Submitted:
06 March 2024
Posted:
07 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Population:
2.2. Bibliographic Search Strategy and Selection:
2.3. Statistical Analysis:
3. Results
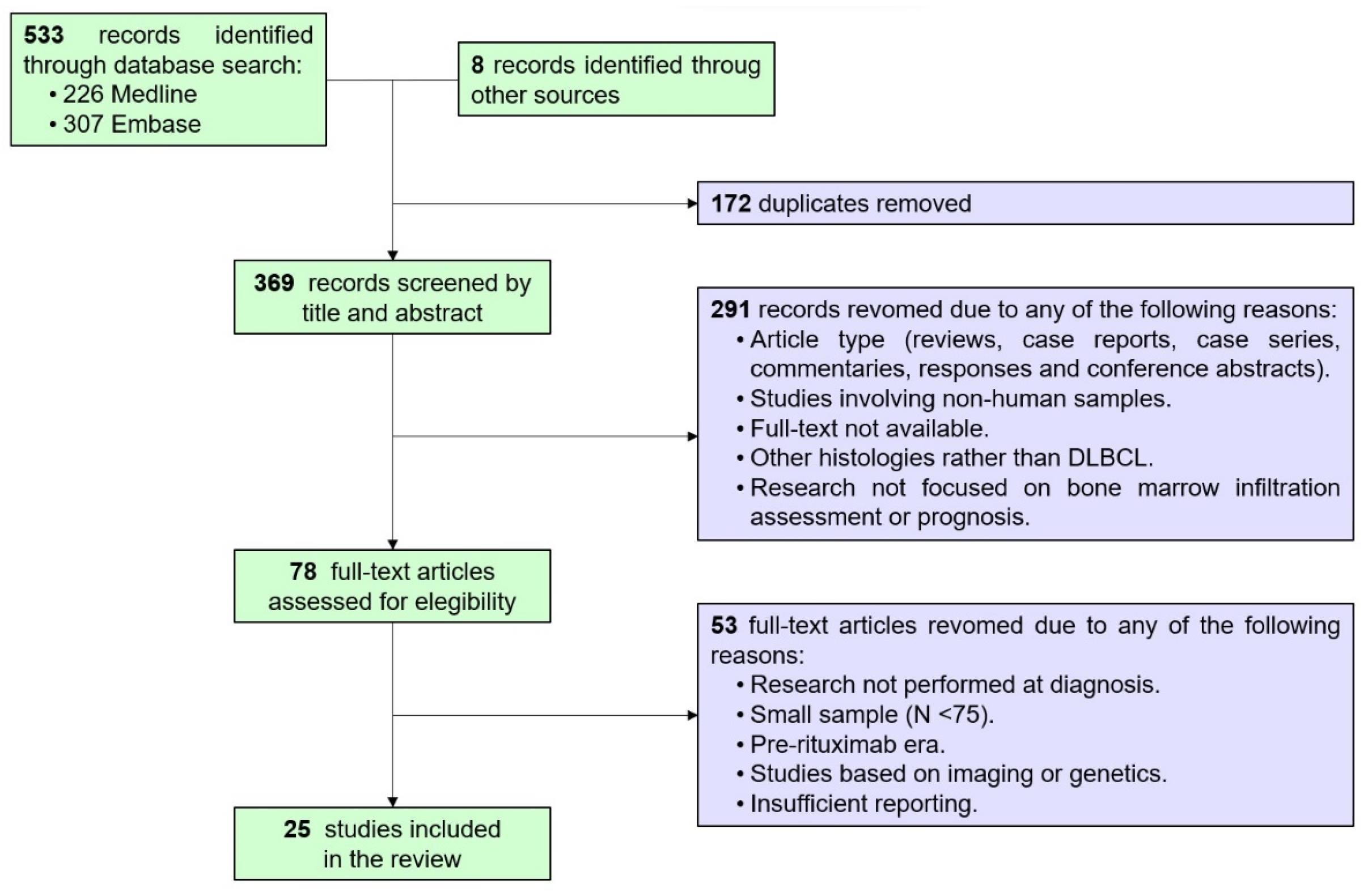
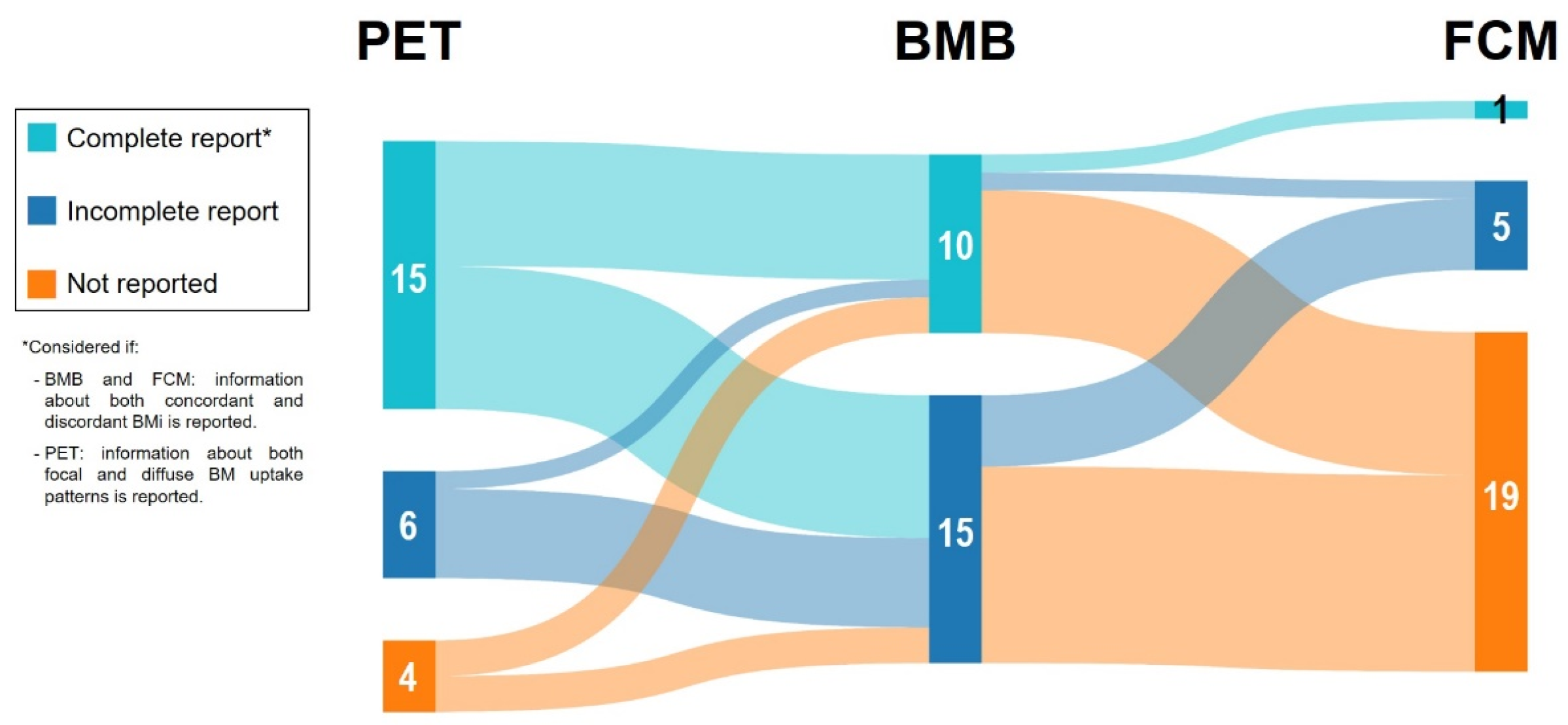
3.1. Description of the Included Studies, Charancteristics of the DLBCL Populations and BM Evaluation:
3.2. Concordance Analysis Among BMB, FCM and PET When Assessing BMi at DLBCL Diagnosis:
3.2.1. Concordance between BMB and FCM in the Setting of DLBCL BMi Assessment:
3.2.2. Concordance between BMB and PET in the Setting of DLBCL BMi Assessment:
3.2.3. Concordance between FCM and PET in the Setting of DLBCL BMi Assessment:
3.3. Diagnostic Accuracy of BMB and PET for Detecting BMi at DLBCL Diagnosis:
3.4. Prognostic Impact of BMi at DLBCL Diagnosis According to BMB, FCM and PET in Comparative Studies:
3.4.1. BMB and FCM Outcomes According to BMi in Newly DLBCL:
3.4.2. BMB and PET Outcomes According to BMi in Newly DLBCL:
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bastos-Oreiro, M.; Muntañola, A.; Panizo, C.; Gonzalez-Barca, E.; de Villambrosia, S.G.; Córdoba, R.; López, J.L.B.; González-Sierra, P.; Terol, M.J.; Gutierrez, A.; Grande, C.; Ramirez, M.J.; Iserte, L.; Perez, E.; Navarro, B.; Gomez, P.; Salar, A.; Luzardo, H.; López, A.; Del Campo, R.; García-Belmonte, D.; Vida, M.J.; Infante, M.; Queizan-Hernandez, J.A.; Novelli, S.; Moreno, M.; Penarrubia, M.; Gómez, J.; Domingo, A.; Donato, E.; Viguria, M.C.; López, F.; Rodriguez, M.J.; Pardal, E.; Noriega, V.; Andreu, R.; Peñalver, J.; Martín, A.; Caballero, D.; López-Guillermo, A. RELINF: Prospective Epidemiological Registry of Lymphoid Neoplasms in Spain. A Project from the GELTAMO Group. Ann Hematol 2020, 99, 799–808. [Google Scholar] [CrossRef]
- Sehn, L.H.; Salles, G. Diffuse Large B-Cell Lymphoma. New England Journal of Medicine 2021, 384, 842–858. [Google Scholar] [CrossRef]
- Lamy, T.; Damaj, G.; Soubeyran, P.; Gyan, E.; Cartron, G.; Bouabdallah, K.; Gressin, R.; Cornillon, J.; Banos, A.; Le Du, K.; Benchalal, M.; Moles, M.-P.; Le Gouill, S.; Fleury, J.; Godmer, P.; Maisonneuve, H.; Deconinck, E.; Houot, R.; Laribi, K.; Marolleau, J.P.; Tournilhac, O.; Branger, B.; Devillers, A.; Vuillez, J.P.; Fest, T.; Colombat, P.; Costes, V.; Szablewski, V.; Béné, M.C.; Delwail, V.; LYSA Group. R-CHOP 14 with or without Radiotherapy in Nonbulky Limited-Stage Diffuse Large B-Cell Lymphoma. Blood 2018, 131, 174–181. [Google Scholar] [CrossRef]
- Poeschel, V.; Held, G.; Ziepert, M.; Witzens-Harig, M.; Holte, H.; Thurner, L.; Borchmann, P.; Viardot, A.; Soekler, M.; Keller, U.; Schmidt, C.; Truemper, L.; Mahlberg, R.; Marks, R.; Hoeffkes, H.-G.; Metzner, B.; Dierlamm, J.; Frickhofen, N.; Haenel, M.; Neubauer, A.; Kneba, M.; Merli, F.; Tucci, A.; de Nully Brown, P.; Federico, M.; Lengfelder, E.; di Rocco, A.; Trappe, R.; Rosenwald, A.; Berdel, C.; Maisenhoelder, M.; Shpilberg, O.; Amam, J.; Christofyllakis, K.; Hartmann, F.; Murawski, N.; Stilgenbauer, S.; Nickelsen, M.; Wulf, G.; Glass, B.; Schmitz, N.; Altmann, B.; Loeffler, M.; Pfreundschuh, M.; FLYER Trial Investigators; German Lymphoma Alliance. Four versus Six Cycles of CHOP Chemotherapy in Combination with Six Applications of Rituximab in Patients with Aggressive B-Cell Lymphoma with Favourable Prognosis (FLYER): A Randomised, Phase 3, Non-Inferiority Trial. Lancet 2019, 394, 2271–2281. [Google Scholar] [CrossRef]
- Tilly, H.; Morschhauser, F.; Sehn, L.H.; Friedberg, J.W.; Trněný, M.; Sharman, J.P.; Herbaux, C.; Burke, J.M.; Matasar, M.; Rai, S.; Izutsu, K.; Mehta-Shah, N.; Oberic, L.; Chauchet, A.; Jurczak, W.; Song, Y.; Greil, R.; Mykhalska, L.; Bergua-Burgués, J.M.; Cheung, M.C.; Pinto, A.; Shin, H.-J.; Hapgood, G.; Munhoz, E.; Abrisqueta, P.; Gau, J.-P.; Hirata, J.; Jiang, Y.; Yan, M.; Lee, C.; Flowers, C.R.; Salles, G. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. New England Journal of Medicine 2022, 386, 351–363. [Google Scholar] [CrossRef]
- Bobillo, S.; Khwaja, J.; Ferreri, A.J.M.; Cwynarski, K. Prevention and Management of Secondary Central Nervous System Lymphoma. Haematologica 2023, 108, 673–689. [Google Scholar] [CrossRef]
- Tilly, H.; Gomes da Silva, M.; Vitolo, U.; Jack, A.; Meignan, M.; Lopez-Guillermo, A.; Walewski, J.; André, M.; Johnson, P.W.; Pfreundschuh, M.; Ladetto, M.; ESMO Guidelines Committee. Diffuse Large B-Cell Lymphoma (DLBCL): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol 2015, 26 (Suppl. 5), v116–v125. [Google Scholar] [CrossRef]
- Bain, B.J. Morbidity Associated with Bone Marrow Aspiration and Trephine Biopsy - a Review of UK Data for 2004. Haematologica 2006, 91, 1293–1294. [Google Scholar]
- Brunetti, G.A.; Tendas, A.; Meloni, E.; Mancini, D.; Maggiore, P.; Scaramucci, L.; Giovannini, M.; Niscola, P.; Cartoni, C.; Alimena, G. Pain and Anxiety Associated with Bone Marrow Aspiration and Biopsy: A Prospective Study on 152 Italian Patients with Hematological Malignancies. Ann Hematol 2011, 90, 1233–1235. [Google Scholar] [CrossRef]
- Berthet, L.; Cochet, A.; Kanoun, S.; Berriolo-Riedinger, A.; Humbert, O.; Toubeau, M.; Dygai-Cochet, I.; Legouge, C.; Casasnovas, O.; Brunotte, F. In Newly Diagnosed Diffuse Large B-Cell Lymphoma, Determination of Bone Marrow Involvement with 18 F-FDG PET/CT Provides Better Diagnostic Performance and Prognostic Stratification Than Does Biopsy. J Nucl Med 2013, 54, 1244–1250. [Google Scholar] [CrossRef]
- El-Galaly, T.C.; Gormsen, L.C.; Hutchings, M. PET/CT for Staging; Past, Present, and Future. Semin Nucl Med 2018, 48, 4–16. [Google Scholar] [CrossRef]
- Adams, H.J.A.; Kwee, T.C. Do Not Abandon the Bone Marrow Biopsy yet in Diffuse Large B-Cell Lymphoma. J Clin Oncol 2015, 33, 1217. [Google Scholar] [CrossRef]
- Adams, H.J.A.; Kwee, T.C. Increased Bone Marrow FDG Uptake at PET/CT Is Not a Sufficient Proof of Bone Marrow Involvement in Diffuse Large B-Cell Lymphoma. Am J Hematol 2015, 90, E182–E183. [Google Scholar] [CrossRef]
- Avigdor, A. Staging DLBCL: Bone Marrow Biopsy or PET-CT? Blood 2013, 122, 4–5. [Google Scholar] [CrossRef]
- Adams, H.J.A.; Kwee, T.C.; de Keizer, B.; Fijnheer, R.; de Klerk, J.M.H.; Nievelstein, R.A.J. FDG PET/CT for the Detection of Bone Marrow Involvement in Diffuse Large B-Cell Lymphoma: Systematic Review and Meta-Analysis. Eur J Nucl Med Mol Imaging 2014, 41, 565–574. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, M.; Liu, J.; Huang, G. Prognostic Value of Bone Marrow FDG Uptake Pattern of PET/CT in Newly Diagnosed Diffuse Large B-Cell Lymphoma. J. Cancer 2018, 9, 1231–1238. [Google Scholar] [CrossRef]
- Elstrom, R.L.; Tsai, D.E.; Vergilio, J.-A.; Downs, L.H.; Alavi, A.; Schuster, S.J. Enhanced Marrow [18F]Fluorodeoxyglucose Uptake Related to Myeloid Hyperplasia in Hodgkin’s Lymphoma Can Simulate Lymphoma Involvement in Marrow. Clin Lymphoma 2004, 5, 62–64. [Google Scholar] [CrossRef]
- Akin, O.; Brennan, S.B.; Dershaw, D.D.; Ginsberg, M.S.; Gollub, M.J.; Schöder, H.; Panicek, D.M.; Hricak, H. Advances in Oncologic Imaging: Update on 5 Common Cancers. CA Cancer J Clin 2012, 62, 364–393. [Google Scholar] [CrossRef]
- Jerusalem, G.; Beguin, Y.; Najjar, F.; Hustinx, R.; Fassotte, M.F.; Rigo, P.; Fillet, G. Positron Emission Tomography (PET) with 18F-Fluorodeoxyglucose (18F-FDG) for the Staging of Low-Grade Non-Hodgkin’s Lymphoma (NHL). Ann Oncol 2001, 12, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Cerci, J.J.; Györke, T.; Fanti, S.; Paez, D.; Meneghetti, J.C.; Redondo, F.; Celli, M.; Auewarakul, C.; Rangarajan, V.; Gujral, S.; Gorospe, C.; Campo, M.V.; Chung, J.-K.; Morris, T.P.; Dondi, M.; Carr, R. Combined PET and Biopsy Evidence of Marrow Involvement Improves Prognostic Prediction in Diffuse Large B-Cell Lymphoma. J Nucl Med 2014, 55, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Pfistner, B.; Juweid, M.E.; Gascoyne, R.D.; Specht, L.; Horning, S.J.; Coiffier, B.; Fisher, R.I.; Hagenbeek, A.; Zucca, E.; Rosen, S.T.; Stroobants, S.; Lister, T.A.; Hoppe, R.T.; Dreyling, M.; Tobinai, K.; Vose, J.M.; Connors, J.M.; Federico, M.; Diehl, V.; International Harmonization Project on Lymphoma. Revised Response Criteria for Malignant Lymphoma. J Clin Oncol 2007, 25, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Quintario, M.A.; Gomez, P.; Yuste-Del Pozo, V.; Valencia-Mesa, A.L.; Sosa, G.; Ricard, P.; Hijas-Gómez, A.I.; Pinedo, F.; Arguelles, M. Bone Marrow Trephine Biopsy Involvement by Lymphoma: Pattern of Involvement and Concordance with Flow Cytometry, in 10 Years from a Single Institution. Clin Transl Oncol 2016, 18, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Talaulikar, D.; Dahlstrom, J.E. Staging Bone Marrow in Diffuse Large B-Cell Lymphoma: The Role of Ancillary Investigations. Pathology 2009, 41, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Pittaluga, S.; Tierens, A.; Dodoo, Y.L.; Delabie, J.; De Wolf-Peeters, C. How Reliable Is Histologic Examination of Bone Marrow Trephine Biopsy Specimens for the Staging of Non-Hodgkin Lymphoma? A Study of Hairy Cell Leukemia and Mantle Cell Lymphoma Involvement of the Bone Marrow Trephine Specimen by Histologic, Immunohistochemical, and Polymerase Chain Reaction Techniques. Am J Clin Pathol 1999, 111, 179–184. [Google Scholar] [CrossRef]
- Tierens, A.M.; Holte, H.; Warsame, A.; Ikonomou, I.M.; Wang, J.; Chan, W.C.; Delabie, J. Low Levels of Monoclonal Small B Cells in the Bone Marrow of Patients with Diffuse Large B-Cell Lymphoma of Activated B-Cell Type but Not of Germinal Center B-Cell Type. Haematologica 2010, 95, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Stacchini, A.; Demurtas, A.; Godio, L.; Martini, G.; Antinoro, V.; Palestro, G. Flow Cytometry in the Bone Marrow Staging of Mature B-Cell Neoplasms. Cytometry B Clin Cytom 2003, 54, 10–18. [Google Scholar] [CrossRef]
- Shim, H.; Oh, J.-I.; Park, S.H.; Jang, S.; Park, C.-J.; Huh, J.; Suh, C.; Chi, H.-S. Prognostic Impact of Concordant and Discordant Cytomorphology of Bone Marrow Involvement in Patients with Diffuse, Large, B-Cell Lymphoma Treated with R-CHOP. J Clin Pathol 2013, 66, 420–425. [Google Scholar] [CrossRef]
- Sehn, L.H.; Scott, D.W.; Chhanabhai, M.; Berry, B.; Ruskova, A.; Berkahn, L.; Connors, J.M.; Gascoyne, R.D. Impact of Concordant and Discordant Bone Marrow Involvement on Outcome in Diffuse Large B-Cell Lymphoma Treated With R-CHOP. JCO 2011, 29, 1452–1457. [Google Scholar] [CrossRef]
- Chung, R.; Lai, R.; Wei, P.; Lee, J.; Hanson, J.; Belch, A.R.; Turner, A.R.; Reiman, T. Concordant but Not Discordant Bone Marrow Involvement in Diffuse Large B-Cell Lymphoma Predicts a Poor Clinical Outcome Independent of the International Prognostic Index. Blood 2007, 110, 1278–1282. [Google Scholar] [CrossRef]
- Yao, Z.; Deng, L.; Xu-Monette, Z.Y.; Manyam, G.C.; Jain, P.; Tzankov, A.; Visco, C.; Bhagat, G.; Wang, J.; Dybkaer, K.; Tam, W.; Hsi, E.D.; Van Krieken, J.H.; Ponzoni, M.; Ferreri, A.J.M.; Møller, M.B.; Winter, J.N.; Piris, M.A.; Fayad, L.; Liu, Y.; Song, Y.; Orlowski, R.Z.; Kantarjian, H.; Medeiros, L.J.; Li, Y.; Cortes, J.; Young, K.H. Concordant Bone Marrow Involvement of Diffuse Large B-Cell Lymphoma Represents a Distinct Clinical and Biological Entity in the Era of Immunotherapy. Leukemia 2018, 32, 353–363. [Google Scholar] [CrossRef]
- Chigrinova, E.; Mian, M.; Scandurra, M.; Greiner, T.C.; Chan, W.C.; Vose, J.M.; Inghirami, G.; Chiappella, A.; Baldini, L.; Ponzoni, M.; Ferreri, A.J.M.; Franceschetti, S.; Gaidano, G.; Tucci, A.; Facchetti, F.; Lazure, T.; Lambotte, O.; Montes-Moreno, S.; Piris, M.A.; Nomdedeu, J. Fr.; Uccella, S.; Rancoita, P.M.V.; Kwee, I.; Zucca, E.; Bertoni, F. Diffuse Large B-cell Lymphoma with Concordant Bone Marrow Involvement Has Peculiar Genomic Profile and Poor Clinical Outcome. Hematological Oncology 2011, 29, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-J.; Park, S.-H.; Park, P.-W.; Seo, Y.-H.; Kim, K.-H.; Seo, J.-Y.; Jeong, J.-H.; Kim, M.J.; Ahn, J.-Y.; Hong, J. Prognostic Impact of Concordant and Discordant Bone Marrow Involvement and Cell-of-Origin in Korean Patients with Diffuse Large B-Cell Lymphoma Treated with R-CHOP. J Clin Pathol 2015, 68, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, M.; El-Galaly, T.C.; Hutchings, M.; Hansen, J.W.; Loft, A.; Johnsen, H.E.; Iyer, V.; Wilson, D.; Sehn, L.H.; Savage, K.J.; Connors, J.M.; Gascoyne, R.D.; Johansen, P.; Clasen-Linde, E.; Brown, P.; Villa, D. The Value of Routine Bone Marrow Biopsy in Patients with Diffuse Large B-Cell Lymphoma Staged with PET/CT: A Danish-Canadian Study. Annals of Oncology 2016, 27, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Soydal, C.; Koksoy, E.B.; Yasar, A.; Turgal, E.; Erdogan, B.D.; Akbulut, H.; Kucuk, N.O. Prognostic Importance of Bone Marrow Uptake on Baseline 18F-FDG Positron Emission Tomography in Diffuse Large B Cell Lymphoma. Cancer Biother Radiopharm 2016, 31, 361–365. [Google Scholar] [CrossRef]
- Khan, A.B.; Barrington, S.F.; Mikhaeel, N.G.; Hunt, A.A.; Cameron, L.; Morris, T.; Carr, R. PET-CT Staging of DLBCL Accurately Identifies and Provides New Insight into the Clinical Significance of Bone Marrow Involvement. Blood 2013, 122, 61–67. [Google Scholar] [CrossRef]
- Hong, J.; Lee, Y.; Park, Y.; Kim, S.G.; Hwang, K.H.; Park, S.H.; Jeong, J.; Kim, K.-H.; Ahn, J.Y.; Park, S.; Park, J.; Lee, J.H. Role of FDG-PET/CT in Detecting Lymphomatous Bone Marrow Involvement in Patients with Newly Diagnosed Diffuse Large B-Cell Lymphoma. Ann Hematol 2012, 91, 687–695. [Google Scholar] [CrossRef]
- Bo, G.; Ran, Q.; Yang, G.Z.; Fen, L.Y.; Lei, G.; Rong, H.W. Diagnostic Efficacy of 18F-FDG PET/CT in Detecting Bone Marrow Infiltration in Patients with Newly Diagnosed Diffuse Large B-Cell Lymphoma. Biomed Environ Sci 2023, 36, 510–516. [Google Scholar] [CrossRef]
- Han, E.J.; O, J.H.; Yoon, H.; Ha, S.; Yoo, I.R.; Min, J.W.; Choi, J.-I.; Choi, B.-O.; Park, G.; Lee, H.H.; Jeon, Y.-W.; Min, G.-J.; Cho, S.-G. Comparison of FDG PET/CT and Bone Marrow Biopsy Results in Patients with Diffuse Large B Cell Lymphoma with Subgroup Analysis of PET Radiomics. Diagnostics 2022, 12, 222. [Google Scholar] [CrossRef]
- Kim, M.; Ahn, S.-Y.; Ahn, J.-S.; Song, G.-Y.; Jung, S.-H.; Lee, J.-J.; Kim, H.-J.; Lee, J.H.; Shin, M.-G.; Song, S.Y.; Yang, D.-H. Diagnostic Accuracy and Prognostic Relevance of Immunoglobulin Heavy Chain Rearrangement and 18F-FDG-PET/CT Compared With Unilateral Bone Marrow Trephination for Detecting Bone Marrow Involvement in Patients With Diffuse Large B-Cell Lymphoma. J Korean Med Sci 2022, 37, e2. [Google Scholar] [CrossRef]
- Okamoto, H.; Uoshima, N.; Muramatsu, A.; Isa, R.; Fujino, T.; Matsumura-Kimoto, Y.; Tsukamoto, T.; Mizutani, S.; Shimura, Y.; Kobayashi, T.; Kawata, E.; Uchiyama, H.; Kuroda, J.; Kyoto Clinical Hematology Study Group Investigators. Combination of Bone Marrow Biopsy and Flow Cytometric Analysis: The Prognostically Relevant Central Approach for Detecting Bone Marrow Invasion in Diffuse Large B-Cell Lymphoma. Diagnostics 2021, 11, 1724. [Google Scholar] [CrossRef]
- Lim, C.H.; Hyun, S.H.; Cho, Y.S.; Choi, J.Y.; Lee, K.-H. Prognostic Significance of Bone Marrow 2-[18F]-Fluoro-2-Deoxy-d-Glucose Uptake in Diffuse Large B-Cell Lymphoma: Relation to Iliac Crest Biopsy Results. Clinical Radiology 2021, 76, 550.e19–550.e28. [Google Scholar] [CrossRef]
- Saiki, Y.; Tomita, N.; Uchida, A.; Uemura, Y.; Suzuki, Y.; Hirakawa, T.; Kato, M.; Hoshikawa, M.; Kawano, T.; Nakamura, N.; Miura, I.; Arai, A. Biopsy Remains Indispensable for Evaluating Bone Marrow Involvement in DLBCL Patients despite the Use of Positron Emission Tomography. Int J Hematol 2021, 113, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Martín-Moro, F.; Piris-Villaespesa, M.; Marquet-Palomanes, J.; García-Cosío, M.; Villarrubia, J.; Lario, A.; García, I.; Michael, B.; Roldán, E.; García-Vela, J.A.; Lopez-Jiménez, J. Bone Marrow Infiltration by Flow Cytometry at Diffuse Large B-cell Lymphoma NOS Diagnosis Implies Worse Prognosis without Considering Bone Marrow Histology. Cytometry Part B Clinical 2020, 98, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabbagh, A.; Ibrahim, F.; Szabados, L.; Soliman, D.S.; Taha, R.Y.; Fernyhough, L.J. The Role of Integrated Positron Emission Tomography/Computed Tomography (PET/CT) and Bone Marrow Examination in Staging Large B-Cell Lymphoma. Clin Med Insights Oncol 2020, 14, 117955492095309. [Google Scholar] [CrossRef] [PubMed]
- Min, G.-J.; Jeon, Y.-W.; Park, S.-S.; Shin, S.-H.; Yahng, S.-A.; Yoon, J.-H.; Lee, S.-E.; Cho, B.-S.; Eom, K.-S.; Kim, Y.-J.; Lee, S.; Kim, H.-J.; Min, C.-K.; Kim, D.-W.; Lee, J.-W.; Cho, S.-G. Poor Prognosis in Patients with Diffuse Large B Cell Lymphomas with Bone Marrow Involvement Possessing Chromosomal Abnormalities, despite Aggressive Treatment. Ann Hematol 2020, 99, 557–570. [Google Scholar] [CrossRef]
- Kandeel, A.A.; Hussein, M.; Zidan, L.; Younis, J.; Edesa, W.; Alsayed, Y. Diagnostic Performance of 18F-2-Fluoro-2-Deoxy-D-Glucose PET/Computerized Tomography in Identifying Bone Marrow Infiltration in New Patients with Diffuse Large B-Cell Lymphoma and Hodgkin Lymphoma. Nuclear Medicine Communications 2020, 41, 269–279. [Google Scholar] [CrossRef]
- Alonso-Álvarez, S.; Alcoceba, M.; García-Álvarez, M.; Blanco, O.; Rodríguez, M.; Baile, M.; Caballero, J.C.; Dávila, J.; Vidriales, M.B.; Esteban, C.; Arias, P.; Díaz, L.G.; Tamayo, P.; Caballero, M.D.; Gutiérrez, N.C.; González, M.; Martín, A. Biological Features and Prognostic Impact of Bone Marrow Infiltration in Patients with Diffuse Large B-Cell Lymphoma. Cancers 2020, 12, 474. [Google Scholar] [CrossRef]
- Wang, J.; Kim, D.; Kang, W.J.; Cho, H. Prognostic Value of Bone Marrow F-18 FDG Uptake in Patients with Advanced-Stage Diffuse Large B-Cell Lymphoma. Nucl Med Mol Imaging 2020, 54, 28–34. [Google Scholar] [CrossRef]
- Greenbaum, U.; Levi, I.; Madmoni, O.; Lior, Y.; Al-Athamen, K.; Perry, Z.H.; Hatzkelzon, L.; Shubinsky, G. The Prognostic Significance of Bone Marrow Involvement in Diffuse Large B Cell Lymphoma According to the Flow Cytometry. Leukemia & Lymphoma 2019, 60, 2477–2482. [Google Scholar] [CrossRef]
- Chen-Liang, T.; Martín-Santos, T.; Jerez, A.; Rodríguez-García, G.; Senent, L.; Martínez-Millán, C.; Muiña, B.; Orero, M.; Teruel, A.; Martín, A.; Gómez-Espuch, J.; Kennedy, K.; Benet, C.; Raya, J.M.; Fernández-González, M.; De La Cruz, F.; Guinot, M.; Villegas, C.; Ballester, I.; Baile, M.; Moya, M.; López-Jiménez, J.; Frutos, L.; Navarro, J.L.; Uña, J.; Fernández-López, R.; Igua, C.; Contreras, J.; Sánchez-Vañó, R.; Cozar, M.D.P.; Tamayo, P.; Mucientes, J.; Sánchez-Blanco, J.J.; Pérez-Ceballos, E.; Ortuño, F.J. Bone Marrow Biopsy Superiority over PET / CT in Predicting Progression-free Survival in a Homogeneously-treated Cohort of Diffuse Large B-cell Lymphoma. Cancer Medicine 2017, 6, 2507–2514. [Google Scholar] [CrossRef]
- Vishnu, P.; Wingerson, A.; Lee, M.; Mandelson, M.T.; Aboulafia, D.M. Utility of Bone Marrow Biopsy and Aspirate for Staging of Diffuse Large B Cell Lymphoma in the Era of Positron Emission Tomography With 2-Deoxy-2-[Fluorine-18]Fluoro-Deoxyglucose Integrated With Computed Tomography. Clinical Lymphoma Myeloma and Leukemia 2017, 17, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-H.; Sun, J.; Wang, L.; Fan, L.; Chen, Y.-Y.; Qu, X.-Y.; Li, T.-N.; Li, J.-Y.; Xu, W. Prognostic Significance of Bone Marrow Infiltration Detected by PET-CT in Newly Diagnosed Diffuse Large B Cell Lymphoma. Oncotarget 2016, 7, 19072–19080. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.J.A.; Kwee, T.C.; Fijnheer, R.; Dubois, S.V.; Nievelstein, R.A.J.; De Klerk, J.M.H. Bone Marrow 18 F-fluoro-2-deoxy- d -glucose Positron Emission Tomography/Computed Tomography Cannot Replace Bone Marrow Biopsy in Diffuse Large B-cell Lymphoma. American J Hematol 2014, 89, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Maruoka, H.; Nasu, K.; Tabata, S.; Kurata, M.; Matsushita, A.; Imai, Y.; Takahashi, T.; Ishikawa, T. Impact of Occult Bone Marrow Involvement on the Outcome of Rituximab plus Cyclophosphamide, Doxorubicin, Vincristine and Prednisone Therapy for Diffuse Large B-Cell Lymphoma. Leukemia & Lymphoma 2013, 54, 2645–2653. [Google Scholar] [CrossRef]
- Cortés-Romera, M.; Sabaté-Llobera, A.; Mercadal-Vilchez, S.; Climent-Esteller, F.; Serrano-Maestro, A.; Gámez-Cenzano, C.; González-Barca, E. Bone Marrow Evaluation in Initial Staging of Lymphoma: 18F-FDG PET/CT Versus Bone Marrow Biopsy. Clinical Nuclear Medicine 2014, 39, e46–e52. [Google Scholar] [CrossRef] [PubMed]
- Wolach, O.; Fraser, A.; Luchiansky, M.; Shapiro, C.; Radnay, J.; Shpilberg, O.; Lishner, M.; Lahav, M. Can Flow Cytometry of Bone Marrow Aspirate Predict Outcome of Patients with Diffuse Large B Cell Lymphoma? A Retrospective Single Centre Study. Hematological Oncology 2015, 33, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-C.; Chung, Y.; Jang, S.; Park, C.-J.; Chi, H.-S.; Huh, J.; Suh, C.; Shim, H. Prognostic Impact of Germinal Center B-Cell-like and Non-Germinal Center B-Cell-like Subtypes of Bone Marrow Involvement in Patients with Diffuse Large B-Cell Lymphoma Treated with R-CHOP. Medicine 2018, 97, e13046. [Google Scholar] [CrossRef]
- Kremer, M.; Spitzer, M.; Mandl-Weber, S.; Stecker, K.; Schmidt, B.; Höfler, H.; Quintanilla-Martínez, L.; Fend, F. Discordant Bone Marrow Involvement in Diffuse Large B-Cell Lymphoma: Comparative Molecular Analysis Reveals a Heterogeneous Group of Disorders. Lab Invest 2003, 83, 107–114. [Google Scholar] [CrossRef]
- Wang, Y.; Link, B.K.; Witzig, T.E.; Maurer, M.J.; Allmer, C.; King, R.L.; Feldman, A.L.; Habermann, T.M.; Ansell, S.M.; Slager, S.L.; Cerhan, J.R.; Nowakowski, G.S. Impact of Concurrent Indolent Lymphoma on the Clinical Outcome of Newly Diagnosed Diffuse Large B-Cell Lymphoma. Blood 2019, 134, 1289–1297. [Google Scholar] [CrossRef]
- El-Azony, A.; Basha, M.A.A.; Almalki, Y.E.; Abdelmaksoud, B.; Hefzi, N.; Alnagar, A.A.; Mahdey, S.; Ali, I.M.; Nasr, I.; Abdalla, A.A.E.-H. M.; Yousef, H.Y.; Zaitoun, M.M.A.; Elsayed, S.B.; Nada, M.G.; Amin, M.I.; Hassan, R.M.; Ali, S.A.; Dawoud, T.M.; Aly, S.A.; Algazzar, Y.H.; Abdelhamed, H. The Prognostic Value of Bone Marrow Retention Index and Bone Marrow-to-Liver Ratio of Baseline 18F-FDG PET/CT in Diffuse Large B-Cell Lymphoma. Eur Radiol 2023. [Google Scholar] [CrossRef]
- Ke, Q.; Liao, C.-C.; Tan, X.-H.; Guo, B.-P.; Cen, H.; Li, L.-Q. Diagnostic Accuracy of Pelvic Magnetic Resonance Imaging for the Assessment of Bone Marrow Involvement in Diffuse Large B-Cell Lymphoma. PLoS One 2021, 16, e0252226. [Google Scholar] [CrossRef]
- Adams, H.J.A.; Kwee, T.C.; Lokhorst, H.M.; Westerweel, P.E.; Fijnheer, R.; Kersten, M.J.; Verkooijen, H.M.; Stoker, J.; Nievelstein, R.A.J. Potential Prognostic Implications of Whole-Body Bone Marrow MRI in Diffuse Large B-Cell Lymphoma Patients with a Negative Blind Bone Marrow Biopsy. J Magn Reson Imaging 2014, 39, 1394–1400. [Google Scholar] [CrossRef]
- Cho, Y.A.; Yang, W.I.; Song, J.; Min, Y.H.; Yoon, S.O. The Prognostic Significance of Monoclonal Immunoglobulin Gene Rearrangement in Conjunction with Histologic B-cell Aggregates in the Bone Marrow of Patients with Diffuse Large B-cell Lymphoma. Cancer Medicine 2016, 5, 1066–1073. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.J.; Kang, H.J.; Kim, J.S.; Eom, H.S.; Kim, T.M.; Yoon, S.-S.; Suh, C.; Lee, D.S.; Korean Society of Hematology Lymphoma Working Party. Clinical Significance of Cytogenetic Aberrations in Bone Marrow of Patients with Diffuse Large B-Cell Lymphoma: Prognostic Significance and Relevance to Histologic Involvement. J Hematol Oncol 2013, 6, 76. [Google Scholar] [CrossRef]
| Ref.* | Region | Study type | Period | N | DLBCL population characteristics | Bone marrow assessment | |||||||||||||||
| Male/female ratio | Median age (range) | COO† | Ann Arbor | IPI | Frontline | Follow-up (months) | Histology | Flow cytometry | PET-FDG | ||||||||||||
| Not specified or global | Concordant | Discordant | Not specified or global | Concordant | Discordant | Not specified or global | Focal | Diffuse | |||||||||||||
| Bo et al. [37] | China | Retrospective | 2019-2022 | 102 | 1.2 | 29.4% ≥60yo | GCB: 35%Non-GCB: 65% | I-II: 14%III: 0%IV: 86% | 3-5: 28% | - | - | 21% (28% after second punction) | - | - | 18% (24% after second punction) | - | - | 26% | 23% | 3% | |
| Han et al. [38] | South Korea | Retrospective | 2014-2020 | 328 | 1.3 | 59 (44-74) | - | I-II: 52%III-IV: 48% | 3-5: 41% | - | - | 14% | - | - | - | - | - | 18% | 11% | 7% | |
| Kim et al. [39] | South Korea | Prospective | 2017-2018 | 94 | 1.8 | 66 (24-85) | - | I-II: 47%III-IV: 53% | 3-5: 61% | R-CHOP | 35 (23-47) | 10% | 6% | 3% | - | - | - | 17% | 12% | 5% | |
| Okamoto et al. [40] | Japan | Retrospective | 2012-2018 | 221 | 1.6 | 72 (26-97) | - | I-II: 42%III-IV: 58% | Poor R-IPI 53% | R-CHOP or R-CHOP-like 83% | 31 (N=184 subcohort R-CHOP or R-CHOP-like) | 8% | - | - | 12% | - | - | - | - | - | |
| Lim et al. [41] | South Korea | Retrospective | 2009-2014 | 512 | 1.2 | 57 (47-67) | - | I-II: 56%III-IV: 44% | 3-5: 32% | R-CHOP | 52 (1-127) | 12% (bilateral) | 11% | 1% | - | - | - | 13% | 8% | 2% | |
| 2% heterogeneous | |||||||||||||||||||||
| Saiki et al. [42] | Japan | Retrospective | 2008-2017 | 84 | 1.2 | 70 (19-86) | - | I-II: 47%III-IV: 53% | - | Mostly R-CHOP | - | 26% | 19% | 7% | - | - | - | 19% | 8% | 11% | |
| Martin-Moro et al. [43] | Spain | Retrospective | 2013-2017 | 82 (38 PET data) | 1.2 | 63 (33-85) | GCB: 49%Non-GCB: 51% | I-II: 50%III-IV: 50% | aaIPI 2-3: 41% | R-CHOP or R-CHOP-like | 33 (NR) | 13% | - | - | 24% | - | - | 16% | - | - | |
| Al-Sabbagh et al. [44] | Qatar | Retrospective | 2013-2017 | 89 | 2.6 | 48 (18-77) | - | I-II: 39%III-IV: 61% | - | - | - | 13% | - | - | - | - | - | 26% (focal and heterogeneous) | - | - | |
| Min et al. [45] | South Korea | Retrospective | 2009-2016 | 600 | 1.4 | 59 (17-88) | GCB: 36%Non-GCB: 64% | I-II: 44%III-IV: 56% | 3-5: 50% | R-CHOP | 50 (0.2-123) | 15%(bilateral) | - | - | - | - | - | 16% | 10% | 3% | |
| Both 3% | |||||||||||||||||||||
| Kandeel et al. [46] | Egypt | Retrospective | 2015-2018 | 88 | 0.7 | 50 (21-70) | GCB: 43%Non-GCB: 57% | I-II: 14%III-IV: 86% | - | Mostly R-CHOP | 11 (2-20) | 25% | - | - | - | - | - | - | 30% | - | |
| Alonso-Alvarez et al. [47] | Spain | Retrospective | 1999-2014 | 232 | 1 | 66% >60yo | GCB: 37%Non-GCB: 63% | I-II: 29%III-IV: 71% | Poor R-IPI: 41% | 81% R-CHOP or R-CHOP-like | 58 (1-152) | 25% | 16% | 9% | 25% | 10% | 15% | - | - | - | |
| Wang et al. [48] | South Korea | Retrospective | 2011-2017 | 140 | 1.4 | 65 (22-86) | GCB: 9%Non-GCB: 91% | I-II: 0%III-IV: 100% | >3: 45% | R-CHOP | 49 (1-98) | 36%(bilateral) | - | - | - | - | - | - | 31% | - | |
| Greenbaum et al. [49] | Israel | Retrospective | 2005-2014 | 81 | 1.5 | 65 (23-87) | - | I-II: 38%III-IV: 62% | Median 3 | 91% R-CHOP | - | 26% | - | - | 63% | - | - | - | - | - | |
| Chen et al. [16] | China | Retrospective | 2007-2016 | 193 | 0.9 | 58 (14-87) | GCB: 32%Non-GCB:68% | I-II: 44%III-IV: 56% | 3-5: 43% | R-CHOP | 30 (12-124) | 7% | - | - | - | - | - | 24% | 15% | 9% | |
| Chen-Liang et al. [50] | Spain | Retrospective | 2007-2015 | 268 | 1 | 61 (18-85) | - | I-II: 25%III-IV: 75% | 3-5: 42% | 76% R-CHOP | 25 (1-91) | 13% | - | - | - | - | - | 22% | 17% | 6% | |
| Vishnu et al. [51] | USA | Retrospective | 2004-2013 | 99 | 1.7 | 62 (24-88) | - | - | Poor R-IPI: 24% | - | 91 (28-140) | 14% | - | - | - | - | - | 24% | - | - | |
| Alzahrani et al. [33] | Canada and Denmak | Retrospective | 2007-2013 | 530 | 1.2 | 65 (16-90) | - | I-II: 37%III-IV: 63% | 3-5: 43% | - | 24 (3-78) | 16% | 10% | 7% | - | - | - | - | 28% | - | |
| Liang et al. [52] | China | Retrospective | 2005-2014 | 169 | 1.3 | 55 (18-85) | GCB: 40%Non-GCB: 60% | I-II: 36%III-IV: 64% | 4-5: 17% | R-CHOP (60%) or DA-EPOCH-R (40%) | 38 (12-113) | 12% (some bilateral) | - | - | - | - | - | 21% | 20% | 2% | |
| Cerci et al. [20] | Brazil, Chile, Hungary, India, Italy, South Korea, Philippines, and Thailand | Prospetive | 2008-2011 | 327 | 1.1 | 55 (IQR 44-63) | - | I-II: 36%III-IV: 64% | 3-5: 34% | R-CHOP recommended | 35 (NR) | 11% | - | - | - | - | - | 26% | 21% | 6% | |
| Adams et al. [53] | The Netherlands | Retrospective | 2007-2013 | 78 | 1.2 | 69 (33-88) | - | I-II: 23%III-IV: 77% | NCCN-IPI >3: 71% | 91% R-CHOP | 28 (5-74) | 21% (3% undetermined) | 14% | 4% | - | - | - | 44% | 39% | 5% | |
| Arima et al. [54] | Japan | Retrospective | 2006-2011 | 96‡ | 1.5 | 69 (22-89) | - | I-II: 39%III-IV: 61% | 3-5: 52% | R-CHOP | 36 (1-72) | 20% | 15% | 5% | 28% | - | - | - | - | - | |
| Berthet et al. [10] | France | Retrospective | 2006-2011 | 133 | 1 | 57 (18-87) | - | I-II: 26%III-IV: 74% | 3-5: 40% | R-CHOP or R-CHOP-like | 24 (1-67) | 6% | 4% | 2% | - | - | - | 32% | 24% | 8% | |
| Khan et al. [35] | United Kingdom | Retrospective | 2005-2012 | 130 | 1.5 | 59 (22-87) | - | I-II: 45%III-IV: 55% | 3-5: 40% | 95% R-CHOP | - | 11% | 11% | 0% | - | - | - | 25% | 21.5% | 1.5% | |
| Both 2% | |||||||||||||||||||||
| Hong et al. [36] | South Korea | Retrospective | 2007-2011 | 89 | 0.8 | 59 (26-83) | GCB: 49%Non-GCB: 51% | I-II: 47%III-IV: 52% | Poor R-IPI: 35% | R-CHOP | 16 (NR) | 16%(bilateral) | 10% | 6% | - | - | - | 19% | 11% | 8% | |
| Cortes Romera et al. [55] | Spain | - | 2004-2010 | 84 | 1 | 63 (19-78) | - | I-II: 50%III-IV: 50% | - | R-CHOP | NR (9-34) | - | 19% | - | - | - | - | 29% | 23% | 6% | |
| Ref. | Concordant results | Discordant results | Cohen’s kappa index | ||||||||||||
| BMB+/FCM+ | BMB+/PET+ | FCM+/PET+ | BMB-/FCM- | BMB-/PET- | FCM-/PET- | BMB+/FCM- | BMB+/PET- | BMB-/FCM+ | BMB-/PET+ | FCM+/PET- | FCM-/PET+ | BMB/FCM | BMB/PET | FCM/PET | |
| Bo et al. [37] | - | 16% (23% after second punction) | 14% (20% after second punction) | - | 71% | 72% | - | 5% | - | 10% (3% after second punction) | 4% | 12% (6% after second punction) | - | 0.59 (0.8 after second punction) | 0.54 (0.74 after second punction) |
| Han et al. [38] | - | 7% | - | - | 76% | - | - | 6% | - | 11% | - | - | - | 0.36* | - |
| Kim et al. [39] | - | 5% | - | - | 79% | - | - | 4% | - | 12% | - | - | - | 0.32* | - |
| Okamoto et al. [40] | 3% | - | - | 83% | - | - | 5% | - | 9% | - | - | - | 0.25 | - | - |
| Lim et al. [41] | - | 7% | - | - | 83% | - | - | 5% | - | 6% | - | - | - | 0.51* | - |
| Saiki et al. [42] | - | 10% | - | - | 64% | - | - | 17% | - | 10% | - | - | - | 0.26* | - |
| Martín-Moro et al. [43] | 13% | 5% | 8% | 76% | 79% | 71% | 0% | 5% | 15% | 11% | 13% | 8% | 0.65* | 0.31* | 0.30* |
| Al-Sabbagh et al. [44] | - | 12% | - | - | 73% | - | - | 1% | - | 13% | - | - | - | 0.55* | - |
| Min et al. [45] | - | 9% | - | - | 76% | - | - | 7% | - | 8% | - | - | - | 0.43* | - |
| Kandeel et al. [46] | - | 14% | - | - | 59% | - | - | 11% | - | 16% | - | - | - | 0.31* | - |
| Alonso-Alvarez et al. [47] | 18% | - | - | 69% | - | - | 6% | - | 7% | - | - | - | 0.65* | - | - |
| Wang et al. [48] | - | 21% | - | - | 54% | - | - | 14%† | - | 10% | - | - | - | 0.46* | - |
| Greenbaum et al. [49] | 26% | - | - | 37% | - | - | 0% | - | 37% | - | - | - | 0.4* | - | - |
| Chen et al. [16] | - | 7% | - | - | 76% | - | - | 1% | - | 17% | - | - | - | 0.36* | - |
| Chen-Liang et al. [50] | - | 9% | - | - | 74% | - | - | 4% | - | 13% | - | - | - | 0.41* | - |
| Vishnu et al. [51] | - | 12% | - | - | 74% | - | - | 2% | - | 12% | - | - | - | 0.55* | - |
| Alzahrani et al. [33] | - | 9% | - | - | 66% | - | - | 7% | - | 18% | - | - | - | 0.30* | - |
| Liang et al. [52] | - | 11% | - | - | 78% | - | - | 1% | - | 10% | - | - | - | 0.59* | - |
| Cerci et al. [20] | - | 8% | - | - | 71% | - | - | 3% | - | 19% | - | - | - | 0.31* | - |
| Adams et al. [53] | - | 14% | - | - | 50% | - | - | 6% | - | 29% | - | - | - | 0.22* | - |
| Arima et al. [54] | 18% | - | - | 70% | - | - | 2% | - | 10% | - | - | - | 0.66* | - | - |
| Berthet et al. [10] | - | 5% (4% subcohort PET focal) | - | - | 67% (74% subcohort PET focal) | - | - | 1% (2% subcohort PET focal) | - | 27% (20% subcohort PET focal) | - | - | - | 0.19* (0.23* subcohort PET focal) | - |
| Khan et al. [35] | - | 9% | - | - | 73% | - | - | 2% | - | 16% | - | - | - | 0.42* | - |
| Hong et al. [36] | - | 8% | - | - | 73% | - | - | 8% | - | 11% | - | - | - | 0.34* | - |
| Cortes Romera et al. [55] | - | 18% | - | - | 70% | - | - | 1% | - | 11% | - | - | - | 0.68* | - |
| Ref. | Definition of true positive BMi | Sensitivity (CI 95%) | Specificity (CI 95%) | Positive/Negative predictive value | Youden’s index / Diagnostic Accuracy | ||||||||
| BMB | PET | PET focal | BMB | PET | PET focal | BMB | PET | PET focal | BMB | PET | PET focal | ||
| Bo et al. [37] | After first direct BM study (BMB and FCM) | - | 62% (43-78) | - | - | 93% (86-97) | - | - | - | - | - | 0.55% (YI) | - |
| After second direct BM study (BMB and FCM) | - | 92% (76-98) | - | - | - | - | - | - | - | 0.86% (YI) | - | ||
| Lim et al. [41] | Billateral BMB | - | 59% (NR) | - | - | 94% | - | - | 55%/95% | - | - | 90% (DA) | - |
| Saiki et al. [42] | BMB | - | 36% (NR) | - | - | 87% (NR) | - | - | 50%/79% | - | - | - | - |
| Al-Sabbagh et al. [44] | BMBorPET + any of guided biopsy confirmation / MRI / focal uptake / FDG disappearance with treatment | 50% (29-71) | 96% (79-100) | - | 100% (94-100) | 100% (95-100) | - | 100%/84% | 100%/98% | - | 87% (DA) | 99% (DA) | - |
| Min et al. [45] | Billateral BMB | - | 52% | - | - | 91% | - | - | - | - | - | - | - |
| Kandeel et al. [46] | BMBorPET + any of concordant morphologic changes by CT / FDG disappearance with treatment / concordant FDG progression on follow-up | 69% (NR) | - | 67% (NR) | 100% (NR) | - | 90% (NR) | 100%/85% | - | 77%/84% | 89% (DA) | - | 82% (DA) |
| Chen et al. [16] | BMB or PET focal | 44% (NR) | 88% (NR) | - | - | - | - | NR/90% | NR/98% | - | 91% (DA) | 98% (DA) | - |
| Chen-Liang et al. [50] | BMB or PET | 40% (27-53) | 69% (52-85) | - | 95% (91-98) | 85% (80-89) | - | NR/85% | NR/95% | - | 83% (DA) / 0.4 (YI) | 83% (DA) / 0.5 (YI) | |
| Vishnu et al. [51] | BM aspirate and trephine biopsy | - | 86% (56-97) | - | - | 86% (76-92) | - | - | 50%/98% | - | - | 86% (DA) | |
| Alzahrani et al. [33] | BMB or PET | 48% (41-56) | - | 81% (74-86) | - | - | - | NR/79% | - | NR/91% | - | - | - |
| BMB | - | - | 60% (49-70) | - | - | 79% (75-83) | - | - | 36%/91% | - | - | - | |
| BMB concordant | - | - | 77% (63-87) | - | - | 79% (75-83) | - | - | 29%/97% | - | - | - | |
| PET focal | 36% (28-44) | - | - | 91% (88-94) | - | - | 60%/79% | - | - | - | - | - | |
| Berthet et al. [10] | BMBorPET focal + confirmed byguided biopsy / targeted MRI / after chemotherapy by concomitant disappearance of uptake | 24% (9-39) | - | 94% (86-100) | 100% (100-100) | - | 99% (97-100) | 100%/80% | - | 97%/98% | 81% (DA) | - | 98% (DA) |
| Khan et al. [35] | BMB or PET | 40% (NR) | 94% (NR) | - | 100% (NR) | 100% (NR) | - | NR | NR | - | 84% (DA) | 99% (DA) | - |
| Cortes Romera et al. [55] | BMB | - | 95% | - | - | 86% | - | - | 54%/99% | - | - | 87% (DA) | |
| Ref. | N | Adverse factor related to BM assessment (compared with its complementary good prognosis reference in each case) | Survival endpoints | ||||||
| Event-free survival or progression-free survival | Overall survival | ||||||||
| Prognosis | UV HR (CI 95%) | MV HR (CI 95%) | Prognosis | UV HR (CI 95%) | MV HR (CI 95%) | ||||
| Okamoto et al.* [40] | 184 (subcohort R-CHOP or R-CHOP-like) | BMB+ | 20% (2-year)C-index 0.68 | 4.3 (2.1-8.8) | 2.3 (1.3-6.5)1 | 24% (2-year)C-index 0.74 | 5.2 (2.6-10.5) | 3 (1.3-6.8)1 | |
| FCM+ | C-index 0.70 | 3.1 (1.6-5.8) | 2.8 (1.4-5.2)1 | C-index 0.74 | 2.2 (1.1-4.5) | 1.9 (0.9-3.7)1 | |||
| Lim et al.‡ [41] | 512 | BMB+ | - | 3.1 (1.8-5.4) | 1.7 (1.1-2.6)2 | 52% (2-year)37% (5-year) | - | - | |
| PET | PET+ | 29% (2-year) | 2.8 (1.6-4.7) | 1.7 (1.1-2.6)2 | - | - | - | ||
| PET+ focal | - | 2.1 (1.1-3.9) | - | - | - | - | |||
| PET+ diffuse | - | 3.1 (0.8-11) | - | - | - | - | |||
| PET+ heterogeneous | - | 4.2 (1-16.9) | - | - | - | - | |||
| Martin-Moro et al.* [43] | 82 | BMB+/FCM+ | 27% (18-month) | 2.2 (1.4-3.3) | - | 55% (18-month) | 1.9 (1.2-3) | - | |
| BMB-/FCM+ | 23% (18-month) | 4.9 (1.7-14.2) | - | 46% (18-month) | 4.4 (1.5-12.4) | - | |||
| FCM+ | - | 4.8 (2.3-10) | 1.9 (1.3-2.9)32 (1.3-3.1)4 | - | 3.8 (1.8-8.3) | 1.7 (1.1-2.7)31.7 (1.1-2.7)4 | |||
| Min et al.* [45] | 600 | BMB+ | - | - | - | 56% (4-year) | - | 1 (0.8-1.3)5 | |
| PET+ | 43% (4-year) | - | - | 65% (4-year) | - | - | |||
| Kandeel et al.• [46] | 88 | BMB+ | 64% (18-month) | 1.4 (0.5-3.5) | - | - | - | - | |
| PET+ focal | 73% (18-month) | 1 (0.4-2.5) | - | - | - | - | |||
| Alonso-Alvarez et al.* [47] | 189 (subcohort R-CHOP or R-CHOP-like) | Concordant | BMB+ concordant | 32% (5-year) | - | 2.2 (1.1-4.3)6 | 51% (5-year) | - | 1.6 (0.7-3.4)6 |
| BMB+ concordant GCB | 25% (5-year) | - | 2.9 (1-8.7)7 | 38% (5-year) | - | 1.2 (0.3-4.4)7 | |||
| BMB+ concordant non-GCB | 33% (5-year) | - | 3 (1.4-6.4)7 | 49% (5-year) | - | 1.6 (0.7-3.9)7 | |||
| Discordant | BMB/FCM+ discordant | 62% (5-year) | - | 1.5 (0.7-3)6 | 73% (5-year) | - | 1.5 (0.7-3.2)6 | ||
| BMB/FCM+ discordant GCB | 76% (5-year) | - | 0.7 (0.1-3)7 | 76% (5-year) | - | 0.7 (0.1-3.4)7 | |||
| BMB/FCM+ discordant non-GCB | 46% (5-year) | - | 1.9 (0.9-4.2)7 | 63% (5-year) | - | 1.6 (0.6-3.7)7 | |||
| Wang et al.† [48] | 140 (all cases advanced stage) | BMB+ | - | 2.3 (1.4-3.9) | 2.3 (1.4-3.9)8 | - | 1.7 (1-2.8) | Not significant8 | |
| PET+ focal | - | 2.1 (1.3-3.6) | Not significant8 | - | 1.8 (1.1-3) | 1.9 (1.1-3.1)8 | |||
| BMB+/PET+ | 7 months (median) | - | - | 12 months (median) | - | - | |||
| BMB+/PET- | 14 months (median) | - | - | 27 months (median) | - | - | |||
| BMB-/PET+ | 26 months (median) | - | - | 31 months (median) | - | - | |||
| Greenbaum et al.† [49] | 81 | BMB+/FCM+ | 67 months (median)47% (1-year)36% (5-year)24% (7-year) | - | - | 54 months (median)43% (1-year)32% (5-year)32% (7-year) | - | - | |
| BMB-/FCM+ | 77 months (median)61% (1-year)56% (5-year)49% (7-year) | - | - | 77 months (median)60% (1-year)55% (5-year)54% (7-year) | - | - | |||
| FCM+ | - | - | 2.6 (1-6.8)9 | - | - | 1.4 (0.7-3)9 | |||
| Chen et al.† [16] | 193 | BMB+ | 61% (3-year) | 1.8 (0.7-4.6) | - | 61% (3-year) | 4 (1.3-11.9) | - | |
| PET+ focal | 33% (3-year) | 4.4 (2.4-8.2) | 2.3 (1.1-4.7)10 | 69% (3-year) | 3.7 (1.5-9.3) | Not significant10 | |||
| PET+ diffuse | 81% (3-year) | 0.9 (0.3-2.4) | - | 94% (3-year) | 0.6 (0.1-4.3) | - | |||
| BMB-/PET+ focal | 0% (3-year) | - | - | 77% (3-year) | - | - | |||
| BMB-/PET+ diffuse | 90% (3-year) | - | - | 100% (3-year) | - | - | |||
| Chen-Liang et al.* [50] | 203 (subcohort R-CHOP) | BMB+ | - | P <0.001 | 3.6 (1.7-7.6)11 | - | P = 0.326 | ||
| PET+ | - | P = 0.121 | P >0.1511 | - | P = 0.018 | P >0.1511 | |||
| Vishnu et al. [51] | 99 (not reported treatment approach) | BMB+ | - | - | - | 65 months (median)80% (2-year)66% (5-year) | - | - | |
| PET+ | - | - | - | 67 months (median)83% (2-year)79% (5-year) | - | - | |||
| Alzahrani et al.* [33] | 256 (subcohort Ann Arbor IV) | BMB+ or PET+ | 57% (2-year) | - | - | 65% (2-year) | - | - | |
| PET+ | 53% (2-year) | - | - | 63% (2-year) | - | - | |||
| Liang et al.† [52] | 169 | BMB+ | - | 4.5 (2.5-8) | NS12 | - | 6.2 (3.1-12.7) | NS12 | |
| PET+ | - | 4 (2.3-6.6) | NS12 | - | 6.7 (3.4-13.3) | 2.9 (1.2-7)12 | |||
| 68 (subcohort Ann Arbor IV) | PET+ | 29% (3-year) | - | - | 44% (3-year) | - | - | ||
| Cerci et al.• [20] | 327 | BMB+ | 56% (2-year) | 2.2 (1.3-3.3) | - | 68% (2-year) | - | - | |
| BMB+/PET+ | 45% (2-year) | 2.7 (1.5-4.8) | 1.6 (0.8-3.1)13 | 55% (2-year) | 3.9 (1.9-8.1) | 2.3 (1-5)13 | |||
| BMB+/PET+ focal | 46% (2-year) | 2.5 (1.1-5.5) | - | 57% (2-year) | 3 (1.1-8.4) | - | |||
| BMB+/PET- | 80% (2-year) | - | 1.1 (0.4-3.2)13 | 100% (2-year) | - | 0.5 (0.1-3.7)13 | |||
| BMB-/PET+ | 81% (2-year) | - | 0.7 (0.4-1.3)13 | 88% (2-year) | - | 0.7 (0.3-1.7)13 | |||
| BMB-/PET+ focal | 78% (2-year) | - | - | 87% (2-year) | - | ||||
| Adams et al.* [53] | 71 (subcohort R-CHOP) | BMB+ | - | 3.2 (1.3-7.6) | 3.3 (1.3-8.6)14 | - | 3.5 (1.3-9.3) | 4.5 (1.6-12.4)14 | |
| PET+ | - | 0.9 (0.4-2.1) | - | - | 0.7 (0.3-2.2) | - | |||
| Berthet et al.* [10] | 133 | BMB+ | 38% (2-year) | 4.9 (1.6-14.6) | 2..2 (0.8-6)15 | 63% (2-year) | 4.1 (1.4-12.4) | 2.7 (0.9-8.2)15 | |
| PET+ | 63% (2-year) | 2.9 (1.2-7) | 2.5 (1.2-5.3)15 | 76% (2-year) | 2.8 (1.2-6.8) | 2.2 (0.9-5.3)15 | |||
| Khan et al.† [35] | 44 (subcohort Ann Arbor IV) | BMB+ | - | 3.7 (1.6-8.8) | - | - | 3.9 (1.5-10) | - | |
| PET+ | - | 0.8 (0.3-2) | - | - | 0.9 (0.3-2.5) | - | |||
| PET+/BMB- | - | 0.4 (0.1-1.2) | - | - | 0.4 (0.1-1.6) | - | |||
| Hong et al.* [36] | 89 | BMB+ | 37% (2-year) | - | - | 36% (2-year) | 7.9 (3.2-19.6) | 8.7 (3.2-23.5)16 | |
| PET+ | 63% (2-year) | - | - | 59% (2-year) | 2 (0.8-5.3) | - | |||
| BMB+/PET+ | 38% (2-year) | - | - | 38% (2-year) | - | - | |||
| BMB+/PET- | 36% (2-year) | - | - | 36% (2-year) | - | - | |||
| BMB-/PET+ | 79% (2-year) | - | - | 73% (2-year) | - | - | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





