1. Introduction
Beer, a globally cherished alcoholic libation, occupies a distinguished status among the most favored beverages. Its origins date back approximately 6000-8000 years, where early renditions likely boasted a sweet, malty essence complemented by infusions of herbs, spices, or fruits—deviating significantly from the contemporary brew we recognize today [
1].
Across epochs, an array of beer styles has unfolded, spanning from the crisp and invigorating pilsners to the robust and malty porters, and the opaque, hop-laden India pale ales (IPAs). Despite the kaleidoscope of hues and flavor profiles, modern beer varieties derive their essence from the alcoholic fermentation of a must concocted with four primary raw components: malted and unmalted cereals, water, hops (
Humulus lupulus L.), and yeast [
2]. In 2018, global beer production peaked at nearly two billion hectolitres, as documented by statista.com. The surge in independent microbreweries, particularly in Italy’s Calabria region, has spurred a renaissance of experimentation with ingredients and brewing methodologies. This innovative wave has birthed a new era of beers—sophisticated, intricate, and bursting with flavor. As a result, this upsurge in a myriad of beer offerings has sparked increased curiosity and enthusiasm for specialty brews, emphasizing a growing need for accurate and dependable flavour profiling techniques.
The chemical makeup of beer, influencing its taste, aroma, and color, undergoes dynamic transformations based on the diversity of raw materials and production methods [
3]. Delving into the flavors of beer at the chemical level proves to be a nuanced task, given the intricate nature of this beverage—a complex amalgamation of various elements such as carbohydrates, proteins, microbes, secondary metabolites, sulfur dioxide, and ethanol. The palate of beer is shaped by a myriad of compounds, with polyphenols playing a significant role, sourced 80% from cereals and 20% from hops [
4].
Phenols, pivotal not only in defining beer’s taste but also in enhancing its long-term stability, play a crucial role in the fermentation product’s preservation. Notably, polyphenols like flavon-3-oils exhibit a natural ability to chelate metals within the solution. Simultaneously, various phenolic families act as inhibitors during oxidative processes, curbing the generation of diketones, sulfur compounds, aldehydes, and low molecular weight fatty acids—molecules implicated in the beer maturation process [
5,
6].
Another class of influential molecules shaping beer’s aroma, flavor, and shelf life is found in the bitter acids of hops. Concentrating in the resin of hops, these bitter acids come in two categories: α-acids or humulons (humulone, cohumulone, adhumulone as primary, and prehumulone, posthumulone as secondary) and β-acids or lupulones (lupulone, colupulone, adlupulone as primary, and prelupulon, postlupulon as secondary) [
7,
8,
9].
During the boiling of the must and preceding the fermentation phase, these bitter acids undergo isomerization, a process influenced by various factors such as the quality, quantity, aging degree, and form (cone, pellet, or plug) of the hops. Additionally, the duration and temperature of boiling, pH, and the presence of bivalent cations in the must impact the isomerization yield [
7,
8,
9]. Humulons, through isomerization, give rise to iso-α-acids or iso-humulons in cis and trans forms, exhibiting a heightened bitterness effect compared to their precursors. In contrast, lupolones remain unaltered during isomerization, forming cyclic and epoxy compounds [
10].
Beers with elevated levels of bitter acids, such as Indian Pale Ale (IPA) and American Pale Ale (APA), are often perceived as less bitter by consumers compared to beers with a moderate bitter acid content, like Bitter and Strong Bitter. This intriguing phenomenon arises from the specific type of bitter acids present in beer and the balancing influence of phenols on the bitter taste [
1,
3,
11].
Moreover, the bitter acids found in hops have garnered attention for their potential antiviral, antibacterial, and anti-inflammatory properties [
12,
13,
14,
15].
The polyphenolic profile of craft beers, primarily influenced by barley and hops, serves as a key indicator not only of nutritional and antioxidant quality but also of colloidal stability and the beer’s ability to interact with proteins. Importantly, this profile profoundly shapes sensory characteristics such as color, aroma, and flavor. Hence, it becomes imperative to establish a rapid, straightforward, and cost-effective method readily available in standard analysis laboratories to accurately trace the polyphenolic fingerprint of beers. Traditionally, total phenols and total bitter acids in beers are quantified using distinct spectrophotometric methods developed by the American Society of Brewing Chemists (ASBC) and European Brewery Convention (EBC) [
3,
16,
17,
18,
19].
Chromatographic methods, designed for the identification and quantification of phenolic acids and flavonoids, have also been developed [
4,
20,
21,
22].
While some methods involve liquid-liquid extraction (LLE) with organic solvents [
23,
24], others opt for sample injection without treatment, capturing only a subset of phenols [
25,
26]. Few studies exist for the identification of different bitter acids in beers using chromatographic methods, and these typically employ liquid-liquid extraction (LLE) with organic solvents [
27,
28,
29,
30]. Only one published work addresses the qualitative identification of prenylflavonoids and bitter acids in beers [
31].
This study aimed to develop and validate an ultra-high-performance liquid chromatography method coupled with a photodiode array detector (UHPLC-PDA) for the simultaneous analysis of diverse phenolic classes and the numerous bitter acids present in beers, employing a rapid and simple sample preparation. To the best of the authors’ knowledge, there is only one analytical method in the literature for simultaneous phenol and bitter acid identification in beers [
32] using liquid chromatography-quadrupole time-of-flight mass spectrometry (LC-MS). Despite LC-MS’s high selectivity and specificity, HPLC-PAD proves to be a more accessible choice for small laboratories, offering comprehensive assessment capabilities for specific chemical compounds. This makes HPLC-PAD the preferred option for delving into the extensive chemical variations seen in the products produced by today’s craft brewing industry.
The polyphenolic composition of beer can undergo substantial changes due to the use of specific cereals, hops, and “characterizing foods” such as fruits, herbs, and juices employed in the production of flavored beers. Beers crafted with citrus juices, like those with bergamot, or with grape must (Italian Grape Ale or IGA), exhibit phenols absent in traditional beers, originating from these “characterizing foods.” Employing advanced analytical techniques to explore characterizing profiles in the brewing industry holds the potential to facilitate the development of new flavors, bolster quality control procedures, and provide brewers with a deeper understanding of how ingredients and processes shape their final products. This not only expands the market appeal of beers but also offers a more profound insight into their chemical profiles.
The study aimed to conduct a simultaneous analysis of IAAs and phenolic compounds in beer using a straightforward HPLC-PAD procedure, leveraging the resulting chemical profiles to assess the ability to discriminate the polyphenolic profile and flavor of beers with different styles yet all flavored with bergamot.
2. Materials and Methods
Ultrapure water was obtained using a Milli-Q system (Millipore, Milan, Italy), formic acid and acetonitrile (ACN) with HPLC grade of purity were purchased from Sigma-Aldrich (Milan, Italy), while the phenol standards (p-Coumaric acid, 4-Hydroxybenzoic acid, Caffeic acid, Ethylgallate, Ferulic acid, Kampferol, Naringin, Protocatechuic acid, Rutin, Syrengin acid, Vanillic acid, Hesperidin, Sinensetin, Neodiosmin, Neoeriocitrin, Hesperetin, (-)Epicatechin, Eriocetrin, Isorhamnetin, Myricetin, Neohesperidin, Diosmin, Narirutin, Rhamnetin, Tangeretin, Apigenin, Chlorogenic acid, Nobiletin, Naringenin) were purchased from Extrasynthese (Genay Cedex, France). The bitter acid standards (ICE-4, ICS-I4, ICS-R3, ICS-H2 and ICS-T3) were obtained from the American Society of Brewing Chemists (ASBC) (St. Paul, MN 55121, USA). ICE-4 contains Cohumulone 10.98%, Colupulone 3.02%, Humulone/Adhumulone 31.60%, Hupulone/Adlupulone 13.52%, with total α-acids 42.58% and total β-acids 26.54%. ICS-I4 contains trans-Isocoumulone, trans-Isohumulone and trans-Isoadhumulone with total trans-iso-α-acids 65.2% ICS-R3 containing cis-ρ-Isocoumulones, cis-ρ-Isohumulones and cis-ρ-Isoadhumulones with total cis-ρ-iso-α-acids 65%. ICS-H2 containing cis-Hexaidroisocoumuloni, cis-Hexaidroisoumuloni and cis-Hexaidroisoadhumuloni with total cis-Hexahydro-iso-α-acids 65.7%. ICS-T3 contains cis-trans-Tetrahydroisocoumulones, cis-trans-Tetraidroisohumuloni and cis-trans-Tetrahydroisoadhumulones with total cis-trans-Tetrahydro-iso-α-acids 99.4%. The bitter acid standards purchased are all those currently available on the market. For bitter unit: hydrochloric acid and isooctane were purchased from Sigma Aldrich. For total phenols: carboxymethyl cellulose, Sodium salt, Ethylenediaminetetraacetic acid, disodium salt dihydrate, Ammonium iron (III) citrate, 25% ammonia solution, were purchased from Sigma.
2.1. Sample Preparation and Analysis
The beers have been created using different recipes, which are listed below along with the codes used for their identification:
- ❖
CBS1: water, hops, barley malt and bergamot peels
- ❖
CBS2: water, hops, barley malt, wheat, and bergamot peels
- ❖
CBS3: water, hops, barley malt, oat flakes, honey, bergamot peels, black pepper, coriander
- ❖
CBS4: water, hops, barley malt, rye and bergamot
Barley malt, yeasts, hops and bergamot were the same for the preparation of all the three beers. The beers differentiated only for cereals and flavoring. This is in order to verify the potential of the new protocols developed both for the study of polyphenolic fingerprinting. All beer samples were placed in a refrigerator at 4°C in the dark and then degassed by magnetic stirring (500rpm) for 8h. Subsequently the beer samples were filtered through a 0.45μm regenerated cell membrane filter (Aisino Corporation) and analyzed immediately after degasification pretreatment to reduce experimental errors caused by temperature and instrument instability.
2.2. Bitter Unit and Total Phenols by UV-VIS
For the determination of bitter unit, a standard method EBC was used (Analytica-EBC, Section 9 Beer, Method 9.6.) For the determination of total phenols, a standard method EBC was used (RIF Analytica-EBC, Section 9 Beer, Method 9.11) [
33]
2.3. UHPLC-PDA Instrumentation
The analyzes were carried out with a Shimadzu Nexera UHPLC-PDA system (Shimadzu, Kyoto, Japan), composed of a controller (CBM-20A), a degasser (DGU-20A5R), dual-plunger parallel-flow pumps (LC-30AD), an autosampler (SIL-30AC), a column oven (CTO-20AC), a photodiode detector (SPD-M30A). LC data processing was performed with LC solution software (Version 5.71, Shimadzu).
2.4. UHPLC-PDA Condition
The analytical conditions used for the analyzes have been optimized to obtain the best chromatographic separation for the classes of molecules considered, phenols and bitter acids. Ten microliters of degassed and microfiltered sample were injected without performing preliminary extraction procedures. The chromatographic separation was carried out with a Kinetex C18 column (50 mm × 3 mm × 1.7 μm d.p) and a Kinetex C18 pre-column, the columns are manufactured by Phenomenex (Torrance, California, United States). The oven temperature was set at 40°C, a flow rate of 0.6 mL/min was used with mobile phases composed of water with 0.1% formic acid (v/v) (mobile phase A) and acetonitrile with 0.1% formic acid (v/v) (mobile phase B). The gradient used was: 5 minutes with 1% B (isocratic mode), 15 minutes from 1% to 30% B, 3.5 minutes from 30% to 47% B (gradient mode for the separation of polyphenols), 30 seconds from 47% at 60% B, 2 minutes with 60% B (isocratic mode for the separation of bitter acids), washing the system with 100% B and reconditioning with 1% B. The photodiode detector was set with 8 nm divided width, 256 spectrum resolution, 40 Hz sampling rate, 40°C cell temperature and 190-450 nm analysis range.
3. Statistical Analysis
The data were analyzed using XLSTAT software (Version 2022.4.5, Addinsoft, Paris, France). All the data were subjected to analysis of variance (ANOVA). ANOVA was applied to the antioxidant profile. The means were separated using the Tukey test only when the F-test for treatments and interactions was significant at the p ≤ 0.05 probability level. Principal component analysis (PCA) was conducted using PCA-XLSTAT software version 2015.5 by Addinsoft, Paris, France, to assess datasets of antioxidant compounds, bitter units and total phenols.
3. Results
3.1. Validation Method for Polyphenols
Seven concentration levels of the polyphenolic standards were prepared with methanol from a 1000 mg/L stock solution with concentration range of 0.5-120 mg/L. Five analyzes were performed for each concentration level with the HPLC-PDA system under optimized chromatographic conditions. Seven-level calibration curves were constructed using the least squares method by obtaining the equations of the regression lines (Table S1). Mandel’s test confirmed the linearity of each calibration curve in the considered range. The limit of quantification (LoQ) and limit of detection (LoD) (Table S1) were calculated by multiplying the standard deviation (SD) of the lowest level of the calibration curve (n = 7) ten and three times, respectively, and dividing the result for the slope of the calibration curve. The repeatability and reproducibility values (Table S1) were expressed as percentage coefficient of variation (CV%) and calculated using the average of the areas of the lowest level of the calibration curve (n=7) divided by the corresponding standard deviations. Finally, retention time, instrumental recovery and percentage relative standard deviation (RSD%) were determined using the fourth level (n=4) of each calibration curve (Table S2).
3.2. Identification of Bitter Acids
Five bitter acid standards (ICE-4, ICS-I4, ICS-R3, ICS-H2, ICS-T3) were analyzed individually by the method described and all compounds in the standards were separated to best of instrumental capability. UHPLC system with sub-2 core-shell column allowed to separate compounds with very similar structure such as cis-trans pairs (trans-tetrahydroisoCohumulone/cis-tetrahydroiso Cohumulone and trans-tetrahydroiso Humulone/cis-tetrahydroiso Humulone). Some analytes coelute due to the very similar chemical structure such as trans-iso Adhumulone and cis-ρ-iso Adhumulone, while Adlupulone/Lupulone and Adhumulone/Humulone coelute because they are quite apolar and a reverse phase isocratic elution does not allow their separation. These coelutions are reported in several publications [
28,
29,
30] and also by the producers of the standards (Table S1). The compounds identified for each standard, and related coelutions, have been reported in Table S3.
4. Discussion
The method for simultaneous identification of polyphenols and bitter acids has undergone refinement through meticulous optimization of various analytical parameters. This optimization process encompassed crucial aspects such as the selection of columns, acidifiers for the mobile phase, flow rate, and oven temperature. In previous literature, C18 columns were a prevalent choice for chromatographically separating polyphenols [
34,
35,
36,
37] and bitter acids [
38]. Our investigation revealed that the Kinetex C18 50 mm × 3 mm × 1.7 μm d.p. column outperformed the Kinetex C18 100 mm × 2.1 mm × 2.6 μm d.p. column, primarily attributable to the utilization of sub-2 core-shell particles as the stationary phase. Subsequently, two different acidifiers were scrutinized for mobile phase acidification. While formic acid is conventionally employed to enhance the chromatographic separation of polyphenols [
20], phosphoric acid is preferred for bitter acid analysis [
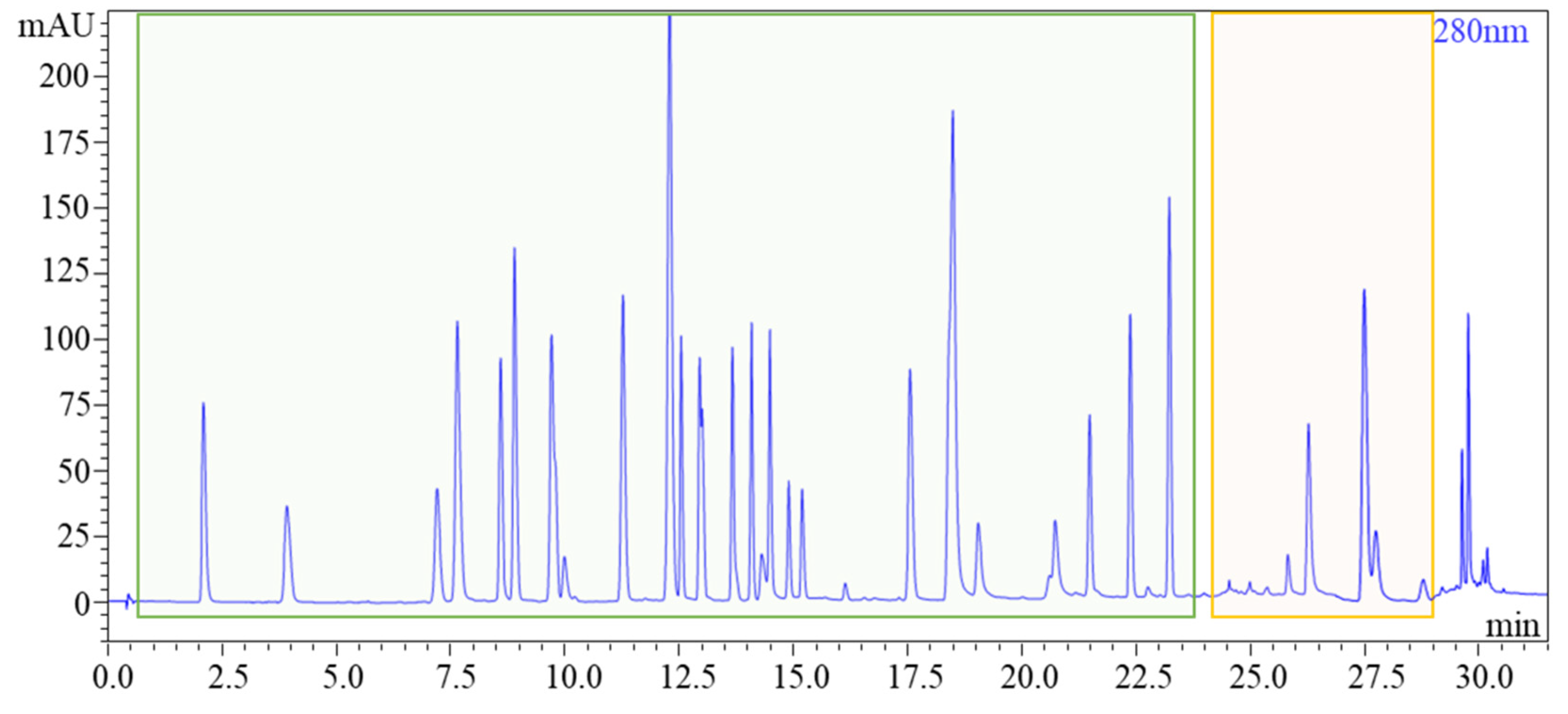
28]. Significantly, when both acids were used at a 0.1% v/v concentration, no substantial differences were observed, leading to the selection of formic acid as the optimal acidifier. Following this decision, parameters such as flow rate, oven temperature, and injection volume underwent meticulous fine-tuning for optimal performance.To further enhance chromatographic separation, we refined the mobile phase composition, implementing a gradient mode for polyphenols and an isocratic mode for bitter acids. This strategic adjustment allowed for the creation of a chromatogram neatly divided into two distinct sections: the green segment representing polyphenols and the orange segment representing bitter acids (see
Figure 1). Our method achieved remarkable elution times, with approximately 24 minutes for polyphenols and 5 minutes for bitter acids. This represents a significant reduction in analysis times compared to existing liquid chromatography systems documented in the literature [
4,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
39], underscoring the efficiency and speed of our approach. To assess potential interferences in compound quantification stemming from direct beer injection, we conducted a thorough evaluation of matrix effects (ME). Introducing variability, we incorporated two phenols with distinct polarities, namely 4-Hydroxybenzoic acid and Tangeretin, as internal standards (IS) into both blonde beers, characterized by a simple polyphenolic composition, and dark beer, known for its complex polyphenolic makeup [
2,
3]. Matrix calibration curves were meticulously established at four levels, covering n=1 and n=7 of the solvent calibration curves, following the methodology outlined by Gosetti et al. [
40]. ME was computed using the formulas outlined by Gosetti et al. and Trufelli et al. [
40,
41], leveraging the slopes of the calibration curves in both solvent and matrix. The resulting values are meticulously presented in Table S4. Notably, the matrix effect observed for both types of beer falls into the suppression category (as indicated in Table S4), yet remains well within the acceptable range of 80% to 120% [
42], affirming the robustness of our method in handling complex matrices. This extensive study on matrix effects (ME) robustly affirms that the direct injection of beer, while not considering potential coelutions, does not significantly interfere with the quantification of polyphenols. This underscores the reliability of our method in maintaining accuracy amidst the complexity of beer matrices.
4.1. Qualitative and Quantitative Analysis
Phenolic acids, renowned for their antioxidant properties, play a pivotal role in mitigating the adverse effects of oxidative stress. In the evaluation of beer production and marketing quality, the polyphenolic composition emerges as a critical benchmark [
8]. It’s crucial to recognize that the type and quantity of phenolic compounds exert a profound influence on various facets of beer, encompassing taste, aroma, color, colloidal stability, foam retention, and shelf-life. Beer, a rich source of diverse phenolic compounds, is primarily categorized into phenolic acids, tannins, flavones, and flavonols [
9]. Among these, phenols in alcoholic beers act as protective agents, shielding yeast from stress induced by high ethanol levels, akin to the role resveratrol plays in wine [
13]. This highlights that phenolic compounds not only undergo changes during brewing but actively shape the brewing process.
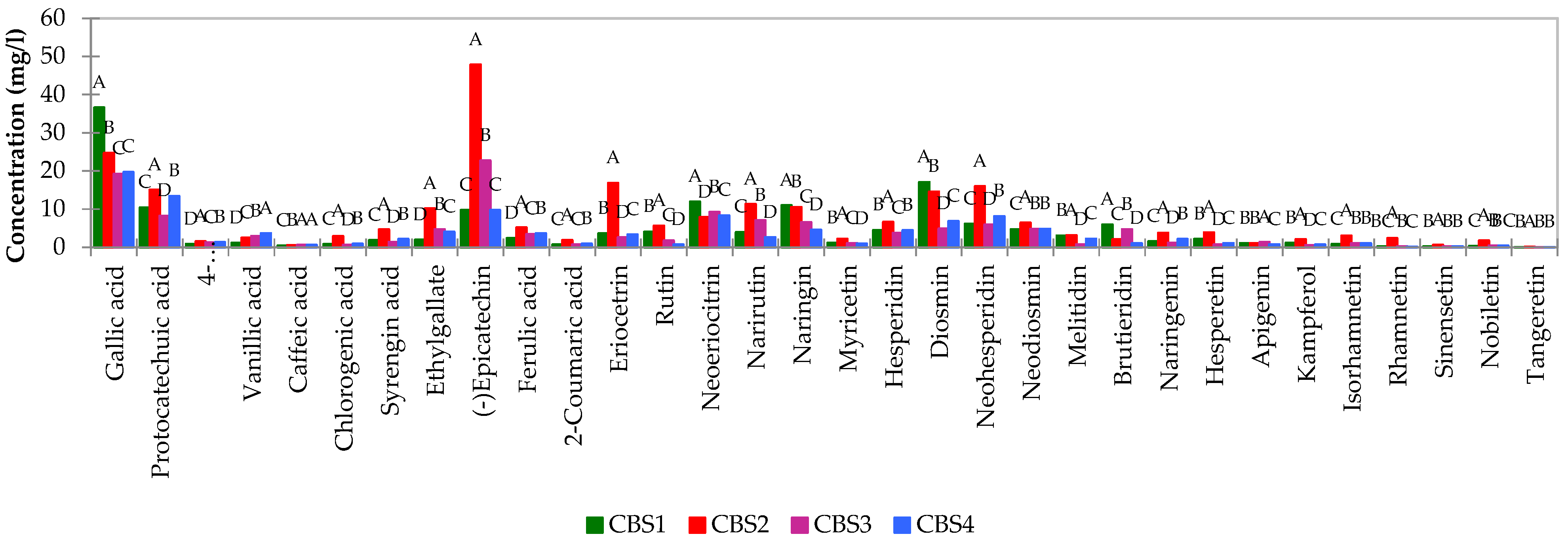
Concerning the qualitative and quantitative analysis of polyphenols in craft beers, noteworthy differences surfaced. Total concentrations of polyphenols, as determined by HPLC-PAD, were compared to assess relative phenolic content between beer styles. The two IPA beers exhibited higher concentrations of polyphenols, with an average amount of 238.91 mg/l for CBS2, enriched with wheat and bergamot peels, compared to 151.75 mg/l for CBS1 without wheat. The ALE beer (CBS4), enriched with rye and bergamot peels, demonstrated the lowest overall phenolic content among the analyzed beers. Notably, epicatechin and gallic acid emerged as the predominant phenolic compounds across all beer styles. In particular, CBS1 stood out for its elevated concentration of gallic acid, averaging at 36.87 mg/l, while IPA CBS2 exhibited higher levels of (-) epicatechin, reaching an average value of 47.87 mg/l. This value significantly surpassed other IPA variants such as CBS1 (9.75 mg/l) and was notably higher than the ale (9.71 mg/l) and blanche (22.68 mg/l). The IPA (CBS2) beer displayed a pronounced expression of flavonoids, as evidenced by
Figure 2.
In this particular beer variety, Eriocetrin, Diosmin, Neohesperidin, Narirutin, and Naringin stood out as notably abundant compounds. The IPA style (CBS1 and CBS2) distinguished itself with a higher concentration of these compounds, with naringin, narirutin, and neohesperidin particularly prominent in wheat beer compared to other beer styles. Moreover, IPA (CBS2) exhibited the highest levels of Gallic acid, Protocatechuic acid, and ethylgallate. CBS2 also showcased a heightened concentration of chlorogenic acid compared to other beers, suspected to have originated from the distinctive brewing ingredients. Significantly, previous studies have identified chlorogenic acid as a major phenolic acid present in oranges and other citrus fruits [
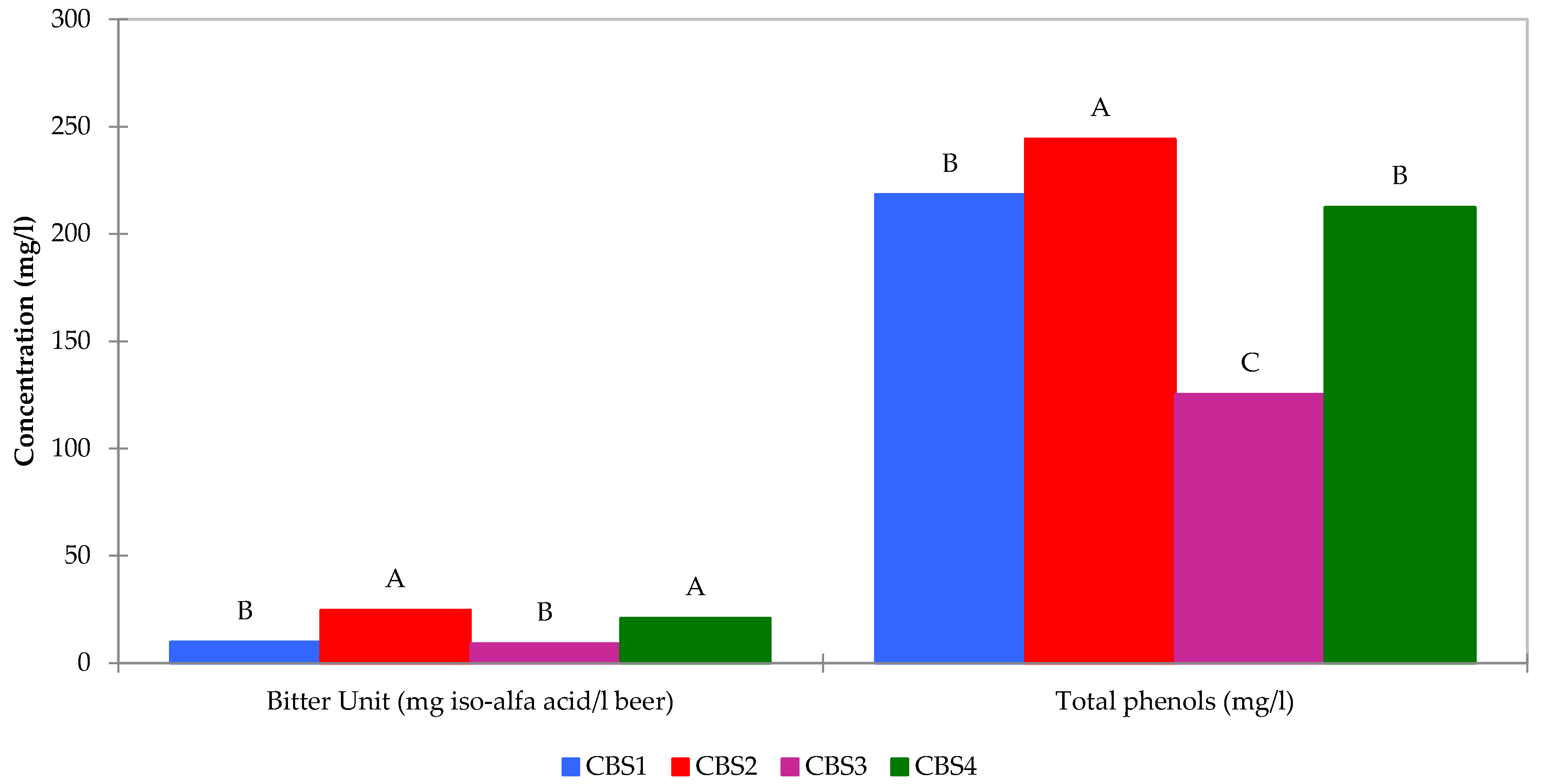
32]. Total phenols and bitter units were also determined for these beers, integral components of the broader quality control processes in the brewing industry aimed at maintaining consistency in taste and characteristics. Bitter Units (BU) or International Bitterness Units (IBU) serve as a metric for gauging the bitterness or hoppy flavor in beer. This value quantifies the amount of bitter compounds, primarily iso-alpha acids derived from hops in the beer. The measurement and control of both Bitter Units (IBU) and total phenols in beer are crucial for brewers to ensure the desired flavor profile and quality of their products. It plays a pivotal role in discriminating between different styles of beer, contributing to both positive and negative characteristics in terms of flavor and aroma. This comprehensive assessment remains essential for brewers to uphold the distinctive qualities of their beer varieties (
Figure 3
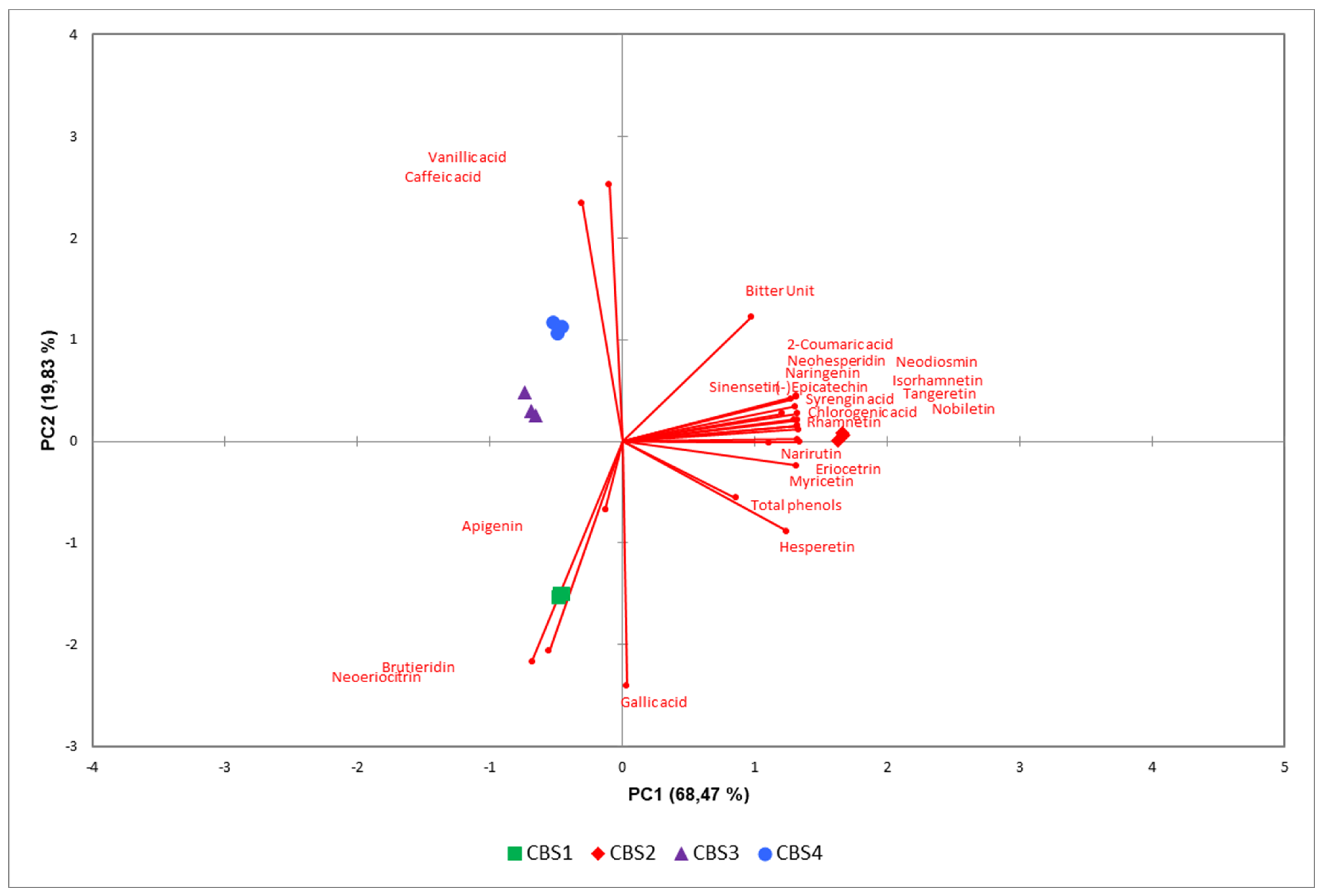
The evaluation of samples aimed to assess whether the style of beer could be effectively classified based on phenolic content. The principal component analysis (PCA) model successfully differentiated between the various beers (
Figure 4). PC1, which accounts for 68.47% of the total variance. Notably, the two IPAs (CBS1 and CBS2) emerged as the most distinct beer styles, clearly separated in a three-dimensional space, as depicted in the figure. Conversely, CBS3 and CBS4 were discriminated but exhibited proximity and occupied a similar region of space. To understand the variables contributing most to the observed patterns, a loading plot was applied. This graphical representation visually depicts the relationships between variables and principal components, facilitating the interpretation of complex datasets. Each polyphenolic compound is represented as a point in a scatter plot, its position determined by its correlation with the underlying components. Apigenin, brutieridin, and neoritrocin closely align with CBS1, showcasing a strong association with this beer (
Figure 4). On the other hand, vanillic acid and caffeic acid distinctly discriminate CBS3 and CBS4. Bitter units, total phenols, and other polyphenolic compounds exhibit a robust association with CBS2 (
Figure 4). Variables positioned farther away denote weaker relationships. Furthermore, the phenolic content in beer is contingent on the types of barley and hops used in production. Despite hops containing a substantial amount of phenols (up to 4% of dry matter) compared to barley (up to 0.1%), it is noteworthy that, on average, four-fifths of the phenols in beer originate from malt or other mashed cereals due to their significantly higher initial content. Our findings revealed variations linked to the diverse malts and cereals employed, indicating that the presence of bergamot peels had no discernible impact on phenolic compound expression. However, it did influence the flavonoid content.
5. Conclusions
In conclusion, this study harnessed advanced analytical techniques, such as UHPLC-PDA, to unveil the distinctive fingerprint of craft beers. We successfully developed and validated a swift, uncomplicated sample preparation method, enabling the simultaneous analysis of diverse phenolic classes and numerous bitter acids in beers. Leveraging the resulting chemical profiles, we meticulously evaluated our method’s efficacy in discriminating the polyphenolic profile of beers with different styles, all flavored with bergamot. Employing statistical methods, we not only discerned variations among beer styles but also traced the compounds responsible for distinguishing these typologies. This not only contributes to quality assurance for confirming beer styles but also advances our understanding of the intricate chemistry influencing beer quality. The significance of this research lies in providing a deeper insight into the nuanced chemistry governing beer quality. The application of a streamlined analytical protocol, requiring equipment that offers extensive information on beer chemistry but remains more accessible than LC-MS in terms of cost and complexity, holds tremendous potential. This approach can deliver valuable insights for both brewers and beer enthusiasts, contributing to the ongoing quest for enhanced beer quality and flavor characterization.
Author Contributions
Conceptualization: Mariateresa Russo, Adele Muscolo; Methodology: Rosa Di Sanzo, Sonia Carabetta and Salvatore Fuda; Software: Rosa Di Sanzo, Sonia Carabetta, Tomas Branyik; Validation: Adele Muscolo, Mariateresa Russo; Formal Analysis: Pietro Andronaco, Fabio Salafia and Francesco Canino; Investigation: Sonia Carabetta, Pietro Andronaco and Rosa Di Sanzo; Writing—original draft preparation: Adele Muscolo; Writing—review and editing: Mariateresa Russo; Project administration: Mariateresa Russo; Funding Acquisition: Mariateresa Russo. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Next Generation EU - Italian NRRP, Mission 4, Component 2, Investment 1.5, call for the creation and strengthening of ‘Innovation Ecosystems’, building ‘Territorial R&D Leaders’ (Directorial Decree n. 2021/3277) - project Tech4You - Technologies for climate change adaptation and quality of life improvement, n. ECS0000009. This work reflects only the authors’ views and opinions, neither the Ministry for University and Research nor the European Commission can be considered responsible for them.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Damerow, P. Sumerian Beer: The Origins of Brewing Technology in Ancient Mesopotamia. Cuneiform Digital Library Journal 2012, 1–20. [Google Scholar]
- Anderson, H. E.; Santos, I. C.; Hildenbrand, Z. L.; Schug, K.A. A review of the analytical methods used for beer ingredient and finished product analysis and quality control. Analytica Chimica Acta 2019, 1085, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Papaziam, C. Beer Styles: Their Origins and Classification Handbook of Brewing. In Handbook of brewing, 2nd ed; Taylor and Francis: London, 2006. [Google Scholar]
- Habschie, K.; Lončarić, A.; Mastanjević, K. Screening of Polyphenols and Antioxidative Activity in Industrial Beers. Foods 2020, 9, 238. [Google Scholar] [CrossRef]
- Aron, P. M.; Shellhammer, T. H. A Discussion of Polyphenols in Beer Physical and Flavour Stability. Journal of the Institute of Brewing 2010, 116, 369–380. [Google Scholar] [CrossRef]
- Habschied, K.; Mastanjević, K. Maintaining the Quality Control of Beer. In Proceedings of the 30th International Conference on Organization and Technology of Maintenance (OTO 2021) Lecture Notes in Networks and Systems; Springer International Publishing. [CrossRef]
- Vanderhaegen Bart, Neven Hedwig, Verachtert Hubert, Derdelinck Guy The chemistry of beer aging – a critical review. Food Chemistry 2006, 95, 357–381.
- Jaskula, B.; Kafarski, P.; Aerts, G.; De Cooman, L. A Kinetic Study on the Isomerization of Hop α-Acids. J. Agric. Food Chem. 2008, 56, 6408–6415. [Google Scholar] [CrossRef] [PubMed]
- Malowicki, M.G.; Shellhammer, T.H. ; Isomerization and Degradation Kinetics of Hop (Humulus lupulus) Acids in a Model Wort-Boiling System. J. Agric. Food Chem. 2005, 53, 4434–4439. [Google Scholar] [CrossRef]
- Hao, J.; Speers, R.A.; Fan, H.; Deng, Y.; Dai, Z. A Review of Cyclic and Oxidative Bitter Derivatives of Alpha, Iso-Alpha and Beta-Hop Acids. Journal of the American Society of Brewing Chemists 2020. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Guido, L. F. A review on the fate of phenolic compounds during malting and brewing: Technological strategies and beer styles. Food Chemistry 2022, 372. [Google Scholar] [CrossRef]
- Fuchimoto, J.; Kojima, T.; Okabayashi, T.; Masaki, T.; Ogasawara, N.; Obata, K.; Nomura, K.; Hirakawa, S.; Kobayashi, N.; Shigyo, T.; Yokota, S.; Fujii, N.; Tsutsumi, H.; Himi, T.; Sawad, N. Humulone suppresses replication of respiratory syncytial virus and release of IL-8 and RANTES in normal human nasal epithelial cells. Med Mol Morphol 2013, 46, 203–209. [Google Scholar] [CrossRef]
- Bohr, G.; Gerhäuser, C.; Knauft, J.; Zapp, J.; Becker, H. Anti-inflammatory Acylphloroglucinol Derivatives from Hops (Humulus lupulus). Nat. Prod. 2005, 68, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Goldberg, D.H.; Gerhard, J. Contributions to the antimicrobial spectrum of hop constituents. Medicinal Plants 2004, 58, 230–238. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Virani, S.; Zavro, M.; Haas, G.J. Inhibition of Streptococcus mutans and Other Oral streptococci by hop (Humulus lupulus L.) constituents. Economic Botany 2003, 57, 118–125. [Google Scholar] [CrossRef]
- Oladokun, O.; Tarrega, A.; James, S.; Smart, K.; Hort, J.; Cook, D. The impact of hop bitter acid and polyphenol profiles on the perceived bitterness of beer. Food Chemistry 2016, 2016. 205, 212–220. [Google Scholar] [CrossRef]
- Benard, M. Determination of Repeatability and Reproducibility of EBC Accepted Methods: V – Beer. Journal of the Institute of Brewing 2000, 106, 3. [Google Scholar] [CrossRef]
- Jurić, A.; Ćorić, N.; Odak, A.; Herceg, Z.; Tišma, M. Analysis of total polyphenols, bitterness and haze in pale and dark lager beers produced under different mashing and boiling conditions. Journal of the Institute of Brewing 2015, 121, 4. [Google Scholar] [CrossRef]
- Piazzon, A.; Forte, M. , Nardini, M. Characterization of Phenolics Content and Antioxidant Activity of Different Beer Types. J. Agric. Food Chem. 2010, 58, 10677–10683. [Google Scholar] [CrossRef] [PubMed]
- 20. Quifer-Rada Paola, Vallverdú-Queralt Anna, Martínez-Huélamo Miriam, Chiva-Blanch Gemma, Jáuregui Olga, Estruch Ramon, Lamuela-Raventós Rosa. A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC–ESI-LTQ-Orbitrap-MS). Food Chemistry 2015, 169, 336–343.
- Dvořáková, M.; Hulín, P.; Karabín, M.; Dostálek, P. Determination of Polyphenols in Beer by an Effective Method Based on Solid-Phase Extraction and High Performance Liquid Chromatography with Diode-Array Detection. Czech J. Food Sci. 2007, 25, 182–188. [Google Scholar] [CrossRef]
- Montanari, L.; Perretti, G.; Natella, F.; Guidi, A.; Fantozzi, P. Organic and Phenolic Acids in Beer. Food Science and Technology 1999, 32, 535–539. [Google Scholar] [CrossRef]
- Mcmurrough, I.; Roche, G. P.; Cleary, K.G. Phenolic Acids in Beers and Worts. Journal of the Institute of Brewing 1984, 90, 3. [Google Scholar] [CrossRef]
- Garcia-Sanchez, F.; Carnero, C.; Heredia, A. Fluorometric determination of p-coumaric acid in beer. Agric. Food Chem. 1988, 36, 80–82. [Google Scholar] [CrossRef]
- McMurrough, I.; Madigan, D.; Donnelly, D.; Hurley, J.; Doyle, A.; Hennigan, G.; McNulty, N.; Smyth, R. M. , Control of Ferulic Acid and 4-Vinyl Guaiacol in Brewing. J. Insl.Brew 1996, 102, 327–332. [Google Scholar] [CrossRef]
- Andersen, M.L.; Skibsted, L.H. Modification of the Levels of Polyphenols in Wort and Beer by Addition of Hexamethylenetetramine or Sulfite during Mashing. J. Agric. Food Chem. 2001, 49, 5232–5237. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P. J.; Guido, L.F.; Cruz, J.M.; Barros, A.A. Analysis of xanthohumol and isoxanthohumol in different hop products by liquid chromatography-diode array detection-electrospray ionization tandem mass spectrometry. Journal of Chromatography A 2007, 1150, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Jaskula, B.; Goiris, K.; De Rouck, G.; Aerts, G.; De Cooman, L. Enhanced Quantitative Extraction and HPLC Determination of Hop and Beer Bitter Acids. Journal of the Institute of Brewing 2007, 13, 381–390. [Google Scholar] [CrossRef]
- Baker, G.A.; Danenhower, T. M.; Force, L. J.; Petersen, K. J. , Betts, T.A. HPLC Analysis of α- and β-Acids in Hops. J. Chem. Educ. 2008, 85, 954. [Google Scholar] [CrossRef]
- Oladokun, O.; Smart, K.; Cook, D. An improved HPLC method for single-run analysis of the spectrum of hop bittering compounds usually encountered in beers. Journal of the Institute of Brewing 2016, 122, 11–20. [Google Scholar] [CrossRef]
- Kaom, T.H.; Wu, G.Y. ; Simultaneous determination of prenylflavonoid and hop bitter acid in beer lee by HPLC-DAD-MS. Food Chemistry 2013, 141, 1218–1226. [Google Scholar]
- Anderson, H. E.; Liden, T.; Berger, B.K.; Schu, K. A. Target profiling of beer styles by their iso-α-acid and phenolic content using liquid chromatography–quadrupole time-of-flight–mass spectrometry. Journal of Separation Science 2021, 44, 2764–2772. [Google Scholar] [CrossRef]
- Simon, M.; Collin, S. Why Oxidation Should Be Still More Feared in NABLABs: Fate of Polyphenols and Bitter Compounds. Beverages 2022, 8, 61. [Google Scholar] [CrossRef]
- Quifer-Rada, P.; Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R. A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC–ESI-LTQ-Orbitrap-MS). Food Chemistry 2015, 169, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Floridi, S.; Montanari, L.; Marconi, O.; Fantozzi, P. Determination of free phenolic acids in wort and beer by coulometric array detection. Journal of Agriculture and Food Chemistry 2003, 51, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- García, A.A.; Grande, B.C.; Gádara, J.S. Development of rapid method based on solid-phase extraction and liquid chromatography with ultraviolet absorbance detection for determination of polyphenols in alcohol-free beers. Journal of Chromatography A 2004, 1054, 175–180. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández, O.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Rocchetti, G.; Lorenzo, J. M. Determination of Polyphenols Using Liquid Chromatography–Tandem Mass Spectrometry Technique (LC–MS/MS): A Review. Antioxidants 2020, 9, 479. [Google Scholar] [CrossRef] [PubMed]
- Oladokun, O.; Tarrega, A.; James, S.; Smart, K.; Hort, J.; Cook, D. The impact of hop bitter acid and polyphenol profiles on the perceived bitterness of beer. Food Chemistry 2016, 205, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Ghiselli, A. Determination of free and bound phenolic acids in beer. Food Chemistry 2004, 84, 137–143. [Google Scholar] [CrossRef]
- Gosetti, F.; Mazzucco, E.; Zampieri, D.; Gennaro, M. C. Signal suppression/enhancement in high-performance liquid chromatography tandem mass spectrometry. Journal of Chromatography A 2010, 1217, 3929–3937. [Google Scholar] [CrossRef]
- Trufelli, H.; Palma, P.; Famiglini, G.; Cappiello, A. An overview of matrix effects in liquid chromatography–mass spectrometry. Mass Spectrometry Reviews 2011, 30, 3. [Google Scholar] [CrossRef]
- Arena, K.; Cacciola, F.; Rigano, F.; Dugo, P.; Mondello, L. Evaluation of matrix effect in one-dimensional and comprehensive two-dimensional liquid chromatography for the determination of the phenolic fraction in extra virgin olive oils. Journal of Separation Science 2020, 43, 1781–1789. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).