Submitted:
07 March 2024
Posted:
07 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Setting and Ethics
2.2. Study Population and Data Collection
2.2.1. Laboratory Analysis
2.2.2. Molecular Analyses
2.2.3. Bone Marrow Biopsy and Pathological Analysis
2.2.4. Smoking Status
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
ORCID iDs
References
- Sung, H.J. A diagnostic roadmap for polycythemia. Korean J Med. 2020, 95, 27–30. [Google Scholar] [CrossRef]
- Yalcinkaya, R.; Zenciroglu, A. Evaluation of Neonatal Polycythemia in Terms of Gestational Age, Hematocrit, and Platelet Levels. Türkiye Çocuk Hast Derg 2022, 16, 495–500. [Google Scholar] [CrossRef]
- Kim M. J.; Kwon S. S.; Ji Y. S.; Lee M. Y.; Kim K. H.; Lee N.; Park S. K.; Won J. H.; Yoon S. Y. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as new possible minor criteria for diagnosis of polycythemia vera. Int J Lab Hematol. 2023, 45, 853–859. [CrossRef]
- Barbui T.; Thiele J.; Gisslinger H.; Kvasnicka H. M.; Vannucchi A. M.; Guglielmelli P.; Orazi A.; Tefferi A. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 2018, 8, 15. [CrossRef]
- Krečak, I.; Holik, H.; Zekanović, I.; Morić Perić, M.; Marketin, T.; Coha, B.; Gverić-Krečak, V.; Vodanović, M.; Lucijanić, M. Thrombotic risk in secondary polycythemia resembles low-risk polycythemia vera and increases in specific subsets of patients. Thromb Res. 2022, 209, 47–50. [Google Scholar] [CrossRef]
- Krečak, I.; Holik, H.; Morić Perić, M.; Zekanović, I.; Coha, B.; Gverić-Krečak, V.; Lucijanić, M. High platelet-to-lymphocyte ratio may differentiate polycythemia vera from secondary polycythemia. Wien Klin Wochenschr. 2022, 134, 483–486. [Google Scholar] [CrossRef]
- Birgegård, G.; Wide, L. Serum erythropoietin in the diagnosis of polycythaemia and after phlebotomy treatment. Br J Haematol. 1992, 81, 603–606. [Google Scholar] [CrossRef]
- Mossuz, P.; Girodon, F.; Donnard, M.; Latger-Cannard, V.; Dobo, I.; Boiret, N.; Lecron, J.C.; Binquet, C.; Barro, C.; Hermouet, S.; et al. Diagnostic value of serum erythropoietin level in patients with absolute erythrocytosis. Haematologica. 2004, 89, 1194–1198. [Google Scholar]
- Lupak, O.; Han, X.; Xie, P.; Mahmood, S.; Mohammed, H.; Donthireddy, V. The role of a low erythropoietin level for the polycythemia vera diagnosis. Blood Cells Mol Dis. 2020, 80, 102355. [Google Scholar] [CrossRef]
- Mendez Luque L. F.; Blackmon A. L.; Ramanathan G.; Fleischman A. G. Key Role of Inflammation in Myeloproliferative Neoplasms: Instigator of Disease Initiation, Progression. and Symptoms. Curr Hematol Malig Rep. 2019, 14, 145–153. [CrossRef]
- Yalcinkaya, A.; Samadi, A.; Lay, I.; Unal, S.; Sabuncuoglu, S.; Oztas, Y. Oxysterol concentrations are associated with cholesterol concentrations and anemia in pediatric patients with sickle cell disease. Scand J Clin Lab Invest. 2019, 79, 381–387. [Google Scholar] [CrossRef]
- Hasselbalch, H.C.; Holmström, M.O. Perspectives on interferon-alpha in the treatment of polycythemia vera and related myeloproliferative neoplasms: minimal residual disease and cure? Semin Immunopathol. 2019, 41, 5–19. [Google Scholar] [CrossRef]
- Jomrich, G.; Gruber, E.S.; Winkler, D.; Hollenstein, M.; Gnant, M.; Sahora, K.; Schindl, M. Systemic Immune-Inflammation Index (SII) Predicts Poor Survival in Pancreatic Cancer Patients Undergoing Resection. J Gastrointest Surg. 2020, 24, 610–618. [Google Scholar] [CrossRef]
- Chen, L.; Kong, X.; Wang, Z.; Wang, X.; Fang, Y.; Wang, J. Pre-treatment systemic immune-inflammation index is a useful prognostic indicator in patients with breast cancer undergoing neoadjuvant chemotherapy. J Cell Mol Med. 2020, 24, 2993–3021. [Google Scholar] [CrossRef]
- Tong Y. S.; Tan J.; Zhou X. L.; Song Y. Q.; Song Y. J. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med. 2017, 15, 221. [CrossRef]
- Yatabe, S.; Eto, K.; Haruki, K.; Shiba, H.; Kosuge, M.; Ohkuma, M.; Ito, D.; Takeda, Y.; Sugano, H.; Sasaki, S.; et al. Signification of Systemic Immune-Inflammation Index for prediction of prognosis after resecting in patients with colorectal cancer. Int J Colorectal Dis. 2020, 35, 1549–1555. [Google Scholar] [CrossRef]
- Ersal T.; Özkocaman V.; Pınar İ. E.; Yalçın C.; Orhan B.; Candar Ö.; Çubukçu S.; Koca T. G.; Hunutlu F. Ç.; Yavuz Ş. Systemic inflammatory indices for predicting prognosis of myelofibrosis. Sci Rep. 2023, 13, 12539. [CrossRef]
- Yalcinkaya R.; Öz F. N.; Durmuş S. Y.; Fettah A.; Kaman A.; Teke T. A.; Örün U. A.; Tanır G. Is There a Role for Laboratory Parameters in Predicting Coronary Artery Involvement in Kawasaki Disease? Klin Padiatr. 2022, 234, 382–387. [CrossRef]
- Baxter E. J.; Scott L. M.; Campbell P. J.; East C.; Fourouclas N.; Swanton S.; Vassiliou G. S.; Bench A. J.; Boyd E.M.; Curtin N.; et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005, 365, 1054–1061. [CrossRef]
- Spivak, J.L. Polycythemia vera. Curr Treat Options Oncol. 2018, 19, 1–14. [Google Scholar] [CrossRef]
- Krečak, I.; Lucijanić, M. Can we use platelet-to-lymphocyte ratio (PLR) to differentiate JAK2-unmutated erythrocytosis from polycythemia vera? Eur J Intern Med. 2023, 108, 120–121. [Google Scholar] [CrossRef]
- Kwon S. S.; Yoon S. Y.; Jeong S. Y.; Lee M. Y.; Kim K. H.; Lee N.; Won J. H. Neutrophil-lymphocyte ratio and carotid plaque burden in patients with essential thrombocythemia and polycythemia vera. Nutr Metab Cardiovasc Dis. 2022, 32, 1913–1916. [CrossRef]
- Yang, R.; Chang, Q.; Meng, X.; Gao, N.; Wang, W. Prognostic value of systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018, 9, 3295. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.-R.; Xu, Y.; Sun, Y.-F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.-M.; Qiu, S.-J.; Zhou, J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Stefaniuk, P.; Szymczyk, A.; Podhorecka, M. The Neutrophil to Lymphocyte and Lymphocyte to Monocyte Ratios as New Prognostic Factors in Hematological Malignancies - A Narrative Review. Cancer Manag Res. 2020, 12, 2961–2977. [Google Scholar] [CrossRef]
- Sun, J.; Chen, X.; Gao, P.; Song, Y.; Huang, X.; Yang, Y.; Zhao, J.; Ma, B.; Gao, X.; Wang, Z. Can the Neutrophil to Lymphocyte Ratio Be Used to Determine Gastric Cancer Treatment Outcomes? A Systematic Review and Meta-Analysis. Dis Markers. 2016, 2016, 7862469. [Google Scholar] [CrossRef]
- Ethier J. L.; Desautels D.; Templeton A.; Shah P. S.; Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017, 19, 2. [CrossRef]
- Krečak, I.; Holik, H.; Morić Perić, M.; Zekanović, I.; Coha, B.; Valovičić Krečak, M.; Gverić-Krečak, V.; Lucijanić, M. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as prognostic biomarkers in polycythemia vera. Int J Lab Hematol. 2022, 44, e145–e148. [Google Scholar] [CrossRef]
- Krečak, I.; Lucijanić, M. Platelet-to-lymphocyte ratio and accelerated atherosclerosis in essential thrombocythemia and polycythemia vera. Nutr Metab Cardiovasc Dis. 2022, 32, 2010–2011. [Google Scholar] [CrossRef]
- Lucijanic, M.; Cicic, D.; Stoos-Veic, T.; Pejsa, V.; Lucijanic, J.; Fazlic Dzankic, A.; Vlasac Glasnovic, J.; Soric, E.; Skelin, M.; Kusec, R. Elevated Neutrophil-to-Lymphocyte-ratio and Platelet-to-Lymphocyte Ratio in Myelofibrosis: Inflammatory Biomarkers or Representatives of Myeloproliferation Itself? Anticancer Res. 2018, 38, 3157–3163. [Google Scholar]
- Zhou D.; Chen W.; Cheng H.; Qiao J. L.; Zhu L. L.; Li Z. Y.; Xu K. L. Clinico-hematological profile and thrombotic/hemorrhagic events in 150 chinese patients with essential thrombocythemia. Leuk Res. 2018, 69, 1–6. [CrossRef]
- Carobbio A.; Vannucchi A. M.; De Stefano V.; Masciulli A.; Guglielmelli P.; Loscocco G. G.; Ramundo F.; Rossi E.; Kanthi Y.; Tefferi A.; et al.Neutrophil-to-lymphocyte ratio is a novel predictor of venous thrombosis in polycythemia vera. Blood Cancer J. 2022, 12, 28. [CrossRef]
- Lai, H.; Tu, Y.; Zhang, S.; Liao, C.; Tu, H.; Li, J. Association of inflammation and abnormal lipid metabolism with risk of thrombosis and thrombosis progression in patients with polycythemia vera: a retrospective study. Ann Hematol. 2023, 102, 3413–3426. [Google Scholar] [CrossRef]
- Kocak, M.Z.; Dağlı, M.; Ünlü, A. The ratio of platelet/lymphocyte, the ratio of neutrophil/lymphocyte and some haemogram parameters related to thrombosis in essential thrombocytosis and polycythaemia vera. Biomed Res. 2017, 28, 3036–3039. [Google Scholar]
- Hacibekiroglu, T.; Akinci, S.; Basturk, A.; Inal, B.; Guney, T.; Bakanay, S.M.; Dilek, I. Evaluation of Inflammation Parameters in Philadelphia Negative Chronic Myeloproliferative Neoplasia Patients. Asian Pac J Cancer Prev. 2015, 16, 5159–5162. [Google Scholar] [CrossRef]
| Diagnosis | |||
| Secondary polycythemia (n=84) | Polycythemia vera (n=145) | p | |
| Age (n=229) | 44.67 ± 15.59 | 56.78 ± 13.30 | <0.001a |
| Sex (n=229) | |||
| Male | 68 (80.95%) | 96 (66.21%) | 0.026c |
| Female | 16 (19.05%) | 49 (33.79%) | |
| Splenomegaly (n=227) | 0 (0.00%) | 39 (27.08%) | <0.001c |
| Smoking status (n=201) | |||
| Non-smoker | 34 (41.98%) | 70 (58.33%) | 0.060c |
| Ex-smoker | 12 (14.81%) | 10 (8.33%) | |
| Smoker | 35 (43.21%) | 40 (33.33%) | |
| WBC (x103) (n=229) | 8.11 (6.72 - 9.93) | 10.80 (8.47 - 12.56) | <0.001b |
| RBC (x106) (n=229) | 5.90 ± 0.52 | 6.60 ± 1.05 | <0.001a |
| Hemoglobin (g/dL) (n=229) | 18.02 ± 1.03 | 17.95 ± 1.73 | 0.696a |
| Hematocrit (%) (n=229) | 52.72 ± 3.77 | 55.31 ± 5.92 | <0.001a |
| MCV (fL) (n=229) | 89.19 ± 5.32 | 85.12 ± 9.20 | <0.001a |
| Lymphocyte (x103) (n=229) | 2.43 (2.12 - 2.90) | 2.06 (1.65 - 2.66) | <0.001b |
| Neutrophil (x103) (n=229) | 4.58 (3.70 - 6.49) | 7.27 (5.38 - 8.95) | <0.001b |
| Eosinophil (x103) (n=229) | 0.16 (0.09 - 0.26) | 0.27 (0.18 - 0.42) | <0.001b |
| Platelet (x103) (n=229) | 228.5 (195.5 - 273.5) | 407 (301 - 615) | <0.001b |
| LDH (mg/dL) (n=224) | 197 (166 - 221) | 260 (209 - 345) | <0.001b |
| Erythropoietin (mU/mL) (n=218) | 8.00 (6.20 - 11.50) | 2.10 (1.20 - 4.25) | <0.001b |
| NLR (n=229) | 1.92 (1.51 - 2.35) | 3.29 (2.40 - 4.88) | <0.001b |
| PLR (n=229) | 94.37 (78.72 - 114.06) | 216.85 (136.65 - 290.42) | <0.001b |
| SII (x103) (n=229) | 432.33 (335.97 - 582.93) | 1479.11 (872.41 - 2526.75) | <0.001b |
| JAK2 V617F positivity (n=227) | 0 (0.00%) | 126 (86.90%) | <0.001c |
| JAK2 exon 12 positivity (n=67) | 0 (0.00%) | 4 (16.67%) | 0.014d |
| Thrombosis history (n=229) | 13 (15.48%) | 37 (25.52%) | 0.108c |
| Bone marrow biopsy (n=229) | |||
| No CMPD findings | 84 (100.00%) | 0 (0.00%) | <0.001e |
| PV findings | 0 (0.00%) | 142 (97.93%) | |
| Post-polycythemia MF | 0 (0.00%) | 3 (2.07%) | |
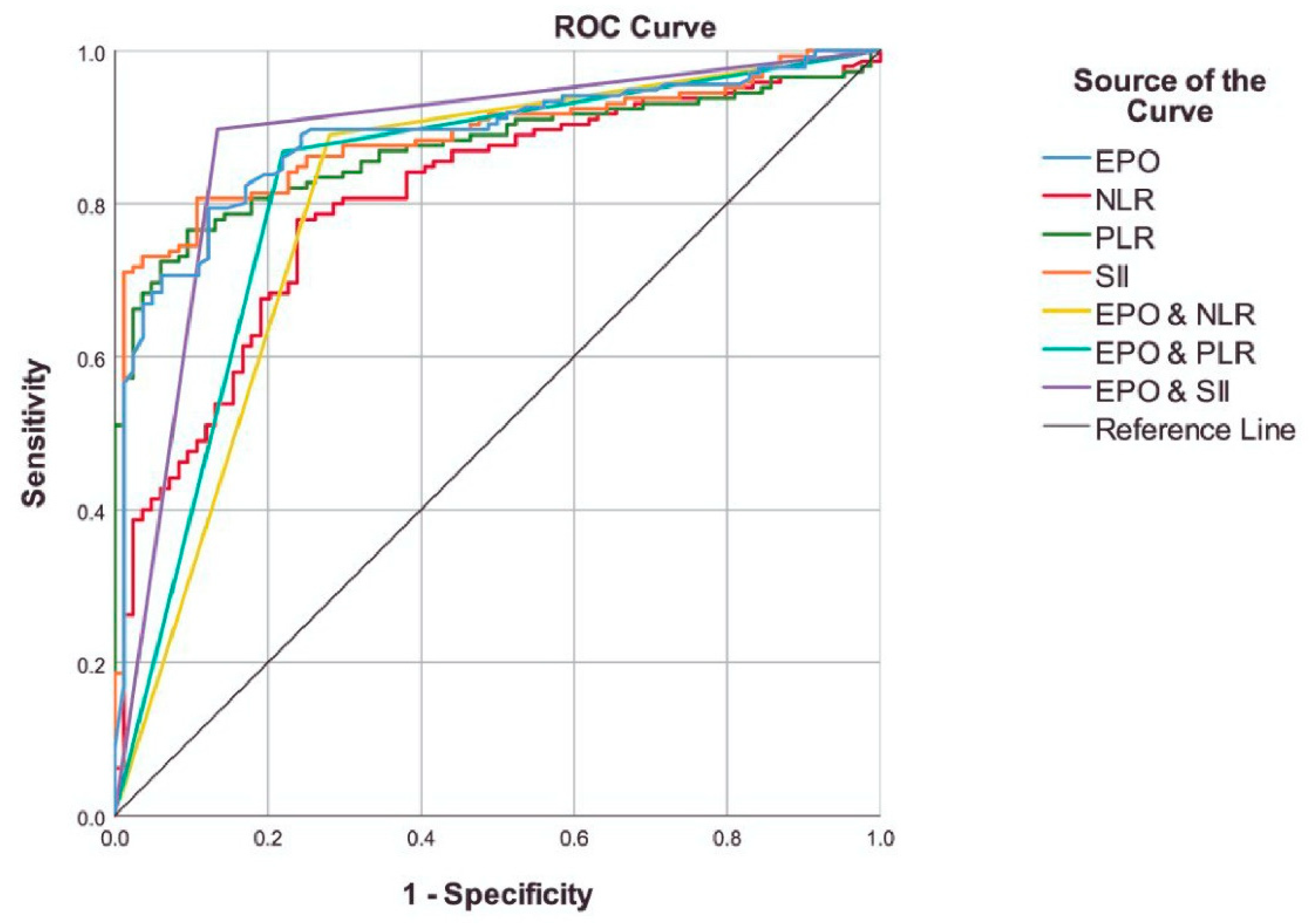
| Cut-off | Sensitivity | Specificity | Accuracy | PPV | NPV | AUC (95% CI) | pa | pb | |
|---|---|---|---|---|---|---|---|---|---|
| EPO | <4.85 | 79.41% | 87.80% | 82.57% | 91.53% | 72.00% | 0.886 (0.841 - 0.931) | <0.001 | - |
| NLR | ≥2.35 | 77.93% | 76.19% | 77.29% | 84.96% | 66.67% | 0.803 (0.745 - 0.861) | <0.001 | 0.018 |
| PLR | ≥135 | 76.55% | 90.48% | 81.66% | 93.28% | 69.09% | 0.871 (0.825 - 0.917) | <0.001 | 0.709 |
| SII | ≥803 | 80.69% | 89.29% | 83.84% | 92.86% | 72.82% | 0.885 (0.841 - 0.929) | <0.001 | 0.934 |
| EPO & NLR† | - | 88.97% | 71.95% | 82.57% | 84.03% | 79.73% | 0.805 (0.739 - 0.870) | <0.001 | 0.010 |
| EPO & PLR† | - | 86.76% | 78.05% | 83.49% | 86.76% | 78.05% | 0.824 (0.762 - 0.886) | <0.001 | 0.055 |
| EPO & SII† | - | 89.71% | 86.59% | 88.53% | 91.73% | 83.53% | 0.881 (0.829 - 0.933) | <0.001 | 0.883 |
| Unadjusted | Adjusted† | |||
| OR (95% CI) | p | OR (95% CI) | p | |
| EPO, <4.85 | 27.771 (12.715 - 60.655) | <0.001 | 29.636 (12.477 - 70.394) | <0.001 |
| NLR, ≥2.35 | 11.300 (5.975 - 21.372) | <0.001 | 8.768 (4.512 - 17.038) | <0.001 |
| PLR, ≥135 | 31.015 (13.611 - 70.673) | <0.001 | 27.572 (11.587 - 65.607) | <0.001 |
| SII, ≥803 | 34.821 (15.568 - 77.887) | <0.001 | 28.109 (12.345 - 64.006) | <0.001 |
| EPO & NLR | 20.693 (10.061 - 42.558) | <0.001 | 19.130 (8.860 - 41.306) | <0.001 |
| EPO & PLR | 23.309 (11.338 - 47.919) | <0.001 | 28.709 (12.493 - 65.973) | <0.001 |
| EPO & SII | 56.247 (24.230 - 130.568) | <0.001 | 48.519 (20.287 - 116.039) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





