1. Introduction
Crohn’s disease (CD) is a progressive, multifactorial, immune-mediated disease characterized by chronic inflammation in any part of the gastrointestinal (GI) tract [
1,
2,
3]. It is thought to occur due to an altered immune response to unknown environmental factors in a genetically predisposed person, with a peak age of onset between 15 and 30 years of age [
3,
4,
5]. The incidence of pediatric-onset CD is rising globally and varies geographically with a recorded incidence in Europe of 0.2-23 per 100,000 people [
5,
6,
7]. Pediatric patients present with a more extensive form of the disease, accompanied by non-specific gastrointestinal symptoms and weight loss, causing a concerning delay in the linear growth of immature children [
2,
5]. The risk of having a disabling condition 5 years after diagnosis is higher in pediatric patients, and associated with psychosocial effects of living with a chronic incurable disease [
1,
6]. The diagnosis of CD is complex and depends upon the combination of clinical signs and symptoms, serologic (fecal calprotectin, C-reactive protein, and others), and endoscopic and histopathological data. These same parameters are used in disease monitoring, as multiple grading scores are developed but not yet part of a standardized practice, especially in pediatric patients [
2,
3,
8]. Although it can arise anywhere from the mouth to the anus, a great proportion of children have the burden of active disease in the upper gastrointestinal tract with various inflammatory patterns on endoscopy and histopathology (HP) [
5]. According to standard criteria, an esophagogastroduodenoscopy (EGDS) with biopsy obtainment is performed in all children at initial evaluation, in addition to the ileo-colonoscopy. On HP analysis, chronic inflammation with non-caseating granulomas is a characteristic feature to define CD. Still, other findings can be highly suggestive of the presence of this condition, such as focally enhanced gastritis and lymphocytic oesophagitis [
9]. Standardized histological grading systems have not yet been developed for pediatric CD [
10]. Upon the completion of the assessment of disease severity and activity, a prediction of progression can be made, and aid in the choice of personalized therapy for every individual patient [
1].
Our study aims to analyze the endoscopic and histopathological findings in children diagnosed with Crohn’s disease and compare the results of the initial and follow-up examinations.
2. Materials and Methods
Our retrospective study included 100 children and adolescents with Crohn’s disease treated at the University Children’s Hospital in Belgrade, Serbia, in the period between January 2016 and December 2023, where the EGDS and ileo-colonoscopy with biopsy was performed. The histopathological sample was analyzed at the Institute of Pathology ‘‘Prof. dr Đorđe Joannović’’, Faculty of Medicine, University of Belgrade. During this period, 68 patients were diagnosed with Crohn’s disease for the first time and 32 patients had already been diagnosed in the past and had a follow-up biopsy. A total of 45 patients had two biopsies during these 8 years, and their endoscopic and HP results were matched and compared. From the medical documentation, patient demographics (age at diagnosis, first and second biopsies, and gender), duration of symptoms before hospitalization, treatment modality, and laboratory values (fecal calprotectin and C-reactive protein) were collected. Four symptoms were recorded during the evaluation: stool changes (frequent bowel movements or diarrhea), abdominal pain, anemia, and weight loss. We described the findings of upper endoscopy and ileo-colonoscopy and classified them as specific (which included either of the following: cobblestone-like mucosa, aphthous lesions, mucosal ulcers, erosions and strictures), non-specific (edema, hyperemia), and normal. In patients with specific upper endoscopic changes, we noted their localization (oesophagus, stomach, and duodenum). At the time of endoscopy, the biopsy set consisted of samples taken from the oesophagus, stomach, duodenum, terminal ileum, cecum, ascending colon, transverse colon, descending colon, sigmoid colon, and rectum. The slides were stained with hematoxylin and eosin [H&E] and evaluated by a pathology specialist. The HP findings were further classified appropriately, with an emphasis on those that were specific (chronic inflammation with non-caseous granulomas) and highly suggestive of CD (focally enhanced gastritis and lymphocytic oesophagitis). Regarding biopsies taken during ileo-colonoscopy, we noted whether signs of CD were detected and their localization and consequently classified the findings into 3 categories (ileocecal, colorectal, and CD of the entire lower GI tract). We also noted the Global Histologic Disease Activity Score (GHAS) for the terminal ileum and colon, which was scored independently by a pathology specialist. It consisted of 8 items, scored individually for the terminal ileum and colon. The score range was from 0, which indicated no disease activity, to 14, which represented severe disease activity. Furthermore, a value of ≤4 implied mild, 5-9 moderate, and ≥10 severe disease activity. Statistical analysis was performed in EZR (R package version 0.1.4). We have compared nominal variables using the Chi-squared (ꭓ2) test for independent, and Mc Nemar’s test for dependent variables, and the results were represented as medians and 1st-3rd quartiles. Numerical valuables were evaluated using Wilcoxon’s test and represented as mean values with standard deviation. Statistical significance was set at p<0.05.
3. Results
The median age of patients was 13 years and 8 months at the time of diagnosis, 14 years at the time of the first analyzed biopsy, and 15 years and 7 months on the second biopsy. Five of our patients can be classified into the very early onset inflammatory bowel disease (VEO-IBD) category, i.e., patients diagnosed before the age of 6. The male-to-female ratio was 1.68 (62 boys and 38 girls). The median duration of symptoms before diagnosis was 5 weeks (0.2-60 weeks). The most frequent presenting symptoms were stool changes (65.52%), followed by abdominal pain (41.86%), weight loss (35.23%), and anemia (11.49%). We compared the frequencies of symptoms in time of the first and second biopsy obtention which are shown in
Figure 1. A statistically significant decrease in anamnestic data regarding stool changes (
p<0.001), weight loss (
p<0.001), and abdominal pain (
p<0.001) was observed.
Immunosuppressive therapy was the most common drug modality (54) of our patients, followed by immunotherapies that included either infliximab or adalimumab (44) and corticosteroids (27). A smaller proportion of patients were treated with other types of medications (anti-inflammatories: 12, proton pump inhibitors: 10, antibiotics: 14 patients).
At the time of the first visit, 58/60 patients had elevated fecal calprotectin levels. The value of this parameter decreased significantly between the first (median 1000 μg/g) and the second examination (298.9 μg/g) (Wilcoxon’s test, p<0.001). The mean value of the first C-reactive protein (CRP) was 44.89±47.2 mg/L (median 25 mg/L) and the second was 32.45±44.22 mg/L (median 15.1 mg/L) with no statistical difference.
We compared endoscopic findings at the upper and lower endoscopy in patients with CD. On EGDS, 32 (36.59%) patients had specific, 20 (24.39%) had non-specific findings, and 30 (36.59%) were normal. Of patients who had specific findings, 50% were in the stomach, 28.57% in the duodenum, and 21.43% in the oesophagus. On colonoscopy, 48 (64.86%) patients had specific, 16 (21.62%) had non-specific findings and 10 (13.51%) were normal. We have compared and presented the simultaneous findings on both endoscopies (
ꭓ2=9.1,
p=0.059) in
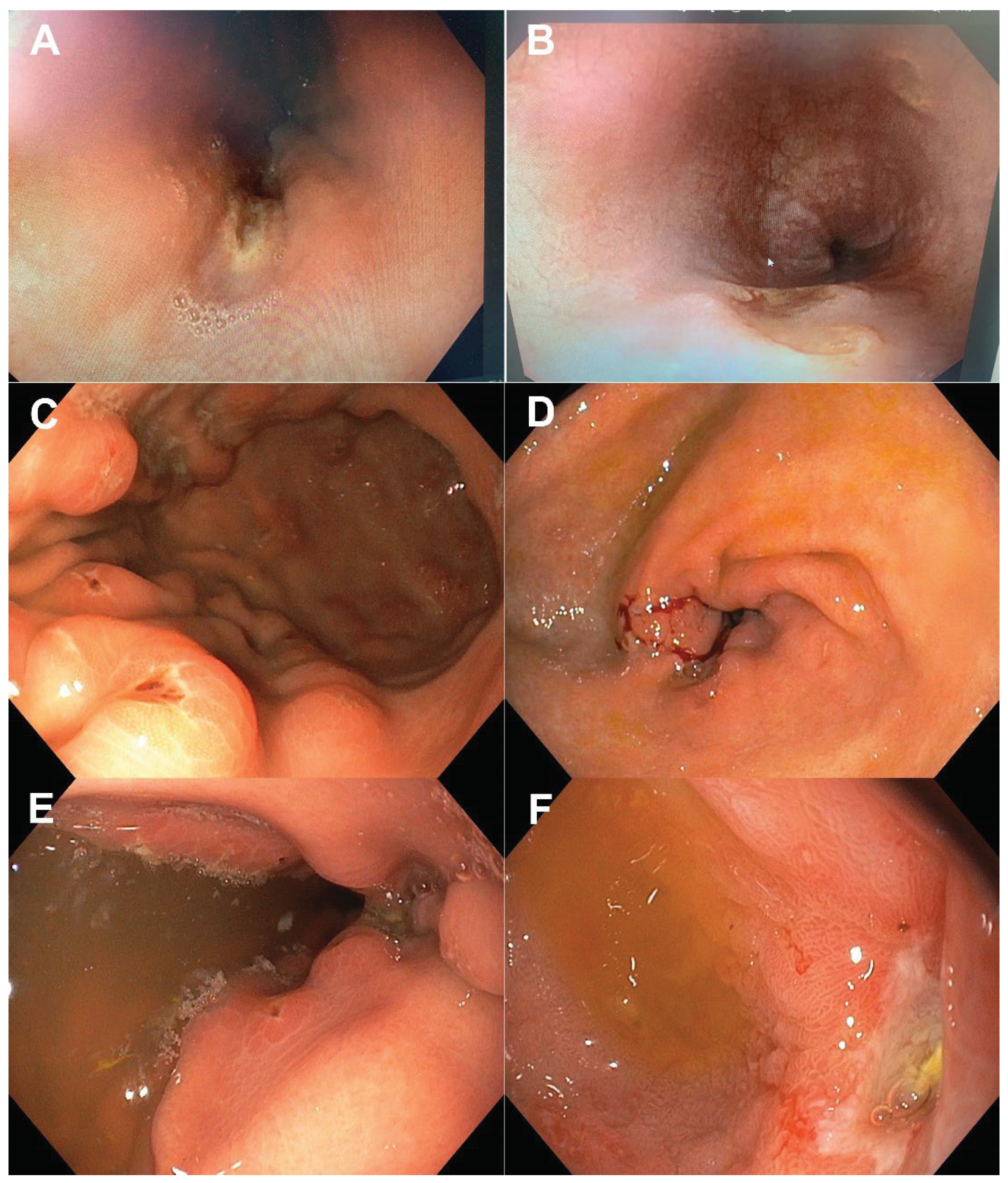
Table 1. Nineteen patients had specific changes on both colonoscopy and EGDS. Typical endoscopic findings suggestive of CD in the proximal GI tract are shown in
Figure 2.
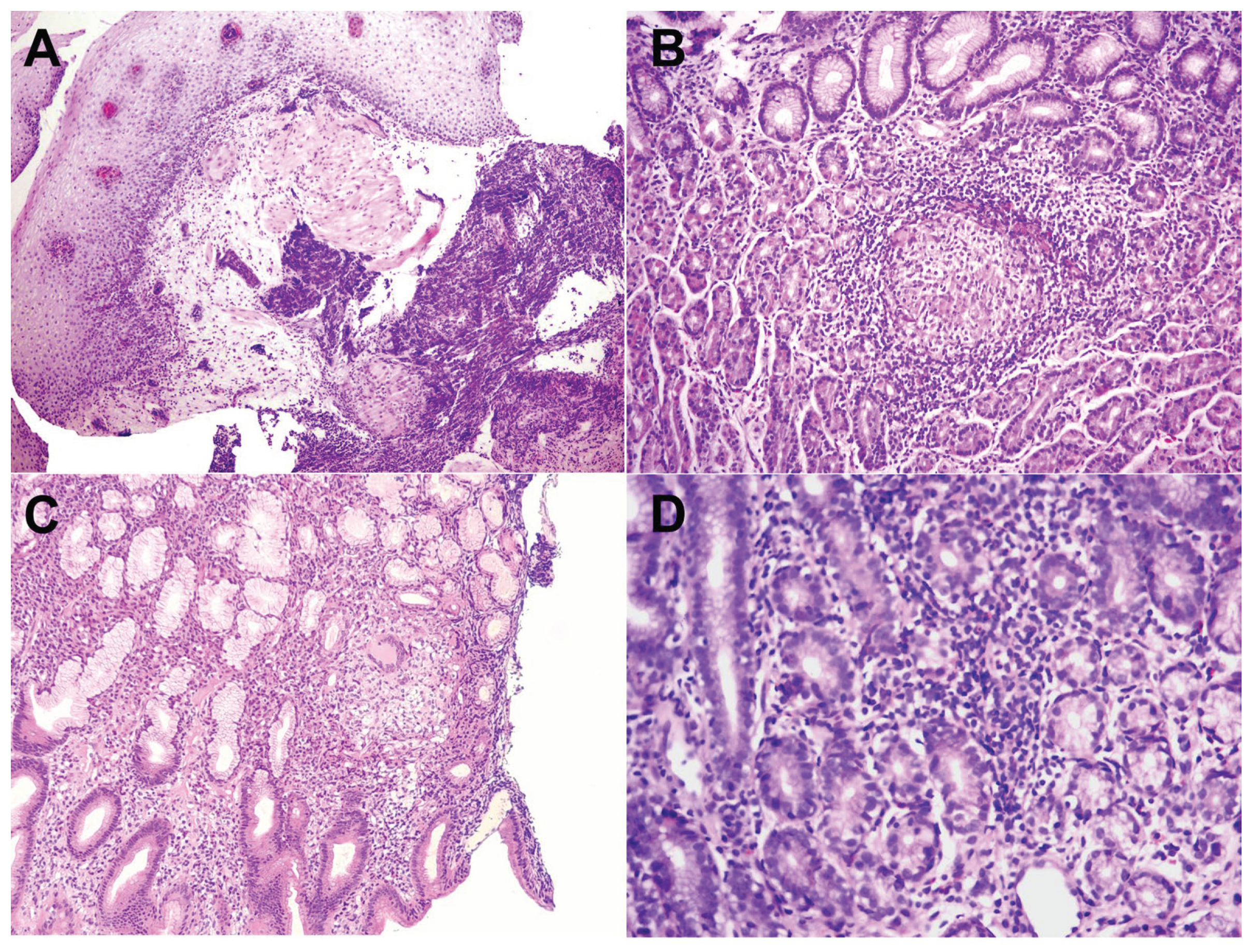
We further analyzed the histopathological profile of each biopsy. The diagnosis of CD was noted with the presence of chronic inflammation with non-caseous granulomas. A total of 31 patients had characteristic signs of CD in the first biopsy taken from the upper GI tract: 13 in the stomach, 5 in the oesophagus, 6 in the duodenum, 2 in the oesophagus and duodenum, 2 in the stomach and oesophagus, and 3 in the stomach and duodenum. Highly suggestive findings alone had 23 patients (17 focally enhanced gastritis and 6 lymphocytic oesophagitis) (
Figure 3).
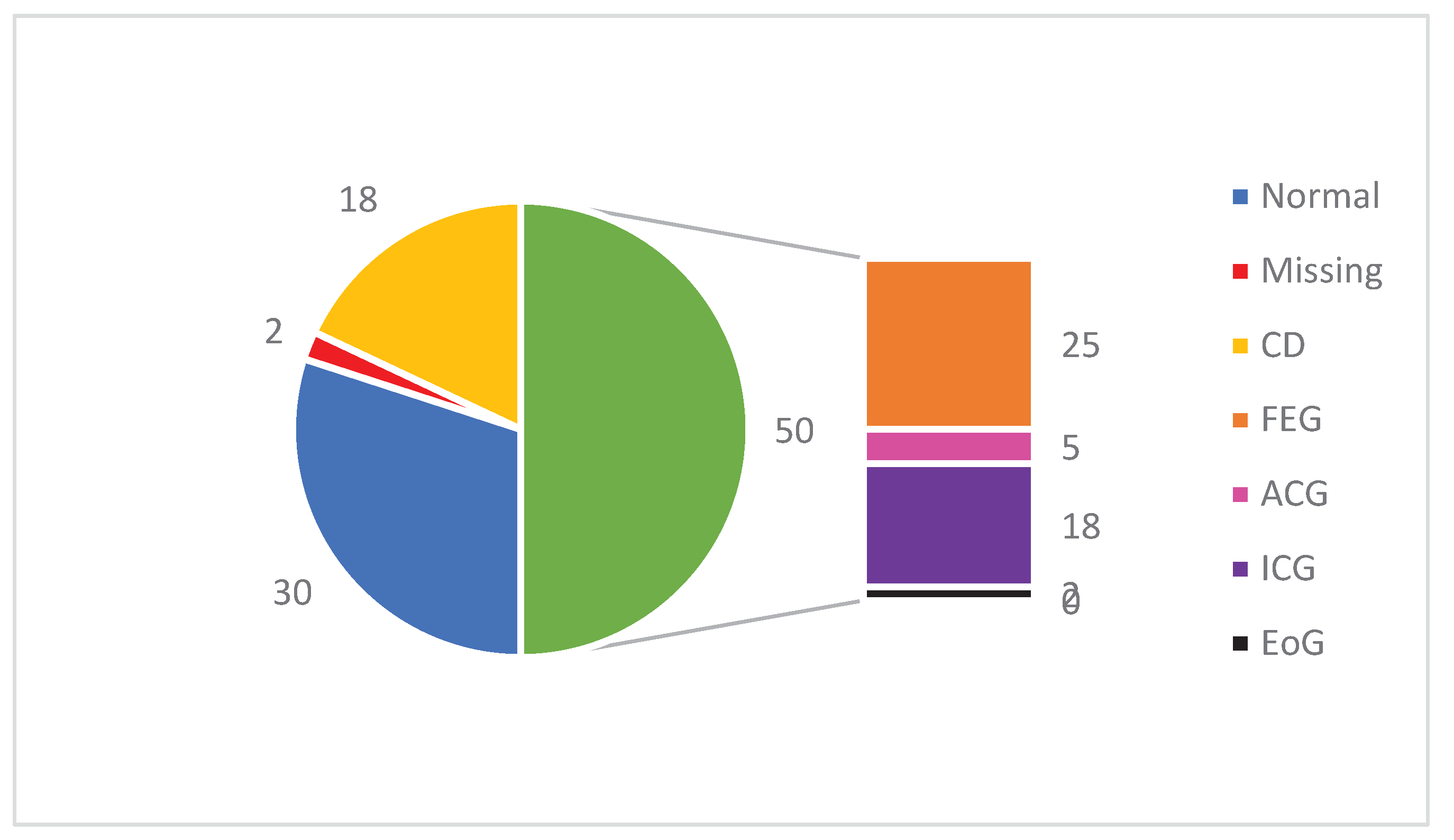
The pathohistological findings of the first gastric biopsy are presented in
Figure 4. Signs of CD were found in 18 patients (18%) and a normal gastric wall had 30 (30%). Focally enhanced gastritis (FEG) had 25 patients, active chronic gastritis (ACG) 5, inactive chronic gastritis (ICG) 18, and an elevated number of eosinophils in the gastric mucosa (EoG) 2 of our patients.
On the second gastric biopsy, 1 (2.22%) patient had signs of CD, 22 (48.89%) had normal findings, 8 (17.78%) FEG, 13 (28.89%) ICG and 1 (2.22%) EoG. There was no statistically significant difference between the first and second biopsies (Mc Nemar’s test, p=0.178).
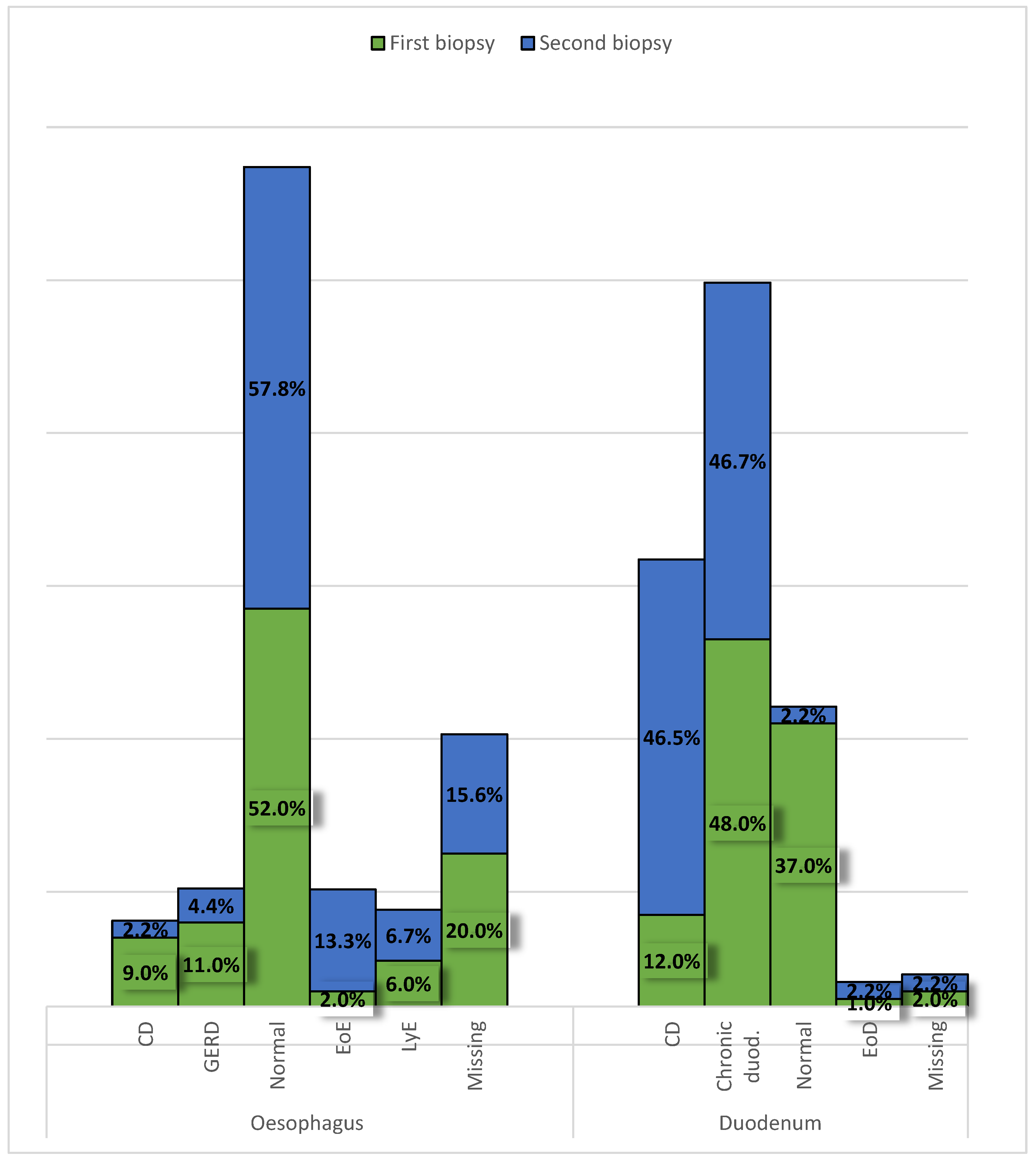
Specific signs of CD were found in 9 (9%) oesophageal and 12 (12%) duodenal biopsies. A normal biopsy had 52 patients in their oesophagus and 37 in the duodenum. In the oesophagus, signs of gastroesophageal reflux disease were seen in 11 patients, lymphocytic esophagitis in 6, and eosinophilic oesophagitis in 2 patients. Chronic duodenitis was recorded in 48 and an elevated number of eosinophils in the duodenal mucosa in 1 patient. In twenty patients a biopsy of the oesophagus was not obtained and in 2 of the duodenum (
Figure 5). No significant changes were observed between the first and second biopsies in the oesophagus or duodenum.
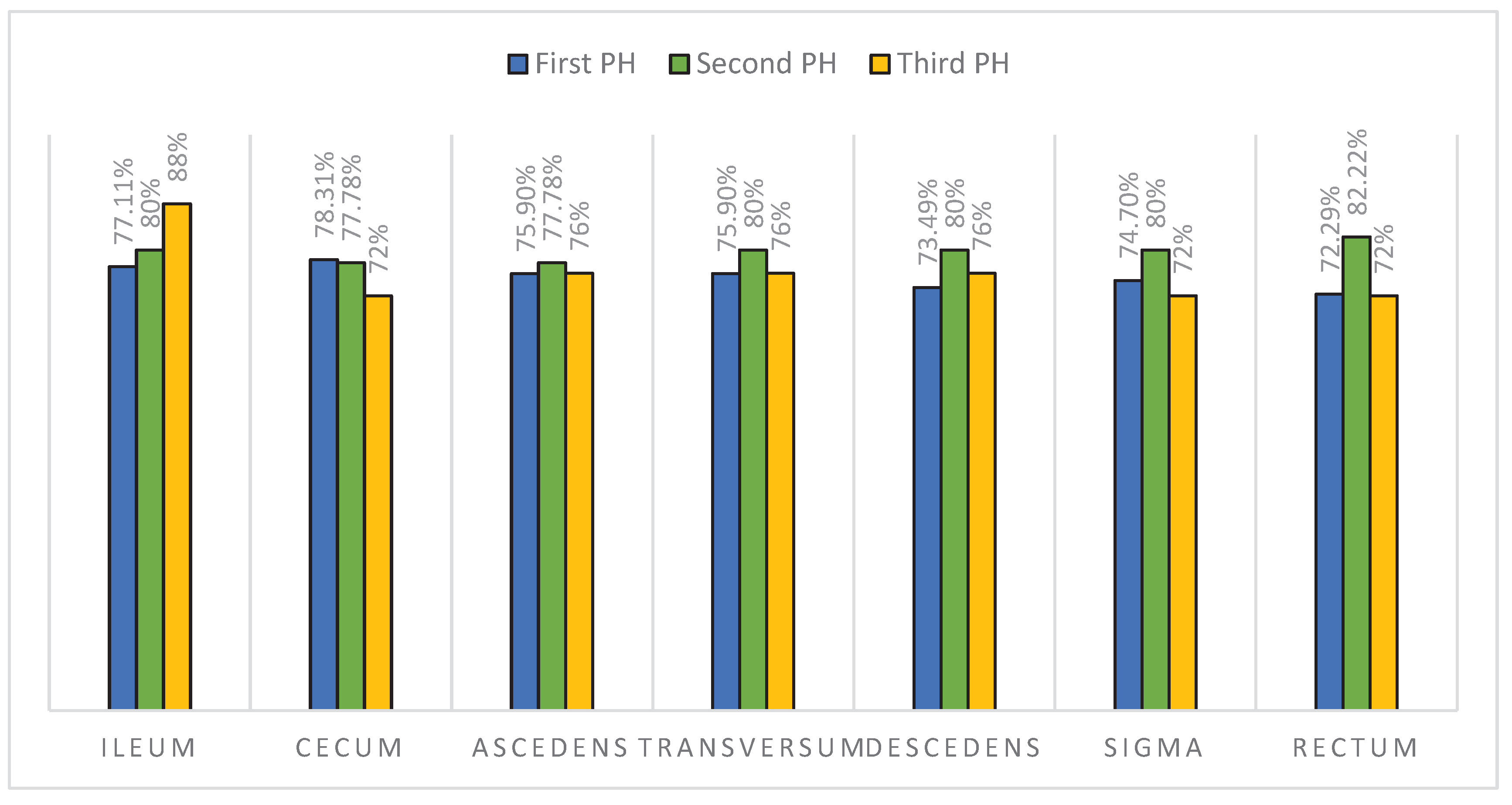
In
Figure 6 we have compared the findings between the first and second biopsy results for each segment of the lower GI tract and found that the signs of CD were significantly increased in the second biopsy in the descending colon (
p=0.02) and rectum (
p=0.035). On the first biopsy, 73.49% of patients had signs of CD in the descending colon and 72.29% in the rectum. On the second biopsy, 80% of patients developed signs of CD in the descending colon and 82.22% in the rectum. We have further grouped the findings into: only ileocecal, only colorectal, and those with signs of CD throughout the entire lower GI (pancolitis). Within these three groups, no statistical differences between the first and second biopsies were found (Mc Nemar’s test, ileocecal
p=0.257, colorectal
p=0.317, pancolitis
p=0.166). Of the patients diagnosed for the first time with CD, 68% had specific findings in the entire lower GI tract.
The median value of the GHAS for the terminal ileum was 3 (0-14) and for the colon was 7 (0-13). In the terminal ileum 13 (28.89%) patients had a GHAS of 0, 12 (26.67%) ≤4, 14 (31.11%) from 5 to 9, and 6 (13.33%) ≥10. In the colon 1 (1.72%) patient had a GHAS of 0, 21 (36.21%) ≤4, 25 (43.1%) from 5 to 9, and 11 (18.96%) ≥10.
4. Discussion
Inflammatory bowel disease (IBD) can occur at any age, but almost a third of patients are diagnosed in the period of childhood or adolescence. The diagnosis of this condition in children is different from the adult forms, as they are often diagnosed before the development of disease complications [
2,
6]. In our study, the median age of diagnosis was 13 years and 8 months, and the disease was more frequent in males (62%), both characteristics consistent with older-onset IBD in children [
10]. The duration of signs and symptoms before diagnosis was 5 weeks (to a maximum of 5 months) which is less than in a Swiss IBD cohort which found the diagnostic delay of an average of 3 months in pediatric patients [
11]. The dissimilarities in presentation in CD are one of the reasons for this diagnostic delay, but most commonly involves non-specific gastrointestinal symptoms such as abdominal pain, diarrhea, nausea, vomiting, or hematochezia [
2,
4]. Our study showed that stool changes were the most common presenting symptoms (65.52%) and that they, along with abdominal pain and weight loss, significantly subsided at the time of a follow-up visit. Weight loss is a particularly concerning clinical sign because it can lead to a defect in linear growth, which is seen in CD in up to 40% of pediatric patients. This is because of the effects of chronic gastrointestinal inflammation accompanied by malabsorption, increased metabolic demand, poor oral intake, and the adverse effects of corticosteroid and immunosuppressant therapy. Along with clinical signs, laboratory values are used for aiding the diagnosis of CD, but even so, up to 20% of children have normal marker levels [
2]. Fecal calprotectin, a cytosolic protein released by neutrophils, is a stool marker of intestinal inflammation used for the assessment of disease activity and progression and is a screening and exclusion tool for suspected IBD patients when values exceed 50-100 μg/g and ≤40 μg/g, respectively [
12,
13]. In our study, at the index visit 58 out of 60 patients had elevated values of fecal calprotectin, with a median value of 1000 μg/g which significantly declined in time of the second visit (298.9 μg/g), similar to other studies [
3]. CRP, an acute-phase protein, is frequently elevated in pediatric patients and has also been shown to aid in the diagnosis and prognosis of CD because it correlates well with the degree of the inflammatory process seen in this condition [
1,
14]. In our study, only 19 patients had the information on CRP values in the medical reports, and the average value was 44.89±47.2 mg/L, which shows great variability but is similar to other studies [
3]. The median value declined between the first and second patient visits (from 25 to 15.1 mg/L), however this was not statistically significant. Although both fecal calprotectin and CRP are used in the evaluation of CD, they are not specific and do not correlate well with small-bowel CD which should be mentioned [
1]. For small bowel CD a magnetic resonance enterography is used [
2]. The treatment of CD depends on the extent and severity of the disease [
1]. The main therapeutic goal in pediatric CD is achieving and maintaining clinical remission of the disease, as well as reaching mucosal healing, decreasing symptom occurrence, improving life quality, and preventing linear growth defects while keeping the adverse drug effects to a minimum [
4]. The most common drug regimen used in our patients was immunosuppressants (azathioprine or methotrexate), which is a common first-line therapy choice used for induction and maintenance of CD remission. Methotrexate is an anti-folate agent while azathioprine is a purine analogue [
7]. Immunotherapy was the second most prevalent treatment, i.e., Infliximab and Adalimumab (chimeric monoclonal IgG antibodies to TNFα, a prominent gastrointestinal pro-inflammatory cytokine) both approved for use in pediatric patients with excellent success rates [
4]. We will also mention that 5-ASA compounds, which are one of the earliest approved drugs for CD management, were a therapy choice for 12 of our patients. They are used in patients with mild-to-moderate disease activity, as they function as gastrointestinal anti-inflammatories and aminosalicylates [
7].
Endoscopy is a gold standard for the diagnosis and evaluation of the disease activity, as EGDS and ileo-colonoscopy enable the macroscopic evaluation of the intestinal mucosa and histopathological biopsy analysis [
2,
3]. A total of 19% of patients had specific changes on both the upper and lower endoscopies. Specific findings on EGDS and ileo-colonoscopy had 36.6% and 64.9% of patients, respectively. The percentage of our patients with these findings on upper endoscopy was larger than the European CD registries report (9-24%), which shows that a properly performed EGDS is a good diagnostic tool for CD [
15,
16]. Abuquteish et al. [
5] reported that the most frequent changes seen on endoscopy are in the stomach, followed by the oesophagus and duodenum. In our study, the stomach was the site of the most frequent macroscopic changes (50%) as well, followed by the duodenum (28.57%) and oesophagus (21.43%). This shows the importance of routine endoscopic procedures in the diagnosis and monitoring of CD, as it can serve in the assessment of disease progression and suggest underlying pathology.
With further evaluation of the biopsies obtained during EGDS, diagnostic signs of CD (chronic inflammation with non-caseating granulomas) were found in 18% of patients in the stomach, 9% in the oesophagus, and 12% in the duodenum. Compared with the EUROKIDS registry [
17], the incidence of granulomas in our study was higher in all segments of the upper GI tract: the stomach (11.5%), oesophagus (4.7%), and duodenum (3.3%). Histologically, non-caseating granulomas consist of 5 or more epithelioid histiocytes and/or multinucleated giant cells and are the distinguishing feature between CD and ulcerative colitis [
5,
10].
Histopathological findings suggestive, but not characteristic of CD are focally enhanced gastritis and lymphocytic oesophagitis. Focally enhanced gastritis was seen in 25% of our patients in the first and 28.89% in the second biopsy, while the reported frequency in literature is up to 50%. Children with this finding are 15 times more likely to be diagnosed with either CD or ulcerative colitis. It is defined as a focal pit inflammation comprised of lymphocytes and histiocytes most commonly seen in the gastric antrum. These patients are also more likely to have signs of CD somewhere else in the gastrointestinal tract, so further endoscopic and HP evaluation is mandatory [
18,
19]. Lymphocytic oesophagitis (LyE), another histopathological finding highly suggestive of pediatric CD, is diagnosed when more than 20 intraepithelial lymphocytes are found in one high-power field (HPF), with no significant number of granulocytes. This diagnosis is not yet standardized, as lymphocytes are normally found in the oesophageal mucosa, mainly in the peripapillary epithelium (10-12/HPF). In our study, the prevalence of LyE was 6% in the first and 6.7% in the second biopsy, which was similar to a study conducted by Sutton et al
. [
20]. In the duodenum, the most frequent non-specific finding seen in patients with CD is chronic duodenitis whose prevalence ranges from 33-48% [
5]. In our study, chronic duodenitis was the most commonly seen finding on both the first (48%) and second (46.7%) duodenal biopsies. We also found it interesting that eosinophilic oesophagitis was present in half (52%) and an elevated number of eosinophils in the duodenum in a third (37%) of our patients. Eosinophils play an important role in both pro-inflammatory and anti-inflammatory processes, depending on the extent of the infiltration, and in some cases indicate disease remission [
10].
Regarding the HP findings on the ileo-colonoscopy, almost 2/3 (63.9%) of patients had complete ileo-colonic disease, and limited ileocecal and colorectal disease had 16.9% and 6%, respectively. These results differ from a study [
10], which reported a lower GI involvement in older-onset pediatric CD patients of 9%, 18%, and 0% regarding the limited ileocecal, colonic, and ileocolonic disease, respectively. We have observed that CD changes worsened significantly in the rectum and descending colon between the first and second biopsies, which indicates a predominance of disease progression in the left colon. In the groups of patients diagnosed for the first time with CD, 68% had specific findings in the entire lower GI tract, which shows that the HP analysis of CD should always include both upper and lower GI tract biopsies.
The most commonly used index for the assessment of disease activity in the terminal ileum and colon is GHAS, which was frequently noted in pathology reports in our study.) [
21]. Our study demonstrated a median low GHAS in the terminal ileum (GHAS=3), and a high value in the colon (GHAS =7). Many patients (28.89%) had no disease activity in the terminal ileum. In both the terminal ileum and colon the highest proportion of patients (31.1% and 43.1%, respectively) had a moderate GHAS (ranging from 5 to 9).
The variability of HP findings in our study shows unpredictability in the biopsy results in pediatric patients with CD, and the need to find a more standardized approach to the disease diagnosis and distinguish findings that are highly suggestive of CD and whose appearance prompts a more detailed assessment of the entire GI tract of affected patients to reach the diagnosis with minimal delay time.
The limitations of our study are incomplete information regarding fecal calprotectin (n=60) and CRP (n=19) values and a smaller number of patients in the follow-up biopsy category in comparison to the index biopsy group (n=100/45). The strengths of this study are the inclusion of both endoscopic and histopathologic findings of CD and the subclassification of the results.
5. Conclusions
Our study showed that more than a third of patients had specific endoscopic (36.59%) and histopathologic (32%) findings in the upper GI tract, and an additional 23% had HP findings highly suggestive of CD. We demonstrated the importance of regular endoscopic and histopathological assessments of pediatric CD patients. Additionally, risk factors for disease progression and dietary data should be included in future work, for a better understanding of the complexity of CD.
Author Contributions
Conceptualization, D.P. and R.J.; methodology, D.P., N.R., I.M., and R.J.; formal analysis, D.P., J.J., M.Đ. and R.J.; investigation, D.P., J.J., N.R., I.M, M.R., N.P., I.Đ., Z.L., and R.J.; writing—original draft preparation, D.P., J.J., M.Đ., N.R., I.M., M.R., N.P., Z.L., I.Đ., and R.J..; writing—review and editing, D.P., J.J., R.J., N.R. and I.M.; supervision, R.J., I.M. and N.R.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethical Committee of the University Children’s Hospital in Belgrade, Serbia (017 14/32, 27 February 2020).
Informed Consent Statement
Patient consent was waived because patients cannot be identified from anonymized data used in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Agrawal M, Spencer EA, Colombel JF, Ungaro RC. Approach to the Management of Recently Diagnosed Inflammatory Bowel Disease Patients: A User’s Guide for Adult and Pediatric Gastroenterologists. Gastroenterology 2021; 161(1):47-65. [CrossRef]
- Subedi S, Behrle Yardley AL, Shapiro JM. Inflammatory Bowel Disease in Children and Adolescents. R I Med J (2013). 2022; 105(10):8-13.
- Metwally RH. Can Neutrophil/Lymphocyte Ratio Assess Inflammatory Bowel Disease Activity and Severity in Children? Turk J Gastroenterol. 2022;33(12):1058-1061.
- Conrad MA, Kelsen JR. The Treatment of Pediatric Inflammatory Bowel Disease with Biologic Therapies. Curr Gastroenterol Rep 2020; 22(8):36. [CrossRef]
- Abuquteish D, Putra J. Upper gastrointestinal tract involvement of pediatric inflammatory bowel disease: A pathological review. World J Gastroenterol 2019; 25(16):1928-1935. [CrossRef]
- Kuenzig ME, Fung SG, Marderfeld L, Mak JWY, Kaplan GG, Ng SC et al. Twenty-first Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology. 2022; 162(4):1147-1159.e4. [CrossRef]
- Gurram B, Patel AS. Recent advances in understanding and managing pediatric inflammatory bowel disease. F1000Res. 2019; 8:F1000 Faculty Rev-2097. [CrossRef]
- Shaoul R, Day AS. An Overview of Tools to Score Severity in Pediatric Inflammatory Bowel Disease. Front Pediatr 2021; 9:615216. [CrossRef]
- Basturk A, Artan R, Yılmaz A, Gelen MT. Gastritis Associated with Initially Pediatric Crohn’s Disease and Ulcerative Colitis. Pediatr Gastroenterol Hepatol Nutr 2018; 21:163-169. [CrossRef]
- Conrad MA, Carreon CK, Dawany N, Russo P, Kelsen JR. Distinct Histopathological Features at Diagnosis of Very Early Onset Inflammatory Bowel Disease. J Crohns Colitis 2019; 13(5):615-625. [CrossRef]
- Schoepfer A, Santos J, Fournier N, et al. Systematic analysis of the impact of diagnostic delay on bowel damage in paediatric versus adult onset Crohn’s disease. J Crohns Colitis 2019; 13:1334–1342. [CrossRef]
- Kennedy NA, Jones GR, Plevris N, et al. Association between level of fecal calprotectin and progression of Crohn’s disease. Clin Gastroenterol Hepatol 2019; 17:2269–2276.e4. [CrossRef]
- Crawford E, Gestrich C, Malay S, Young D, Perry S, Splawski J, et al. Association of Fecal Calprotectin With Endoscopic and Histologic Activity in Pediatric Inflammatory Bowel Disease. JPGN Rep 2021; 2(4):e129. [CrossRef]
- Glapa-Nowak A, Szczepanik M, Banaszkiewicz A, Kwiecień J, Szaflarska-Popławska A, Grzybowska-Chlebowczyk U, et al. C-Reactive Protein/Albumin Ratio at Diagnosis of Pediatric Inflammatory Bowel Disease: A Retrospective Multi-Center Study. Med Sci Monit 2022; 28:e937842. [CrossRef]
- Kovacs M, Muller KE, Arato A, Lakatos PL, Kovacs JB, Varkonyi A et al. Diagnostic yield of upper endoscopy in paediatric patients with Crohn’s disease and ulcerative colitis. Subanalysis of the HUPIR registry. J Crohns Colitis 2012; 6:86-94.
- de Bie CI, Buderus S, Sandhu BK, de Ridder L, Paerregaard A, Veres G, et al.. Diagnostic workup of paediatric patients with inflammatory bowel disease in Europe: results of a 5-year audit of the EUROKIDS registry. J Pediatr Gastroenterol Nutr 2012; 54:374-380.
- Kim ES, Kwon Y, Choe YH, Kim MJ. Upper gastrointestinal tract involvement is more prevalent in Korean patients with pediatric Crohn’s disease than in European patients. Sci Rep 2020; 10(1):19032. [CrossRef]
- Putra J, Ornvold K. Focally enhanced gastritis in children with inflammatory bowel disease: a clinicopathological correlation. Pathology 2017; 49:808-810. [CrossRef]
- Ushiku T, Moran CJ, Lauwers GY. Focally enhanced gastritis in newly diagnosed pediatric inflammatory bowel disease. Am J Surg Pathol 2013; 37:1882-1888. [CrossRef]
- Sutton LM, Heintz DD, Patel AS, Weinberg AG. Lymphocytic esophagitis in children. Inflamm Bowel Dis 2014; 20(8):1324-8.
- Gong W, Guo K, Zheng T, Fang M, Xie H, Li W, et al. Correlation between endoscopic and histological validated scoring indices in Crohn’s disease. Dig Liver Dis 2019; 51(6):812-817. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).