Introduction
Hypertension (HTN) is a very common disease worldwide and a strong risk factor for cardiovascular diseases and a plethora of conditions, including atrial fibrillation (AF), perioperative ischemia, coronary artery disease (CAD), rehospitalization, kidney disease, valvular diseases, acute aortic syndromes (like aortic dissection, intramural hematoma, or aortic ulcer) [
1]. Although the cutoff criteria for hypertension vary between the American and European guidelines, there is substantial similarity in most recommendations for the management of hypertension and its complications [
2,
3,
4]. Among hypertension-derived complications, hypertensive heart disease (HHD) has been widely recognised and refers to disturbed cardiac structure and function, affecting the left ventricle (LV), left atrium (LA), and coronary arteries as a result of prolonged exposition to high blood pressure (BP) [
5]. Nevertheless, there is no universal consensus on the definition of HHD and therefore, HHD diagnosis is based on the clinical history, and the imaging modalities, like echocardiography and functional or anatomical tests for ischemia, to identify all the possible changes in the myocardium and coronary arteries.
From the pathophysiological perspective, the unmanaged high BP induces high afterload and high LV filling pressures leading to structural changes such as left ventricular hypertrophy (LVH) and fibrosis, and LA enlargement [
6]. Besides this, HHD relates to systolic and diastolic LV dysfunction or a combination of them. HTN stands out as a primary risk factor for heart failure (HF) development. Recent evidence suggests the co-existence of HTN in 76% of newly diagnosed HF cases [
7], and individuals with HTN have an almost twofold higher lifetime risk of HF development compared to those with normal BP [
8]. HTN stands out as the predominant and highly impactful morbidity in heart failure with preserved ejection fraction (HFpEF), with a prevalence of 80% in the Get with the Guidelines (GWTG) initiative. The most formidable challenge in the diagnosis and management of HfpEF, lies within its high incidence, affecting high percentage of individuals [
9,
10]. Moreover, numerous epidemiological studies have unveiled the link between HTN and coronary artery disease (CAD), a significant HF risk factor
3. Individuals with HHD face an elevated risk of developing CAD affecting both large and small arteries (microvascular disease). Notably, the INTERHEART study demonstrated that 25% of the population-attributable risk for a myocardial infarction can be attributed to HTN [
11].
Despite the interplay between HTN and CAD, one of the most frequent challenges in clinical practice is to diagnose HHD in patients without underlying CAD. Echocardiography is a easily performed, cheap, repeatable and accessible modality to estimate the cardiac structure and function [
12]. During the working-up diagnosis of HHD, several classic echocardiographic indices (e.g. LVH) have been proposed. Besides this, novel echocardiographic parameters (e.g. speckle tracking) may be useful to identify cardiac changes due to HHD at an early stage. A prompt therapy may inhibit cardiac remodeling and attenuate cardiovascular risk [
13].
The purpose of this paper is to provide an overview of the classic and novel echocardiographic findings and their role in the diagnosis and prognosis HHD without CAD. An objective knowledge of the potentiality and challenges of this imaging modality might promptly detect HHD, prevent its progression to HF or even reverse the HF.
Pathophysiology
Chronic arterial HTN trigger pathological processes, leading to structural and functional disturbances in the myocardium, like hypertrophy, fibrosis, and ischemia [
14,
15]. Chronic or sustained arterial HTN, leads to pressure overload of the left cardiac cavities, induce change in gene expression and confers simultaneous hypertrophy of the myocyte and changes in the extracellular matrix. Structurally, the LV walls become thicker, with or without simultaneous increase in absolute myocardial mass, as a compensatory mechanism to pressure overload. Depending on whether there is LV dilation, four patterns of hypertrophy are distinguished: eccentric non-dilated, eccentric dilated, concentric non-dilated, and concentric dilated [
16]. Non-hemodynamic factors, such as the renin-angiotensin-aldosterone system, demographic factors (gender and ethnicity), obesity, and genetics, play a significant role in myocardial thickening. It is worth noting that it is not fully understood why patients with HHD develop a specific LVH pattern.
Another integral element of HHD is the myocardial fibrosis [
17], either interstitial (reactive, diffuse) or replacement (reparative). Myocardial reactive fibrosis involves the accumulation of fibrous connective tissue in the interstitial and perivascular spaces without a significant loss of myocardial cells. Replacement fibrosis, on the other hand, consists of scar tissue formed due to the loss of myocardial cells and their replacement by connective tissue following myocardial infarction or myocardial death from other causes [
18]. It is still unclear whether those two types of myocardial fibrosis represent distinct processes, as they may occur simultaneously [
6]. Hemodynamic factors, such as chronic pressure overload causing myocardial stress or injury, lead to increased collagen production (type I, type III) in the myocardium as a reparative mechanism. Non-hemodynamic factors, such as the renin-angiotensin-aldosterone system, also play a significant role in myocardial fibrosis [
6].
HHD eventually may progress towards HF, where the initial myocardial hypertrophy and fibrosis result in myocardium stiffening and systolic and diastolic LV dysfunction. Eccentric hypertrophy is more likely to lead to HF with reduced ejection fraction (HFrEF), while concentric hypertrophy may lead to HF with preserved ejection fraction (HFpEF). Additionally, changes in the LA morphology begin in the early stages of HHD despite the normal LV [
18]. In particular, microstructural changes precede macroscopic alterations (LA dilatation), resulting in a functionally impaired atrium despite its normal size. LA remodeling in HHD is once again the result of both hemodynamic factors (increased afterload) and non-hemodynamic factors (neurohormonal activation) [
19]. The increased LV afterload leads to elevated filling pressures within the left atrium, consequently increasing its wall tension. As an adaptation, LA may reshape affecting first its contractility. Non-hemodynamic factors, such as the activation of renin-angiotensin-aldosterone system, the release of natriuretic peptides, and endothelin-1, also play a significant role. Those factors trigger inflammatory cells accumulation, leading to fibrosis and further remodeling of the LA. Additionally, there is an increase in sympathetic nervous system activation due to neurohormonal activation. In the context of HHD, the involvement of norepinephrine plays a pivotal role in the pathophysiological cascade. The sequence begins with increased sympathetic nerve activity, particularly in young hypertensive individuals, leading to elevated heart rate, cardiac output, renal vascular resistance, and blood pressure. Norepinephrine, along with other humoral factors like angiotensin II, contributes to a hemodynamic profile that induces adaptive changes in cardiac structure, ultimately culminating in hypertensive left ventricular (LV) hypertrophy. The significance of norepinephrine becomes apparent as its high levels or interactions with humoral factors potentiate structural changes in the heart [
20].
Despite the acknowledged importance of norepinephrine in the development of LV hypertrophy, there exists a notable discrepancy in the effects of antiadrenergic drugs on regression. While α1-adrenergic blockers, such as doxazosin, demonstrate favorable effects on reversing LV hypertrophy, the clinical consequences may be not always beneficial with reducing LA mass, as evidenced by an increased risk of HF incidence during treatment [
21].
AF is frequently observed not only in patients with dilated LA, but also when atrial fibrosis is present. Speckle tracking and cardiac magnetic resonance have clarified this point [
22]. It is worth mentioning that several mechanisms have been proposed to explain how systemic HTN leads to remodeling of the right ventricle (RV), and fewer have been proposed for the right atrium (RA). However, the whole process is not yet fully understood. The same pathophysiological steps present in the left cavities are involved in the right cavities as well: hypertrophy of myocardial cells, fibrosis, and alterations in the composition of the extracellular matrix. The RV myocardium becomes thicker, dilated, accompanied by RA dysfunction [
22]. Finally, persistent HTN leads to arteriosclerosis of the vessels in circulation, as well as microvascular rarefaction. The co-existence of hypertrophic myocardium and arterial stiffness increases the LV afterload and precipitates the LV remodeling affecting the overall systolic and diastolic function of the heart.
Classical Echocardiographic Indices
Echocardiography plays a pivotal role in identifying structural changes in individuals with HTN, leading to the development of HHD.
Table 1 summarizes the challenges and the prognostic value of proposed parameters.
Left Ventricular Hypertrophy
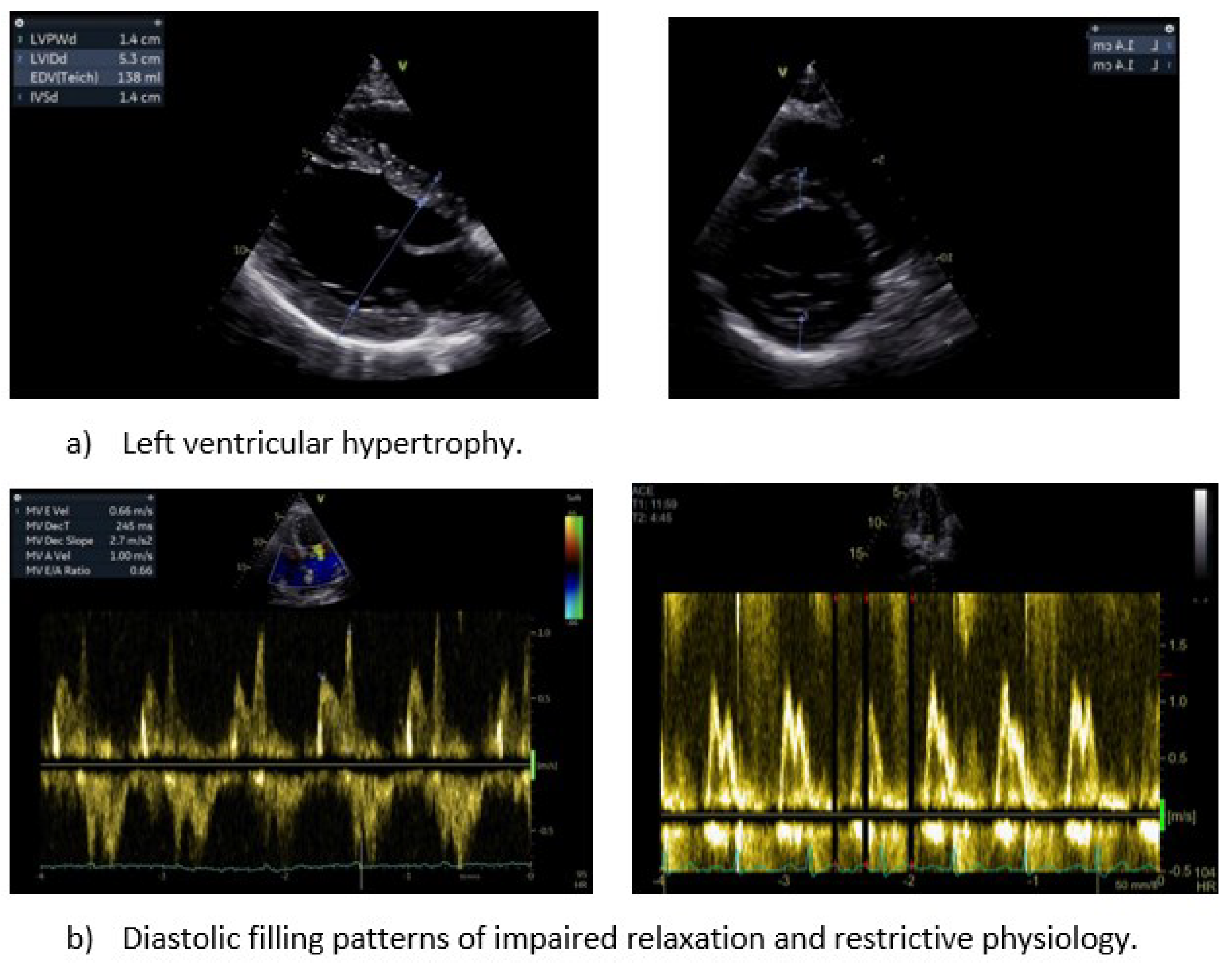
LVH is a common manifestation of HDD and defined as LV thickening (≥12mm) or increased LV mass [
23]. LVH has been long used as a risk factor of individuals without known cardiovascular disease. Hence, its detection stratifies the cardiovascular risk of patients with and without HHD and has considerable impact on patient’s management and prognosis. Both LV thickening and increased LV mass rely on linear measurements of LV dimensions on 2-dimensional (2D) images, while LV mass calculation uses geometric assumptions regarding the LV shape [
24]. The echocardiography image quality is of paramount importance for measurements. Moreover, there is a high intra-observer and inter-observer variability even with the high image resolution 2D echocardiography. The presence of moderator band, different sites for dimension measurements in parasternal long-axis (PLAX) and parasternal short-axis (PSAX) views and the miscalculation of wall thickness at the papillary muscles origin may attenuate the reliability of measurements. Thus, the 2D echocardiographic assessment of LV mass has significant drawbacks and tends to overestimate LV mass in comparison to cardiac magnetic resonance (CMR) imaging [
25]. This is the case when image quality is suboptimal. The recent advances in 3D echocardiography may compensate the abovementioned limitations, with less overestimation of LV mass than CMR [
26]. However, an optimal 2D image quality is a prerequisite for valid 3D echocardiography, while it is difficult to detect changes in LV mass along time. Therefore, a technically perfect echocardiographic examination from an experienced operator is deemed necessary.
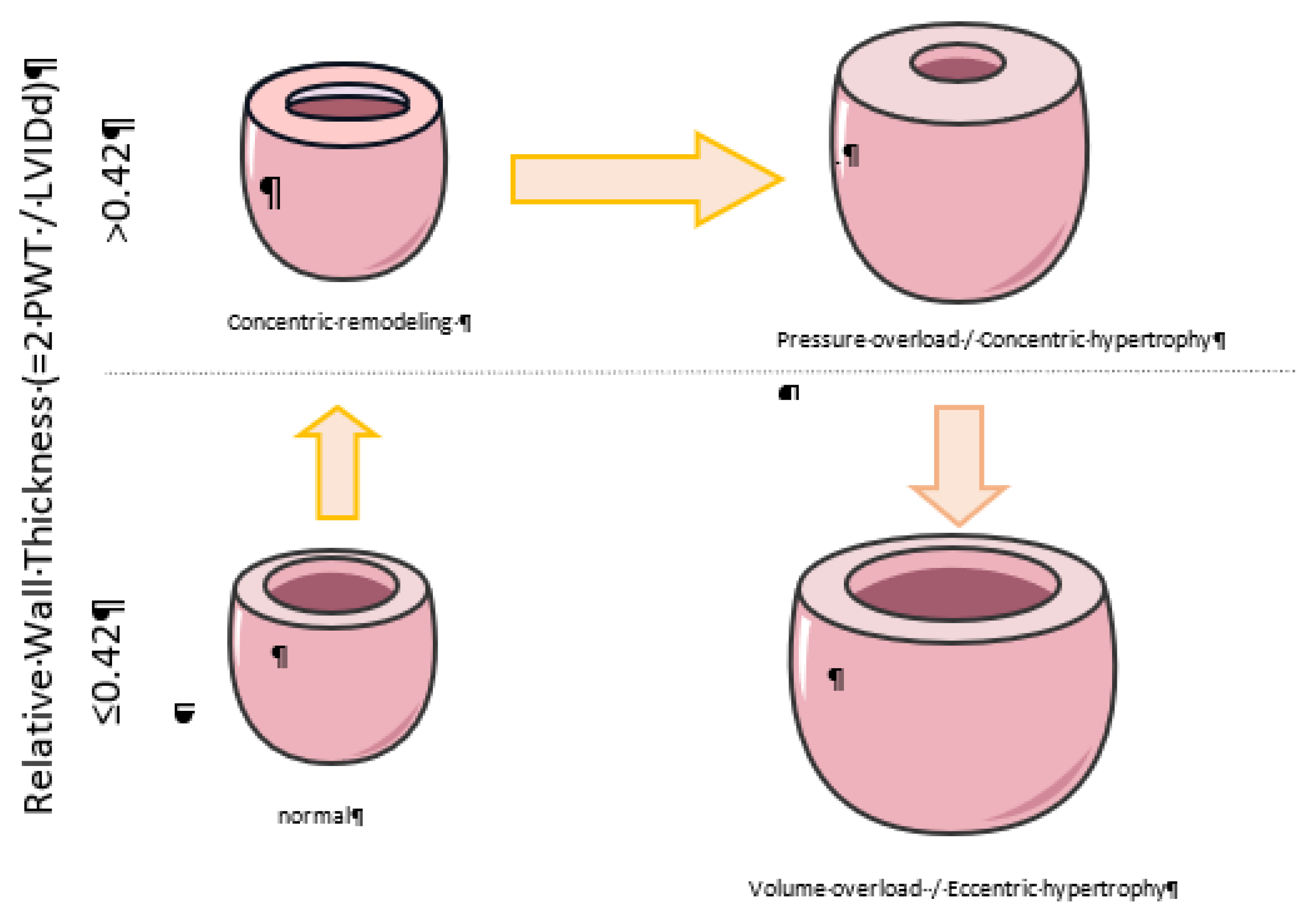
Three main patterns of LVH have been first described: (a) concentric hypertrophy, (b) eccentric hypertrophy, and (c) concentric remodeling
6. The first two, concentric and eccentric LVH, are the two main patterns of LVH. That classification is based on three parameters with their cut-off values: 1) the relative wall thickness (RWT ≥ 0.42), 2) the left ventricular mass index [LVMI≥95 g/m2 (female) and ≥115 g/m2 (male)], 3) end-diastolic diameter [LVEDD ≥ 5.3 cm (female) and ≥ 5.8 cm (male)] in hypertensive individuals, enhancing clinical understanding, and guiding appropriate interventions. By adding the end-diastolic volume refines the classification of LVH in four distinct patterns: eccentric non-dilated, eccentric dilated, concentric non-dilated, and concentric dilated hypertrophy (
Figure 1)
Since LV mass relates to body size, indexing values to body surface area (BSA) has been used in population-based studies for reference values. The LV mass indexed by BSA underestimates the prevalence of LVH in obese patients [
27]. An alternative to traditional BSA indexing is using height alone for adjusting cardiac parameter measurements, particularly relevant in HHD assessments. This method aims to provide a more nuanced evaluation by considering an individual's height as the sole determinant, addressing variations in body size not fully captured by BSA. Height indexing enhances precision in evaluating cardiac structure and function, providing a more individualized assessment of dimensions. Utilizing height as a reference in obese patients acknowledges the significance of differentiating between overall body size and its composition. This approach enhances the ability to gain a more accurate understanding of cardiac health within this specific population [
28,
29]. The cutoff values, based on height alone, for left ventricular mass index are established at equal to or greater than 45 g/m².7 in females and equal to or greater than 49 g/m².7 in males.
The prognostic value of LVH is unambiguous. The pattern of increased LV mass has an added value to LVH in general and hypertensive population [
30,
31]. Since 1990s, it has been demonstrated that LVH is a strong factor for cardiovascular mortality [
32]. 10 years follow-up of hypertensive patients have outlined the prognostic power of LVH and ventricular arrhythmias [
33]. An indexed LV mass has better prognostic ability and may be preferred instead of absolute values of LVH. However, it is subject of debate to use either BSA or height for LV mass indexing. Another proof of the prognostic impact of LVH derives from studies using anti-hypertensive medications. The targeted LVH regression has been accompanied with cardiovascular benefits [
34].
Left Ventricular Systolic Function
The conventional assessment of left ventricular systolic function relies on metrics like LV ejection fraction (LVEF) and endocardial fractional shortening (FS). However, their application, primarily at the endocardial surface, sparks concern about their relevance in the context of LVH. It becomes apparent that these measurements may tend to overstate LV systolic function. This phenomenon can be ascribed to cross-fiber shortening, where myocardial fibers not only shorten along their direction but also perpendicular to it [
35]. This unique dual direction shortening allows the LV wall to contract in multiple dimensions, resulting in considerable thickening despite reduced shortening in specific myocardial segments, particularly evident in hypertrophic left ventricles. Interestingly, the impact of cross-fiber shortening diminishes in the myocardial midwall [
36].
A rising embraced metric is to enter stress-corrected midwall fractional shortening. This can provide a more nuanced reflection of systolic function, particularly beneficial in cases of LVH. Unlike left ventricular endocardial FS, this parameter paints a more accurate picture [
37]. The LV systolic function assessment may face challenges, especially in patients with inadequate quality of acoustic windows, like obese individuals or those with chronic obstructive pulmonary disease. Unfortunately, overweight or obesity are common predisposition factors of HHD, preventing the echocardiographic early diagnosis. Moreover, regional wall motion abnormalities is another obstacle faced in systolic function variation. Measurements taken from specific views of the left ventricle (parasternal axis) do not align overall ventricular function. Instead of them, Simpson biplane technique is the most reliable, but it is still based on geometrical assumptions and faces important limitations. In particular, its accuracy is compromised with off-axis or foreshortened imaging planes. In the latter case, an obscured true apex may introduce potential errors, especially when wMA are present (e.g. apical infarcts) [
38]. All echocardiographic methods rely on good acoustic windows for clear delineation of the blood/endocardial border, vital for accurate measurement/tracing. Large studies have reported the failure of the modified Simpson method to determine LVEF in a significant proportion of participants (31–38%) due to poor image quality, blurred visualization of endocardial borders leading to potential inaccuracies. The alternative administration of contrast agents has proven effective in improving LVEF determination in patients with challenging acoustic windows [
39].
Additionally, in terms of accuracy, studies have consistently shown that echocardiography tends to underestimate the LVEF when compared to the more advanced imaging capabilities of CMR [
40]. A reduced LVEF, or even a low normal LVEF has been long associated with poor prognosis in HHD, which outlines the necessity to get accurate measurements and intensive therapy. In the context of HFrEF, the prognosis is reduced proportionally to the degree of LV systolic impairment [
41].
Left Atrium Dilatation
In the context of HHD, LA enlargement signifies an early and prevalent structural change. Interestingly, the specific geometric pattern of the LVH does not affect the size of the LA in the initial stages of essential hypertension, whether expressed in terms of diameter or volume. What emerges as a critical determinant of LA enlargement, independent of potential confounding factors, is the left ventricular mass index (LVMI) [
42]. The greatest anterior-posterior dimension at the level of the aortic root of LA has long been discussed as an unambiguous index of LA enlargement. To quantify LA volume, methods such as area-length or modified Simpson's are employed, typically normalized for BSA and presented as mL/m² (left atrial volume index - LAVI). The normal range of LAVI extends up to 34 mL/m².
A notable challenge in evaluating the LA stems from the potential inaccuracies in echocardiographic measurements. LA anterior-posterior diameter can be obtained from the PLAX or PSAX views and it is highly dependent on perpendicularity of axis. Suboptimal views, angled views or bad visualization of the posterior wall may lead to miscalculation of LA dimension. The assessment of LAVI necessitates the employment of two orthogonal echocardiographic views (apical 4 chamber and apical 2 chamber view). To refine the LA length, align it perpendicular to the midpoint between the mitral annular plane and the midpoint of the LA superior wall. When appropriately captured, the LA length should exhibit minimal variation, ideally not exceeding 5 mm, between the 4-chamber and 2-chamber views [
43]. Ensuring the avoidance of foreshortening in the long axis of the LA is critical for maintaining precision in the evaluation process [
44]. Accurate assessment of the LA requires calculating the maximum LA area and ensure optimal evaluation of all LA walls. It is equally imperative to carefully navigate around the confluence of pulmonary veins and the left atrial appendage during this process which may influence the final measurements [
45].
Comparative studies between Transthoracic echocardiography (TTE) and CMR have consistently demonstrated the underestimation of both LA and RA volumes in TTE. The disparity, statistically significant in mean values, was not attributed to interobserver differences, clinical events, or image quality. These findings are in agreement with previous studies, revealing CMR's tendency to overestimate atrial volumes. Discrepancies between imaging modalities may stem from poor endocardial definition and foreshortening in echocardiography, particularly evident in standard apical views, impacting the lateral atrial walls. Less accurate delineation of pulmonary veins and appendices may be the case of echocardiography, as highlighted by area discrepancies being more frequent than length discrepancies [
46].
Notably, the LAVI has been related to mortality risk, regardless of the left ventricular geometry, in a substantial cohort of patients exhibiting preserved LV systolic function [
47,
48]. LA enlargement has been considered as a bad prognostic factor in patients with HHD and HFrEF [
49]. On the other hand, it may be a consequence of HFpEF which interplays with AF occurrence and other co-morbidities [
50]. Recent studies indicate that measuring minimal left atrial volume (LAVmin) at LV end-diastole, when the left atrium is directly exposed to LV end-diastolic pressure, may offer a closer correlation to LV filling pressure and clinical outcomes compared to maximal left atrial volume (LAVmax) [
51,
52].
This suggests that LAVmin could be a more effective marker for LA structural remodeling. It's noteworthy that while the prognostic value of LAVmax in patients with HFpEF is contentious, there is limited data on the prognostic value of LAVmin in HFpEF patients, necessitating further research [
53,
54,
55,
56]. The adaptation to BSA leads to the calculation of the LAVmin index (LAVImin), whose power to predict HF hospitalization appears to be notably stronger for individuals without a history of AF compared to those with AF, especially when juxtaposed with LAVImax
58.
Diastolic Dysfunction
Mitral inflow measurements play a crucial role in assessing diastolic function. Parameters such as, E and A velocity, their ratio (E/A), the deceleration time of E velocity, and the isovolumic relaxation time provide valuable insights. Notably, in hypertensive individuals, a normal in-treatment transmitral flow pattern serves as an indicator of a lower risk for HF, irrespective of BP levels. Although the implementation of antihypertensive therapy in patients with LVH improves mitral inflow patterns, this has not been correlated with a reduction in cardiovascular morbidity and mortality [
57]. On the other hand, high E/A ratio has been associated with grade III diastolic dysfunction indicating high LV filling pressures and poor prognosis, especially when HF has been already developed [
58,
59].
The main limitation of echocardiography in diastolic dysfunction assessment is the clustering of numerous parameters and the inaccuracy in blood flow calculation. The proper alignment of the ultrasound beam is pivotal, given that Doppler tends to significantly underestimate flow velocities when the angle of interrogation exceeds 30°. To counteract the impact of transducer angulation, it is imperative to align the Doppler sample as parallel as possible with blood flow, ensuring optimal waveform morphology and maximal diastolic flow velocities. Color flow echocardiography may guide the alignment of sample volume parallel to flow [
60]. The placement of pulsed-Doppler sample at the tips of the mitral leaflets (4 chamber view) is a prerequisite for the estimation of the left atrioventricular gradient, which may be miscalculated if it is placed at the mitral valve annulus level. Using the lowest filter setting is advised to capture the full velocity profile [
61].
The pulsed Tissue Doppler-derived E′ velocity of the mitral annulus is an essential component in the evaluation of cardiac diastolic function. A decline in septal (<7cm/s) and lateral e' (<10cm/s) and the increased average ratio (E/e’ ≥14) signal compromised LV relaxation, albeit with certain limitations, like limited mitral annulus movement, calcification, or prosthetic valves (aortic or mitral). It is worth emphasizing the predictive capability of the E/E′ ratio for primary cardiac events in a hypertensive population without established cardiac disease [
62]. The additional assessment of late (atrial) diastolic velocity (A′), may be influenced by LA function and LV end-diastolic pressure, but its diagnostic and prognostic values have not been established.
The evaluation of pulmonary venous flow pattern, when obtainable, has been implicated as an independent diagnostic criterion and a valid predictor of CVD events in essential hypertension. A high S/D ratio per se is independently associated with an increased cardiovascular disease risk in hypertensive patients [
63]. Furthermore, the concomitant high pulmonary venous systolic-to-diastolic wave ratio (S/D, normal values: male < 1.51, female < 1.66) and a low E/A ratio exerts predictive value in HHD
65. In TTE S, D flow data are usually acquired from the orifice of the right upper pulmonary vein in the apical 4-chamber view. Color flow Doppler should be employed to accurately place the sample volume 1 to 2 cm into the pulmonary vein, acknowledging that the far-field nature of the structure may limit the quality of the recording [
64]. Overall, the echocardiographic assessment of diastolic function has several limitations, including dependence on number of Doppler measurements, potential interobserver variability and acoustic window challenges in hypertensive population. In contrast, CMR has the advantage of precise volumetric measurements, tissue velocity mapping, independent of acoustic window quality [
65].
Figure 2.
Classical echocardiographic indices of hypertensive heart disease.
Figure 2.
Classical echocardiographic indices of hypertensive heart disease.
RV Systolic Function
RV systolic dysfunction is not only a recognized marker of adverse prognosis in HF but is also linked to poor prognosis in HHD [
66]. All the following parameters have been associated with negative prognosis in the whole spectrum of cardiac diseases. On the other hand, RV measurements are usually influenced by volume load or are based on geometrical assumptions which attenuate the accuracy of RV systolic function estimation.
Echocardiographic evaluation of the RV systolic function in HHD involves a comprehensive assessment of various parameters of the right cavities: tricuspid annular plane systolic excursion (TAPSE), systolic velocity of the tissue doppler of the tricuspid valve (TV) annulus, right ventricular ejection fraction (RVEF), Fractional Area Change (FAC), pulmonary artery systolic pressure (PASP), size of RV ventricle, right ventricular systolic pressure (RVSP). A comprehensive echocardiographic assessment and early detection of right cavities dysfunction may contribute to risk stratification and tailored clinical management of individuals with HHD [
67].
The TAPSE method stands out for its simplicity and reproducibility, but it’s susceptible to variations in load and angle. This technique involves measuring edge-to-edge the excursion of tricuspid annulus during systole with a swift sweep speed of ≤100 mm/s. In cases of severe tricuspid regurgitation, TAPSE may exhibit pseudo-normalization due to volume overloading [
68]. In parallel, the tissue Doppler RV systolic wave velocity may assist to assess the longitudinal function of the basal RV free wall, lacking a comprehensive evaluation of global RV function. Similar to TAPSE, it's also angle- and load-dependent, requiring precise alignment of the ultrasound beam with the lateral TV annulus, essentially capturing only the longitudinal function of the RV base [
69].
The assessment of RV size and systolic function through conventional echocardiography should be conducted in all patients with HHD, considering RV loading conditions. It is crucial to employ a multi-parametric approach and utilize various echocardiographic views to ensure accurate evaluation, especially when there is a discrepancy between different echocardiographic parameters [
70]. This comprehensive approach enhances the precision and reliability of the interpretation of findings related to RV size and function.
HFpEF is acknowledged as a contributor to pulmonary hypertension (PH) [
71]. The estimation of PH relies on the detection of the tricuspid regurgitant jet, to calculate the RA-RV gradient and leads to the calculation of the right ventricle systolic pressure (RVSP) through the simplified Bernoulli equation [
72]. However, if tricuspid regurgitation is absent, as is occasionally encountered, the assessment of RVSP becomes challenging through conventional echocardiography. It's crucial to acknowledge that RVSP estimation is subject to assumptions, such as the absence of right ventricular outflow tract obstruction and accurate assessment of right atrial pressure [
73]. The co-existence of RV systolic dysfunction and PH leads to underestimation of RVSP due to 2 important limitations: the arbitrary set of RA pressure (RAP) levels and the calculation of trans-tricuspid pressure gradient which is usually severely attenuated in severe TR. Despite the great advantage of echocardiography to easily estimate the RVSP in a non-invasive way, this remains an indirect measurement of pulmonary pressures providing a likelihood of PH and not a firmed diagnosis. A detailed assessment often necessitates additional testing, particularly right heart catheterization, to provide more accurate and comprehensive insights into pulmonary hemodynamics [
74].
Calculation of the RVEF and FAC is based on assumptions as well and demands meticulous manual tracings of the RV endocardial edge at both end-systole and end-diastole, and it should include papillary muscles, trabeculations, and the moderator band [
75]. 3D echocardiography has emerged as a solution to the inherent limitations of 2D echocardiography. It surpasses these constraints in RVEF calculation, and it is considered the gold standard for global RV function evaluation. Notably, the 3D calculation of RVEF closely aligns to CMR relative measurements. It's crucial to know that accurate 3D echocardiography is strongly dependent on high-quality imaging, invariable heart rates, specialized and expensive software, and demands substantial time and expertise [
76].
Stress Echocardiography
The interplay between HF with preserved Ejection Fraction (HFpEF) and HHD is complex. Both are characterized by exertional dyspnea and effort intolerance due to elevated LV filling pressure [
77]. HFpEF may be not clinically apparent, posing challenges to diagnosis based solely on symptoms and standard evaluations. Diastolic dysfunction is the first manifestation of this pathological process, discernible in early, mild arterial hypertension even before the development of LVH. HFpEF is not uncommon among patients with HHD, but surprisingly hypertensive medications do not considerably alleviate symptoms or alter prognosis. Up to now the diagnosis of HFpEF is challenging and most recently diastolic stress echocardiography (DSTE) has been proposed in cases where diastolic dysfunction at rest is mild (grade I) [
78]. On top of the resting echocardiography parameters and natriuretic peptides levels, an abnormal response during exercise test is defined as E/e′ >15 and an increase in TRVmax>3.4m/s. According to the consensus statement, DSTE may be useful to confirm or reject HFpEF diagnosis in patients at intermediate risk. However, recent questions have arisen about the reliability and technical feasibility of using this parameter during physical activity [
79]. The differentiation of dyspnea among elderly requires a non-invasive test for HFpEF diagnosis, as patients without exercise-related diastolic dysfunction may not benefit from specific treatments [
80]. Despite DSTE's potential, the absence of diagnostic and prognostic validation, the limited expertise capacity and the required equipment restrict its widespread use [
81].
A cardiopulmonary exercise test (CPET) evaluates the cardiopulmonary system by observing how the cardiovascular and respiratory systems respond to the energy demands of muscle contraction during physical exercise. The choice of exercise modalities, such as a bicycle ergometer or treadmill, and specific protocols is personalized based on factors such as the patient's fitness level, health, weight, age, under the guidance of the requesting physician [
82].
The combined exercise stress echocardiography (ESE) and cardiopulmonary exercise test (CPET) constitute a diagnostic approach aiming to unmask HFpEF in patients with hypertension. It's noteworthy that physical exercise has the capacity to stress cardiopulmonary homeostasis and reveal pathological hemodynamic changes that may not be apparent at rest. In addition to the aforementioned changes during DSTE, lower peak values of oxygen consumption (VO2) and end-tidal carbon dioxide (PetCO2), along with a higher ventilatory equivalent for carbon dioxide (VE/VCO2) slope, are independent predictors of masked HFpEF [
83,
84]
. This comprehensive diagnostic approach enables timely intervention and personalized management strategies [
85,
86]. Notably, CPET-ESE has recently demonstrated additional predictive value in patients with subclinical HF compared to the two techniques used separately [
87]. Among its limitations, CPET-ESE is a more time-intensive and expensive procedure, requiring specialized equipment and personnel. Careful patient selection is critical, guided by clinical judgment. Therefore, hypertensive individuals exhibiting symptoms and signs indicative of HFpEF, should initially undergo a comprehensive evaluation that encompasses standard rest echocardiography and the measurement of natriuretic peptides and then if it is medically deemed necessary further exercise testing [
88]. Overall, the diagnosis of HFpEF in the context of HHD may require multimodality imaging approach to unmask symptoms and detect hemodynamic changes during exertion associated with elevated LV filling pressures [
89].
Novel Echocardiographic Indices
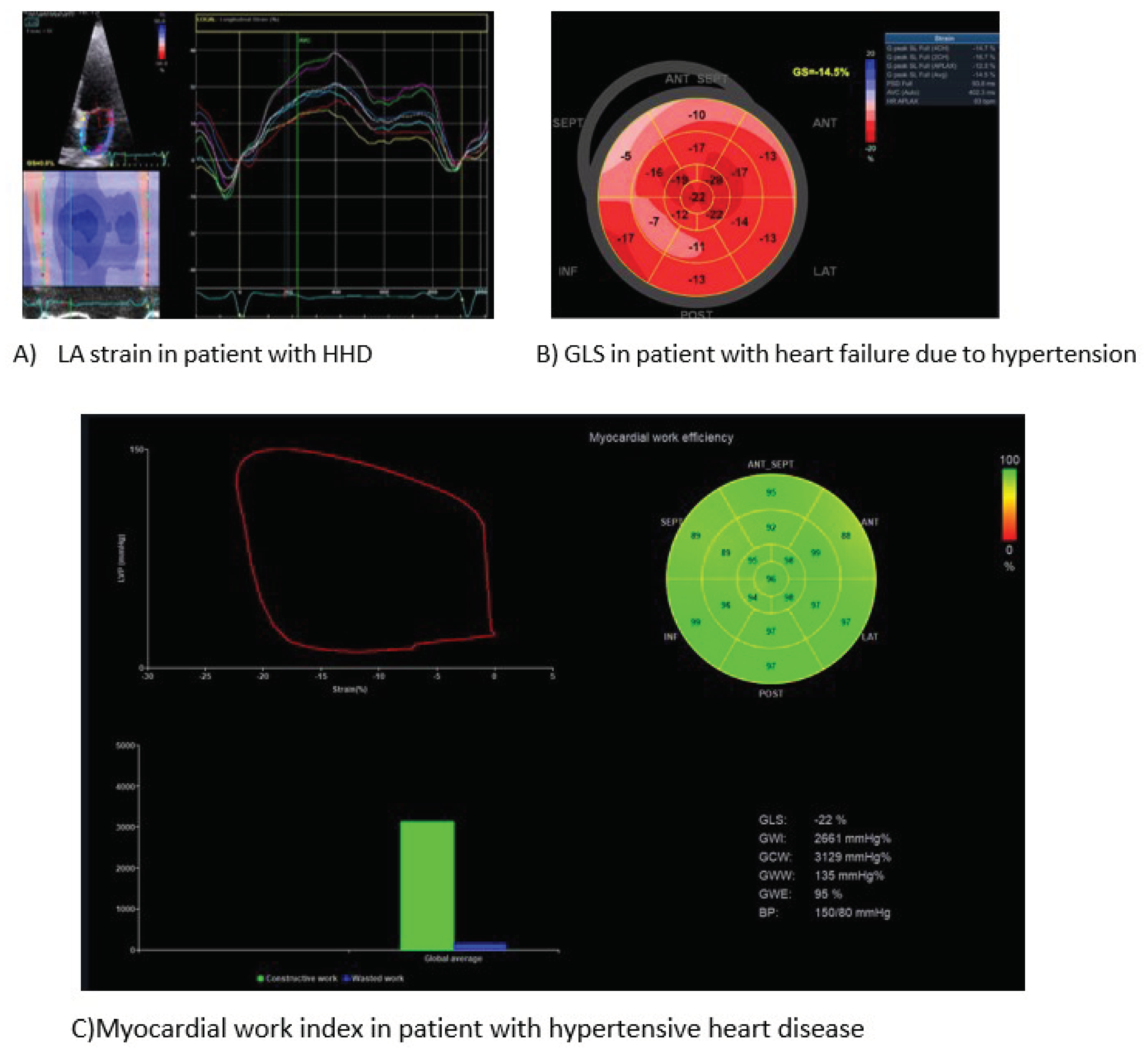
Speckle Tracking of Left Ventricle
Myocardial strain and its sensitive index of myocardial deformation, global longitudinal strain (GLS), provides a widely accepted, accurate, repeatable evaluation of LV systolic function [
90]. The sub-endocardial layer of LV supports predominantly the longitudinal mechanics, whereas the mid-wall and sub-epicardium contribute mostly to the circumferential, rotational, and twisting motions. HTN is well-known that severely suppresses the LV myocardial shortening in the longitudinal direction, than in the circumferential direction [
91]. Impaired myocardial deformation has been long associated with HHD [
92]. Moreover, speckle tracking has the potential to differentiate various types of LVH diseases [
93]. The adverse structural cardiac remodelling in HHD involves the expansion of cardiomyocytes and accumulation of fibrosis in the extracellular matrix. Speckle tracking in echocardiography has the advantage of detecting a decline in myocardial strain in patients with HHD and normal LVEF before apparent structural changes, unravelling subtle impairment of systolic function [
94]. A recent meta-analysis of 6 studies reported the clinical value of GLS to detect subclinical cardiac damage in patients with masked hypertension [
95]. However, the myocardial strain is load dependent. Hence, an increase in afterload may lead to a decreased GLS, thereby attenuating the accuracy of research results [
96]. A compensatory modality using myocardial strain with a limited influence of blood pressure on its results is myocardial work index [
97]. This is a non-invasive index, based on the speckle tracking technique, to assess myocardial deformation incorporating the left ventricular pressure. Growing evidence supports the potentiality of myocardial work index to assist the diagnostic algorithm of HHD [
98,
99]. A growing number of studies suggests that speckle tracking performs better than LVEF, since it may uncover subtle LV systolic dysfunction in hypertensive patients, before LVEF starts declining [
100].
In addition to the diagnostic value, the contribution of GLS to prognosis has been proved in a wide a spectrum of cardiomyopathies, however its application in risk stratification of patients with HHD has not been validated [
101]. Future studies will assess the efficacy of speckle tracking to predict cardiac organ damage and determine the anti-hypertensive regimen [
102]. The introduction of 3D-speckle tracking echocardiography may further increase the sensitivity of echocardiography to detect regional alterations of longitudinal strain and area strain. With optimal echocardiographic views, the 3D speckle tracking may assist to better quantify the degree of myocardial damage [
103]. It is a promising technique which requires further investigation.
Speckle Tracking of Left Atrium
Most recently, researchers have hypothesized the LA dysfunction before structural changes become evident, like LA dilatation [
104]. All three phases of LA function (reservoir, conduit and booster pump) functions have been found significantly impaired in HTN patients than healthy controls [
105]. Classical (E/E’, LA size) and novel indices (reservoir strain (LASr), conduit strain, and contraction strain) of LA myocardial deformation have been associated with HTN [
106]. Notably, LA reservoir and booster pump strain seem to correlate with GLS in hypertensive patients. Changes in LA deformation are more sensitive than other indices, since acute blood pressure lowering in patients with hypertensive urgency improves promptly LA strain [
107]. Unambiguously, alterations in LA mechanics come up early in the course of HHD development [
108]. Up to now, the evidence of the diagnostic power and to a lesser extent of the prognostic value of LA strain in HHD is not robust [
109]. More studies are required to verify the cut-off values f LA strain. In all cases, speckle tracking implementation has a prerequisite of good quality views of LA in order to achieve accurate and repeatable measurements (
Figure 3).
Recently, both TTE and CMR, stand as indispensable tools for cardiac evaluation, each presenting distinctive advantages. Echocardiography, with its real-time imaging capabilities and widespread accessibility, emerges as a dynamic and cost-effective option, capable of assessing valve function and chamber dimensions. Its bedside utility and portability enhance its versatility in various clinical scenarios. Conversely, CMR, renowned for superior spatial resolution and tissue characterization, offers unparalleled insights into cardiac anatomy and function. Despite the higher costs and specialized requirements associated with CMR, its ability to provide detailed information about myocardial tissue texture and views of high imaging quality make it a valuable complement to echocardiography. The selection between these modalities hinges on clinical nuances, specific diagnostic needs, and available resources, often prompting a synergistic integration for a comprehensive cardiac assessment. The technological advances in echocardiography open new roads in its clinical application and future research will clarify its diagnostic and prognostic power.
Conclusions
HHD as a consequence of high-pressure overload on the heart has been increasingly recognized comprising an emerging cardiac disease. It presumably consists of a clinical entity with unfavourable prognosis beyond CAD or kidney function impairment. Echocardiography is a first-line modality for HHD diagnosis clustering a number of parameters of cardiac morphology and function in patients with persistent hypertension. The absence of a single, gold-standard diagnostic parameter has compromised all estimations about the incidence, evolution and prognosis of HHD. The addition of novel indices, like speckle tracking of the left ventricle and left atrium, 3D volume evaluation, myocardial work to classical echocardiographic parameters, may provide more accurate and reproducible diagnostic and prognostic data in those patients. Till now, their use is still underappreciated, but they have the potential to objectively define the HHD and estimate its prognosis.
Author Contributions
N.K. Conceptualization Methodology Writing – Review & Editing Supervision A.M. Methodology, Writing – Original Draft Preparation N.H. Writing – Original Draft Preparation, Resources, Investigation I.K. Writing – Original Draft Preparation, Resources M.M. Methodology, Supervision, Writing – Review & Editing.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fuchs FD, Whelton PK. High Blood Pressure and Cardiovascular Disease. Hypertension 2020; 75(2): 285-92. [CrossRef]
- Stergiou GS, Palatini P, Parati G, et al. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens 2021; 39(7): 1293-302. [CrossRef]
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018; 36(10): 1953-2041.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. Journal of the American College of Cardiology 2018; 71(19): e127-e248.
- Drazner MH. The progression of hypertensive heart disease. Circulation 2011; 123(3): 327-34.
- Nemtsova V, Vischer AS, Burkard T. Hypertensive Heart Disease: A Narrative Review Series—Part 1: Pathophysiology and Microstructural Changes. Journal of Clinical Medicine 2023; 12(7): 2606. [CrossRef]
- Conrad N, Judge A, Tran J, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018; 391(10120): 572-80.
- Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. The Lancet 2014; 383(9932): 1899-911.
- Kuan V, Denaxas S, Patalay P, et al. Identifying and visualising multimorbidity and comorbidity patterns in patients in the English National Health Service: a population-based study. Lancet Digit Health 2023; 5(1): e16-e27. [CrossRef]
- Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012; 126(1): 65-75.
- Yusuf S, Hawken S, Ôunpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. The Lancet 2004; 364(9438): 937-52.
- Kadoglou NPE, Papadopoulos CH, Papadopoulos KG, et al. Updated knowledge and practical implementations of stress echocardiography in ischemic and non-ischemic cardiac diseases: An expert consensus of the Working Group of Echocardiography of the Hellenic Society of Cardiology. Hellenic Journal of Cardiology 2022; 64: 30-57. [CrossRef]
- Chang D, Xu TT, Zhang SJ, et al. Telmisartan ameliorates cardiac fibrosis and diastolic function in cardiorenal heart failure with preserved ejection fraction. Exp Biol Med (Maywood) 2021; 246(23): 2511-21. [CrossRef]
- Nemtsova V, Vischer AS, Burkard T. Hypertensive Heart Disease: A Narrative Review Series-Part 1: Pathophysiology and Microstructural Changes. J Clin Med 2023; 12(7). [CrossRef]
- Nwabuo CC, Vasan RS. Pathophysiology of Hypertensive Heart Disease: Beyond Left Ventricular Hypertrophy. Curr Hypertens Rep 2020; 22(2): 11. [CrossRef]
- Kishio Kuroda TSK, Atsushi Amano. Hypertensive cardiomyopathy: A clinical approach and literature review. World Journal of Hypertension 2015; 5(2): 41-52.
- Díez J, González A, López B, Querejeta R. Mechanisms of disease: pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat Clin Pract Cardiovasc Med 2005; 2(4): 209-16. [CrossRef]
- López B, González A, Ravassa S, et al. Circulating Biomarkers of Myocardial Fibrosis: The Need for a Reappraisal. J Am Coll Cardiol 2015; 65(22): 2449-56.
- Nemtsova V, Burkard T, Vischer AS. Hypertensive Heart Disease: A Narrative Review Series-Part 2: Macrostructural and Functional Abnormalities. J Clin Med 2023; 12(17). [CrossRef]
- Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F, Esler MD. Relation Between Cardiac Sympathetic Activity and Hypertensive Left Ventricular Hypertrophy. Circulation 2003; 108(5): 560-5. [CrossRef]
- Clinical Implications of Recent Findings from the Antihypertensive and Lipid-Lowering Treatment To Prevent Heart Attack Trial (ALLHAT) and Other Studies of Hypertension. Annals of Internal Medicine 2001; 135(12): 1074-8.
- Tadic M, Cuspidi C. Right Ventricle in Arterial Hypertension: Did We Forget Something? J Clin Med 2022; 11(21).
- Ismail TF, Frey S, Kaufmann BA, et al. Hypertensive Heart Disease-The Imaging Perspective. J Clin Med 2023; 12(9). [CrossRef]
- Zhou D, Yan M, Cai A, et al. Prognostic implications of left ventricular hypertrophy defined by the thresholds from the international and Chinese guidelines. J Clin Hypertens (Greenwich) 2023; 25(7): 628-37. [CrossRef]
- Armstrong AC, Gidding S, Gjesdal O, Wu C, Bluemke DA, Lima JA. LV mass assessed by echocardiography and CMR, cardiovascular outcomes, and medical practice. JACC Cardiovasc Imaging 2012; 5(8): 837-48. [CrossRef]
- Takeuchi M, Nishikage T, Mor-Avi V, et al. Measurement of left ventricular mass by real-time three-dimensional echocardiography: validation against magnetic resonance and comparison with two-dimensional and m-mode measurements. J Am Soc Echocardiogr 2008; 21(9): 1001-5. [CrossRef]
- Murdolo G, Angeli F, Reboldi G, et al. Left ventricular hypertrophy and obesity: only a matter of fat? High Blood Press Cardiovasc Prev 2015; 22(1): 29-41.
- Moukarzel J, Guevara E, Casciaro ME, Guilenea FN, Pascaner AF, Craiem D. Echocardiographic Measurements of Left Heart Chamber Size in a Large Cohort of Subjects: Comparison of Body Surface Area and Height Indexing to Account for Effects of Obesity. J Am Soc Echocardiogr 2022; 35(11): 1159-67.e2. [CrossRef]
- Cuspidi C, Meani S, Negri F, et al. Indexation of left ventricular mass to body surface area and height to allometric power of 2.7: is the difference limited to obese hypertensives? J Hum Hypertens 2009; 23(11): 728-34. [CrossRef]
- Lundin M, Heiberg E, Nordlund D, Gyllenhammar T, Steding-Ehrenborg K, Engblom H, Carlsson M, Atar D, van der Pals J, Erlinge D, Borgquist R, Khoshnood A, Ekelund U, Nickander J, Themudo R, Nordin S, Kozor R, Bhuva AN, Moon JC, Maret E, Caidahl K, Sigfridsson A, Sörensson P, Schelbert EB, Arheden H, Ugander M. Prognostic utility and characterization of left ventricular hypertrophy using global thickness. Sci Rep. 2023 Dec 20;13(1):22806. [CrossRef]
- Stewart MH, Lavie CJ, Shah S, Englert J, Gilliland Y, Qamruddin S, Dinshaw H, Cash M, Ventura H, Milani R. Prognostic Implications of Left Ventricular Hypertrophy. Prog Cardiovasc Dis. 2018 Nov-Dec;61(5-6):446-455. [CrossRef]
- Bayés-Genís A, Guindo J, Viñolas X, Tomás L, Elosua R, Duran I, Bayés de Luna A. Cardiac arrhythmias and left ventricular hypertrophy in systemic hypertension and their influences on prognosis. Am J Cardiol. 1995 Nov 2;76(13):54D-59D. [CrossRef]
- Saadeh AM, Jones JV. Predictors of sudden cardiac death in never previously treated patients with essential hypertension: long-term follow-up. J Hum Hypertens. 2001 Oct;15(10):677-80. [CrossRef]
- Chu HW, Hwang IC, Kim HM, Park J, Choi H, Choi HM, Yoon YE, Cho GY. Age-dependent implications of left ventricular hypertrophy regression in patients with hypertension. Hypertens Res. 2024 Jan 18. [CrossRef]
- Rademakers FE, Rogers WJ, Guier WH, et al. Relation of regional cross-fiber shortening to wall thickening in the intact heart. Three-dimensional strain analysis by NMR tagging. Circulation 1994; 89(3): 1174-82. [CrossRef]
- Aurigemma GP, Silver KH, Priest MA, Gaasch WH. Geometric changes allow normal ejection fraction despite depressed myocardial shortening in hypertensive left ventricular hypertrophy. Journal of the American College of Cardiology 1995; 26(1): 195-202. [CrossRef]
- Park K, Chang S-A, Kim H-K, et al. Normal Ranges and Physiological Changes of Midwall Fractional Shortening in Healthy Korean Population. kcj 2010; 40(11): 587-92. [CrossRef]
- Yu EH, Sloggett CE, Iwanochko RM, Rakowski H, Siu SC. Feasibility and accuracy of left ventricular volumes and ejection fraction determination by fundamental, tissue harmonic, and intravenous contrast imaging in difficult-to-image patients. J Am Soc Echocardiogr 2000; 13(3): 216-24.
- Nagy A, Borbás S, Lengyel M. Measurement of left ventricular volumes and ejection fraction after intravenous contrast agent administration using standard echocardiographic equipment. Echocardiography 2000; 17(5): 433-7.
- Margossian R, Schwartz ML, Prakash A, et al. Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study). Am J Cardiol 2009; 104(3): 419-28. [CrossRef]
- Pranata R, Tondas AE, Yonas E, Vania R, Yamin M, Chandra A, Siswanto BB. Differences in clinical characteristics and outcome of de novo heart failure compared to acutely decompensated chronic heart failure - systematic review and meta-analysis. Acta Cardiol. 2021 Jun;76(4):410-420. [CrossRef]
- Tsioufis C, Taxiarchou E, Syrseloudis D, et al. Left ventricular mass but not geometry determines left atrial size in the early stages of hypertension. Journal of Human Hypertension 2009; 23(10): 674-9. [CrossRef]
- Thomas L, Muraru D, Popescu BA, et al. Evaluation of Left Atrial Size and Function: Relevance for Clinical Practice. J Am Soc Echocardiogr 2020; 33(8): 934-52. [CrossRef]
- Parajuli P, Ahmed AA. Left Atrial Enlargement. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; 2023.
- Nistri S, Galderisi M, Ballo P, et al. Determinants of echocardiographic left atrial volume: implications for normalcy. European Journal of Echocardiography 2011; 12(11): 826-33. [CrossRef]
- Whitlock M, Garg A, Gelow J, Jacobson T, Broberg C. Comparison of left and right atrial volume by echocardiography versus cardiac magnetic resonance imaging using the area-length method. Am J Cardiol 2010; 106(9): 1345-50. [CrossRef]
- Tsang TSM, Abhayaratna WP, Barnes ME, et al. Prediction of Cardiovascular Outcomes With Left Atrial Size. Journal of the American College of Cardiology 2006; 47(5): 1018-23.
- Patel DA, Lavie CJ, Milani RV, Ventura HO. Left Atrial Volume Index Predictive of Mortality Independent of Left Ventricular Geometry in a Large Clinical Cohort With Preserved Ejection Fraction. Mayo Clinic Proceedings 2011; 86(8): 730-7. [CrossRef]
- Rossi A, Dini FL, Agricola E, Faggiano P, Benfari G, Temporelli PL, Cucco C, Scelsi L, Vassanelli C, Ghio S. Left atrial dilatation in systolic heart failure: a marker of poor prognosis, not just a buffer between the left ventricle and pulmonary circulation. J Echocardiogr. 2018 Dec;16(4):155-161. [CrossRef]
- Gehlken C, Screever EM, Suthahar N, van der Meer P, Westenbrink BD, Coster JE, Van Veldhuisen DJ, de Boer RA, Meijers WC. Left atrial volume and left ventricular mass indices in heart failure with preserved and reduced ejection fraction. ESC Heart Fail. 2021 Aug;8(4):2458-2466. [CrossRef]
- Russo C, Jin Z, Homma S, et al. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart 2012; 98(10): 813. [CrossRef]
- Russo C, Jin Z, Homma S, et al. LA phasic volumes and reservoir function in the elderly by real-time 3D echocardiography: normal values, prognostic significance, and clinical correlates. JACC: Cardiovascular Imaging 2017; 10(9): 976-85.
- Zile MR, Gottdiener JS, Hetzel SJ, et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation 2011; 124(23): 2491-501. [CrossRef]
- Burke MA, Katz DH, Beussink L, et al. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circulation: Heart Failure 2014; 7(2): 288-99. [CrossRef]
- Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. Journal of the American College of Cardiology 2009; 53(13): 1119-26.
- Shin SH, Claggett B, Inciardi RM, et al. Prognostic value of minimal left atrial volume in heart failure with preserved ejection fraction. Journal of the American Heart Association 2021; 10(15): e019545. [CrossRef]
- Leache L, Gutiérrez-Valencia M, Finizola RM, et al. Pharmacotherapy for hypertension-induced left ventricular hypertrophy. Cochrane Database of Systematic Reviews 2021; (10).
- Hansen S, Brainin P, Sengeløv M, Jørgensen PG, Bruun NE, Olsen FJ, Fritz-Hansen T, Schou M, Gislason G, Biering-Sørensen T. Prognostic utility of diastolic dysfunction and speckle tracking echocardiography in heart failure with reduced ejection fraction. ESC Heart Fail. 2020 Feb;7(1):147-157. [CrossRef]
- Lin TT, Wang YC, Juang JJ, Hwang JJ, Wu CK. Application of the newest European Association of Cardiovascular Imaging Recommendation regarding the long-term prognostic relevance of left ventricular diastolic function in heart failure with preserved ejection fraction. Eur Radiol. 2020 Jan;30(1):630-639. [CrossRef]
- Grodecki PV, Klein AL. Pitfalls in the echo-Doppler assessment of diastolic dysfunction. Echocardiography 1993; 10(2): 213-34. [CrossRef]
- Mantero A, Gentile F, Azzollini M, et al. Effect of sample volume location on Doppler-derived transmitral inflow velocity values in 288 normal subjects 20 to 80 years old: an echocardiographic, two-dimensional color Doppler cooperative study. J Am Soc Echocardiogr 1998; 11(3): 280-8. [CrossRef]
- Sharp ASP, Tapp RJ, Thom SAM, et al. Tissue Doppler E/E′ ratio is a powerful predictor of primary cardiac events in a hypertensive population: an ASCOT substudy. European Heart Journal 2009; 31(6): 747-52. [CrossRef]
- Iwashima Y, Horio T, Kamide K, Rakugi H, Ogihara T, Kawano Y. Pulmonary venous flow and risk of cardiovascular disease in essential hypertension. Journal of Hypertension 2008; 26(4): 798-805. [CrossRef]
- Klein AL, Hatle LK, Burstow DJ, et al. Doppler characterization of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol 1989; 13(5): 1017-26. [CrossRef]
- Ibrahim EH, Dennison J, Frank L, Stojanovska J. Diastolic Cardiac Function by MRI-Imaging Capabilities and Clinical Applications. Tomography 2021; 7(4): 893-914. [CrossRef]
- Ojji DB, Lecour S, Atherton JJ, Blauwet LA, Alfa J, Sliwa K. Right Ventricular Systolic Dysfunction Is Common in Hypertensive Heart Failure: A Prospective Study in Sub-Saharan Africa. PLOS ONE 2016; 11(4): e0153479. [CrossRef]
- Oketona OA, Balogun MO, Akintomide AO, et al. Right ventricular systolic function in hypertensive heart failure. Vascular Health and Risk Management 2017; 13(null): 353-60. [CrossRef]
- Aalen JM, Smiseth OA. Strain identifies pseudo-normalized right ventricular function in tricuspid regurgitation. Eur Heart J Cardiovasc Imaging 2021; 22(8): 876-7. [CrossRef]
- Pavlicek M, Wahl A, Rutz T, et al. Right ventricular systolic function assessment: rank of echocardiographic methods vs. cardiac magnetic resonance imaging. Eur J Echocardiogr 2011; 12(11): 871-80. [CrossRef]
- Surkova E, Cosyns B, Gerber B, Gimelli A, La Gerche A, Ajmone Marsan N. The dysfunctional right ventricle: the importance of multi-modality imaging. European Heart Journal-Cardiovascular Imaging 2022; 23(7): 885-97. [CrossRef]
- Kanwar MK, Tedford RJ, Thenappan T, De Marco T, Park M, McLaughlin V. Elevated pulmonary pressure noted on echocardiogram: a simplified approach to next steps. Journal of the American Heart Association 2021; 10(7): e017684. [CrossRef]
- Ahmed SN, Syed FM, Porembka DT. Echocardiographic evaluation of hemodynamic parameters. Critical care medicine 2007; 35(8): S323-S9. [CrossRef]
- Venkatachalam S, Wu G, Ahmad M. Echocardiographic assessment of the right ventricle in the current era: Application in clinical practice. Echocardiography 2017; 34(12): 1930-47. [CrossRef]
- Ahmed I, Nuri MMH, Zakariyya AN, Ahmad SM, Ahmed M. Correlation between doppler echocardiography and right heart catheterization derived pulmonary artery pressures: impact of right atrial pressures. J Coll Physicians Surg Pak 2016; 26(4): 255-9.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28(1): 1-39.e14. [CrossRef]
- Surkova E, Cosyns B, Gerber B, Gimelli A, La Gerche A, Ajmone Marsan N. The dysfunctional right ventricle: the importance of multi-modality imaging. Eur Heart J Cardiovasc Imaging 2022; 23(7): 885-97. [CrossRef]
- Kadoglou NPE, Papadopoulos CH, Krommydas A. The prognostic value of exercise-induced pulmonary hypertension in asymptomatic patients with primary mitral regurgitation. J Cardiol. 2022 Feb;79(2):306-310. [CrossRef]
- Pieske B, Tschope C, de Boer R, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40(40):3297-3317. [CrossRef]
- Sharifov OF, Gupta H. What Is the Evidence That the Tissue Doppler Index E/e′ Reflects Left Ventricular Filling Pressure Changes After Exercise or Pharmacological Intervention for Evaluating Diastolic Function? A Systematic Review. Journal of the American Heart Association 2017; 6(3): e004766. [CrossRef]
- Prasad SB, Holland DJ, Atherton JJ. Diastolic stress echocardiography: from basic principles to clinical applications. Heart 2018; 104(21): 1739-48. [CrossRef]
- Picano E, Pellikka PA. Stress echo applications beyond coronary artery disease. European Heart Journal 2013; 35(16): 1033-40. [CrossRef]
- Albouaini K, Egred M, Alahmar A, Wright DJ. Cardiopulmonary exercise testing and its application. Postgraduate Medical Journal 2007; 83(985): 675-82. [CrossRef]
- Kadoglou NP, Iliadis F, Angelopoulou N, Sailer N, Fotiadis G, Voliotis K, Vitta I, Liapis CD, Alevizos M. Cardiorespiratory capacity is associated with favourable cardiovascular risk profile in patients with Type 2 diabetes. J Diabetes Complications. 2009 May-Jun;23(3):160-6. [CrossRef]
- Khattab E, Velidakis N, Gkougkoudi E, Kadoglou NPE. Exercise-Induced Pulmonary Hypertension: A Valid Entity or Another Factor of Confusion? Life (Basel). 2023 Jan 3;13(1):128.
- Del Punta L, De Biase N, Balletti A, et al. Arterial Hypertension and Cardiopulmonary Function: The Value of a Combined Cardiopulmonary and Echocardiography Stress Test. High Blood Pressure & Cardiovascular Prevention 2022; 29(2): 145-54. [CrossRef]
- Nedeljkovic I, Banovic M, Stepanovic J, et al. The combined exercise stress echocardiography and cardiopulmonary exercise test for identification of masked heart failure with preserved ejection fraction in patients with hypertension. European Journal of Preventive Cardiology 2020; 23(1): 71-7. [CrossRef]
- Guazzi M, Bandera F, Ozemek C, Systrom D, Arena R. Cardiopulmonary Exercise Testing. Journal of the American College of Cardiology 2017; 70(13): 1618-36.
- Del Punta L, De Biase N, Armenia S, et al. Combining cardiopulmonary exercise testing with echocardiography: a multiparametric approach to the cardiovascular and cardiopulmonary systems. European Heart Journal - Imaging Methods and Practice 2023; 1(1). [CrossRef]
- Ünlü S, Özden Ö, Çelik A. Imaging in Heart Failure with Preserved Ejection Fraction: A Multimodality Imaging Point of View. Card Fail Rev 2023; 9: e04. [CrossRef]
- Kadoglou NPE, Bouwmeester S, de Lepper AGW, de Kleijn MC, Herold IHF, Bouwman ARA, Korakianitis I, Simmers T, Bracke FALE, Houthuizen P. The Prognostic Role of Global Longitudinal Strain and NT-proBNP in Heart Failure Patients Receiving Cardiac Resynchronization Therapy. J Pers Med. 2024 Feb 8;14(2):188.
- Ishizu T, Seo Y, Kameda Y, Kawamura R, Kimura T, Shimojo N, Xu D, Murakoshi N, Aonuma K. Left ventricular strain and transmural distribution of structural remodeling in hypertensive heart disease. Hypertension. 2014 Mar;63(3):500-6. [CrossRef]
- Sun JP, Xu TY, Ni XD, Yang XS, Hu JL, Wang SC, Li Y, Bahler RC, Wang JG. Echocardiographic strain in hypertrophic cardiomyopathy and hypertensive left ventricular hypertrophy. Echocardiography. 2019 Feb;36(2):257-265. [CrossRef]
- Afonso L, Kondur A, Simegn M, Niraj A, Hari P, Kaur R, Ramappa P, Pradhan J, Bhandare D, Williams KA, Zalawadiya S, Pinheiro A, Abraham TP. Two-dimensional strain profiles in patients with physiological and pathological hypertrophy and preserved left ventricular systolic function: a comparative analyses. BMJ Open. 2012 Aug 17;2(4):e001390. [CrossRef]
- Ballo P, Nistri S, Cameli M, Papesso B, Dini FL, Galderisi M, Zuppiroli A, Mondillo S; Working Group Nucleus on Echocardiography of the Italian Society of Cardiology. Association of left ventricular longitudinal and circumferential systolic dysfunction with diastolic function in hypertension: a nonlinear analysis focused on the interplay with left ventricular geometry. J Card Fail. 2014 Feb;20(2):110-20. [CrossRef]
- Cuspidi C, Gherbesi E, Faggiano A, Sala C, Grassi G, Tadic M. Unmasking left ventricular systolic dysfunction in masked hypertension: looking at myocardial strain. A review and meta-analysis. J Hypertens. 2023 Feb 1;41(2):344-350. [CrossRef]
- Edwards NFA, Scalia GM, Shiino K, Sabapathy S, Anderson B, Chamberlain R, Khandheria BK, Chan J. Global Myocardial Work Is Superior to Global Longitudinal Strain to Predict Significant Coronary Artery Disease in Patients With Normal Left Ventricular Function and Wall Motion. J Am Soc Echocardiogr. 2019 Aug;32(8):947-957. [CrossRef]
- Boe E, Skulstad H, Smiseth OA. Myocardial work by echocardiography: a novel method ready for clinical testing. Eur Heart J Cardiovasc Imaging. (2019) 20:18–20. [CrossRef]
- Zhao Q, Cui C, Li Y, Liu Y, Huang D, Wang Y, Hu Y, Liu R, Zhu H, Liu L. Evaluation of myocardial work in patients with hypertrophic cardiomyopathy and hypertensive left ventricular hypertrophy based on non-invasive pressure-strain loops. Front Cardiovasc Med. 2022 Jul 26;9:767875. [CrossRef]
- Huang J, Yang C, Yan ZN, Fan L, Ni CF. Global myocardial work: A new way to detect subclinical myocardial dysfunction with normal left ventricle ejection fraction in essential hypertension patients: Compared with myocardial layer-specific strain analysis. Echocardiography. 2021 Jun;38(6):850-860. [CrossRef]
- Cuspidi C, Gherbesi E, Faggiano A, Sala C, Carugo S, Tadic M. Early Left Ventricular Dysfunction and Non-Dipping: When Ejection Fraction is Not Enough. A Meta-Analysis of Speckle tracking Echocardiography Studies. Am J Hypertens. 2023 Feb 13;36(2):109-119. [CrossRef]
- Mordi IR, Singh S, Rudd A, Srinivasan J, Frenneaux M, Tzemos N, Dawson DK. Comprehensive Echocardiographic and Cardiac Magnetic Resonance Evaluation Differentiates Among Heart Failure With Preserved Ejection Fraction Patients, Hypertensive Patients, and Healthy Control Subjects. JACC Cardiovasc Imaging. 2018 Apr;11(4):577-585. [CrossRef]
- Cameli M, Lembo M, Sciaccaluga C, Bandera F, Ciccone MM, D'Andrea A, D'Ascenzi F, Esposito R, Evola V, Liga R, Mandoli GE, Palmiero P, Santoro C, Scicchitano P, Sorrentino R, Zito A, Pedrinelli R, Mondillo S, Mattioli AV, Galderisi M; Working Groups of Echocardiography and Arterial Hypertension of Italian Society of Cardiology (SIC). Identification of cardiac organ damage in arterial hypertension: insights by echocardiography for a comprehensive assessment. J Hypertens. 2020 Apr;38(4):588-598. [CrossRef]
- Kwon A, Ihm SH, Park CS. Morning blood pressure surge in the early stage of hypertensive patients impacts three-dimensional left ventricular speckle tracking echocardiography. Clin Hypertens. 2021 Aug 15;27(1):16. [CrossRef]
- Stefani LD, Trivedi SJ, Ferkh A, Emerson P, Marschner S, Gan G, Altman M, Thomas L. Left atrial mechanics evaluated by two-dimensional strain analysis: alterations in essential hypertension. J Hypertens. 2024 Feb 1;42(2):274-282. [CrossRef]
- Li H, Wang H, Wang T, Jin C, Lu M, Liu B. Different phenotype of left atrial function impairment in patients with hypertrophic cardiomyopathy and hypertension: comparison of healthy controls. Front Cardiovasc Med. 2023 May 10;10:1027665. [CrossRef]
- Cai J, Liang Z, Feng W, Long H. Correlation between left atrial strain and left ventricular diastolic function in hypertensive patients. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023 Jun 28;48(6):846-851. [CrossRef]
- Asarcikli LD, Can F, Guvenc TS, Sert S, Osken A, Dayi SU. The effect of urgent blood pressure reduction on left atrial strain in patients with hypertensive attack : Blood pressure lowering affects LA strain. Int J Cardiovasc Imaging. 2023 Jul;39(7):1221-1230.
- Cameli M, Mandoli GE, Loiacono F, Dini FL, Henein M, Mondillo S. Left atrial strain: a new parameter for assessment of left ventricular filling pressure. Heart Fail Rev. 2016 Jan;21(1):65-76. [CrossRef]
- Miyoshi H, Oishi Y, Mizuguchi Y, Iuchi A, Nagase N, Ara N, Oki T. Association of left atrial reservoir function with left atrial structural remodeling related to left ventricular dysfunction in asymptomatic patients with hypertension: evaluation by two-dimensional speckle-tracking echocardiography. Clin Exp Hypertens. 2015;37(2):155-65. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).