Submitted:
06 March 2024
Posted:
08 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
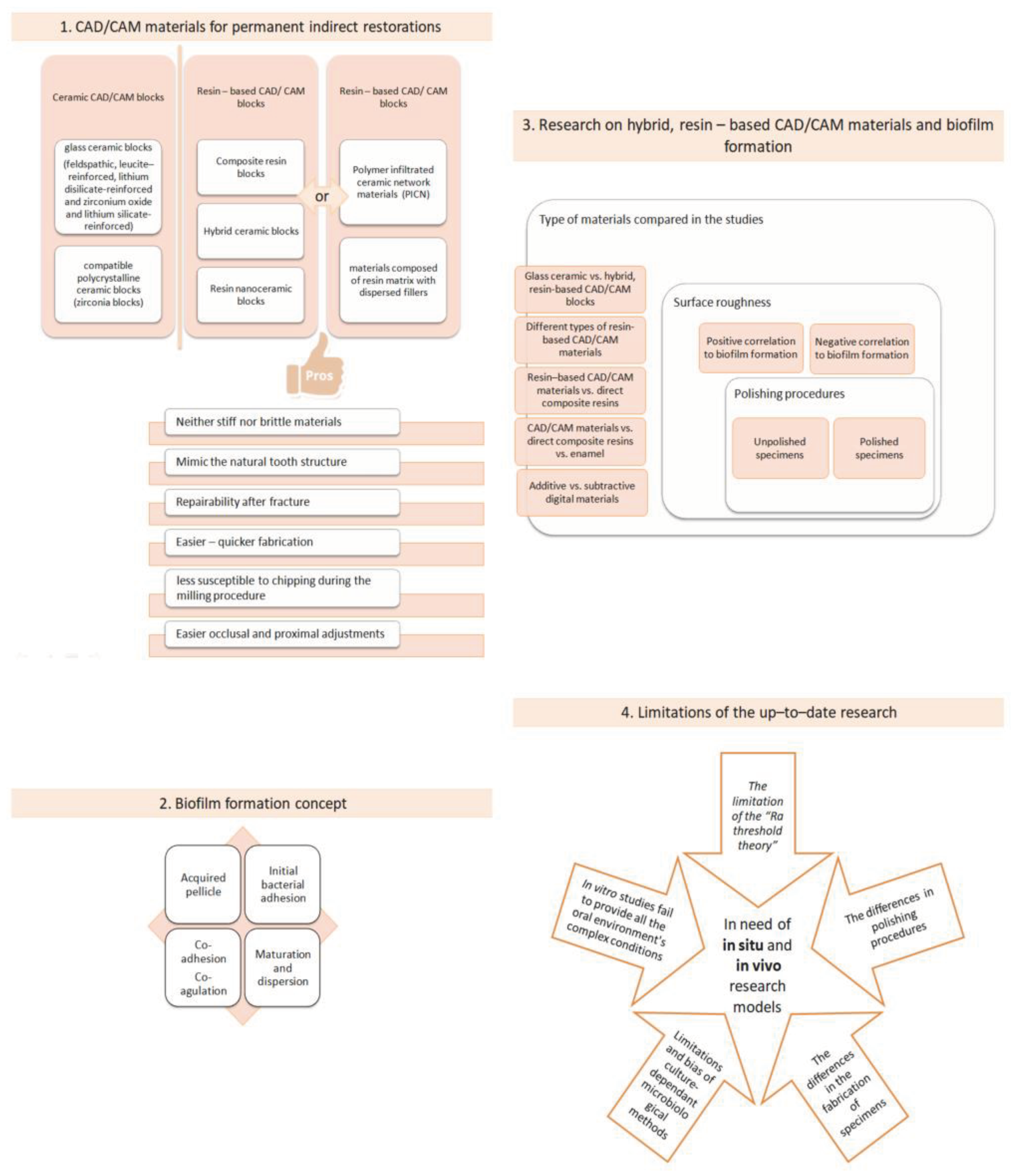
2.“. Hybrid”, Resin–Based Materials in the Digital Dentistry Era
3. The Concept of Biofilm Formation
3. Research on Biofilm Formation on Resin–Based, Hybrid CAD/CAM Materials
6. Limitations of the up–to–Date Research
- [1]
- The Ra threshold theory of 0, 2 μm. In several studies, that incorporate CAD/CAM samples in their protocols, with initial Sa values of samples greater than 0, 2 μm, a positive correlation between surface roughness and bacterial attachment has been found [69,70,77]. Additionally, it is further demonstrated that surface roughness has an insignificant effect on bacterial adhesion when the Sa values of the tested specimens are below this threshold [98]. In the research protocol of Ionescu et al in 2020, were surface roughness values (Sa) were less than 0, 2 μm no strong correlation between Sa and bacterial adhesion was present [73]. Interestingly, in some research protocols with Sa values greater than the 0, 2 μm threshold, no correlation between the two investigated factors has been observed [76,79], and in other research where the Sa values were lower than the established threshold, strong correlation between surface roughness and biofilm adhesion has been demonstrated [74,78]. This fact highlights the potential influence of additional factors, such as polishing procedures, chemical composition, and topography, on bacterial adhesion’s outcome. Moreover, a systematic review by Duetra et al in 2018 [99] concluded that the impact of roughness on bacterial adhesion is not related to a roughness threshold but rather to a range of surface roughness, which is wide and material–dependent. The majority of in vitro studies evaluating either the surface roughness as a single parameter or the relationship between surface roughness and bacterial colonization use only the Sa value, which is a single height parameter of a surface and not further spatial, functional, and hybrid (e.g. developed interfacial area ratio, Sdr) parameters, which may give a greater insight on surface texture and bacterial colonization.
- [2]
- The polishing procedure may affect on bacterial adhesion on resin–based CAD/CAM materials for indirect restorations.
- [3]
- The chemical and topographical microstructure of the hybrid, resin–based CAD/CAM materials.
- [4]
- The lack of standardization on the fabrication of the specimens.
- [5]
- The biofilm assessment method
7. Conclusions
8. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Watts, D.C.; Marouf, A.S.; Al-Hindi, A.M. Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dent Mater. 2003, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Azeem, R.A.; Sureshbabu, N.M. Clinical performance of direct versus indirect composite restorations in posterior teeth: A systematic review. J Conserv Dent. 2018, 21, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.B.; Wu, D.; Holmes, B.N. An application of nanotechnology in advanced dental materials. J Am Dent Assoc. 2003, 134, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Alzraikat, H.; Burrow, M.F.; Maghaireh, G.A.; Taha, N.A. Nanofilled Resin Composite Properties and Clinical Performance: A Review. Oper Dent. 2018, 43, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Dejak, B.; Młotkowski, A.A. Comparison of stresses in molar teeth restored with inlays and direct restorations, including polymerization shrinkage of composite resin and tooth loading during mastication. Dent Mater. 2015, 31, 77–87. [Google Scholar] [CrossRef]
- Nandini, S. Indirect resin composites. J Conserv Dent. 2010, 13, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Peutzfeldt, A. Indirect Resin and Ceramic Systems. Oper Dent. 2001, 200, 1153–1176. [Google Scholar]
- Burke, E.J.; Qualtrough, A.J. Aesthetic inlays: Composite or ceramic? Br Dent J. 1994, 176, 53–60. [Google Scholar] [CrossRef]
- Ruse, N.D.; Sadoun, M.J. Resin-composite blocks for dental CAD/CAM applications. J. Dent. Res. 2014, 93, 1232–1234. [Google Scholar] [CrossRef]
- van Noort, R. The future of dental devices is digital. Dent Mater 2012, 28, 3–12. [Google Scholar] [CrossRef]
- Fasbinder, D.J. Materials for chairside CAD/CAM restorations. Compend Contin Educ Dent. 2010, 31, 702–709. [Google Scholar] [PubMed]
- Lambert, H.; Durand, J.C.; Jacquot, B.; Fages, M. Dental biomaterials for chairside CAD/CAM: State of the art. J Adv Prosthodont. 2017, 9, 486–495. [Google Scholar] [CrossRef]
- Mörmann, W.H. The evolution of the CEREC system. J Am Dent Assoc. 2006, 137, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.D. Key Parameters of Hybrid Materials for CAD/CAM-Based Restorative Dentistry. Compend Contin Educ Dent. 2016, 37, 638–643. [Google Scholar] [PubMed]
- Palacios, T.; Tarancón, S.; Pastor, J.Y. On the Mechanical Properties of Hybrid Dental Materials for CAD/CAM Restorations. Polymers (Basel). 2022, 14, 3252. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, I.; Kamposiora, P.; Dimitriadis, K.; Papavasiliou, G.; Zinelis, S. In vitro evaluation of CAD/CAM composite materials. J Dent. 2023, 136, 104623. [Google Scholar] [CrossRef]
- Koenig, A.; Schmidtke, J.; Schmohl, L.; Schneider-Feyrer, S.; Rosentritt, M.; Hoelzig, H.; Kloess, G.; Vejjasilpa, K.; Schulz-Siegmund, M.; Fuchs, F.; Hahnel, S. Characterisation of the Filler Fraction in CAD/CAM Resin-Based Composites. Materials (Basel). 2021, 14, 1986. [Google Scholar] [CrossRef]
- Rexhepi, I.; Santilli, M.; D’Addazio, G.; Tafuri, G.; Manciocchi, E.; Caputi, S.; Sinjari, B. Clinical Applications and Mechanical Properties of CAD-CAM Materials in Restorative and Prosthetic Dentistry: A Systematic Review. J. Funct. Biomater. 2023, 14, 431. [Google Scholar] [CrossRef]
- Goujat, A.; Abouelleil, H.; Colon, P.; Jeannin, C.; Pradelle, N.; Seux, D.; Grosgogeat, B. Mechanical properties and internal fit of 4 CAD-CAM block materials. J. Prosthet. Dent. 2018, 119, 384–389. [Google Scholar] [CrossRef]
- Stockl, C.; Hampe, R.; Stawarczyk, B.; Haerst, M.; Roos, M. Macro- and microtopographical examination and quantification of CAD-CAM composite resin 2- and 3-body wear. J. Prosthet. Dent. 2018, 120, 537–545. [Google Scholar] [CrossRef]
- Papathanasiou, I.; Zinelis, S.; Papavasiliou, G.; Kamposiora, P. Effect of aging on color, gloss and surface roughness of CAD/CAM composite materials. J. Dent. 2023, 130, 104423. [Google Scholar] [CrossRef] [PubMed]
- Furtado de Mendonca, A.; Shahmoradi, M.; Gouvea, C.V.D.; De Souza, G.M.; Ellakwa, A. Microstructural and mechanical characterization of CAD/CAM materials for monolithic dental restorations. J. Prosthodont. 2019, 28, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Liebermann, A.; Eichberger, M.; Güth, J.F. Evaluation of mechanical and optical behavior of current esthetic dental restorative CAD/CAM composites. J Mech Behav Biomed Mater. 2015, 55, 1–11. [Google Scholar] [CrossRef]
- Lauvahutanon, S.; Takahashi, H.; Shiozawa, M.; Iwasaki, N.; Asakawa, Y.; Oki, M.; Finger, W.J.; Arksornnukit, M. Mechanical properties of composite resin blocks for CAD/CAM. Dent Mater J. 2014, 33, 705–710. [Google Scholar] [CrossRef]
- Sonmez, N.; Gultekin, P.; Turp, V.; Akgungor, G.; Sen, D.; Mijiritsky, E. Evaluation of five CAD/CAM materials by microstructural characterization and mechanical tests: a comparative in vitro study. BMC Oral Health. 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Kunzelmann, K.H.; Garcia-Godoy, F.; Haberlein, I.; Meier, B.; Frankenberger, R. Determination of caries risk at resin composite margins. Am J Dent 2007, 20, 59–64. [Google Scholar] [PubMed]
- Cazzaniga, G.; Ottobelli, M.; Ionescu, A.; Garcia-Godoy, F.; Brambilla, E. Surface properties of resin-based composite materials and biofilm formation: A review of the current literature. Am J Dent. 2015, 28, 311–320. [Google Scholar] [PubMed]
- Mainjot, A.K.; Dupont, N.M.; Oudkerk, J.C.; Dewael, T.Y.; Sadoun, M.J. From Artisanal to CAD-CAM Blocks: State of the Art of Indirect Composites. J Dent Res. 2016, 95, 487–495. [Google Scholar] [CrossRef]
- Della Bona, A.; Corazza, P.H.; Zhang, Y. Characterization of a polymer-infiltrated ceramic-network material. Dent Mater. 2014, 30, 564–569. [Google Scholar] [CrossRef]
- Marchesi, G.; Camurri Piloni, A.; Nicolin, V.; Turco, G.; Di Lenarda, R. Chairside CAD/CAM Materials: Current Trends of Clinical Uses. Biology (Basel). 2021, 10, 1170. [Google Scholar] [CrossRef]
- Skorulska, A.; Piszko, P.; Rybak, Z.; Szymonowicz, M.; Dobrzyński, M. Review on Polymer, Ceramic and Composite Materials for CAD/CAM Indirect Restorations in Dentistry-Application, Mechanical Characteristics and Comparison. Materials (Basel). 2021, 14, 1592. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Conejo, J. The Current State of Chairside Digital Dentistry and Materials. Dent Clin North Am. 2019, 63, 175–197. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhang, J.F.; Li, S. Polymer Infiltrated Ceramic Hybrid Composites as Dental Materials. Oral Health and Dental Studies. 2018, 1, 2. [Google Scholar] [CrossRef]
- Rocha, M.G.; Oliveira, D.; Sinhoreti, M.A.C.; Roulet, J.F.; Zoidis, P.; Duncan, W. Assessment of CAD/CAM composites classification in abstracts using machine learning. Dent Mater 2023, 39, 60–61. [Google Scholar] [CrossRef]
- Tsitrou, E.A.; Northeast, S.E.; van Noort, R. Brittleness index of machinable dental materials and its relation to the marginal chipping factor. J Dent 2007, 35, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Fasbinder, D.J.; Neiva, G.F. Surface evaluation of polishing techniques for new resilient CAD/CAM restorative materials. J Esthet Restor Dent. 2016, 28, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Coldea, A.; Swain, M.V.; Thiel, N. Mechanical properties of polymer-infiltrated-ceramic-network materials. Dent Mater. 2013, 29, 419–426. [Google Scholar] [CrossRef]
- Nguyen, J.F.; Migonney, V.; Ruse, N.D.; Sadoun, M. Resin composite blocks via high-pressure high-temperature polymerization. Dent Mater. 2012, 28, 529–534. [Google Scholar] [CrossRef]
- Vita Enamic®. Scientific-Technical Documentation; VITA Zahnfabrik, H. Rauter GmbH &. Co. KG: Bad Säckingen, Germany. Available online: https://www.vita-zahnfabrik.com (accessed on 5 July 2022).
- Lava™ Ultimate CAD/CAM Restorative for E4D. Available online: https://multimedia.3m.com/mws/media/756863O/3m-lava-ultimate-cad-cam-restorative-for-e4d-the-edge-you-need.pdf (accessed on 2 March 2024).
- SHOFU Block & Disk HC: Instructions for Use. Available online: https://www.shofu.com/wp-content/uploads/SHOFU-Block-HC-IFU-US.pdf (accessed on 2 March 2024).
- CERASMART GC Dental Product Technical Product Profile; GC Corporation: Tokyo, Japan. Available online: www.gcamerica.com (accessed on 7 July 2022).
- Grandio blocs/Grandio disc–Nano-ceramic hybrid CAD/CAM material: Instructions for use. Available online: https://www.voco.dental/en/portaldata/1/resources/products/instructions-for-use/e1/grandio-blocs_ifu_e1.pdf (accessed on 2 March 2024).
- Brilliant Crios: Instructions for use. Available online: https://products.coltene.com/EN/AG/media/DOC_IFU_30003998-12-22-IFU-BRILLIANT-Crios_IND.pdf?sprache=EN (accessed on 2 March 2024).
- Katana Avencia Blocks SDS. Available online: https://katanaavencia.com/wp-content/uploads/KATANA_AVENCIA_Block_SDS_US.pdf (accessed on 2 March 2024).
- Tetric CAD Instructions for use. Available online: https://www.ivoclar.com/en_li/eifu?brand=Tetric+CAD (accessed on 2 March 2024).
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J Oral Maxillofac Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome - an update for oral healthcare professionals. Br Dent J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Samaranayake, L.; Bandara, N.; Pesee, S. Oral Biofilms: What Are They? In Oral Biofilms and Modern Dental Materials, 1st ed.; Ionescu, A.C., Hahnel, S., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 1–7. [Google Scholar] [CrossRef]
- Ptasiewicz, M.; Grywalska, E.; Mertowska, P.; Korona-Głowniak, I.; Poniewierska-Baran, A.; Niedźwiedzka-Rystwej, P.; Chałas, R. Armed to the Teeth-The Oral Mucosa Immunity System and Microbiota. Int J Mol Sci. 2022, 23, 882. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.J. Biofilm over teeth and restorations: What do we need to know? Dent Mater. 2017, 33, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef]
- Kreth, J.; Herzberg, M.C. Molecular principles of adhesion and biofilm formation. In The root canal biofilm, 1st ed.; Chávez de Paz, L.E., Sedgley, C.M., Kishen, A., Eds.; Springer Nature: Berlin, Germany, 2015; pp. 23–54. [Google Scholar] [CrossRef]
- Enax, J.; Ganss, B.; Amaechi, B.T.; Schulze Zur Wiesche, E.; Meyer, F. The composition of the dental pellicle: an updated literature review. Front Oral Health. 2023, 4, 1260442. [Google Scholar] [CrossRef] [PubMed]
- Kreth, J.; Merritt, J.; Pfeifer, C.S.; Khajotia, S.; Ferracane, J.L. Interaction between the Oral Microbiome and Dental Composite Biomaterials: Where We Are and Where We Should Go. J Dent Res. 2020, 99, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Lindh, L.; Aroonsang, W.; Sotres, J.; Arnebrant, T. Salivary pellicles. Monogr Oral Sci. 2014, 24, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.G.; Aparicio, C. The salivary pellicle on dental biomaterials. Colloids Surf B Biointerfaces. 2021, 200, 111570. [Google Scholar] [CrossRef]
- Chawhuaveang, D.D.; Yu, O.Y.; Yin, I.X.; Lam, W.Y.; Mei, M.L.; Chu, C.H. Acquired salivary pellicle and oral diseases: A literature review. J. Dent. Sci. 2021, 16, 523–529. [Google Scholar] [CrossRef]
- Eliades, G.; Eliades, T.; Vavuranakis, M. General aspects of biomaterial surface alterations following exposure to biologic fluids. In Dental Materials in Vivo: Aging and Related Phenomena, 1st ed.; Eliades, G., Eliades, T., Brantley, W.A., Walts, D.C., Eds.; Quintessence Publishing Co: Chicago, USA, 2003; pp. 3–23. [Google Scholar]
- Sbordone, L.; Bortolaia, C. Oral microbial biofilms and plaque-related diseases: Microbial communities and their role in the shift from oral health to disease. Clin Oral Investig 2003, 7, 181–188. [Google Scholar] [CrossRef]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J Dent Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef]
- Schmalz, G.; Cieplik, F. Biofilms on Restorative Materials. Monogr Oral Sci. 2021, 29, 155–194. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implants Res. 2006, 17, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Bürgers, R.; Krohn, S.; Wassmann, T. Surface Properties of Dental Materials and Biofilm Formation. In Oral Biofilms and Modern Dental Materials, 1st ed.; Ionescu, A.C., Hahnel, S., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 55–70. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J Clin Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front Bioeng Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Auschill, T.M.; Arweiler, N.B.; Brecx, M.; Reich, E.; Sculean, A.; Netuschil, L. The effect of dental restorative materials on dental biofilm. Eur J Oral Sci. 2002, 110, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Padovani, G.; Fúcio, S.; Ambrosano, G.; Sinhoreti, M.; Puppin-Rontani, R. In situ surface biodegradation of restorative materials. Oper Dent. 2014, 39, 349–360. [Google Scholar] [CrossRef]
- Kim, K.H.; Loch, C.; Waddell, J.N.; Tompkins, G.; Schwass, D. Surface Characteristics and Biofilm Development on Selected Dental Ceramic Materials. Int J Dent. 2017, 2017, 7627945. [Google Scholar] [CrossRef]
- Hamerschmitt, R.M.; Tomazinho, P.H.; Camporês, K.L.; Gonzaga, C.C.; da Cunha, L.F.; Correr, G.M. Surface topography and bacterial adhesion of CAD/CAM resin based materials after application of different surface finishing techniques. Braz. J. Oral Sci. 2018, 17, e18135. [Google Scholar] [CrossRef]
- Dobrzynski, M.; Pajaczkowska, M.; Nowicka, J.; Jaworski, A.; Kosior, P.; Szymonowicz, M.; Kuropka, P.; Rybak, Z.; Bogucki, Z.A.; Filipiak, J.; Targonska, S.; Ciupa-Litwa, A.; Han, A.; Wiglusz, R.J. Study of Surface Structure Changes for Selected Ceramics Used in the CAD/CAM System on the Degree of Microbial Colonization, In Vitro Tests. Biomed Res Int. 2019, 12, 9130806. [Google Scholar] [CrossRef]
- Conrads, G.; Wendt, L.K.; Hetrodt, F.; Deng, Z.L.; Pieper, D.; Abdelbary, M.M.H.; Barg, A.; Wagner-Döbler, I.; Apel, C. Deep sequencing of biofilm microbiomes on dental composite materials. J Oral Microbiol. 2019, 11, 1617013. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Hahnel, S.; König, A.; Brambilla, E. Resin composite blocks for dental CAD/CAM applications reduce biofilm formation in vitro. Dent Mater. 2020, 36, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Guerrero, P.; Ortiz-Magdaleno, M.; Urcuyo-Alvarado, M.S.; Cepeda-Bravo, J.A.; Leyva-Del Rio, D.; Pérez-López, J.E.; Romo-Ramírez, G.F.; Sánchez-Vargas, L.O. Effect of dental restorative materials surface roughness on the in vitro biofilm formation of Streptococcus mutans biofilm. Am J Dent. 2020, 33, 59–63. [Google Scholar] [PubMed]
- Engel, A.S.; Kranz, H.T.; Schneider, M.; Tietze, J.P.; Piwowarcyk, A.; Kuzius, T.; Arnold, W.; Naumova, E.A. Biofilm formation on different dental restorative materials in the oral cavity. BMC Oral Health. 2020, 20, 162. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.A.; Beleidy, M.; El-Din, Y.A. Biocompatibility and Surface Roughness of Different Sustainable Dental Composite Blocks: Comprehensive In Vitro Study. ACS Omega. 2022, 7, 34258–34267. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.M.; Farahat, D.S.; Eldars, W.; Osman, M.F. Physico-mechanical properties and bacterial adhesion of resin composite CAD/CAM blocks: An in-vitro study. J Clin Exp Dent. 2022, 14, 413–419. [Google Scholar] [CrossRef]
- Ozarslan, M.; Bilgili Can, D.; Avcioglu, N.H.; Çalışkan, S. Effect of different polishing techniques on surface properties and bacterial adhesion on resin-ceramic CAD/CAM materials. Clin Oral Investig. 2022, 26, 5289–5299. [Google Scholar] [CrossRef]
- Ozer, N.E.; Sahin, Z.; Yikici, C.; Duyan, S.; Kilicarslan, M.A. Bacterial adhesion to composite resins produced by additive and subtractive manufacturing. Odontology. 2023. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J Mater Res Tech 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Cazzaniga, G.; Ottobelli, M.; Ionescu, A.; Garcia-Godoy, F.; Brambilla, E. Surface properties of resin-based composite materials and biofilm formation: A review of the current literature. Am J Dent. 2015, 28, 311–320. [Google Scholar] [PubMed]
- Van Meerbeek, B.; Vargas, M.; Inoue, S.; Yoshida, Y.; Perdigão, J.; Lambrechts, P.; Vanherle, G. Microscopy investigations. Techniques, results, limitations. Am J Dent 2000, 13, 3–18. [Google Scholar]
- Kaczmarek, K.; Leniart, A.; Lapinska, B.; Skrzypek, S.; Lukomska-Szymanska, M. Selected Spectroscopic Techniques for Surface Analysis of Dental Materials: A Narrative Review. Materials 2021, 14, 2624. [Google Scholar] [CrossRef]
- Kaczmarek, K.; Konieczny, B.; Siarkiewicz, P.; Leniart, A.; Lukomska-Szymanska, M.; Skrzypek, S.; Lapinska, B. Surface Characterization of Current Dental Ceramics Using Scanning Electron Microscopic and Atomic Force Microscopic Techniques. Coatings 2022, 12, 1122. [Google Scholar] [CrossRef]
- Sacher, E.; França, R. Surface Analysis Techniques for Dental Materials. Dental Biomaterials 2018, 2, 1–31. [Google Scholar] [CrossRef]
- Liber-Kneć, A.; Łagan, S. Surface Testing of Dental Biomaterials-Determination of Contact Angle and Surface Free Energy. Materials (Basel). 2021, 14, 2716. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; Clement, B.; Wentworth, C.D.; Holmes, A.E. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A mini-review. Res Rev J Eng Technol. 2017, 6, 1–42. [Google Scholar]
- Ionescu, A.; Wutscher, E.; Brambilla, E.; Schneider-Feyrer, S.; Giessibl, F.J.; Hahnel, S. Influence of surface properties of resin-based composites on in vitro Streptococcus mutans biofilm development. Eur J Oral Sci 2012, 120, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Aykent, F.; Yondem, I.; Ozyesil, A.G.; Gunal, S.K.; Avunduk, M.C.; Ozkan, S. Effect of different finishing techniques for restorative materials on surface roughness and bacterial adhesion. J Prosthet Dent 2010, 103, 221–227. [Google Scholar] [CrossRef]
- Ikeda, M.; Matin, K.; Nikaido, T.; Foxton, R.M.; Tagami, J. Effect of surface characteristics on adherence of S. mutans biofilms to indirect resin composites. Dent Mater J 2007, 26, 915–923. [Google Scholar] [CrossRef]
- Ionescu, A.; Brambilla, E.; Wastl, D.S.; Giessibl, F.J.; Cazzaniga, G.; Schneider Feyrer, S.; Hahnel, S. Influence of matrix and filler fraction on biofilm formation on the surface of experimental resin-based composites. J Mater Sci Mater Med 2015, 26, 5372. [Google Scholar] [CrossRef]
- Buergers, R.; Schneider-Brachert, W.; Hahnel, S.; Rosentritt., M.; Handel, G. Streptococcal adhesion to novel low-shrink silorane-based restorative. Dent Mater 2009, 25, 269–275. [Google Scholar] [CrossRef]
- Pereira, C.A.; Eskelson, E.; Cavalli, V.; Liporoni, P.C.S.; Jorge, A.O.; do Rego, M.A. Streptococcus mutans biofilm adhesion on composite resin surfaces after different finishing and polishing techniques. Oper Dent 2011, 36, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.X.; Wang, X.; Gao, F.; Chen, X.; Liang, D.; Li, D. Effects of surface properties of polymer- based restorative materials on early adhesion of Streptococcus mutans in vitro. J. Dent. 2016, 54, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, G.; Ottobelli, M.; Ionescu, A.C.; Paolone, G.; Gherlone, E.; Ferracane, J.L.; Brambilla, E. In vitro biofilm formation on resin-based composites after different finishing and polishing procedures. J Dent. 2017, 67, 43–52. [Google Scholar] [CrossRef]
- Bilgili, D.; Dündar, A.; Barutçugil, Ç.; Tayfun, D.; Özyurt, Ö.K. Surface properties and bacterial adhesion of bulk-fll composite resins. J Dent 2020, 95, 103317. [Google Scholar] [CrossRef] [PubMed]
- Hahnel, S.; Ionescu, A.C.; Cazzaniga, G.; Ottobelli, M.; Brambilla, E. Biofilm formation and release of fluoride from dental restorative materials in relation to their surface properties. J Dent. 2017, 60, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: a review of the literature. Dent Mater 1997, 13, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Dutra, D.; Pereira, G.; Kantorski, K.Z.; Valandro, L.F.; Zanatta, F.B. Does Finishing and Polishing of Restorative Materials Affect Bacterial Adhesion and Biofilm Formation? A Systematic Review. Oper Dent. 2018, 43, 37–52. [Google Scholar] [CrossRef]
- Kara, D.; Tekçe, N.; Fidan, S.; Demirci, M.; Tuncer, S.; Balcı, S. The efects of various polishing procedures on surface topography of CAD/CAM resin restoratives. J Prosthodont 2021, 30, 481–489. [Google Scholar] [CrossRef]
- da Silva, T.M.; Salvia, A.C.R.D.; Carvalho, R.F.; Pagani, C.; Rocha, D.M.; da Silva, E.G. Polishing for glass ceramics: Which protocol? J Prosthodont Res. 2014, 58, 160–70. [Google Scholar] [CrossRef]
- de Oliveira, A.L.B.M.; Domingos, P.A.D.S.; Palma-Dibb, R.G.; Garcia, P.P.N.S. Chemical and morphological features of nanofilled composite resin: influence of finishing and polishing procedures and fluoride solutions. Microsc Res Tech 2012, 75, 212–219. [Google Scholar] [CrossRef]
- Kurt, A.; Cilingir, A.; Bilmenoglu, C.; Topcuoglu, N.; Kulekci, G. Effect of different polishing techniques for composite resin materials on surface properties and bacterial biofilm formation. J Dent 2019, 90, 103199. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.C.; Brambilla, E. Bioreactors: How to Study Biofilms In Vitro. In Oral Biofilms and Modern Dental Materials, 1st ed.; Ionescu, A.C., Hahnel, S., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 37–54. [Google Scholar] [CrossRef]
- Cieplik, F.; Aparicio, C.; Kreth, J.; Schmalz, G. Development of standard protocols for biofilm-biomaterial interface testing. JADA FS 2022, 1, 100008. [Google Scholar] [CrossRef]
- Brown, J.L.; Johnston, W.; Delaney, C.; Short, B.; Butcher, M.C.; Young, T.; Butcher, J.; Riggio, M.; Culshaw, S.; Ramage, G. Polymicrobial oral biofilm models: Simplifying the complex. J Med Microbiol 2019, 68, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, S.S.; Wright, P.; Han, P.; Abdal-Hay, A.; Lee, R.S.B.; Ivanovski, S. Evaluating models and assessment techniques for understanding oral biofilm complexity. MicrobiologyOpen 2023, 12, 1377. [Google Scholar] [CrossRef]
- Darrene, L.N.; Cecile, B. Experimental Models of Oral Biofilms Developed on Inert Substrates: A Review of the Literature. Biomed Res Int 2016, 2016, 7461047. [Google Scholar] [CrossRef]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the human oral microbiome. Arch. Microbiol 2018, 200, 525–540. [Google Scholar] [CrossRef]
| Hybrid, resin–based CAD/CAM material | Description | Manufacturer | Composition |
|---|---|---|---|
| Vita Enamic | Polymer infiltrated ceramic network material (PICN) Hybrid ceramic block |
VITA Zahnfabrik | 86% by weight inorganic fillers (mainly silicon dioxide and aluminum oxide) 14% organic matrix by weight: UDMA and TEGDMA |
| Lava Ultimate | Resin nanoceramic block | 3M ESPE | 80% by weight inorganic fillers (nanomers of silica and zirconia and zirconia and silica nanoclusters of 0.6–10μm) 20% organic matrix: Bis-GMA, UDMA, Bis-EMA and TEGDMA |
| Shofu Block HC | Hybrid ceramic block | Shofu Inc | 61% inorganic fillers (silica powder, zirconium silicate and microfumed silica) Organic matrix: UDMA and TEGDMA |
| Cerasmart | Force–absorbing hybrid ceramic block | GC Dental Products | 71% by weight inorganic fillers ( silica (20nm) and barium glass (300nm)) Organic matrix: Bis–MEPP, UDMA, DMA |
| Grandio Bloc | Nanoceramic hybrid block | VOCO GmbH | 86% by weight inorganic fillers Organic matrix: UDMA and DMA |
| Brilliant Crios | reinforced composite block | Coltene Whaledent AG | 70.7% by weight inorganic fillers (barium glass and amorphous silica) Organic matrix: Cross–Bis–GMA, Bis–EMA and TEGDMA |
| Katana Avencia Block | Hybrid ceramic, composite resin CAD/CAM block | Kuraray Noritake Dental Inc. | 82% by weight inorganic fillers (colloidal silica and aluminum oxide) Organic matrix: UDMA and other methacrylate monomers) |
| Tetric CAD | Composite block | Ivoclar Vivadent AG | 71% by weight barium glass (< 1 µm) and silicon dioxide fillers Organic matrix: cross–linked methacrylates, (Bis–GMA, Bis–EMA, TEGDMA, UDMA) |
| Study/Year | Objective | Types of specimens/Type of control group | Tests | Conclusions |
|---|---|---|---|---|
| Kim et al, 2017 [69] | Evaluation of surface roughness and biofilm formation on CAD/CAM materials before and after polishing | 1)Vita Enamic, Vita Zahnfabrik 2) Lava Ultimate, 3M ESPE 3) Vitablocs Mark II, Vita Zahnfabrik 4) Wieland Reflex Veneering porcelain, Wieland Dental POLISHING PROCEDURES Unpolished specimens (control group) Uniformly polished specimens with diamond burs, finishing burs and extrafine porcelain burs (experimental group) |
1) SEM, CLSM, crystal violet assay for microbial analysis of S. grodonii 2) 3D Slicer software for surface roughness evaluation |
More irregular surface topography in polished specimens compared to controls Greater surface roughness (𝑅𝑎) values in polished CAD/CAM blocks compared to controls. Greater biofilm growth on polished specimens compared to controls |
| Hammerschnitt et al, 2018 [70] | Evaluation of the surface topography and bacterial adhesion CAD/CAM blocks after different surface finishing procedures. |
1) Vita Enamic, Vita Zahnfabrik 2) Lava Ultimate, 3M ESPE POLISHING PROCEDURES 1) no surface finish (control group) 2) diamond bur surface finish 3) polishing system for hybrid ceramics 4) polishing system for ceramics |
1) stylus profilometer for surface roughness evaluation (Ra, Rz, Rq height parameters) 2) Spectrophotometry, CFU/ml, SEM and CSLM for microbial analysis of S. mutans |
Surface roughness and bacterial adhesion are lower on Vita Enamic compared to Lava Ultimate, regardless the finishing procedures The type of material and the finishing techniques have an effect on surface roughness and bacterial adhesion |
| Dobrzynski et al, 2019 [71] | Comparison of biofilm formation on CAD/CAM materials in accordance to their roughness |
1)Vita Enamic, Vita Zahnfabrik 2) IPS Empress, Ivoclar Vivadent 3) IPS Empress Multi, Ivoclar Vivadent 4) IPS emax, Ivoclar Vivadent, before and after sintering POLISHING PROCEDURES unpolished specimens (control group) uniformly polished specimens with 800–1200 grit sandpaper discs (experimental group) |
1) Powder X-ray diffraction pattern (XRPD) and (ATR–FT–IR) for surface topography evaluation 2) contact angle measurement for wettability evaluation 3) fluorescence microscopy and CFU/ml counting for microbial analysis of S. mutans, C. albicans and Lactobacillus rhamnosus |
Non–polished surfaces are more susceptible to biofilm adhesion compared to their polished counterparts. The degree of biofilm formations depends on the tested microbial species |
| Conrads et al, 2019 [72] | Identification and comparison of the oral microbiome on resin–based materials in vivo and in vitro | 1) Grandio flow, Voco GmbH (conventional flowable composite resin) 2) Grandio Bloc, Voco GmbH (resin–based CAD/CAM material) 3) bovine enamel (control group) |
1) for the in situ project: 15 volunteers wore oral splints with slabs of resin–based materials and bovine enamel for 48 hours and Ilumina Miseq Next Generation Sequencing of 16S ribosomal RNA (V1–V2 region) for bacterial identification followed | no significant differences in bacterial colonization for the different dental composites and the control group in vivo |
| Ionescu et al, 2020 [73] | Differences on biofilm formation between indirect CAD/CAM resin–based–composites and their direct resin - based counterparts | 1) Grandio Bloc, VOCO GmbH 2) Lava Ultimate, 3M ESPE 3) Katana Avencia, Kuraray Corp. 4) Vita Enamic, Vita Zahnfabrik 5) Grandio SO, VOCO GmbH 6) Filtek Supreme XTE, 3M ESPE 7) Ionostar Plus, VOCO GmbH (positive control) 8) Human enamel (negative control) POLISHING PROCEDURES All specimens are uniformly finished and polished with silica–alumina grinding papers (600-4000 grit) and stored in artificial saliva |
1) Profilometry in contact mode for surface roughness evaluation (Ra height parameter) 2) SEM/EDX analysis and X-ray diffraction (XRD analysis) for molecular, elemental and structural analysis of the specimens. 3) thermogravimetric analysis (TG) and differential scanning calorimetry (DSC) for quantification of filler content of the specimens. 4) Static, orbital shaking, continuous flow and mixed- plaque formation bioreactors for microbial investigation of S. mutans and mixed plaque biofilm |
CAD/CAM blocks yielded lower S. mutans and mixed-plaque biofilm formation compared to direct resin–based materials No strong correlation between biofilm formation and surface roughness Stronger corellation between biofilm formation, manufacturing techniques and curing processes |
| Contreras - Guererro et al, 2020 [74] | Evaluation of biofilm formation on different dental restorative materials | 1) IPS Emax Press, Ivoclar Vivadent 2) IPS Emax CAD, Ivoclar Vivadent 3) Lava Ultimate, 3M ESPE 4) Vita Enamic, Vita Zahnfabrik 5) 2 conventional composite resins POLISHING PROCEDURES CAD/CAM specimens subjected to sandblasting, polished by sandpaper discs (180-2000 grit), Sof–Lex discs, green stone and rubber points. Composite resins polished with polishing brushes, Sof–Lex discs, diamond paste and cotton tassel |
1) Atomic Force Microscopy for surface roughness evaluation (Ra, Rmax, Rz height parameters) 2) dynamic bioreactor, CLSM analysis and arbitary fluorescence unit counting (AFU) for microbial analysis of S. mutans |
Positive correlation between surface roughness and biofilm formation on ceramic CAD/CAM blocks and composite resins. |
| Engel et al, 2020 [75] | Comparison of biofilm adhesion and formation on different smooth dental restorative materials with human enamel |
1) Ceram X, Dentsply, Sirona 2) IPS emax Press, Ivoclar Vivadent 3) Lava Plus, 3M ESPE 4) Vita Enamic, Vita Zahnfabric 5) metal alloy (CoCrMo) 6) human enamel (control group) POLISHING PROCEDURES finished and polished according to the manufacturers’ instructions |
1) 3D–optical profilometer for surface roughness evaluation (Sa height parameter) 2) SEM analysis and CFU/ml counting for microbiological analysis 3) Mass Spectrometry for species identification |
biofilm maturation on specific restorative materials is influenced by surface properties and material composition Microbiological analysis showed that bacterial strains differed between the materials |
| Hassan et al, 2022 [76] | Evaluation of surface roughness, biofilm formation, cytotoxicity and genotoxicity of 3 resin–based CAD/CAM materials | 1) Vita Enamic, Vita Zahnfabrik 2) Cerasmart, GC 3) Brilliant Crios, Coltene Whaledent AG POLISHING PROCEDURES All specimens are uniformly polished with silicone carbide paper discs up to 1200 grit, diamond grit polishing discs and a diamond polishing paste |
1) non contact optical profilometer + SEM for surface roughness evaluation 2) CFU/ml counting for microbial analysis of S. mutans and Lactobacilli |
Brilliant Crios showed the highest biofilm formation values No statistically significant differences in surface roughness values between groups No statistically significant correlation between surface roughness and bacterial adhesion for all groups |
| Mokhtar et al, 2022 [77] | Comparison of physicomechanical properties and biofilm formation between resin–based hybrid materials | 1) Grandio Blocs, VOCO GmbH 2) Lava Untimate, 3M ESPE POLISHING PROCEDURES Materials were polished according to the manufacturer’s instructions |
1) stylus profilometer for surface roughness evaluation (Ra height parameter) 2) SEM analysis and CFU/ml counting for microbial analysis of S. mutans |
Grandio Blocs showed significantly lower roughness and bacterial adhesion when compared to Lava Ultimate Positive correlation between surface roughness and bacterial adherence for both resin–based CAD/CAM materials. |
| Ozarslan et al, 2022 [78] | Effect of different polishing techniques on surface properties and bacterial adhesion on resin–based CAD/CAM materials | 1) Vita Enamic, Vita Zahnfabrik 2) Lava Ultimate, 3M ESPE 3) Cerasmart, GC POLISHING PROCEDURES 1) non–polished (control group) 2) manually–polished 3) glazed |
1) profilometer in contact mode for surface roughness evaluation (Ra height parameter) 2) Contact angle measurement for surface free energy evaluation 3) SEM/EDS analysis for elemental and topographical evaluation 4) CFU/ml counting and SEM analysis for microbial evaluation of S. mutans |
Non–polished CAD/CAM controls showed the highest surface roughness values Non–polished CAD/CAM controls showed higher bacterial adhesion Positive correlation between polishing procedures, surface properties and bacterial adhesion |
| Ozer et al, 2023 [79] | Evaluation of surface roughness, surface wettability and biofilm formation on CAD/CAM and 3D printed materials for permanent restorations | 1) Vita Enamic, Vita Zahnfabrik 2) Cerasmart, GC Corp. 3) Lava Unltimate, 3M ESPE 4) Varseo Smile Crown Plus, BEGO 5) Saremco Print Crowntech, Saremco dental AG 6) Formlabs 3D Permanent Crown, Formlabs POLISHING PROCEDURES Equally polished with 600–800 grit size silicon carbide discs and aluminum oxide coated discs (Coarse, medium, fine and extrafine discs) |
1) Profilometer in contact mode for surface roughness evaluation (Ra height parameter) 2) Contact angle measurement for surface wettability 3) CFU/ml counting and SEM analysis for microbiological analysis of S. mutans and S. sanguis |
Different digital manufacturing techniques and material compositions affect surface roughness. No statistically signifcant diference between the groups in contact angle values Microbial adhesion varies refarding the bacterial species tested No correlation between surface roughness and bacterial adhesion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





