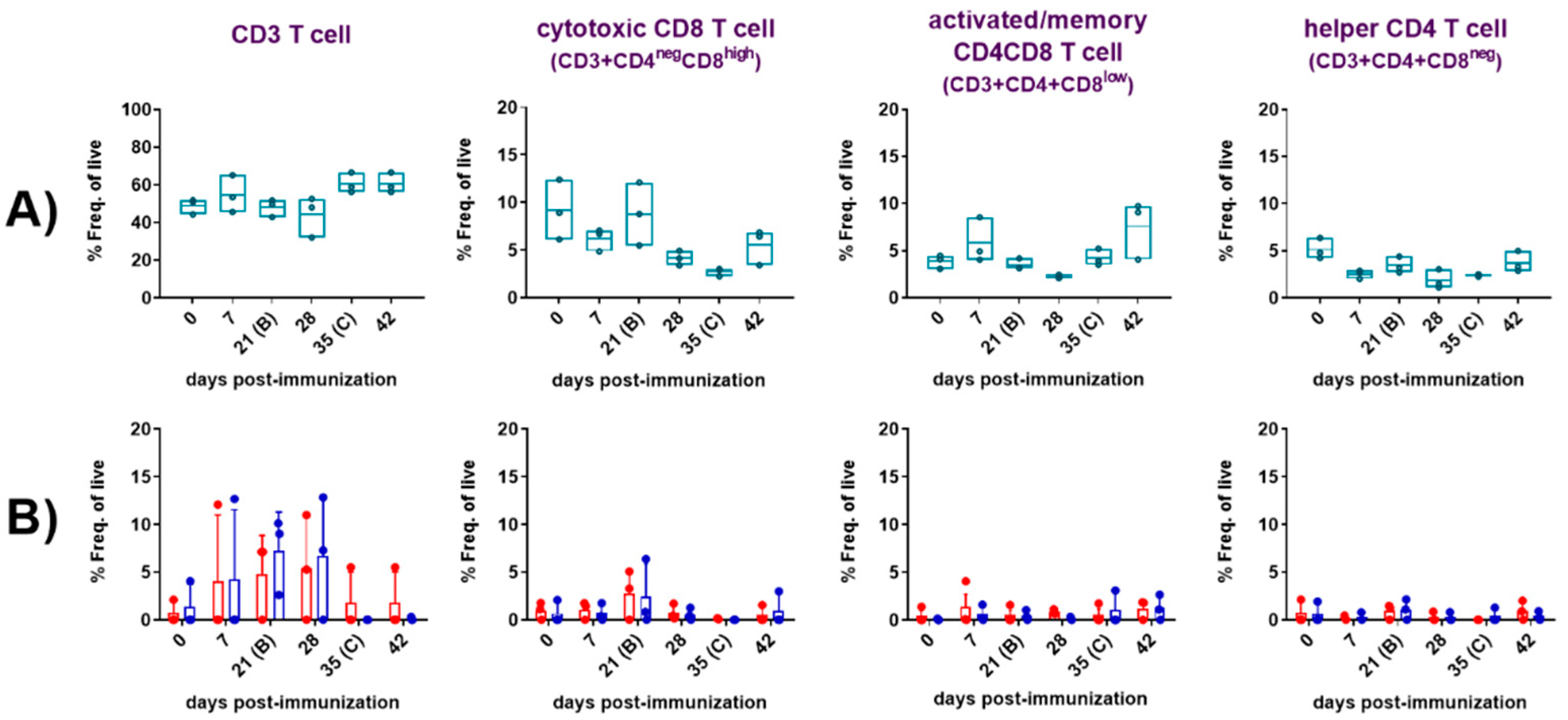
3.3.2. Analysis of Primary Cellular Sources of ASFV-Specific IFNγ and TNFα by Flow Cytometry
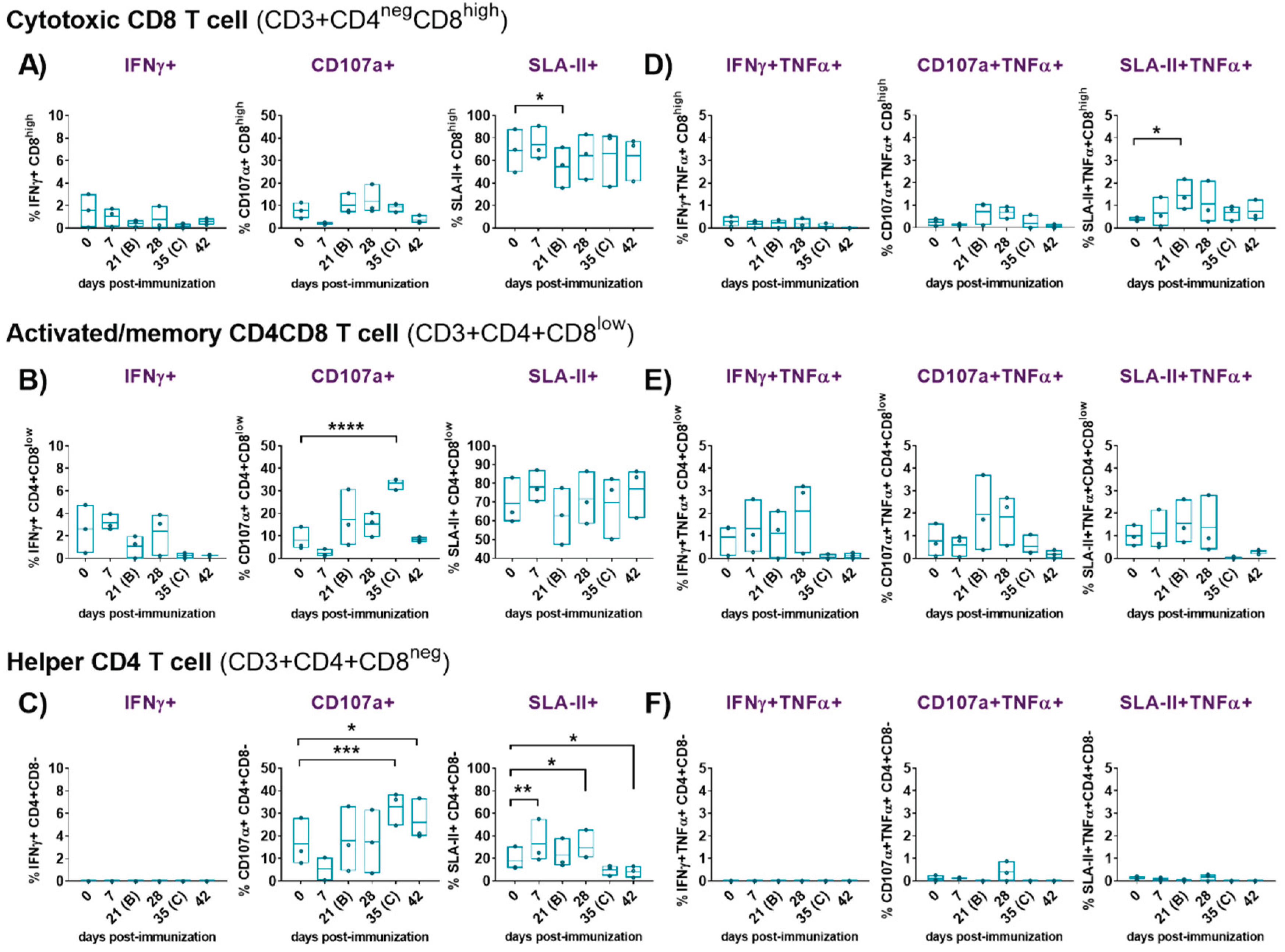
3.3.3. Comprehensive Functional Characterization of ASFV-Induced T-Cell Responses by Flow Cytometry in the Immunized/Challenged Animals
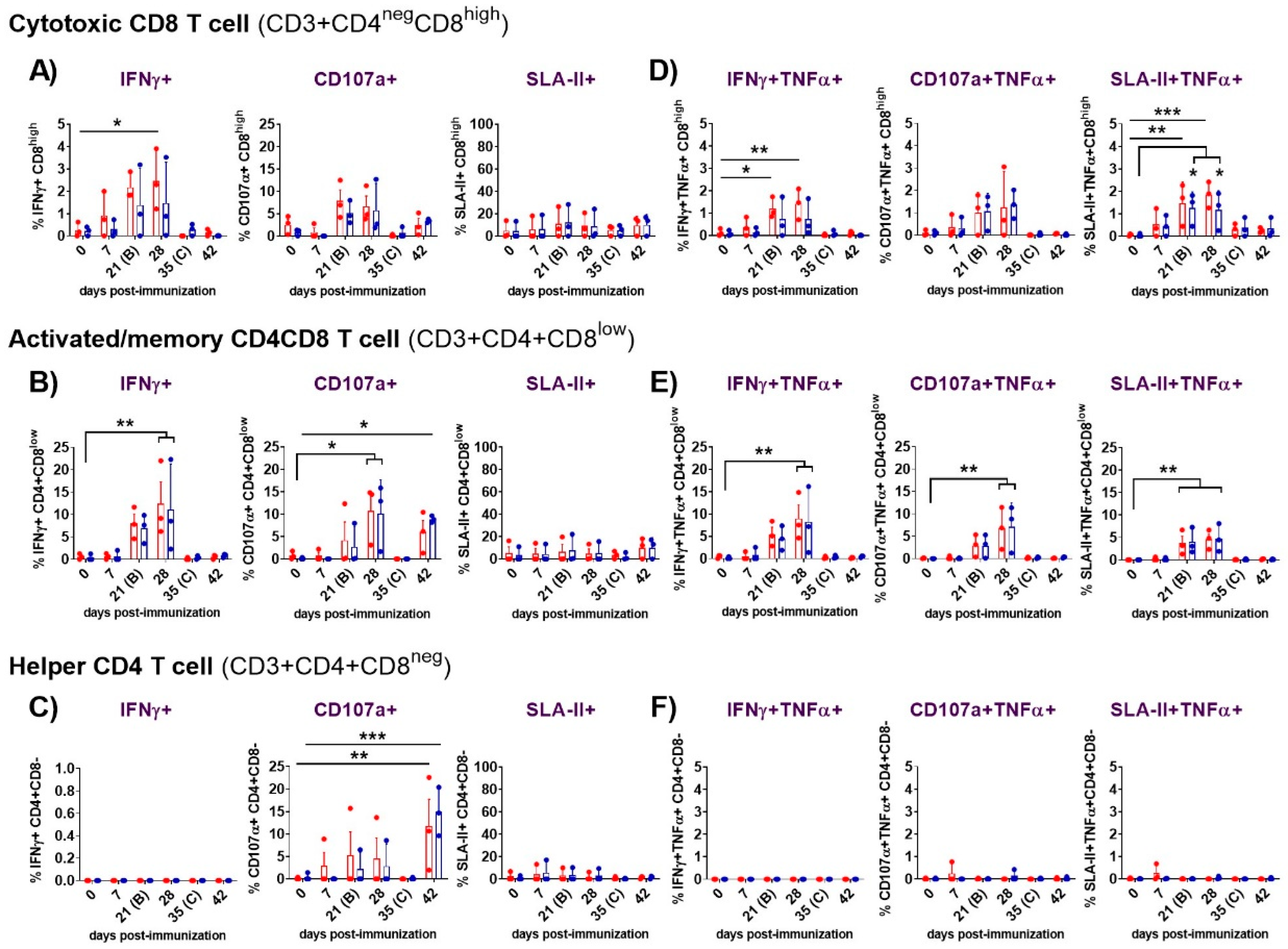
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, M.P.; Tian, K.; Nowotny, N. African Swine Fever, the forgotten pandemic. Transbound Emerg Dis 2021, 68, 2637–2639. [Google Scholar] [CrossRef] [PubMed]
- African swine fever (ASF) - Situation Report 45 - WOAH - World Organisation for Animal Health. 2024.
- Sánchez-Cordón, P.J.; Nunez, A.; Neimanis, A.; Wikström-Lassa, E.; Montoya, M.; Crooke, H.; Gavier-Widén, D. African Swine Fever: Disease Dynamics in Wild Boar Experimentally Infected with ASFV Isolates Belonging to Genotype I and II. Viruses 2019, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Soler, A.; Nurmoja, I.; Cano-Gómez, C.; Cvetkova, S.; Frant, M.; Woźniakowski, G.; Simón, A.; Pérez, C.; Nieto, R.; Arias, M. Dynamics of African swine fever virus (ASFV) infection in domestic pigs infected with virulent, moderate virulent and attenuated genotype II ASFV European isolates. Transbound Emerg Dis 2021, 68, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, E.; Mehinagic, K.; Wüthrich, T.; Hilty, M.; Posthaus, H.; Summerfield, A.; Ruggli, N.; Benarafa, C. The baseline immunological and hygienic status of pigs impact disease severity of African swine fever. PLoS Pathog 2022, 18, e1010522. [Google Scholar] [CrossRef]
- Blome, S.; Gabriel, C.; Beer, M. Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Res 2013, 173, 122–30. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cordón, P.J.; Montoya, M.; Reis, A.L.; Dixon, L.K. African swine fever: A re-emerging viral disease threatening the global pig industry. Vet J 2018, 233, 41–48. [Google Scholar] [CrossRef]
- Rock, D.L. Thoughts on African Swine Fever Vaccines. Viruses 2021, 13, 943. [Google Scholar] [CrossRef]
- Onisk, D.V.; Borca, M.V.; Kutish, G.; Kramer, E.; Irusta, P.; Rock, D.L. Passively transferred African swine fever virus antibodies protect swine against lethal infection. Virology 1994, 198, 350–4. [Google Scholar] [CrossRef]
- Oura, C.A.L.; Denyer, M.S.; Takamatsu, H.; Parkhouse, R.M.E. In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J Gen Virol 2005, 86, 2445–2450. [Google Scholar] [CrossRef]
- King, K.; Chapman, D.; Argilaguet, J.M.; Fishbourne, E.; Hutet, E.; Cariolet, R.; Hutchings, G.; Oura, C.A.; Netherton, C.L.; Moffat, K.; Taylor, G.; Le Potier, M.F.; Dixon, L.K.; Takamatsu, H.H. Protection of European domestic pigs from virulent African isolates of African swine fever virus by experimental immunisation. Vaccine 2011, 29, 4593–600. [Google Scholar] [CrossRef]
- Takamatsu, H.H.; Denyer, M.S.; Lacasta, A.; Stirling, C.M.; Argilaguet, J.M.; Netherton, C.L.; Oura, C.A.; Martins, C.; Rodríguez, F. Cellular immunity in ASFV responses. Virus Res 2013, 173, 110–121. [Google Scholar] [CrossRef]
- Argilaguet, J.M.; Pérez-Martín, E.; López, S.; Goethe, M.; Escribano, J.M.; Giesow, K.; Keil, G.M.; Rodríguez, F. BacMam immunization partially protects pigs against sublethal challenge with African swine fever virus. Antiviral Res 2013, 98, 61–5. [Google Scholar] [CrossRef]
- Lacasta, A.; Ballester, M.; Monteagudo, P.L.; Rodríguez, J.M.; Salas, M.L.; Accensi, F.; Pina-Pedrero, S.; Bensaid, A.; Argilaguet, J.; López-Soria, S.; Hutet, E.; Le Potier, M.F.; Rodríguez, F. Expression library immunization can confer protection against lethal challenge with African swine fever virus. J Virol 2014, 88, 13322–32. [Google Scholar] [CrossRef]
- Schäfer, A.; Franzoni, G.; Netherton, C.L.; Hartmann, L.; Blome, S.; Blohm, U. Adaptive Cellular Immunity against African Swine Fever Virus Infections. Pathogens 2022, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, W.; Jiang, C.; Zhang, X.; Sun, Y.; Liu, R.; Wang, J.; Yang, D.; Zhao, D.; Bu, Z.; He, X. Host Responses to Live-Attenuated ASFV (HLJ/18-7GD). Viruses 2022, 14, 2003. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.; Jurado, C.; Gallardo, C.; Fernández-Pinero, J.; Sánchez-Vizcaíno, J.M. Gaps in African swine fever: Analysis and priorities. Transbound Emerg Dis 2018, 65 Suppl 1, 235–247. [Google Scholar] [CrossRef]
- Gladue, D.P.; Borca, M.V. Recombinant ASF Live Attenuated Virus Strains as Experimental Vaccine Candidates. Viruses 2022, 14, 878. [Google Scholar] [CrossRef] [PubMed]
- Lacasta, A.; Monteagudo, P.L.; Jiménez-Marín, Á.; Accensi, F.; Ballester, M.; Argilaguet, J.; Galindo-Cardiel, I.; Segalés, J.; Salas, M.L.; Domínguez, J.; Moreno, Á.; Garrido, J.J.; Rodríguez, F. Live attenuated African swine fever viruses as ideal tools to dissect the mechanisms involved in viral pathogenesis and immune protection. Vet Res 2015, 46, 135. [Google Scholar] [CrossRef]
- Mulumba-Mfumu, L.K.; Goatley, L.C.; Saegerman, C.; Takamatsu, H.H.; Dixon, L.K. Immunization of African Indigenous Pigs with Attenuated Genotype I African Swine Fever Virus OURT88/3 Induces Protection Against Challenge with Virulent Strains of Genotype I. Transbound Emerg Dis 2016, 63, e323–7. [Google Scholar] [CrossRef]
- Sánchez-Cordón, P.J.; Jabbar, T.; Berrezaie, M.; Chapman, D.; Reis, A.; Sastre, P.; Rueda, P.; Goatley, L.; Dixon, L.K. Evaluation of protection induced by immunisation of domestic pigs with deletion mutant African swine fever virus BeninΔMGF by different doses and routes. Vaccine 2018, 36, 707–715. [Google Scholar] [CrossRef]
- Barasona, J.A.; Gallardo, C.; Cadenas-Fernández, E.; Jurado, C.; Rivera, B.; Rodríguez-Bertos, A.; Arias, M.; Sánchez-Vizcaíno, J.M. First Oral Vaccination of Eurasian Wild Boar Against African Swine Fever Virus Genotype II. Front Vet Science 2019, 6, 137. [Google Scholar] [CrossRef]
- Barasona, J.A.; Cadenas-Fernández, E.; Kosowska, A.; Barroso-Arévalo, S.; Rivera, B.; Sánchez, R.; Porras, N.; Gallardo, C.; Sánchez-Vizcaíno, J.M. Safety of African Swine Fever Vaccine Candidate Lv17/WB/Rie1 in Wild Boar: Overdose and Repeated Doses. Front Immunol 2021, 12, 761753. [Google Scholar] [CrossRef]
- Gallardo, C.; Soler, A.; Rodze, I.; Nieto, R.; Cano-Gómez, C.; Fernandez-Pinero, J.; Arias, M. Attenuated and non-haemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transbound Emerg Dis 2019, 66, 1399–1404. [Google Scholar] [CrossRef]
- Franzoni, G.; Petrini, S.; Mészáros, I.; Dei Giudici, S.; Righi, C.; Olasz, F.; Zinellu, S.; Tamás, V.; Pela, M.; Gallardo, C.; Zádori, Z.; Oggiano, A.; Feliziani, F. Evaluation of Haematological and Immunological Parameters of the ASFV Lv17/WB/Rie1 Strain and Its Derived Mutant Lv17/WB/Rie1/d110-11L against ASFV Challenge Infection in Domestic Pigs. Vaccines 2023, 11, 1277. [Google Scholar] [CrossRef]
- Tamás, V.; Righi, C.; Mészáros, I.; D'Errico, F.; Olasz, F.; Casciari, C.; Zádori, Z.; Magyar, T.; Petrini, S.; Feliziani, F. Involvement of the MGF 110-11L Gene in the African Swine Fever Replication and Virulence. Vaccines 2023, 11, 846. [Google Scholar] [CrossRef]
- Pérez-Núñez, D.; Castillo-Rosa, E.; Vigara-Astillero, G.; García-Belmonte, R.; Gallardo, C.; Revilla, Y. Identification and Isolation of Two Different Subpopulations Within African Swine Fever Virus Arm/07 Stock. Vaccines 2020, 8, 625. [Google Scholar] [CrossRef]
- Carrascosa, A.L.; Bustos, M.J.; de Leon, P. Methods for growing and titrating African swine fever virus: field and laboratory samples. Curr Protoc Cell Biol 2011, Chapter 26, 14.1-26.14.25. [CrossRef]
- Carmina, G.; Nieto, R.; Arias, M. Indirect Immunoperoxidase Test (IPT) for Detection of Antibodies Against African Swine Fever Virus (ASFV) on African Green Monkey Cell Lines (Vero, MS). Methods Mol Biol 2022, 2503, 147–158. [Google Scholar] [CrossRef]
- Gallardo, C.; Soler, A.; Nieto, R.; Sánchez, M.A; Martins, C.; Pelayo, V.; Carrascosa, A.; Revilla, Y.; Simón, A. , Briones, V.; Sánchez-Vizcaíno, J.M.; Arias, M. Experimental Transmission of African Swine Fever (ASF) Low Virulent Isolate NH/P68 by Surviving Pigs. Transbound Emerg Dis 2015, 62, 612-22. [CrossRef]
- Gallardo, C.; Soler, A.; Nieto, R.; Cano, C.; Pelayo, V.; Sánchez, M.A.; Pridotkas, G.; Fernandez-Pinero, J.; Briones, V.; Arias, M. Experimental Infection of Domestic Pigs with African Swine Fever Virus Lithuania 2014 Genotype II Field Isolate. Transbound Emerg Dis 2017, 64, 300–304. [Google Scholar] [CrossRef]
- Sánchez-Cordón, P.J.; Floyd, T.; Hicks, D.; Crooke, H.R.; McCleary, S.; McCarthy, R.R.; Strong, R. , Dixon, L.K.; Neimanis, A.; Wikström-Lassa, E.; Gavier-Widén, D.; Núñez, A. Evaluation of Lesions and Viral Antigen Distribution in Domestic Pigs Inoculated Intranasally with African Swine Fever Virus Ken05/Tk1 (Genotype X). Pathogens 2021, 10, 768. [CrossRef]
- Pedrera, M.; Macchi, F.; McLean, R.K.; Franceschi, V.; Thakur, N.; Russo, L.; Medfai, L.; Todd, S.; Tchilian, E.Z.; Audonnet, J.C.; Chappell, K.; Isaacs, A. , Watterson, D.; Young, P.R.; Marsh, G.A.; Bailey, D.; Graham, S.P.; Donofrio, G. Bovine Herpesvirus-4-Vectored Delivery of Nipah Virus Glycoproteins Enhances T Cell Immunogenicity in Pigs. Vaccines 2020, 8, 115. [CrossRef]
- Terrestrial Manual Online Access - WOAH - World Organisation for Animal Health. 2023.
- Fernández-Pinero, J.; Gallardo, C.; Elizalde, M.; Robles, A.; Gómez, C.; Bishop, R.; Heath, L.; Couacy-Hymann, E.; Fasina, F.O.; Pelayo, V.; Soler, A.; Arias, M. Molecular diagnosis of African Swine Fever by a new real-time PCR using universal probe library. Transbound Emerg Dis 2013, 60, 48–58. [Google Scholar] [CrossRef]
- Franzoni, G.; Kurkure, N.V.; Essler, S.E.; Pedrera, M.; Everett, H.E; Bodman-Smith, K.B.; Crooke, H.R.; Graham, S.P. Proteome-wide screening reveals immunodominance in the CD8 T cell response against classical swine fever virus with antigen-specificity dependent on MHC class I haplotype expression. PloS one 2013, 8, e84246. [Google Scholar] [CrossRef]
- Mokhtar, H.; Pedrera, M. ; Frossard. J.P.; Biffar, L.; Hammer, S.E.; Kvisgaard, L.K.; Larsen, L.E.; Stewart, G.R.; Somavarapu, S.; Steinbach, F.; Graham, S.P. The Non-structural Protein 5 and Matrix Protein Are Antigenic Targets of T Cell Immunity to Genotype 1 Porcine Reproductive and Respiratory Syndrome Viruses. Front Immunol 2016, 7, 40. [Google Scholar] [CrossRef]
- Franzoni, G.; Kurkure, N.V.; Edgar, D.S.; Everett, H.E.; Gerner, W.; Bodman-Smith, K.B.; Crooke, H.R.; Graham, S.P. Assessment of the phenotype and functionality of porcine CD8 T cell responses following vaccination with live attenuated classical swine fever virus (CSFV) and virulent CSFV challenge. Clin Vaccine Immunol 2013, 20, 1604–16. [Google Scholar] [CrossRef] [PubMed]
- Attreed, S.E.; Silva, C. , Abbott, S.; Ramirez-Medina, E.; Espinoza, N.; Borca, M.V.; Gladue, D.P.; Diaz-San Segundo, F. A Highly Effective African Swine Fever Virus Vaccine Elicits a Memory T Cell Response in Vaccinated Swine. Pathogens 2022, 11, 1438. [CrossRef]
- Bosch-Camós, L.; Alonso, U.; Esteve-Codina, A.; Chang, C.Y.; Martín-Mur, B.; Accensi, F.; Muñoz, M.; Navas, M.J.; Dabad, M.; Vidal, E.; Pina-Pedrero, S.; Pleguezuelos, P.; Caratù, G.; Salas, M.L.; Liu, L.; Bataklieva, S.; Gavrilov, B.; Rodríguez, F.; Argilaguet, J. Cross-protection against African swine fever virus upon intranasal vaccination is associated with an adaptive-innate immune crosstalk. PLoS pathog 2022, 18, e1010931. [Google Scholar] [CrossRef] [PubMed]
- Goatley, L.C.; Nash, R.H.; Andrews, C.; Hargreaves, Z.; Tng, P.; Reis, A.L.; Graham, S.P.; Netherton, C.L. Cellular and Humoral Immune Responses after Immunisation with Low Virulent African Swine Fever Virus in the Large White Inbred Babraham Line and Outbred Domestic Pigs. Viruses 2022, 14, 1487. [Google Scholar] [CrossRef]
- Sugimura, T.; Ito, Y.; Tananari, Y.; Ozaki, Y.; Maeno, Y.; Yamaoka, T.; Kudo, Y. Improved antibody responses in infants less than 1 year old using intradermal influenza vaccination. Vaccine 2008, 26, 2700–5. [Google Scholar] [CrossRef]
- Soonawala, D.; Verdijk, P.; Wijmenga-Monsuur, A.J.; Boog, C.J.; Koedam, P.; Visser, L.G.; Rots, N.Y. Intradermal fractional booster dose of inactivated poliomyelitis vaccine with a jet injector in healthy adults. Vaccine 2013, 31, 3688–94. [Google Scholar] [CrossRef] [PubMed]
- Beals, C.R.; Railkar, R.A.; Schaeffer, A.K.; Levin, Y.; Kochba, E.; Meyer, B.K.; Evans, R.K.; Sheldon, E.A.; Lasseter, K.; Lang, N.; Weinberg, A.; Canniff, J.; Levin, M.J. Immune response and reactogenicity of intradermal administration versus subcutaneous administration of varicella-zoster virus vaccine: an exploratory, randomised, partly blinded trial. Lancet Infect Dis 2016, 16, 915–22. [Google Scholar] [CrossRef]
- Schnyder, J.L.; Garcia Garrido, H.M.; De Pijper, C.A.; Daams, J.G.; Stijnis, C.; Goorhuis, A.; Grobusch, M.P. Comparison of equivalent fractional vaccine doses delivered by intradermal and intramuscular or subcutaneous routes: A systematic review. Travel Med Infect Dis 2021, 41, 102007. [Google Scholar] [CrossRef]
- Franzoni, G.; Graham, S.P.; Dei Giudici, S.; Oggiano, A. Porcine Dendritic Cells and Viruses: An Update. Viruses 2019, 11, 445. [Google Scholar] [CrossRef]
- Leitão, A.; Cartaxeiro, C.; Coelho, R.; Cruz, B.; Parkhouse, R.M.E.; Portugal, F.C.; Vigário, J.D.; Martins, C.L.V. The non-haemadsorbing African swine fever virus isolate ASFV/NH/P68 provides a model for defining the protective anti-virus immune response. J Gen Virol 2001, 82, 513–523. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.; Wang, Y.; Wu, S.; Wang, B.; Zhang, J.; Song, X.; Chen, Y.; Lv, P.; Hou, L. Comparative immunogenicity analysis of intradermal versus intramuscular immunization with a recombinant human adenovirus type 5 vaccine against Ebola virus. Front Immunol 2022, 13, 963049. [Google Scholar] [CrossRef] [PubMed]
- Salguero, F.J.; Ruiz-Villamor, E.; Bautista, M.J.; Sánchez-Cordón, P.J.; Carrasco, L.; Gómez-Villamandos, J.C. Changes in macrophages in spleen and lymph nodes during acute African swine fever: expression of cytokines. Vet Immunol Immunopathol 2002, 90, 11–22. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Zhang, Y.; Yang, J.; Wang, L.; Qi, Y.; Han, X.; Zhou, X.; Miao, F.; Chen, T.; Wang, Y.; Zhang, F.; Zhang, S.; Hu, R. Cytokine Storm in Domestic Pigs Induced by Infection of Virulent African Swine Fever Virus. Front Vet Sci 2021, 7, 601641. [Google Scholar] [CrossRef]
- Zakaryan, H.; Cholakyans, V.; Simonyan, L.; Misakyan, A.; Karalova, E.; Chavushyan, A.; Karalyan, Z. A study of lymphoid organs and serum proinflammatory cytokines in pigs infected with African swine fever virus genotype II. Arch Virol 2015, 160, 1407–14. [Google Scholar] [CrossRef]
- Franzoni, G.; Pedrera, M.; Sánchez-Cordón, P.J. African Swine Fever Virus Infection and Cytokine Response In Vivo: An Update. Viruses 2023, 15, 233. [Google Scholar] [CrossRef]
- Barroso-Arévalo, S.; Barasona, J.A.; Cadenas-Fernández, E.; Sánchez-Vizcaíno, J.M. The Role of Interleukine-10 and Interferon-γ as Potential Markers of the Evolution of African Swine Fever Virus Infection in Wild Boar. Pathogens 2021, 10, 757. [Google Scholar] [CrossRef]
- Sánchez-Cordón, P.J.; Jabbar, T.; Chapman, D.; Dixon, L.K.; Montoya, M. Absence of Long-Term Protection in Domestic Pigs Immunized with Attenuated African Swine Fever Virus Isolate OURT88/3 or BeninΔMGF Correlates with Increased Levels of Regulatory T Cells and Interleukin-10. J Virol 2020, 94, e00350–20. [Google Scholar] [CrossRef] [PubMed]
- Gómez del Moral, M.; Ortuño, E.; Fernández-Zapatero, P.; Alonso, F.; Alonso, C.; Ezquerra, A.; Domínguez, J. African swine fever virus infection induces tumor necrosis factor alpha production: implications in pathogenesis. J Virol 1999, 73, 2173–80. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.; O'Donnell, V.; Alfano, M.; Velazquez Salinas, L.; Holinka, L.G.; Krug, P.W.; Gladue, D.P.; Higgs, S.; Borca, M.V. Association of the Host Immune Response with Protection Using a Live Attenuated African Swine Fever Virus Model. Viruses 2016, 8, 291. [Google Scholar] [CrossRef]
- Sánchez-Cordón, P.J.; Chapman, D.; Jabbar, T.; Reis, A.L.; Goatley, L.; Netherton, C.L.; Taylor, G.; Montoya, M.; Dixon, L. Different routes and doses influence protection in pigs immunised with the naturally attenuated African swine fever virus isolate OURT88/3. Antiviral Res 2017, 138, 1–8. [Google Scholar] [CrossRef]
- Saalmüller, A.; Werner, T.; Fachinger, V. T-helper cells from naive to committed. Vet Immunol Immunopathol 2002, 87, 137–45. [Google Scholar] [CrossRef]
- Gerner, W.; Käser, T.; Saalmüller, A. Porcine T lymphocytes and NK cells-an update. Dev Comp Immunol 2009, 33, 310–20. [Google Scholar] [CrossRef]
- Revilla, Y.; Pena, L.; Viñuela, E. Interferon-gamma production by African swine fever virus-specific lymphocytes. Scand J Immunol 1992, 35, 225–30. [Google Scholar] [CrossRef]
- Reis, A.L.; Goatley, L.C.; Jabbar, T.; Sanchez-Cordon, P.J.; Netherton, C.L.; Chapman, D.A.G.; Dixon, L.K. Deletion of the African Swine Fever Virus Gene DP148R Does Not Reduce Virus Replication in Culture but Reduces Virus Virulence in Pigs and Induces High Levels of Protection against Challenge. J Virol 2017, 91, e01428–17. [Google Scholar] [CrossRef] [PubMed]
- Denyer, M.S.; Wileman, T.E.; Stirling, C.M.; Zuber, B.; Takamatsu, H.H. Perforin expression can define CD8 positive lymphocyte subsets in pigs allowing phenotypic and functional analysis of natural killer, cytotoxic T, natural killer T and MHC un-restricted cytotoxic T-cells. Vet Immunol Immunopathol 2006, 110, 279–92. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Díaz, I.; Martín-Valls, G.; Beyersdorf, N. , Mateu, E. Systemic CD4 cytotoxic T cells improve protection against PRRSV-1 transplacental infection. Front Immunol 2023, 13, 1020227. [CrossRef]
- Talker, S.C.; Koinig, H.C.; Stadler, M.; Graage, R.; Klingler, E.; Ladinig, A.; Mair, K.H.; Hammer, S.E.; Weissenböck, H.; Dürrwald, R.; Ritzmann, M.; Saalmüller, A.; Gerner, W. Magnitude and kinetics of multifunctional CD4+ and CD8β+ T cells in pigs infected with swine influenza A virus. Vet Res 2015, 46, 52. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Zani, L.; Pikalo, J.; Hühr, J.; Sehl, J.; Mettenleiter, T.C.; Breithaupt, A.; Blome, S.; Blohm, U. T-cell responses in domestic pigs and wild boar upon infection with the moderately virulent African swine fever virus strain 'Estonia2014'. Transbound Emerg Dis 2021, 68, 2733–2749. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.J.; Cha, S.H.; Grimm, A.L.; Ajithdoss, D.; Rzepka, J.; Chung, G.; Yu, J.; Davis, W.C.; Ho, C.S. Pigs that recover from porcine reproduction and respiratory syndrome virus infection develop cytotoxic CD4+CD8+ and CD4+CD8- T-cells that kill virus infected cells. PloS one 2018, 13, e0203482. [Google Scholar] [CrossRef] [PubMed]
- Preglej, T.; Ellmeier, W. CD4+ Cytotoxic T cells - Phenotype, Function and Transcriptional Networks Controlling Their Differentiation Pathways. Immunol Lett 2022, 247, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, C.; Bustos, M.J.; Granja, A.G.; de León, P.; Sabina, P.; López-Viñas, E.; Gómez-Puertas, P.; Revilla, Y.; Carrascosa, A.L. The African swine fever virus lectin EP153R modulates the surface membrane expression of MHC class I antigens. Arch Virol 2011, 156, 219–34. [Google Scholar] [CrossRef]
| ID domestic pig /Tissue | Pig 6 (D65/30) | Pig 8 (D65/30) |
Pig 9 (D64/29) |
Pig 10 (D64/29) |
|---|---|---|---|---|
| Liver | No Ct | No Ct | No Ct | No Ct |
| Lung | No Ct | No Ct | 33.7 | 35.7 |
| Kidney | 36.98 | No Ct | No Ct | No Ct |
| Heart | No Ct | No Ct | 37.5 | 36.8 |
| Spleen | No Ct | No Ct | No Ct | No Ct |
| Tonsil | No Ct | 37.8 | No Ct | 34 |
| Renal LN* | 39.05 | No Ct | No Ct | No Ct |
| Retropharyngeal LN | No Ct | No Ct | No Ct | No Ct |
| Gastro-hepatic LN | No Ct | No Ct | No Ct | 34.6 |
| Mesenteric LN | No Ct | No Ct | No Ct | 39.2 |
| Mediastinal LN | 33.93 | No Ct | No Ct | 37.8 |
| Inguinal LN | No Ct | No Ct | 38.4 | No Ct |
| Submandibular LN | No Ct | No Ct | No Ct | 36 |
| Splenic LN | No Ct | No Ct | No Ct | 35.3 |
| Popliteal LN | 39.65 | No Ct | 39.6 | 36.3 |
| Bone marrow | No Ct | No Ct | No Ct | No Ct |
| Diaphragm | 39.95 | No Ct | 37.8 | 32.3 |
| Front left IA** | 35.85 | No Ct | No Ct | 34.2 |
| Front right IA | No Ct | No Ct | No Ct | 29.8 |
| Back left IA | No Ct | No Ct | No Ct | 36 |
| Back right IA | No Ct | No Ct | No Ct | 37.3 |
| TOTAL PCR POS. | 6/21 (28.5%) |
1/21 (4,8%) |
5/21 (23.8%) |
14/21 (66.6%) |
| TOTAL VI POS. | 1/21 (4.8%) |
0/21 (0%) |
0/21 (0%) |
5/21 (23.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





