1. Introduction
Vascular disorders are abnormalities in the structure and function of blood vessels. Vascular disorders on the skin are visible as dilated, sometimes cracked capillaries, veins, and arterioles, but also as spots or nodules with an erythematous basis. They arise as a result of pathogenic processes – blood disorders in the vessels or abnormal vascular development [
1]. Normally, blood vessels are formed by two mechanisms: vasculogenesis and angiogenesis. Vasculogenesis is the formation of blood vessels from the cells of the insular endothelium, leading to the blood vessel plexus. Then, the vessels are formed from the vascular endothelium – a process called angiogenesis. Under pathological conditions, it interferes with the process of angiogenesis, called neoangiogenesis, which results in excessive growth of capillaries in the tumour. A key role in the processes mentioned above is the segment of the vascular endothelial growth factor (VEGF) [
2,
3,
4]. In addition, with aging, blood vessels are degraded by the enzyme hyaluronidase, which depolymerizes hyaluronic acid and leads to a weakening of the endothelium and vascular permeability [
5].
Chemical agents that can minimize skin lesions related to vascular abnormalities mostly are highly effective antioxidants and inhibitors of hyaluronidase. Mostly used active substances are compounds from the group of flavonoids and vitamins (e.g., C, K, B3).
The vascular structure, comprising arteries, veins, and capillaries, plays a pivotal role in maintaining physiological homeostasis within the human body. Arteries transport oxygenated blood away from the heart to various tissues and organs, while veins return deoxygenated blood back to the heart. Capillaries, the smallest blood vessels, facilitate nutrient and gas exchange between the bloodstream and surrounding tissues. Structurally, arteries possess thicker walls rich in smooth muscle and elastic fibers, enabling them to withstand high pressure and maintain blood flow. Veins exhibit thinner walls with less muscle and more valves to prevent backflow, aiding in the return of blood to the heart. Capillaries, in contrast, consist of a single layer of endothelial cells, facilitating efficient exchange of gases, nutrients, and waste products. This intricate network of vessels is regulated by complex physiological mechanisms involving neural, hormonal, and local factors to ensure proper distribution of blood throughout the body. Dysfunction in vascular structure and function underlies numerous pathological conditions, including hypertension, atherosclerosis, and peripheral vascular disease, highlighting the critical importance of understanding vascular biology for the advancement of medical interventions and treatments [
6]. This structure is intimately associated with the glycocalyx, a gel-like layer composed of glycoproteins and proteoglycans that coats the luminal surface of endothelial cells lining the blood vessels. The glycocalyx serves as a dynamic interface between the bloodstream and the vessel wall, contributing significantly to vascular function and health. Within arteries and veins, the glycocalyx acts as a protective barrier, preventing the direct contact of circulating blood components with the endothelial cells and underlying tissues. This barrier function is crucial for maintaining vascular integrity and preventing inflammation and thrombosis. Moreover, the glycocalyx participates in the regulation of vascular permeability and endothelial cell signalling. It modulates the passage of nutrients, hormones, and immune cells across the vessel wall, thereby influencing tissue perfusion and immune response. Additionally, the glycocalyx plays a vital role in mechanotransduction, sensing and transducing mechanical forces exerted by blood flow into biochemical signals that regulate vascular tone and remodelling. Furthermore, alterations in the glycocalyx have been implicated in various vascular pathologies, including endothelial dysfunction, atherosclerosis, and diabetic vascular complications. Damage to the glycocalyx, often induced by oxidative stress, inflammation, or hyperglycaemia, compromises its protective and regulatory functions, contributing to vascular injury and disease progression [
7].
Hyaluronic acid is a large, negatively charged glycosaminoglycan that contributes to the hydration, lubrication, and structural integrity of the glycocalyx. Through its ability to interact with water molecules and form a hydrated gel-like matrix, hyaluronic acid helps maintain the thickness and viscoelastic properties of the glycocalyx, crucial for its barrier function and mechanotransduction [
8].
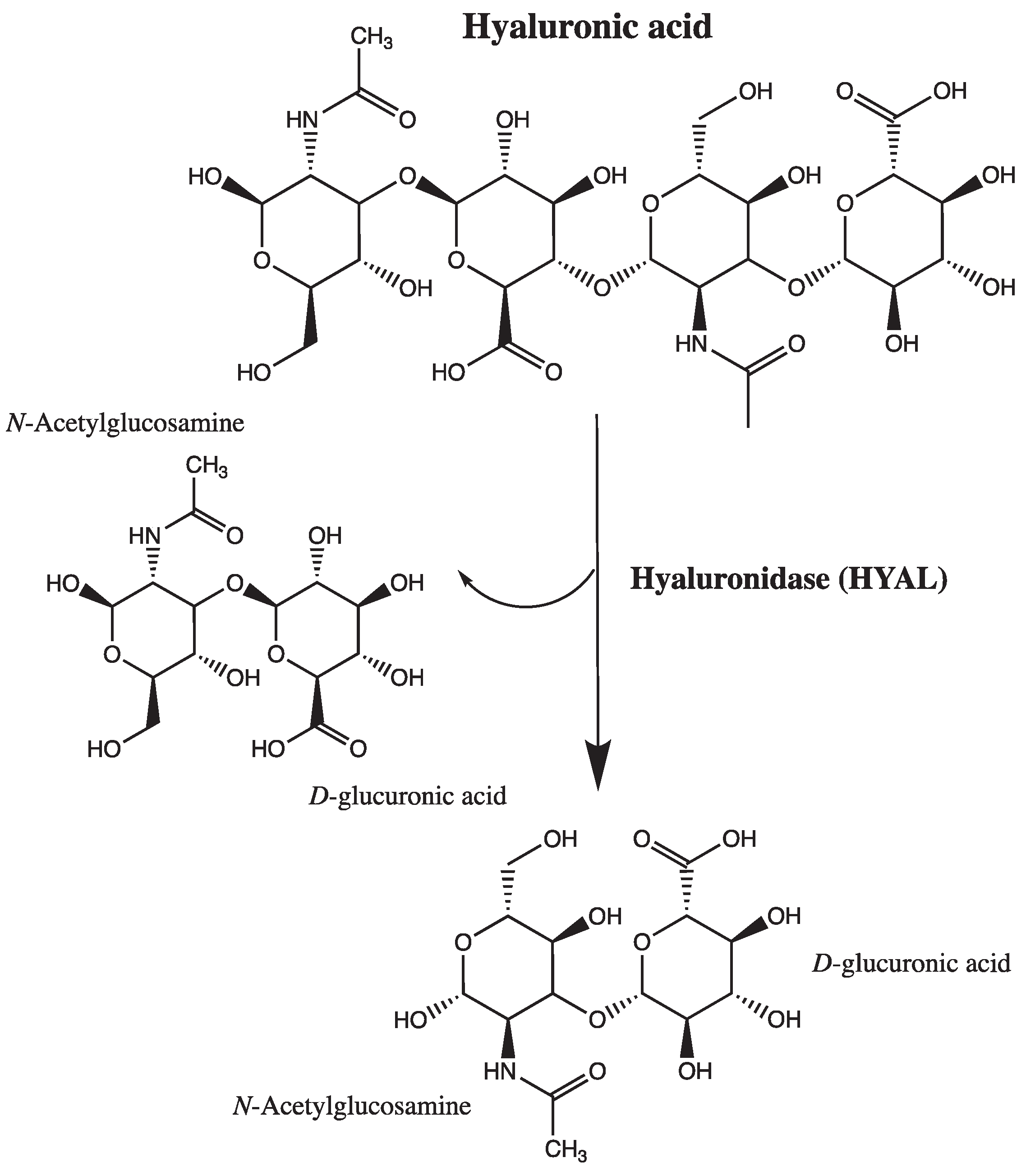
Hyaluronidase is an enzyme of the hydrolase class that depolymerizes the hyaluronic acid located in blood vessel walls. Hyaluronidase is an endoglycosidase that breaks down hyaluronic acid into monosaccharides by cleaving its glycosidic bonds; additionally, to some extent, it also breaks down other acid mucopolysaccharides in the connective tissue [
9]. It is present both in organs and body fluids (e.g., tears, blood). There are six types of hyaluronidases in the human body: hyaluronidases 1-4, encoded by HYAL genes 1-4, PH-20 (SPAM-1), and HYALP1, respectively. Hyaluronidase 1 is present in human organs and acts as the major hyaluronidase in plasma, activated at acidic pH. Hyaluronidase 2, on the other hand, has weaker enzymatic activity than hyaluronidase 1 and degrades only high molecular weight hyaluronic acid. It is mostly used in aesthetic medicine to dissolve tissue fillers based on high molecular weight hyaluronic acid. The role of hyaluronidase 3 remains unknown, as it is found only in the nucleus and bone marrow. Testicular hyaluronidase PH-20 is found on the surface of the human sperm and inner acrosomal membrane and serves to degrade hyaluronic acid in the egg cell during fertilization [
10].
Scheme 1.
Mechanism of action of hyaluronidase. Adapted from Lee A. et al. [
11] and Bala E. et al. [
12].
Scheme 1.
Mechanism of action of hyaluronidase. Adapted from Lee A. et al. [
11] and Bala E. et al. [
12].
Recent studies have shed light on the intricate interplay between hyaluronidase, hyaluronic acid, and the vascular glycocalyx, providing valuable insights into their roles in vascular physiology and pathology. For instance, research by Tarbell and Cancel found that enzymatic degradation of hyaluronic acid by hyaluronidase disrupts the glycocalyx structure, leading to increased vascular permeability and endothelial dysfunction [
13]. Moreover, work by Florian et al. demonstrated that hyaluronidase-mediated degradation of hyaluronic acid fragments can stimulate inflammatory responses and promote atherosclerosis progression in animal models [
14].
Furthermore, clinical studies by Becker et al. [
15] have highlighted the association between elevated hyaluronidase activity and impaired glycocalyx function in patients with cardiovascular diseases, underscoring the potential clinical relevance of targeting hyaluronidase for vascular protection. Additionally, investigations by Slevin et al. [
16] have identified dysregulated hyaluronidase expression in diabetic vasculopathy, suggesting a link between altered hyaluronic acid metabolism and diabetic vascular complications [
15].
These findings collectively emphasize the importance of understanding the dynamic interactions between hyaluronidase, hyaluronic acid, and the glycocalyx in vascular health and disease. Future research efforts aimed at elucidating the molecular mechanisms underlying these interactions may pave the way for the development of novel therapeutic approaches for vascular disorders [
16].
2. Skin Lesions Related to Vascular Disorders
Erythema is a red, delimited lesion, caused by long-term dilatation of capillaries and plasma extravasation caused by inflammatory infiltrates, fading under pressure [
6,
7].
Telangiectasias are enlarged superficial capillaries of the sub-papillary layer of the skin, translucent through the epidermis. They have a blue, brown or light red colour with a diameter of less than 1 mm. They occur singly or in clusters. Probably arising as a result of the backflow of blood from abnormally functioning veins, local increase in venous pressure and unstable walls of small blood vessels in the skin. The initial clinical symptom of telangiectasia is a short-term erythema that becomes permanent over time. Their occurrence is characteristic of people with couperose skin, also called
prerosacea in the literature. Telangiectasia is also one of the first symptoms of emerging venous insufficiency of the lower extremities [
1,
8].
Rosacea is an erythematous dermatosis of variously classified aetiology, belonging to the vasomotor neurosis of the skin. Clinical symptoms include erythematous, papular and pustular lesions on the basis of seborrheic skin with vascular disorders. In the initial stage of the disease, i.e., in
prerosacea we observe the occurrence of paroxysmal erythema, which is fixed over time, along with the presence of telangiectasia. There are four subtypes of rosacea: erythematotelangiectatic (ETR), papulopustural (PPR), phymatous (PRY), and ocular. In the erythematotelangiectatic subtype, erythema and teleangiectasis are observed. On the other hand, in the papulopustural subtype, additionally the papules appear/occur. The aetiology of rosacea seems quite complex, there are three main hypotheses. The first is causing inflammation in blood vessels due to photoaging. The mechanism of photoaging, in which inflammatory factors are released such as matrix metalloproteinases, weakens the walls of blood vessels, leading to the formation of telangiectasia. Moreover, the presence of polymorphism in the VEGF gene, which is a strong mediator of vascular permeability and inflammation, has been demonstrated in people with rosacea [
20]. Another hypothesis explaining the aetiology of rosacea is skin infection with the Gram-negative bacterium
Helicobacter pylori, which lives on the surface of gastric epithelial cells. This is the least likely hypothesis. A meta-analysis [
21] of 42 articles showed a weak association between rosacea and
H. pylori infection as well as a weak effect of anti-
Helicobacter therapy on rosacea. The last hypothesis, the most likely one, explaining the aetiology of dermatosis is skin infection with the
Demodex folliculorum parasite, called Demodex, inhabiting the sebaceous glands of the skin and eyelash hair capsules [
11,
12,
13,
14].
Varicose veins are the permanent, limited extensions of the superficial veins that extend above the skin surface. They belong to the second stage of chronic venous insufficiency. Classification taking into account disposable factors distinguishes between primary and secondary varicose veins. Primary varicose veins are the term used to describe varicose veins of unknown etiological factor, occurring in the normal condition of deep veins. On the other hand, secondary varicose veins are vascular changes that are a complication of a history of thrombosis, insufficiency of deep veins, piercing veins, or arteriovenous fistulas. An important aspect of the pathogenesis of varicose veins is the change in the ratio of type I collagen (which strengthens the vascular walls) to type III collagen (which is responsible for tissue stretching), resulting from disturbance of post-translational or degradation processes. This change reduces the flexibility of the walls. Varicose veins may be accompanied by symptoms such as pain, heaviness in the legs, swelling, tingling, but also bleeding may occur after minor injuries [
1,
14,
15].
Angiomas are vascular changes, the most common benign tumors of developmental age. Caused by excessive growth of endothelial cells of blood vessels. They are characterized by high metabolic activity, with an increased exchange of endothelial cells, mast cells, fibroblasts and macrophages during the period of proliferation. They manifest as erythematosus-vascular lesions, clearly delimited, with increased cohesiveness, usually raised above the skin surface. They concern the dermis and subcutaneous tissue. The clinical appearance of hemangiomas includes single lesions or clusters of erythematous changes on an unchanged substrate, often colloquially referred to as traces of strawberries [
17,
18].
Vascular malformations are a group of non-neoplastic, tumour-like vascular changes resulting from disturbed vascular tissue morphogenesis. They are most often present from birth but may be invisible and reveal themselves later in life. Vascular malformations have been grouped based on a common embryological origin, consisting in the presence of a lining made of a single endothelial cell. Vascular defects are believed to be the result of developmental errors during embryogenesis, which include abnormal signalling processes that control apoptosis, maturation, and vascular cell growth. These errors lead to the cells of the vascular plexus remaining with some degree of differentiation. There are four main categories of vascular malformations based on their flow characteristics:
slow flow: capillary malformation, venous malformation, lymphatic malformation
rapid flow: arteriovenous malformation.
They appear on the skin as extensive, clearly demarcated, erythematous spots [
19,
20].
3. Epidemiology
Vascular lesions, or more precisely skin manifestations of circulatory disorders, are still one of the main therapeutic but also aesthetic problems.
The most common vascular lesions in the population include telangiectasias, varicose veins, rosacea, hemangiomas, and vascular malformations. Hemangiomas occur in approximately 4%-5% of newborns, especially Caucasians, and in up to 30% of children born prematurely. Additionally, 3-9 times more often in girls than in boys. Vascular malformations occur much less frequently, but with the same gender frequency [
33,
34]
The prevalence of venous problems of the lower extremities varies considerably with latitude, with the highest rates reported in Western countries. Varicose veins, estimated to be more common than chronic venous insufficiency, range from <1% to 73% in women, 2% to 56% in men. The main risk factors are age, gender, standing work, pregnancy, and genetic family history of varicose veins [
34].
The epidemiology of telangiectasia was investigated by conducting a large cohort study, determining the prevalence from facial photographs of more than 2000 participants from Northern Europe. This group was predominantly female (58.8%) with a median age of 66.9. The study showed that risk factors for telangiectasia were older age, female gender, smoking - particularly active and prolonged smoking, fair complexion, and high susceptibility to sunburn (phototype I and II on the Fitzpatrick scale). The effect causing the greatest increase in the occurrence of telangiectasias was long-term smoking – increasing the risk by 38.4%. The authors emphasize that facial erythema and telangiectasias are often associated with the erythematous-teleangiectatic subtype of rosacea [
35].
Rosacea is a condition that affects as many as 2 to 22% of the Caucasian population. It appears that the highest risk of rosacea is revealed in Caucasians with fair, sun-sensitive skin (skin phototypes I and II according to the Fitzpatrick scale). However, a recent study shows that rosacea also occurs in phototypes III-VI, but then papulopustular lesions are more frequently observed. The reason for this is the masking of erythema by the pigment of darker phototype skin. A prospective study conducted in Germany in 2016, found an overall prevalence of rosacea of 12%, with erythematous-vascular-pustular and papulopustular subtypes accounting for 9 and 3% respectively. Rosacea occurs with a strong predominance in women and is usually diagnosed after the age of 30.
4. Xanthone Derivatives and Their Vascular Activity
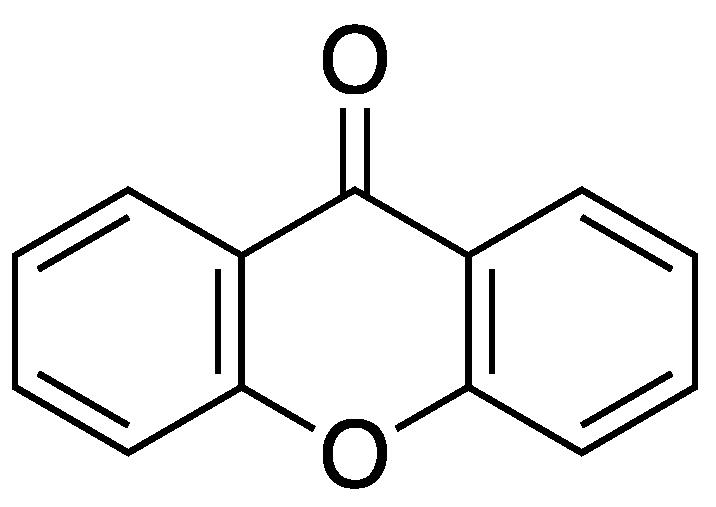
Xanthone (9
H-xanthen-9-one, dibenzo-γ-pyrone) derivatives are oxygen-containing heterocycles (
Figure 1). Many oxygenated heterocycles possess pharmacological activities, and the xanthone class is not an exception [
36]. At present, nearly 1000 naturally occurring xanthone derivatives are known. Each has different substituents at different positions, ultimately leading to a large variety of pharmacological activities [
37]. The multitude of biological activities found for xanthone derivatives include α-glucosidase inhibition, anti-cancer activities, anti-Alzheimer activities, anticonvulsant, anxiolytic, antidepressant, analgesic, antibacterial, antioxidant and anti-inflammatory activities [
39,
40,
41,
42,
43,
44,
45].
4.1. Sealing Blood Vessels Activity
One of the intriguing therapeutic potentials of xanthone derivatives lies in their ability to modulate vascular function, particularly in sealing blood vessels. The integrity of blood vessels is crucial for maintaining proper cardiovascular health, as disruptions in their structure or function can lead to various pathological conditions, including haemorrhage, thrombosis, and atherosclerosis, and connected to the skin telangiectasia and rosacea.
Research has increasingly elucidated the mechanisms by which various xanthone derivatives exert their vascular sealing effects. These mechanisms often involve interactions with key molecular targets involved in vascular homeostasis, such as endothelial cells, smooth muscle cells, and inflammatory mediators. For instance, xanthone derivatives have been shown to enhance endothelial barrier function by regulating the expression of tight junction proteins and inhibiting the production of pro-inflammatory cytokines [
45].
4.1.1. Antiangiogenic Activity
Angiogenesis refers to the formation of new blood vessels from existing ones, and inhibiting this process holds promise in combating cancer. Tumor angiogenesis, a complex series of events involving endothelial cell activation, invasion, migration, proliferation, and the formation of capillary networks, is crucial for cancer progression and metastasis. Without the formation of new blood vessels, tumors typically remain small and are less likely to spread to other parts of the body. Consequently, targeting angiogenesis is a significant strategy in cancer treatment, regarding to the skin lesions as well.
In one of the studies, conducted by Tao Chan et al. a few gambogic acid derivatives were obtained and evaluated against antiangiogenic activity. Four derivatives effectively inhibited the development of new segmental blood vessels in zebrafish assays and showed lower toxicity to zebrafish than GA. They also demonstrated stronger inhibitory effects on the migration and tube formation of HUVECs in vitro compared to GA [
46].
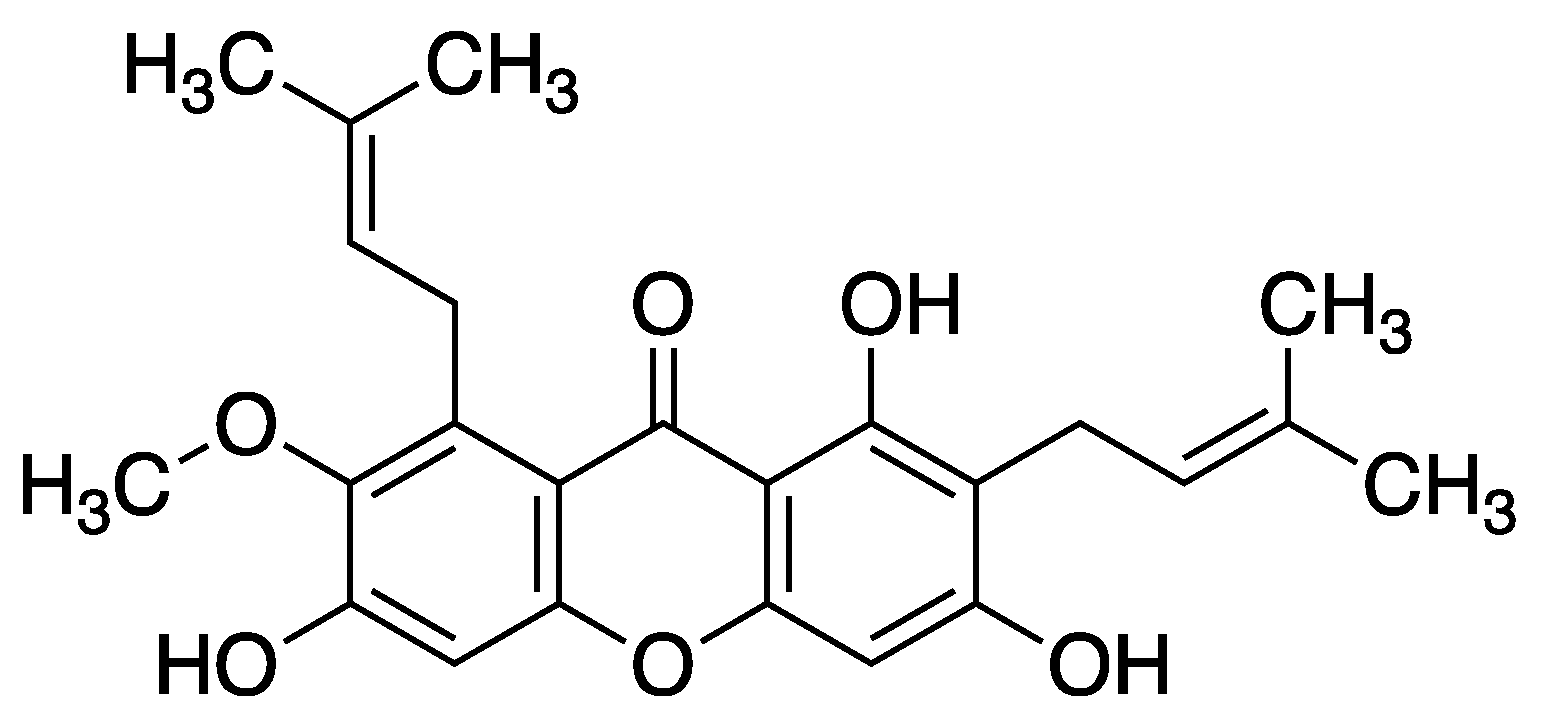
Another xanthone derivative – α-mangostin – exhibits significant inhibitory effects on various aspects of angiogenesis and vascular permeability in retinal endothelial cells (REC). Specifically, it suppresses VEGF-induced permeability increase, proliferation, migration, and tube formation, as well as vascular sprouting in the aortic ring assay. This compound also hampers the phosphorylation of VEGFR2 and ERK1/2-MAPK induced by VEGF. Furthermore, α-mangostin has been found to inhibit ROS formation induced by hypoxia treatment in REC. This suggests its potential in mitigating oxidative stress associated with hypoxia and preventing neovascularization in the retina. The inhibition of VEGF-induced angiogenic responses by α-mangostin is associated with its ability to block VEGFR2 and ERK1/2-MAPK activation. These findings underscore the antioxidant and anti-angiogenic properties of α-mangostin, indicating its potential as a natural therapeutic agent for reducing oxidative stress and preventing retinal neovascularization. Moreover, α-mangostin’s selective inhibition of specific signaling pathways like VEGFR2 and ERK1/2-MAPK without affecting Akt and p38 phosphorylation provides insights into its mechanism of action in regulating angiogenesis and vascular permeability in microvascular endothelial cells. It was successfully demonstrated that α-mangostin possesses both antioxidant and anti-angiogenic properties when applied to retinal endothelial cells (REC). Specifically, our findings reveal that α-mangostin effectively reduces the formation of reactive oxygen species (ROS) triggered by hypoxia treatment in REC, α-mangostin inhibits various angiogenic responses induced by vascular endothelial growth factor (VEGF), such as enhanced REC permeability, proliferation, migration, tube formation, and vascular sprouting in the aortic ring assay, and α-mangostin attenuates the phosphorylation of VEGF receptor 2 (VEGFR2) and ERK1/2-MAPK pathways in REC stimulated by VEGF [
47].
Figure 3.
Structure of α-mangostin (1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methylbut-2-en-1-yl)-xanthone).
Figure 3.
Structure of α-mangostin (1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methylbut-2-en-1-yl)-xanthone).
4.1.2. Endothelial and Mitochondrial Dysfunction
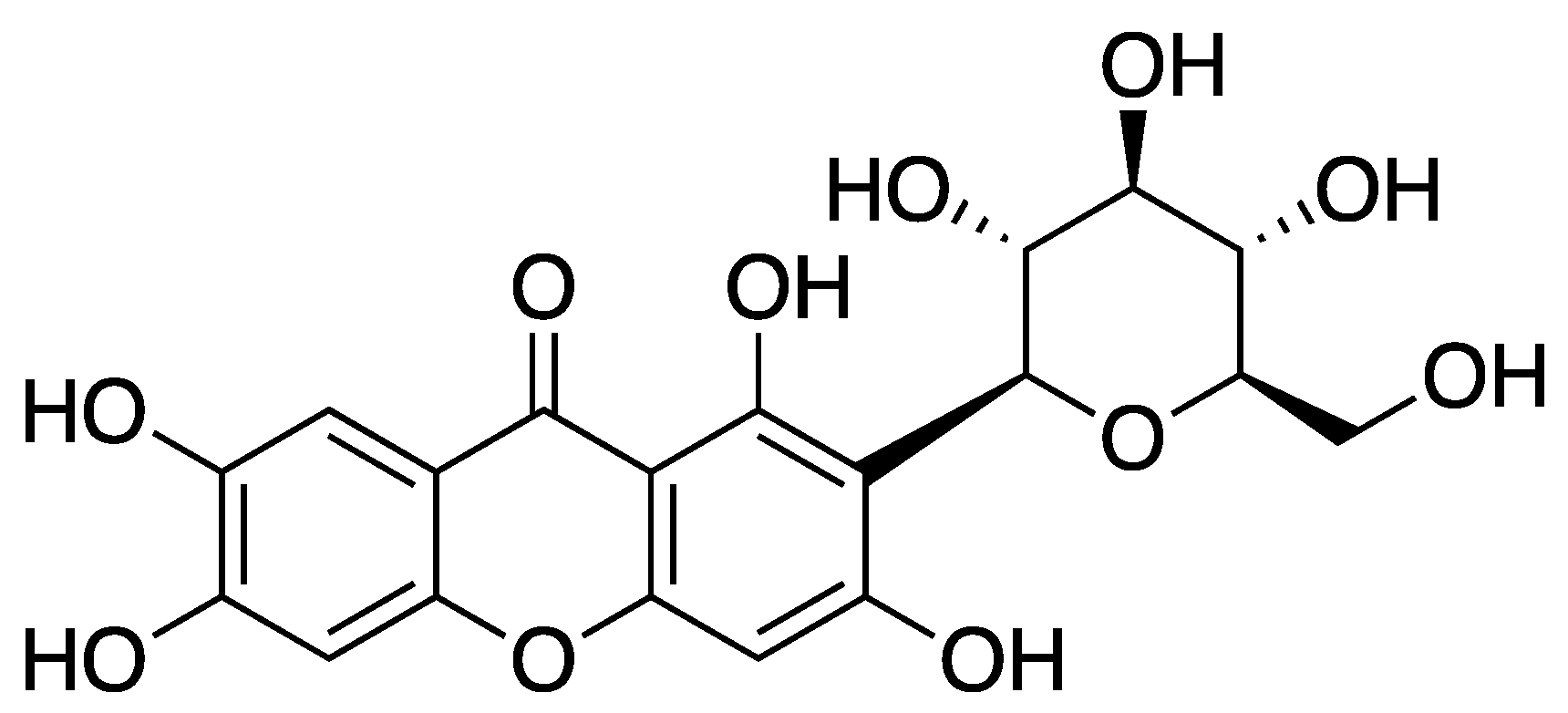
Mitochondrial dysfunction arises from disruptions in mitochondrial HK-II activity. Mangiferin effectively preserves mitochondrial HK-II, thereby safeguarding overall mitochondrial function. In endothelial cells, palmitic acid (PA) stimulation induces oxidative stress, evidenced by heightened ROS production, which is mitigated by mangiferin at concentrations of 1.0 and 10 μM. Considering the vital role of mitochondria in energy production and cell survival, mangiferin’s ability to promote HK-II binding to mitochondria through Akt activation becomes crucial. This mechanism not only protects mitochondrial function in vessel endothelial cells but also suggests a potential therapeutic avenue for mitigating endothelial dysfunction by preserving mitochondrial HK-II via pharmacological Akt activation. Consequently, mangiferin reduces caspase-3 expression and safeguards vessel endothelial integrity by modulating ROS generation and enhancing NO production, as demonstrated
in vivo. The study further underscores the significance of mitochondrial HK-II for maintaining mitochondrial functional integrity in vessel endothelial cells. Through Akt activation, mangiferin fosters HK-II binding to mitochondria, thus ameliorating mitochondrial dysregulation and promoting endothelial cell survival [
48].
Figure 4.
Structure of mangiferin (1,3,6,7-tetrahydroxy-2-((2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)-xanthone).
Figure 4.
Structure of mangiferin (1,3,6,7-tetrahydroxy-2-((2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)-xanthone).
In native, healthy endothelial cells a number of stimuli (
e.g., serotonin from aggregating platelets, sphingosine 1-phosphate, and thrombin) activate eNOS causing the release of NO, which relaxes the vascular smooth muscle that surrounds them. NO, in synergy with prostacyclin, further inhibits platelet aggregation [
49].
It has been reported that endothelial dysfunction is associated with cell apoptosis, migration and then inflammation [
50].
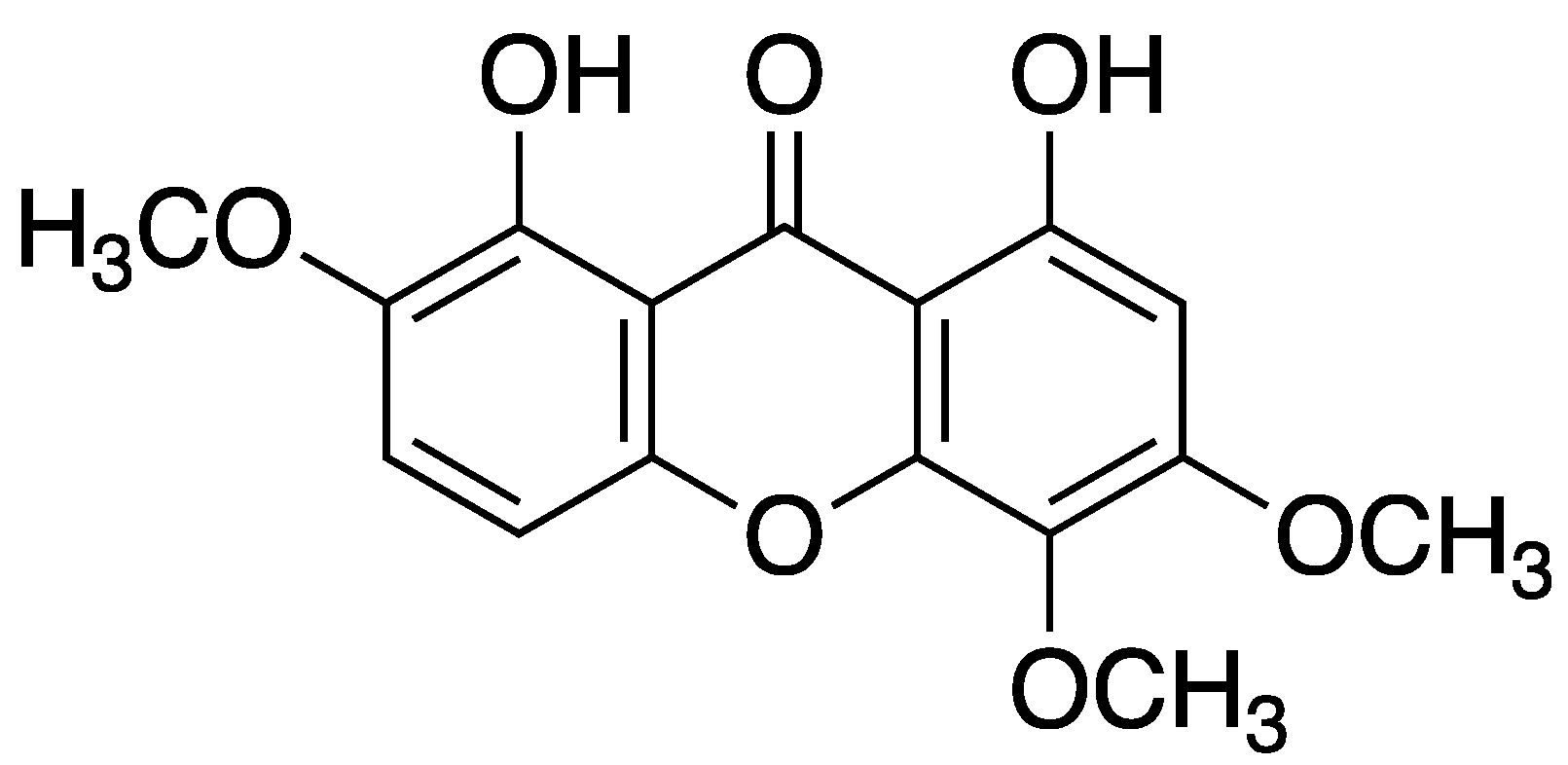
It has been shown that endothelium-dependent vasodilation is attenuated in many risk factors of atherosclerosis, such as hypercholesterolemia, hypertension, and diabetes mellitus, and endothelial dysfunction is recognized as an early event in the pathogenesis of atherosclerosis, which can be visible on the skin like for example xanthomas. Xanthomas are yellowish or orange bumps or nodules that develop under the skin due to the deposition of cholesterol-rich material. Nitric oxide (NO), synthesized from L-arginine by NO synthase (NOS) in endothelial cells, has been thought to play a key role in the maintenance of vascular tone and structure. NO possesses complex cardiovascular actions such as protection of endothelial cells, decrease of the endothelial adhesiveness and inhibition of the adhesion of monocytes to endothelial cells, and it is generally described as an “endogenous antiatherosclerotic molecule”. Recently, it has been found that L-arginine analogs such as asymmetric dimethylarginine (ADMA), which is present in the blood of both humans and animals, can inhibit NOS in vivo and in vitro. ADMA has been shown to concentration-dependently inhibit vasodilator responses to acetylcholine in isolated aortic rings, upregulate expression of MCP-1 and enhance adhesion of monocytes in cultured endothelial cells. There is growing evidence that endothelial dysfunction in some cardiovascular diseases, such as hypercholesterolemia, heart failure and hypertension, is associated with elevation of ADMA levels, and that its levels could predict endothelial dysfunction.
Vasodilator responses to acetylcholine in rings of the isolated thoracic aorta have been shown to be impaired in the presence of lysophosphatidylcholine (LPC), a major component of ox-LDL. Daviditin A significantly attenuated inhibition of endothelium-dependent relaxation by LPC. Moreover, in cultured endothelial cells xanthone derivatives inhibited the increase in the release of LDH, the upregulation of MCP-1 expression and the enhancement of monocytes adhesion concomitantly with a reduction of ADMA levels. These findings suggest that xanthone derivatives protect against endothelial damage induced by high-lipid levels, and that their protective effect on the endothelium is related to a reduction of ADMA concentration [
51].
Figure 5.
Davitin A (1,8-dihydroxy-2,5,6-trimethoxyxanthone) structure.
Figure 5.
Davitin A (1,8-dihydroxy-2,5,6-trimethoxyxanthone) structure.
4.1.3. Inhibition of Hyaluronidase
Inhibition of hyaluronidase by xanthone derivatives is a novel concept, related with permeability through the blood vessels. Only one research was found and had shown that all tested compounds were inactive against hyaluronidase. The structures that were tested was xanthone derivatives with only methoxy, hydroxy or methylbromo moieties [
52]. On the other hand, it was found that
-mangostin showed potent values of inhibition of hyaluronidase (IC
50 23.85 μg*mL
−1) [
53].
Figure 6.
Structure of -mangostin (1,3,6,7-tetrahydroxy-2,8-bis(3-methylbut-2-en-1-yl)-xanthone).
Figure 6.
Structure of -mangostin (1,3,6,7-tetrahydroxy-2,8-bis(3-methylbut-2-en-1-yl)-xanthone).
4.1.2. Diabetic Vascular Complications
Telangiectasia can be also caused by diabetic vascular changes, often followed by microangiopathy.
Diabetic vascular complications represent a significant cause of mortality and morbidity in individuals with diabetes, particularly in those with type 2 diabetes. These complications are marked by endothelial dysfunction, with impaired nitric oxide (NO) production playing a central role. Nitric oxide is crucially synthesized in vascular endothelial cells through the activation of endothelial nitric oxide synthase (eNOS). However, in diabetes, eNOS activity may be compromised due to the accumulation of ceramide. Recent studies have highlighted the potential anti-diabetic properties of α-mangostin.
Research demonstrates that α-mangostin ameliorates impaired endothelium-dependent vasodilation (EDV) in diabetic animals by modulating the aSMase/ceramide pathway and enhancing the eNOS/NO pathway in aortic tissues. Additionally, α-mangostin inhibited the elevated aSMase/ceramide pathway and restored EDV in isolated mouse aortas exposed to high glucose conditions. Moreover, α-mangostin increased eNOS phosphorylation and NO production in aortic tissues treated with high glucose.
Furthermore, α-mangostin normalized the activation of the aSMase/ceramide pathway and improved the eNOS/NO pathway in endothelial cells exposed to high glucose concentrations. Overall, studies reveal that α-mangostin regulates the eNOS/NO pathway and enhances EDV in diabetic mice by inhibiting aSMase activity and reducing endogenous ceramide accumulation. These results provide novel insights into the therapeutic potential of α-mangostin in alleviating endothelial dysfunction associated with diabetes. Additionally, these findings expand upon previous research demonstrating the beneficial effects of inhibiting ceramide synthesis on arterial function in diabetic animals and reversing NO levels in endothelial cells exposed to high glucose [
54].
Figure 7.
Structure of α-mangostin (1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methylbut-2-en-1-yl)-xanthone).
Figure 7.
Structure of α-mangostin (1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methylbut-2-en-1-yl)-xanthone).
4.1.3. Anti-Inflammatory Mechanisms of Xanthone Derivatives
The anti-inflammatory effects of xanthone derivatives are attributed to their ability to modulate key molecular targets involved in the inflammatory process. These targets include pro-inflammatory enzymes such as cyclooxygenase (COX) and lipoxygenase (LOX), as well as inflammatory mediators like cytokines (
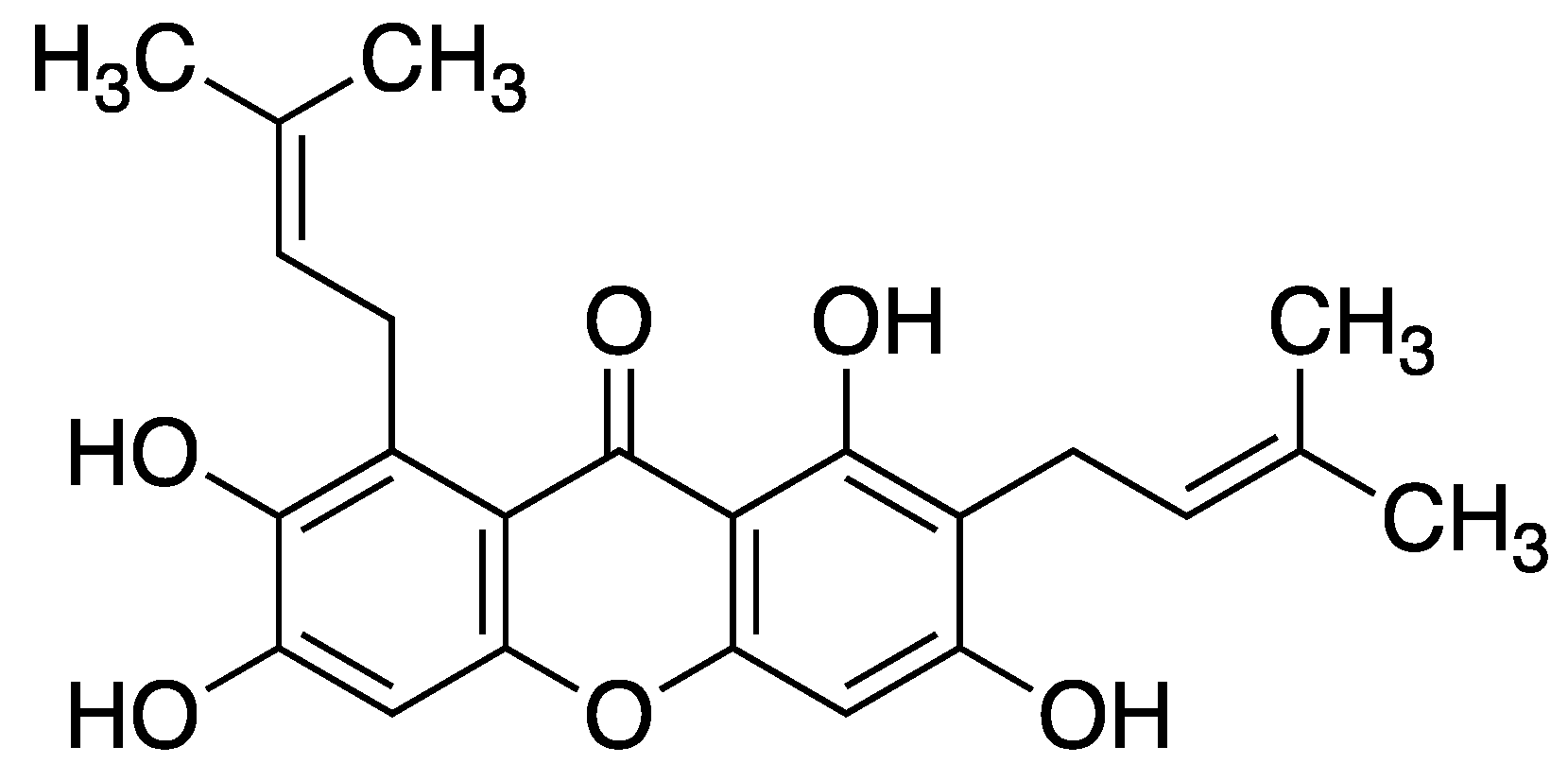
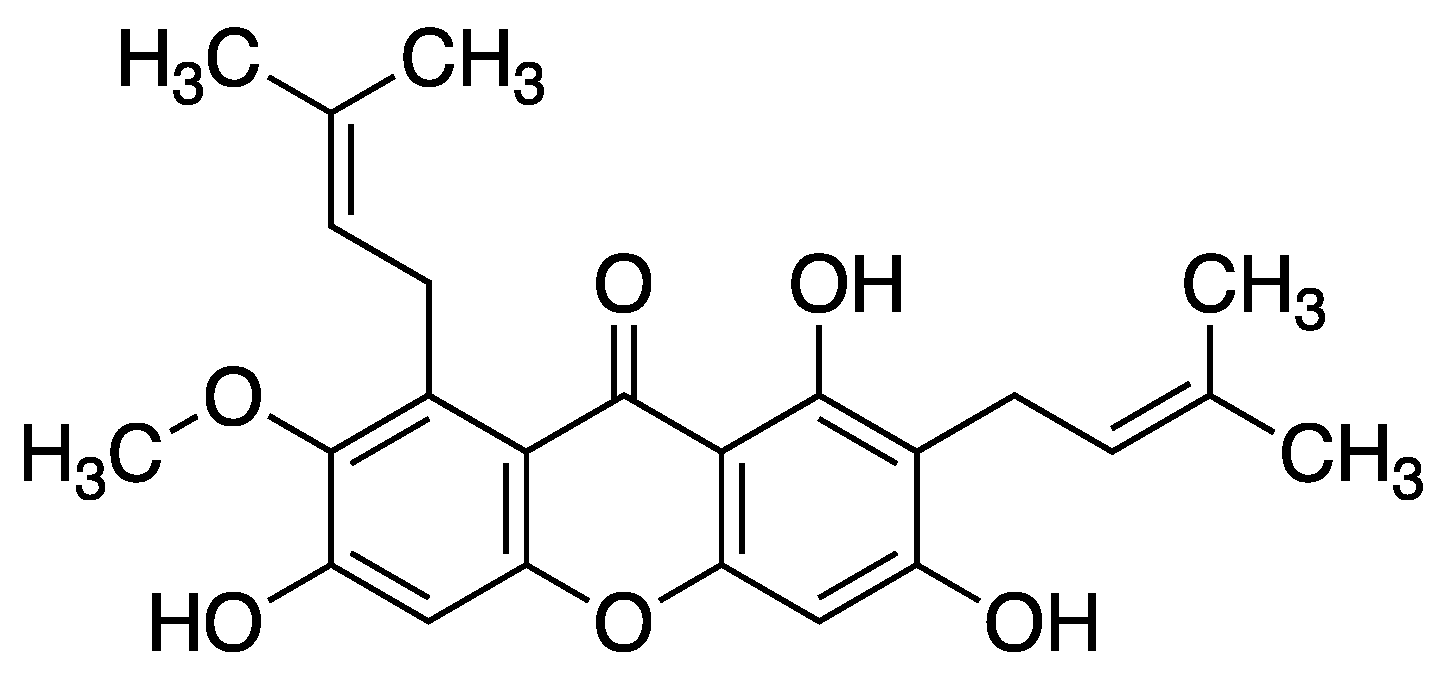
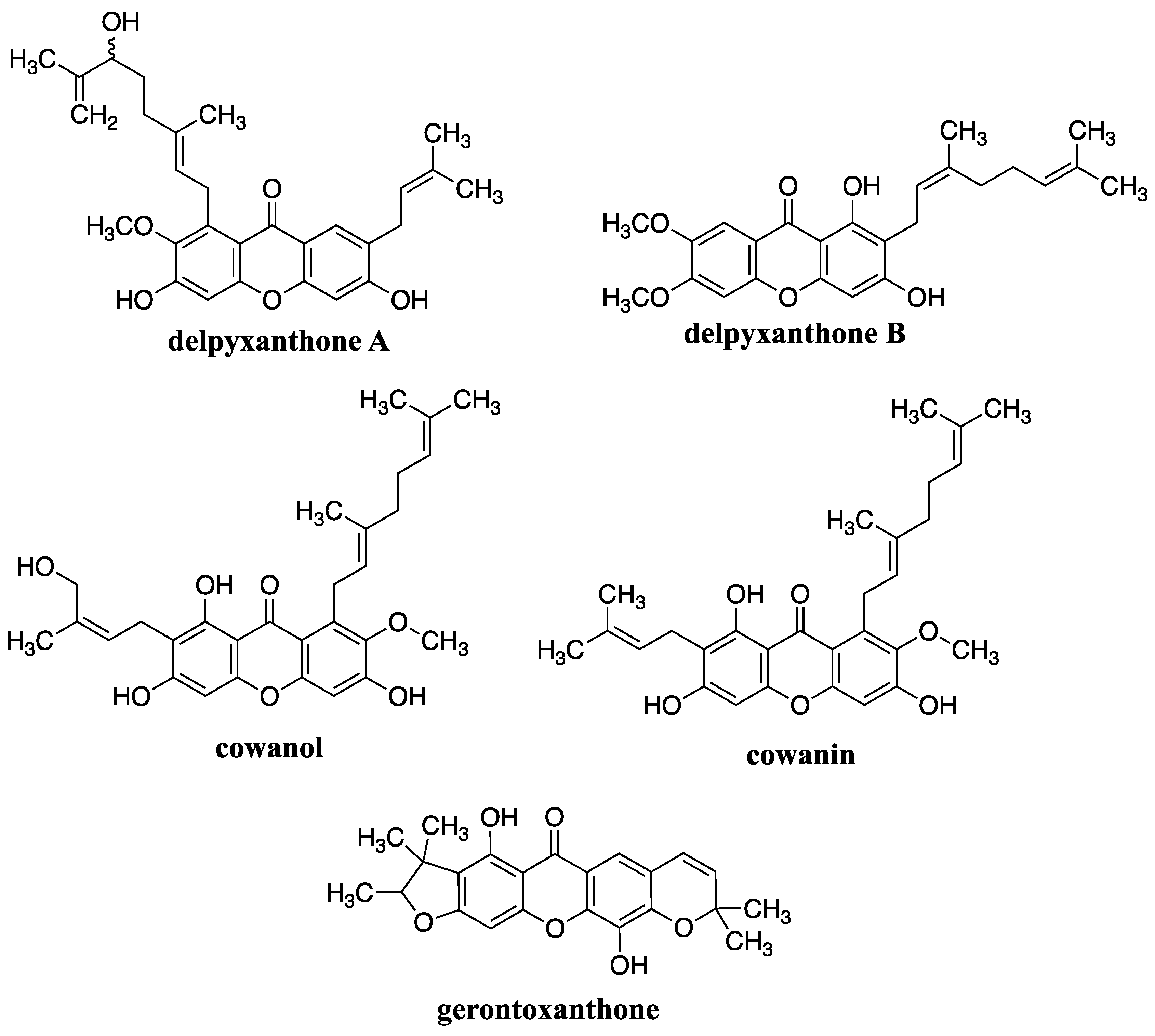
e.g., interleukins IL, tumor necrosis factor-alpha TNF-α), reactive oxygen species (ROS), and nitric oxide (NO). It was shown based on study of isolated xanthone derivatives from the stem bark of
Garcinia delpyana. Followed compounds were isolated, two new ones delpyxanthone A and delpyxanthone B, together with four known ones, gerontoxanthone I, α-mangostin, cowanin and cowanol. Additionally, xanthone derivatives have been shown to influence signalling pathways such as nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinases (MAPKs), which play pivotal roles in orchestrating inflammatory responses. Furthermore, the antioxidant properties of xanthone derivatives contribute to their anti-inflammatory effects by neutralizing ROS and mitigating oxidative stress, a common feature of inflammatory conditions. Additionally, xanthone derivatives may modulate immune responses by regulating the activity of immune cells involved in inflammation, further enhancing their anti-inflammatory actions [
55].
Figure 8.
Structures of xanthone derivatives isolated from Garcinia delpyana.
Figure 8.
Structures of xanthone derivatives isolated from Garcinia delpyana.
Investigations on the potential of
G. mangostana L. pericarps ethanol extracts for acne treatment revealed its anti-inflammatory effects by suppressing the production of pro-inflammatory cytokine, TNF-α. Some research suggests that xanthone derivatives possess antibacterial properties that could be beneficial for treating conditions like acne. Specifically, studies have shown that xanthone derivatives exhibit antibacterial activity against bacteria such as
Propionibacterium acnes and
Staphylococcus epidermidis, which are commonly associated with acne [
56]. Formation of acne it is also associated with one of the stages of rosacea.
4.1.4. Antioxidant Activity
Several studies have investigated the antioxidant activity of xanthone derivatives and have found promising results:
Free radical scavenging: 1,3,7-hydroxyxanthone and other hydroxy xanthone derivatives was shown to effectively scavenge free radicals, thereby reducing oxidative stress and preventing cellular damage as well protected against UV radiation and pollution [
57].
Anti-inflammatory effects, as mentioned above as well: Inflammation is closely linked to oxidative stress, and xanthone derivatives; like for example α-mangostin, cowanol, cowanin have demonstrated anti-inflammatory properties, which may contribute to their overall antioxidant activity.
Neuroprotective effects: Some studies suggest that some xanthone derivatives, like mangiferin may have neuroprotective effects by scavenging free radicals and reducing oxidative damage in the brain, which could potentially help in the prevention or treatment of neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases [
58].
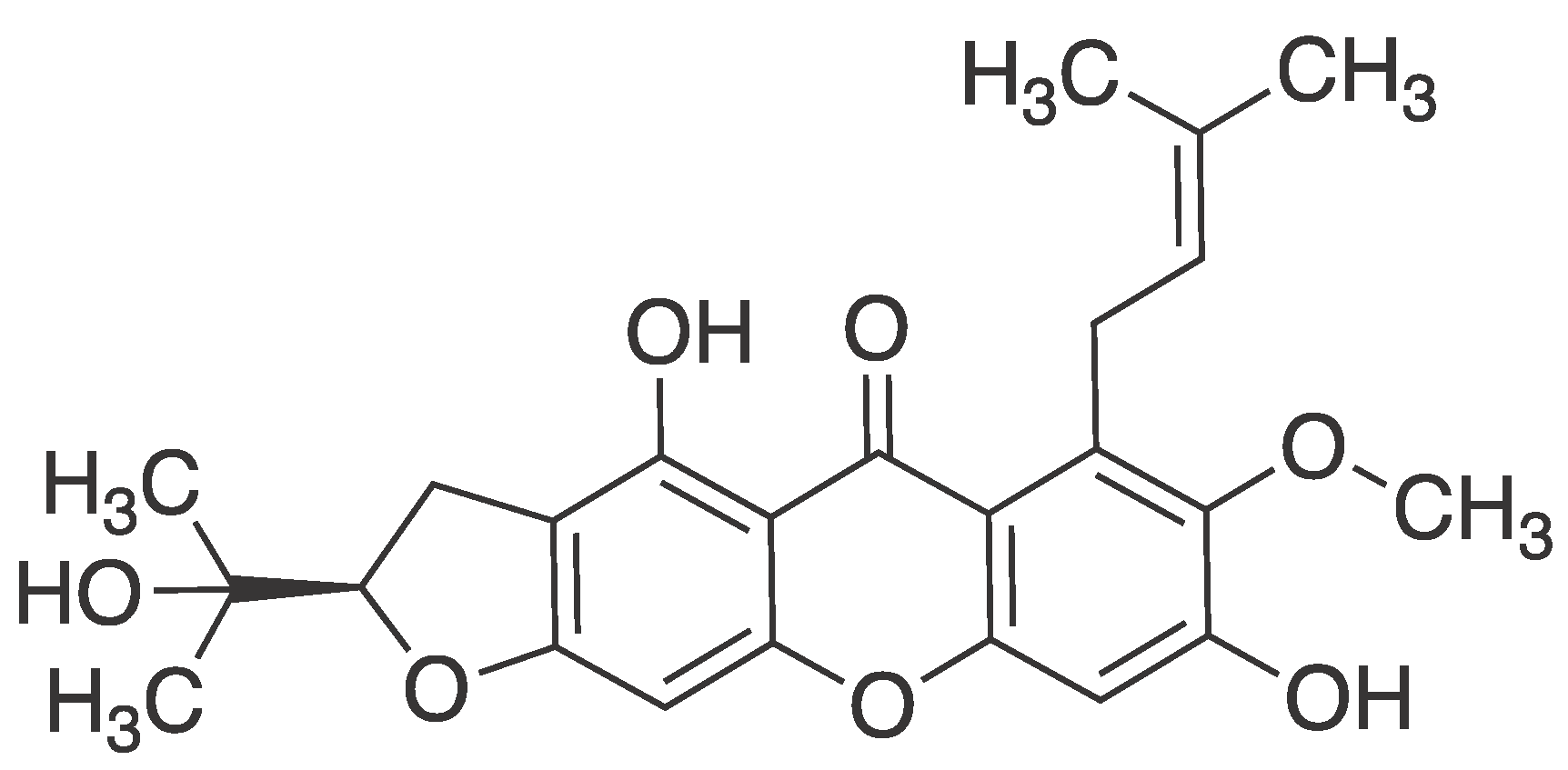
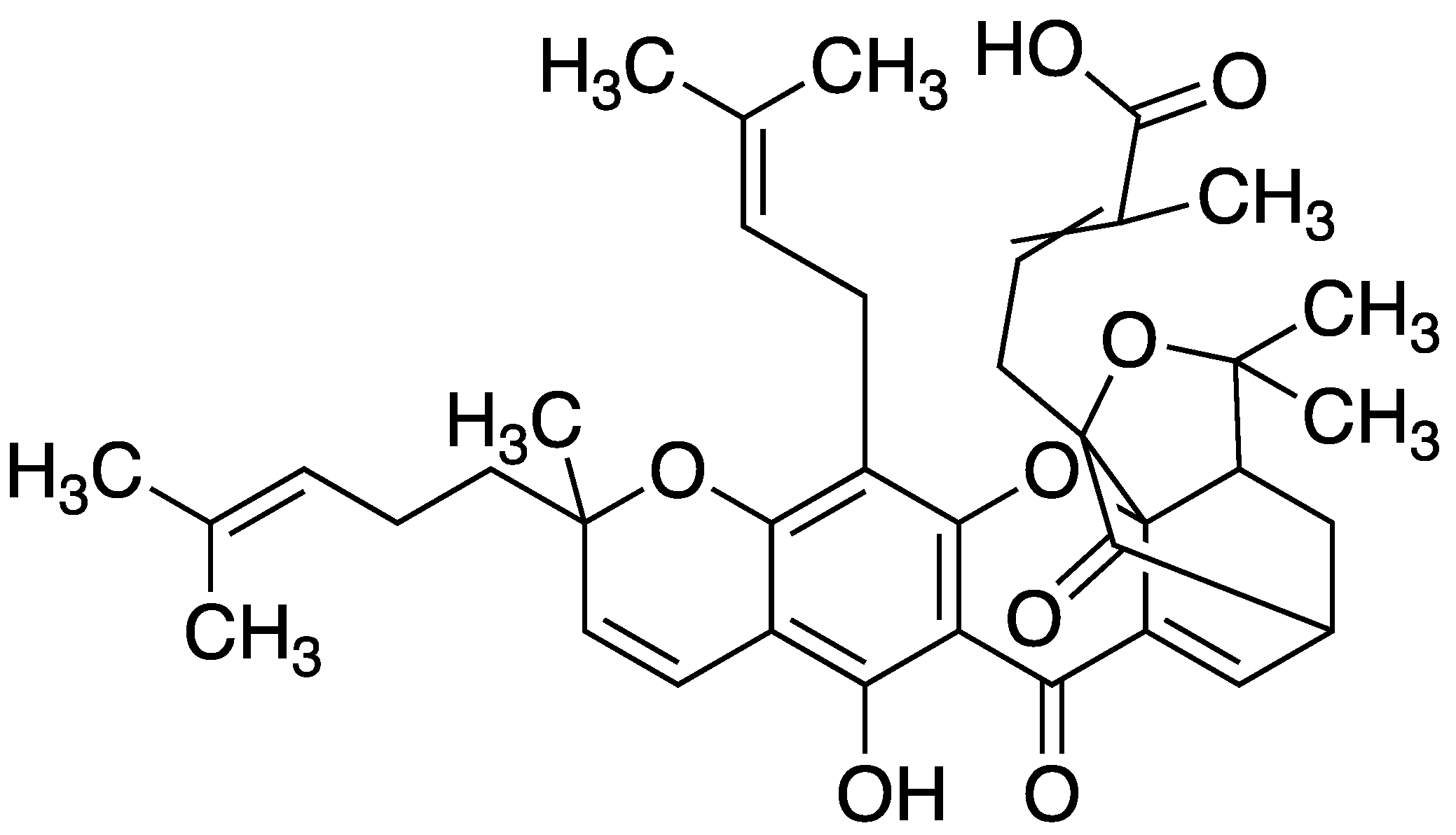
M. Abate et al. study provides novel scientific evidence demonstrating the protective effects of mangostanin, a xanthone derivative isolated from
Garcinia mangostana fruit, on epidermal keratinocytes against oxidative stress-induced damage. Pretreatment with mangostanin significantly attenuated H
2O
2-induced cytotoxicity and reactive oxygen species (ROS) production. Mechanistically, mangostanin inhibited the activation of key signalling pathways including p53, p38 MAPK, ERK, and AKT, as well as the cleavage of Caspase-9 and Caspase-3, while preserving critical cell survival signals such as EGFR and STAT-3. These findings suggest a potential pharmaceutical application for mangostanin in skin protection and aging. However, further in vivo and clinical studies are warranted to validate its efficacy and safety for potential cosmeceutical, pharmacological, or nutraceutical formulations [
59].
Figure 9.
Structure of mangostanin ((R)-4,8-dihydroxy-2-(2-hydroxypropan-2-yl)-7-methoxy-6-(3-methylbut-2-en-1-yl)-2,3-dihydro-5H-furo [3,2-b]xanthen-5-one).
Figure 9.
Structure of mangostanin ((R)-4,8-dihydroxy-2-(2-hydroxypropan-2-yl)-7-methoxy-6-(3-methylbut-2-en-1-yl)-2,3-dihydro-5H-furo [3,2-b]xanthen-5-one).
All of the above mentioned biological activities of xanthone derivatives are connected with the pathogenesis and formation of teleangiectasia and early stages of rosacea.
5. Conclusions
The exploration of xanthone derivatives as potential topical agents for the treatment of telangiectasia and rosacea offers a promising avenue for addressing the unmet needs of patients with these dermatological conditions. Through their multifaceted pharmacological properties, including anti-inflammatory, antioxidant, and antimicrobial effects, xanthone derivatives demonstrate the potential to modulate the underlying pathophysiological mechanisms driving vascular abnormalities and cutaneous inflammation.
Despite the limited number of studies investigating the use of xanthone derivatives specifically for telangiectasia and rosacea, the available evidence suggests encouraging outcomes in terms of efficacy and safety. However, further research is imperative to fully elucidate the therapeutic potential of these compounds and optimize their clinical utility.
Future studies should focus on elucidating the precise mechanisms of action of xanthone derivatives in the context of telangiectasia and rosacea, as well as exploring their synergistic effects with existing treatment modalities. Additionally, efforts to identify the most effective formulations, dosing regimens, and delivery systems for topical application are warranted to maximize therapeutic outcomes and patient compliance, especially evaluating their bioavailability.
Furthermore, rigorous clinical trials are needed to validate the findings from preclinical studies and establish the long-term safety profile of xanthone derivatives in the treatment of telangiectasia and rosacea. Close monitoring of adverse effects and potential drug interactions will be essential to ensure the safety and tolerability of these compounds in clinical practice.
In conclusion, the growing body of evidence supporting the therapeutic potential of xanthone derivatives underscores the importance of continued research in this area. With further investigation and refinement, xanthone-based topical formulations may emerge as valuable additions to the therapeutic armamentarium for telangiectasia and rosacea, offering new hope for patients burdened by these challenging dermatological conditions.
Funding
This research was funded by Jagiellonian University Collegium Medicum, U1C/W42/NO/28.16.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
The data are contained within this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Noszczyk Wojciech, Chirurgia tętnic i żył obwodowych, t. 1, vol. 1. Warszawa: Wydawnictwo Lekarskie PZWL, 2006. Accessed: Apr. 08, 2021. [Online]. Available: http://hanproxy.cm-uj.krakow.pl/han/ibuk/https/libra.ibuk.pl/book/13189.
- S. Patan, “Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling,” Journal of Neuro-Oncology, vol. 50, no. 1–2. pp. 1–15, 2000. [CrossRef]
- G. D. Yancopoulos, M. Klagsbrun, and J. Folkman, “Vasculogenesis, angiogenesis, and growth factors: Ephrins enter the fray at the border,” Cell, vol. 93, no. 5. Elsevier B.V., pp. 661–664, May 29, 1998. [CrossRef]
- E. Swidzińska, W. Naumnik, and E. Chyczewska, “Angiogenesis and neoangiogenesis--the role in lung cancer and other tumors,” Pneumonologia i alergologia polska : organ Polskiego Towarzystwa Ftyzjopneumonologicznego, Polskiego Towarzystwa Alergologicznego, i Instytutu Gruźlicy i Chorób Płuc, vol. 74, no. 4. Pneumonol Alergol Pol, pp. 414–420, 2006. Accessed: Apr. 08, 2021. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/17427152/.
- E. L. Pardue, S. Ibrahim, and A. Ramamurthi, “Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering,” Organogenesis, vol. 4, no. 4. Landes Bioscience, pp. 203–214, 2008. [CrossRef]
- M. K. Pugsley and R. Tabrizchi, “The vascular system. An overview of structure and function,” J Pharmacol Toxicol Methods, vol. 44, no. 2, pp. 333–340, Sep. 2000. [CrossRef]
- C. Frati Munari, “Medical significance of endothelial glycocalyx. Part 2: Its role in vascular diseases and in diabetic complications,” Arch Cardiol Mex, vol. 84, no. 2, pp. 110–116, 2014. [CrossRef]
- P. A. Singleton, “Hyaluronan regulation of endothelial barrier function in cancer,” Adv Cancer Res, vol. 123, pp. 191–209, 2014. [CrossRef]
- H. Jung, “Hyaluronidase: An overview of its properties, applications, and side effects,” Arch Plast Surg, vol. 47, no. 4, p. 297, Jul. 2020. [CrossRef]
- E. Bala, R. Hazarika, P. Singh, M. Yasir, and R. Shrivastava, “A biological overview of Hyaluronidase: A venom enzyme and its inhibition with plants materials,” Mater Today Proc, vol. 5, no. 2, pp. 6406–6412, Jan. 2018. [CrossRef]
- A. Lee, S. E. Grummer, D. Kriegel, and E. Marmur, “Hyaluronidase,” Dermatologic Surgery, vol. 36, no. 7, pp. 1071–1077, Jul. [CrossRef]
- E. Bala, R. Hazarika, P. Singh, M. Yasir, and R. Shrivastava, “A biological overview of Hyaluronidase: A venom enzyme and its inhibition with plants materials,” Mater Today Proc, vol. 5, no. 2, pp. 6406–6412, Jan. 2018. [CrossRef]
- J. M. Tarbell and L. M. Cancel, “The glycocalyx and its significance in human medicine,” J Intern Med, vol. 280, no. 1, pp. 97–113, Jul. 2016. [CrossRef]
- J. A. Florian, J. R. Kosky, K. Ainslie, Z. Pang, R. O. Dull, and J. M. Tarbell, “Heparan sulfate proteoglycan is a mechanosensor on endothelial cells,” Circ Res, vol. 93, no. 10, 2003. [CrossRef]
- B. F. Becker, D. Chappell, and M. Jacob, “Endothelial glycocalyx and coronary vascular permeability: the fringe benefit,” Basic Res Cardiol, vol. 105, no. 6, pp. 687–701, Nov. 2010. [CrossRef]
- M. Slevin et al., “Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways,” Matrix Biol, vol. 26, no. 1, pp. 58–68, Jan. 2007. [CrossRef]
- M. Steinhoff, M. Schmelz, and J. Schauber, “Facial erythema of rosacea – Aetiology, different pathophysiologies and treatment options,” Acta Dermato-Venereologica, vol. 96, no. 5. Medical Journals/Acta D-V, pp. 579–589, 2016. [CrossRef]
- R. J. Rycroft, S. H. Wakelin, and S. J. Robertson, Dermatologia, vol. 1. Warszawa: Wydawnictwo Lekarskie PZWL, 2014. Accessed: Apr. 09, 2021. [Online]. Available: http://hanproxy.cm-uj.krakow.pl/han/ibuk/https/libra.ibuk.pl/book/145065.
- H. Gan, B. Yue, Y. Wang, and Z. Lu, “Treatment of facial telangiectasia with narrow-band intense pulsed light in Chinese patients,” Journal of Cosmetic and Laser Therapy, vol. 20, no. 7–8, pp. 442–446, Nov. 2018. [CrossRef]
- Y. Hayran, I. Lay, M. C. Mocan, T. Bozduman, and S. Ersoy-Evans, “Vascular endothelial growth factor gene polymorphisms in patients with rosacea: A case-control study,” J Am Acad Dermatol, vol. 81, no. 2, pp. 348–354, Aug. 2019. [CrossRef]
- H. R. Jørgensen, A. Egeberg, R. Gideonsson, L. B. Weinstock, E. P. Thyssen, and J. P. Thyssen, “Rosacea is associated with Helicobacter pylori: a systematic review and meta-analysis,” Journal of the European Academy of Dermatology and Venereology, vol. 31, no. 12. Blackwell Publishing Ltd., pp. 2010–2015, Dec. 01, 2017. [CrossRef]
- M. T. Pelle, G. H. Crawford, and W. D. James, “Rosacea: II. Therapy,” Journal of the American Academy of Dermatology, vol. 51, no. 4. Mosby Inc., pp. 499–512, Oct. 01, 2004. [CrossRef]
- Y. A. Wang and W. D. James, “Update on Rosacea Classification and Its Controversies | MDedge Dermatology,” Cutis, vol. 104, no. 01, pp. 70–73, Jul. 2019.
- C. S. Ahn and W. W. Huang, “Rosacea Pathogenesis,” Dermatologic Clinics, vol. 36, no. 2. W.B. Saunders, pp. 81–86, Apr. 01, 2018. [CrossRef]
- U. Wollina, “Is rosacea a systemic disease?,” Clin Dermatol, vol. 37, no. 6, pp. 629–635, Nov. 2019. [CrossRef]
- B. N. Jacobs, E. A. Andraska, A. T. Obi, and T. W. Wakefield, “Pathophysiology of varicose veins,” J Vasc Surg Venous Lymphat Disord, vol. 5, no. 3, pp. 460–467, May 2017. [CrossRef]
- H. Partsch, “Varicose veins and chronic venous insufficiency,” Vasa - Journal of Vascular Diseases, vol. 38, no. 4. pp. 293–301, 2009. [CrossRef]
- T. J. Gampper and R. F. Morgan, “Vascular anomalies: Hemangiomas,” Plastic and Reconstructive Surgery, vol. 110, no. 2. Lippincott Williams and Wilkins, pp. 572–586, 2002. [CrossRef]
- J. M. Zabramski and A. Ahmed, “Hemangiomas,” in Encyclopedia of the Neurological Sciences, Elsevier Inc., 2014, pp. 541–542. [CrossRef]
- Enjolras, “Vascular tumors and vascular malformations: Are we at the dawn of a better knowledge?,” Pediatric Dermatology, vol. 16, no. 3. Pediatr Dermatol, pp. 238–241, 1999. [CrossRef]
- J. A. Cox, E. Bartlett, and E. I. Lee, “Vascular malformations: A review,” Seminars in Plastic Surgery, vol. 28, no. 2. Thieme Medical Publishers, Inc., pp. 58–63, 2014. [CrossRef]
- P. Wójcicki and K. Wójcicka, “Epidemiology, diagnostics and treatment of vascular tumours and malformations,” Advances in Clinical and Experimental Medicine, vol. 23, no. 3. Wroclaw University of Medicine, pp. 475–484, 2014. [CrossRef]
- C. Léauté-Labrèze, J. I. Harper, and P. H. Hoeger, “Infantile haemangioma,” The Lancet, vol. 390, no. 10089. Lancet Publishing Group, pp. 85–94, Jul. 01, 2017. [CrossRef]
- J. L. Beebe-Dimmer, J. R. Pfeifer, J. S. Engle, and D. Schottenfeld, “The epidemiology of chronic venous insufficiency and varicose veins,” Ann Epidemiol, vol. 15, no. 3, pp. 175–184, 2005. [CrossRef]
- S. Mekić et al., “Epidemiology and determinants of facial telangiectasia: a cross-sectional study,” Journal of the European Academy of Dermatology and Venereology, vol. 34, no. 4, pp. 821–826, Apr. 2020. [CrossRef]
- H. Ullah and M. Daglia, “Phytonutrients in the management of glucose metabolism,” The Role of Phytonutrients in Metabolic Disorders, pp. 163–193, Jan. 2022. [CrossRef]
- M. M. M. Pinto, M. E. Sousa, and M. S. J. Nascimento, “Xanthone derivatives: new insights in biological activities,” Curr Med Chem, vol. 12, no. 21, pp. 2517–2538, Oct. 2005. [CrossRef]
- S. Ramakrishnan, S. Paramewaran, and N. M. Nasir, “Synthetic approaches to biologically active xanthones: an update,” Chemical Papers, vol. 75, no. 2, pp. 455–470, Feb. 2021. [CrossRef]
- G. Mazur, I. Skiba-Kurek, E. Karczewska, K. Pańczyk-Straszak, J. Jaworska, and A. M. Waszkielewicz, “Design, synthesis and activity against Staphylococcus epidermidis of 5-chloro-2- or 5-chloro-4-methyl-9H-xanthen-9-one and some of its derivatives,” Chem Biol Drug Des, vol. 97, no. 3, pp. 674–685, Mar. 2021. [CrossRef]
- M. Waszkielewicz et al., “Design, synthesis, and anticonvulsant activity of some derivatives of xanthone with aminoalkanol moieties,” Chem Biol Drug Des, vol. 89, no. 3, pp. 339–352, Mar. 2017. [CrossRef]
- K. Pytka et al., “The antidepressant-like activity of 6-methoxy-2-[4-(2-methoxyphenyl)piperazin-1-yl]-9H-xanthen-9-one involves serotonergic 5-HT1A and 5-HT2A/C receptors activation,” Eur J Pharmacol, vol. 764, pp. 537–546, Jul. 2015. [CrossRef]
- K. Pytka et al., “The antidepressant- and anxiolytic-like activities of new xanthone derivative with piperazine moiety in behavioral tests in mice,” Indian J Pharmacol, vol. 48, no. 3, pp. 286–291, May 2016. [CrossRef]
- N. Szkaradek et al., “Anticonvulsant evaluation of aminoalkanol derivatives of 2- and 4-methylxanthone,” Bioorg Med Chem, vol. 21, no. 5, pp. 1190–1198, Mar. 2013. [CrossRef]
- K. Pytka et al., “HBK-7 - A new xanthone derivative and a 5-HT1A receptor antagonist with antidepressant-like properties,” Pharmacol Biochem Behav, vol. 146–147, pp. 35–43, Jul. 2016. [CrossRef]
- Shagufta and I. Ahmad, “Recent insight into the biological activities of synthetic xanthone derivatives,” Eur J Med Chem, vol. 116, pp. 267–280, Jun. 2016. [CrossRef]
- C. T et al., “Synthesis and antiangiogenic activity of novel gambogic acid derivatives,” Molecules, vol. 17, no. 6, pp. 6237–6248, Jun. 2012. [CrossRef]
- K. Jittiporn et al., “Anti-angiogenic actions of the mangosteen polyphenolic xanthone derivative α-mangostin,” Microvasc Res, vol. 93, p. 72, 2014. [CrossRef]
- J. Song, Y. Li, J. Song, F. Hou, B. Liu, and A. Li, “Mangiferin protects mitochondrial function by preserving mitochondrial hexokinase-II in vessel endothelial cells,” Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, vol. 1863, no. 7, pp. 1829–1839, Jul. 2017. [CrossRef]
- T. Michel and P. M. Vanhoutte, “Cellular signaling and NO production,” Pflugers Arch, vol. 459, no. 6, p. 807, May 2010. [CrossRef]
- Q. Zhao, J. Yang, B. Liu, F. Huang, and Y. Li, “Exosomes derived from mangiferin-stimulated perivascular adipose tissue ameliorate endothelial dysfunction,” Mol Med Rep, vol. 19, no. 6, p. 4797, Jun. 2019. [CrossRef]
- D. J. Jiang, Z. Dai, and Y. J. Li, “Pharmacological effects of xanthones as cardiovascular protective agents,” Cardiovasc Drug Rev, vol. 22, no. 2, pp. 91–102, 2004. [CrossRef]
- G. P. Rosa et al., “Xanthones for melanogenesis inhibition: Molecular docking and QSAR studies to understand their anti-tyrosinase activity,” Bioorg Med Chem, vol. 29, Jan. 2021. [CrossRef]
- W. Widowati et al., “Anti-aging Effects of Mangosteen Peel Extract and Its Phytochemical Compounds: Antioxidant Activity, Enzyme Inhibition and Molecular Docking Simulation,” Trop Life Sci Res, vol. 31, no. 3, p. 127, 2020. [CrossRef]
- M. Jiang, S. Huang, W. Duan, Q. Liu, and M. Lei, “Alpha-mangostin improves endothelial dysfunction in db/db mice through inhibition of aSMase/ceramide pathway,” J Cell Mol Med, vol. 25, no. 7, p. 3601, Apr. 2021. [CrossRef]
- N. T. Nhan, P. H. Nguyen, M. H. Tran, P. D. N. Nguyen, D. T. Tran, and D. C. To, “Anti-inflammatory xanthone derivatives from Garcinia delpyana,” J Asian Nat Prod Res, vol. 23, no. 5, pp. 414–422, 2021. [CrossRef]
- N. V. Gunter, S. S. Teh, Y. M. Lim, and S. H. Mah, “Natural Xanthones and Skin Inflammatory Diseases: Multitargeting Mechanisms of Action and Potential Application,” Front Pharmacol, vol. 11, p. 1, Dec. 2020. [CrossRef]
- N. Ruangsawasdi, N. Boonnak, C. Pruksaniyom, and P. Rodanant, “Xanthones Isolated from Cratoxylum cochinchinensis Reduced Oxidative Stress in Periodontal Ligament Stem Cells,” Int J Mol Sci, vol. 24, no. 19, Oct. 2023. [CrossRef]
- M. P. Phyu and J. Tangpong, “Neuroprotective effects of xanthone derivative of Garcinia mangostana against lead-induced acetylcholinesterase dysfunction and cognitive impairment,” Food and Chemical Toxicology, vol. 70, pp. 151–156, Aug. 2014. [CrossRef]
- M. Abate et al., “Mangostanin, a Xanthone Derived from Garcinia mangostana Fruit, Exerts Protective and Reparative Effects on Oxidative Damage in Human Keratinocytes,” Pharmaceuticals, vol. 15, no. 1, Jan. 2022. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).