Submitted:
08 March 2024
Posted:
08 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnAl-NO3 LDH
2.2. Characterization
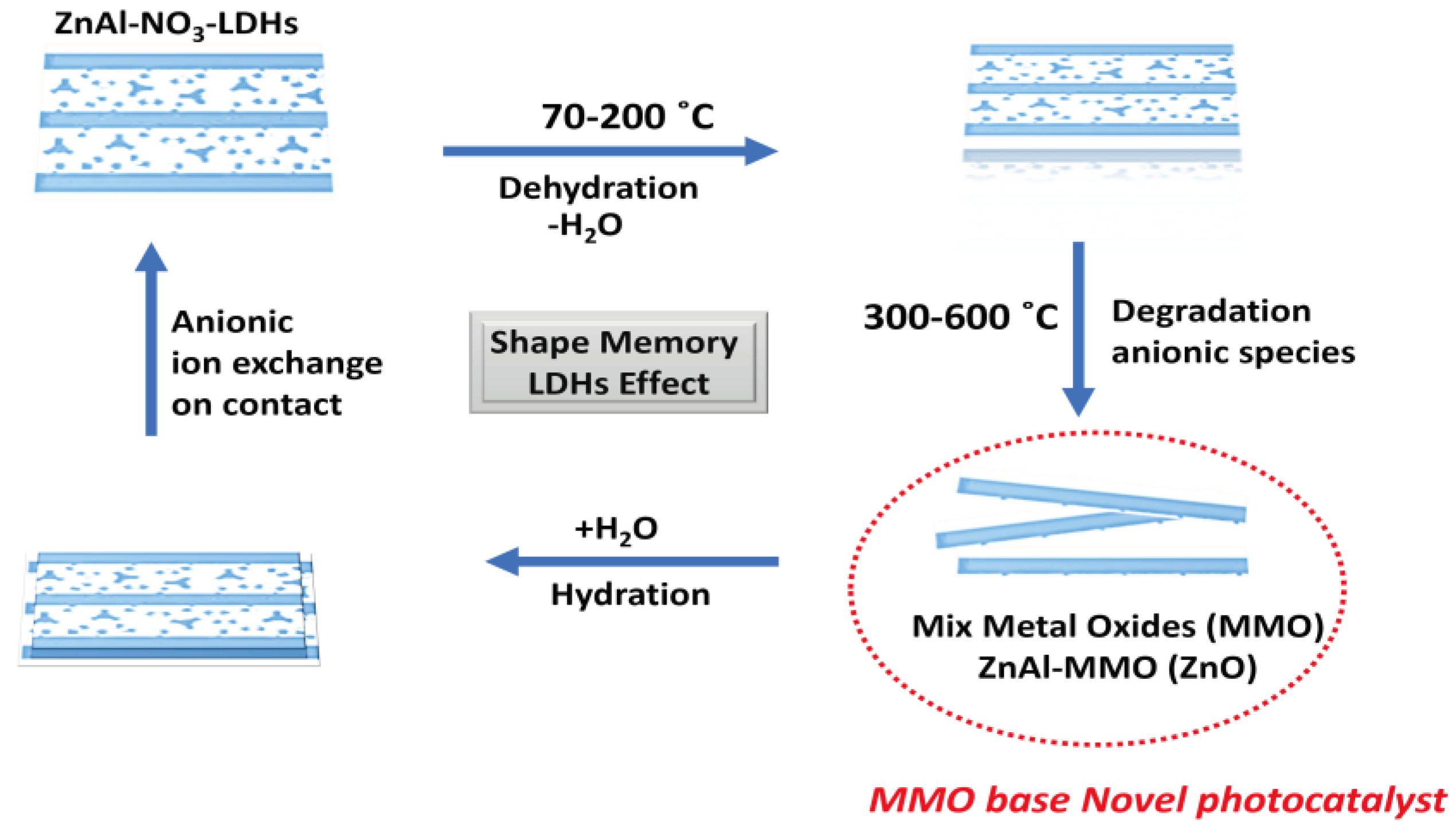
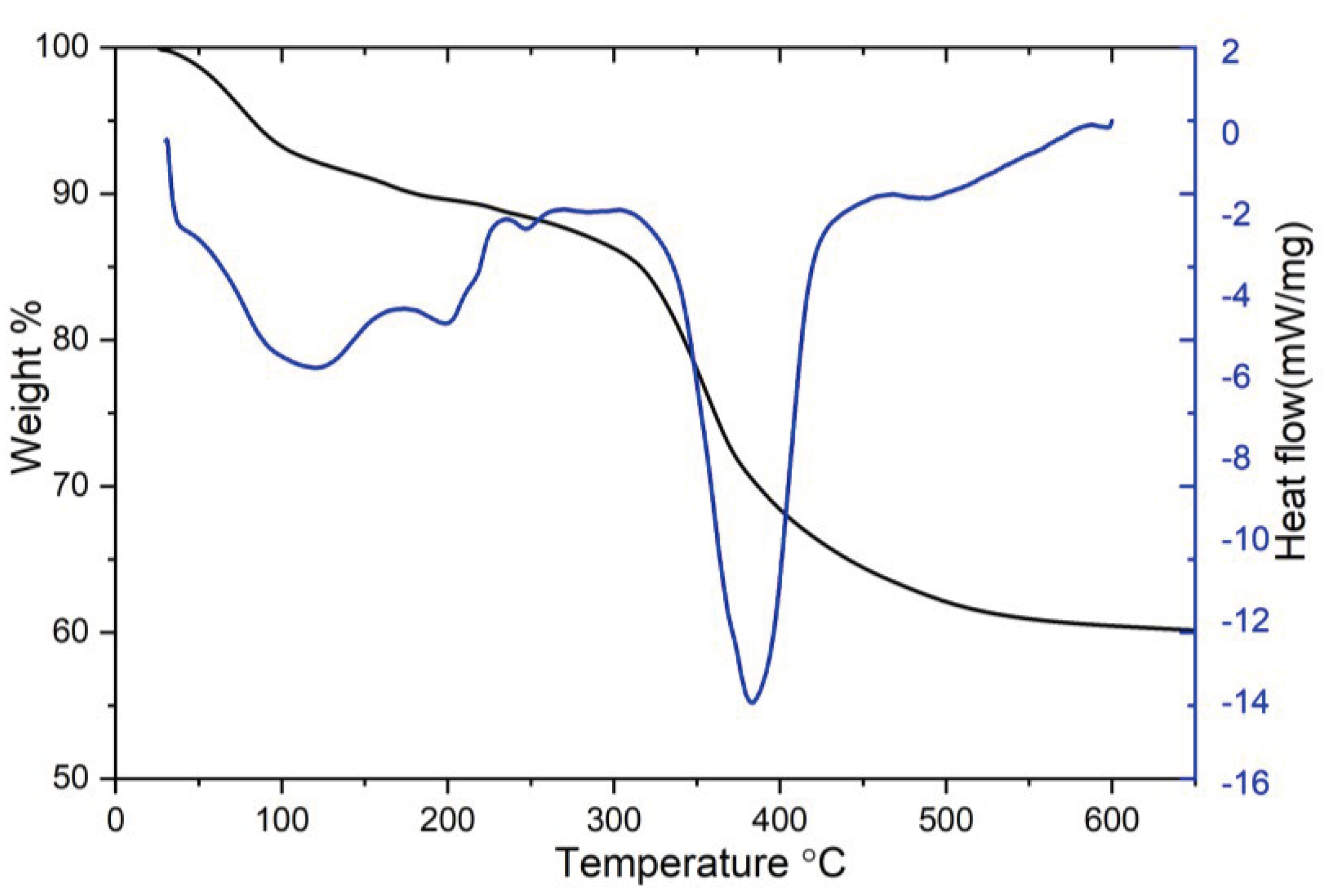
3. Results and Discussion
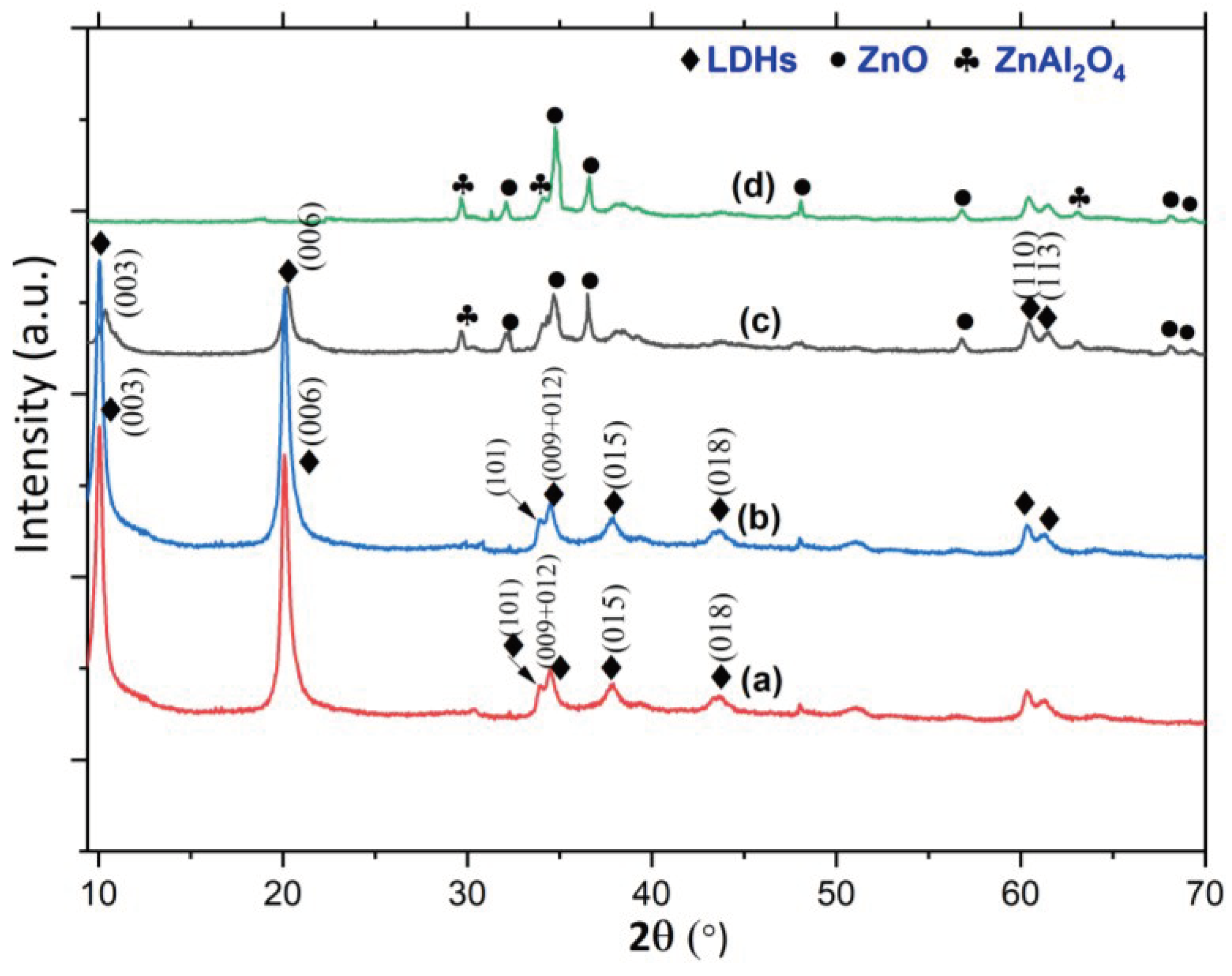
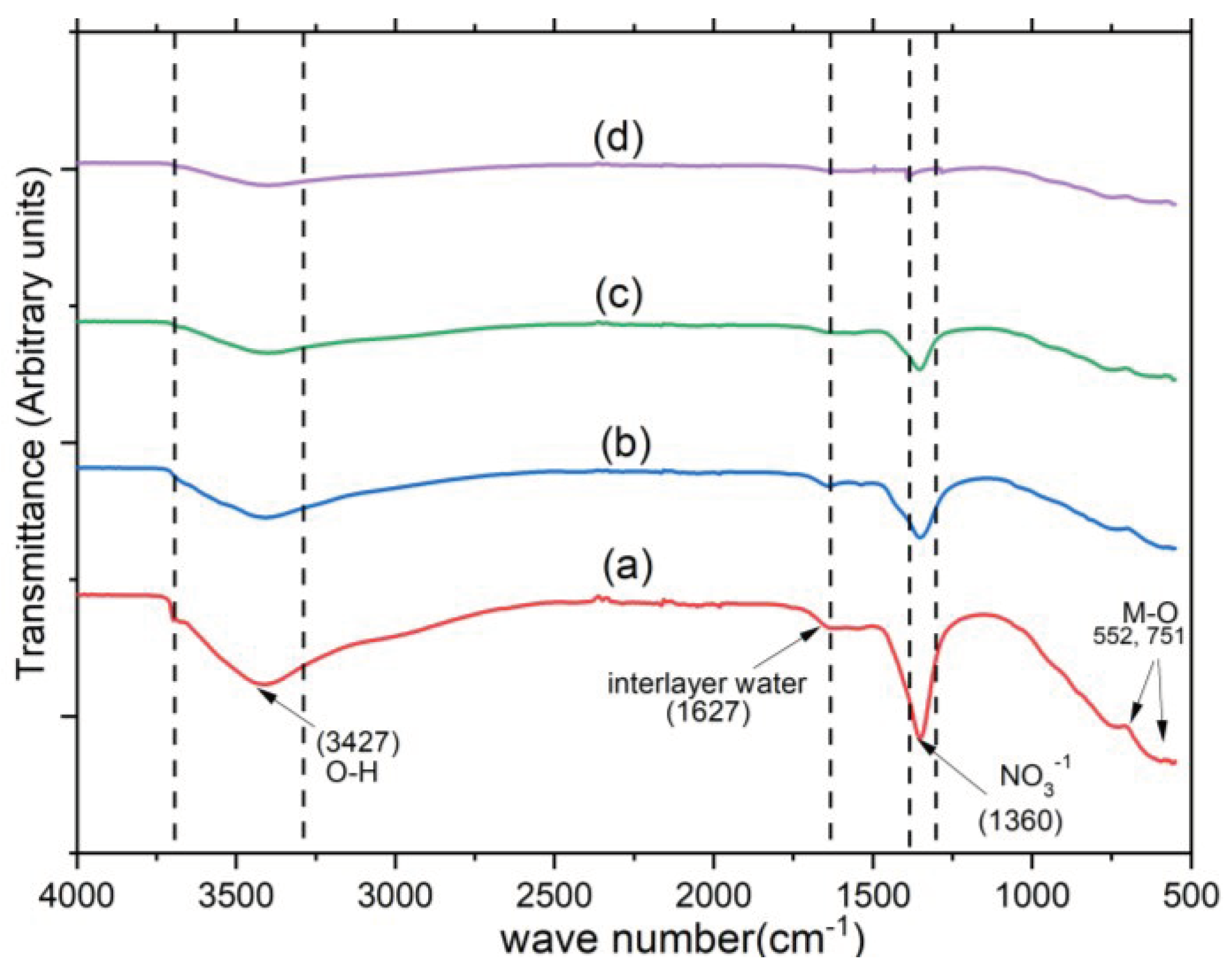
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Lin. Yan.; Haiyang. Hu.; Yun. Hang Hu. Role of ZnO morphology in its reduction and photocatalysis. Appl. Surf. Sci 2020, 502, 144202. [CrossRef]
- Kitiyanan. Athapol.; Supachai. Ngamsinlapasathian.; Soropong. Pavasupree.; Susumu. Yoshikawa. The preparation and characterization of nanostructured TiO2–ZrO2 mixed oxide electrode for efficient dye-sensitized solar cells. J. Solid State Chem 2005, 178, 1044–1048. [CrossRef]
- Fan. Guoli.; Wei. Sun.; Hui. Wang.; Feng. Li. Visible-light-induced heterostructured Zn–Al–In mixed metal oxide nanocomposite photocatalysts derived from a single precursor. J. Chem. Eng 2011, 174, 467–474. [CrossRef]
- Mirzaeifard. Zahra.; Zahra. Shariatinia.; Milad. Jourshabani.; Seyed. Mahmood.; Rezaei. Darvishi. ZnO photocatalyst revisited: effective photocatalytic degradation of emerging contaminants using S-doped ZnO nanoparticles under visible light radiation. Ind. amp; Eng. Chem. Res 2020, 59, 15894–15911. [CrossRef]
- Wachs. Israel. Recent conceptual advances in the catalysis science of mixed metal oxide catalytic materials. Catal. Today 2005, 100, 79–94. [CrossRef]
- Han. Xin.; Yaognag. Li.; Hongzhi. Wang.; Qinghong. Zhang. Controlled preparation of β-Bi 2 O 3/Mg–Al mixed metal oxides composites with enhanced visible light photocatalytic performance. Res. Chem. Intermed 2020, 46, 5009–5021. [CrossRef]
- Zheng. Jiangfu.; Wenbo. Li.; Rongdi. Tang.; Sheng. Xiong.; Daoxin. Gong.; Yaocheng. Deng.; Zhanpeng. Zhou.; Ling. Li.; Long. Su.; Lihua. Yang. Ultrafast photodegradation of nitenpyram by Ag/Ag3PO4/Zn–Al LDH composites activated by persulfate system: removal efficiency, degradation pathway and reaction mechanism. Chemosphere 2022, 292, 133431.
- Shalaby. Nasser.; M. Sayed. Stover ash-extracted mixed oxides surface-doped with Ni for photo-degradation of water organic pollutants. Int. J. Environ. Anal. 2023, 103, 8941–8956. [CrossRef]
- Zheng. Jiangfu.; Changzheng. Fan.; Xiaoming. Li.; Qi. Yang.; Dongbo. Wang.; Abing. Duan.; Jinglin. Ding. Enhanced photodegradation of tetracycline hydrochloride by hexameric AgBr/Zn-Al MMO S-scheme heterojunction photocatalysts: Low metal leaching, degradation mechanism and intermediates. J. Chem. Eng 2022, 446, 137371.
- Zheng. Jiangfu.; Changzheng. Fan.; Xiaoming. Li.; Qi. Yang.; Dongbo. Wang.; Abing. Duan.; Jinglin. Ding. Enhanced photodegradation of tetracycline hydrochloride by hexameric AgBr/Zn-Al MMO S-scheme heterojunction photocatalysts: Low metal leaching, degradation mechanism and intermediates. J. Chem. Eng. 2022, 446, 137371.
- M. Ahsan. Iqbal.; Michele. Fedel. Effect of operating parameters on the structural growth of ZnAl layered double hydroxide on AA6082 and corresponding corrosion resistance properties. JCTR 2019, 16, 1423.
- M. Ahsan. Iqbal.; Luyi. Sun.; Allyson T. Barrett.; Michele. Fedel.; Layered double hydroxide protective films developed on aluminum and aluminum alloys: synthetic methods and anti-corrosion mechanisms. Coatings 2020, 10, 428. [CrossRef]
- Yadav. Dileep Kumar.; Sitharaman. Uma.; Rajamani. Nagarajan. Microwave-assisted synthesis of ternary Li-M-Al LDHs (M= Mg, Co, Ni, Cu, Zn, and Cd) and examining their use in phenol oxidation. Appl. Clay Sci 2022, 228, 106655. [CrossRef]
- Liu. Yi.; Tongwen. Yu.; Rui. Cai.; Yanshuo. Li.; Weishen. Yang.; Jürgen. Caro. One-pot synthesis of NiAl–CO 3 LDH anti-corrosion coatings from CO 2-saturated precursors. RSC Adv 2015, 5, 29552. [CrossRef]
- Zhang. Guanhua.; Xueqiang. Zhang.; Yue. Meng.; Guoxiang. Pan.; Zheming. Ni.; Shengjie. Xia. Layered double hydroxides-based photocatalysts and visible-light driven photodegradation of organic pollutants: A review. J. Chem. Eng 2020, 392, 123684. [CrossRef]
- Razzaq. Abdul.; Shahzad. Ali.; Muhammad. Asif.; Su-Il. In. Layered double hydroxide (LDH) based photocatalysts: An outstanding strategy for efficient photocatalytic CO2 conversion. Catalysts 2020, 10, 1185. [Google Scholar]
- Boumeriame. Hanane.; Eliana S. Da Silva.; Alexey. S. Cherevan.; Tarik. Chafik.; Joaquim. L. Faria.; Dominik. Eder. Layered double hydroxide (LDH)-based materials: A mini-review on strategies to improve the performance for photocatalytic water splitting. J. Energy Chem 2022, 64, 406–431. [CrossRef]
- Bian. Xuanang.; Shuai. Zhang.; Yunxuan. Zhao.; Run. Shi.; Tierui. Zhang. Layered double hydroxide-based photocatalytic materials toward renewable solar fuels production. InfoMat 2021, 3, 719–738. [CrossRef]
- Jiménez-López.; R. Leyva-Ramos.; J. Salazar-Rábago.; A. Jacobo-Azuara.; A. Aragón-Piña. Adsorption of selenium (iv) oxoanions on calcined layered double hydroxides of Mg-Al-CO3 from aqueous solution. Effect of calcination and reconstruction of lamellar structure. Environ. Nanotechnol. Monit. Manag 2021, 16, 100580.
- Wiyantoko. Bayu.; Puji. Kurniawati.; Tri. E. Purbaningtias.; Muhammad. H. Jauhari.; Amri. Yahya.; Muchammad. Tamyiz.; Is. Fatimah.; Ruey-an. Doong. Assessing the effect of calcination on adsorption capability of Mg/Al layer double hydroxides (LDHs). MRX 2022, 9, 035505.
- Zeng. Bin.; Qingqing. Wang.; Liwu. Mo.; Fei. Jin.; Jun. Zhu.; Mingshu. Tang. Synthesis of Mg-Al LDH and its calcined form with natural materials for efficient Cr (VI) removal. J. Environ. Chem. Eng 2022, 10, 108605. [CrossRef]
- M. Ahsan. Iqbal.; Michele. Fedel. Ordering and disordering of in situ grown MgAl-layered double hydroxide and its effect on the structural and corrosion resistance properties. Int. J. Miner. Metall 2019, 26, 1570. [CrossRef]
- M. G Suárez-Quezada.;., J. Romero-Ortiz.; E. Samaniego-Benítez.; V. Suárez.; A. Mantilla. H2 production by the water splitting reaction using photocatalysts derived from calcined ZnAl LDH. Fuel 2019, 240, 262. [CrossRef]
- Seftel. E. M.; E. Popovici.; M. Mertens.; K. De Witte.; G. Van Tendeloo.; P. Cool.; E. F. Vansant. Zn–Al layered double hydroxides: Synthesis, characterization and photocatalytic application. Microporous Mesoporous Mater 2008, 113, 296. [CrossRef]
- Elhalil. A.; R. Elmoubarki.; A. Machrouhi.; M. Sadiq.; M. Abdennouri.; S. Qourzal.; N. Barka. Photocatalytic degradation of caffeine by ZnO-ZnAl2O4 nanoparticles derived from LDH structure. J. Environ. Chem. Eng 2017, 5, 3719. [CrossRef]
- Zhang. Zhe.; Zhong. Hua.; Jihui. Lang.; Yuxin. Song.; Qi. Zhang.; Qiang. Han.; Hougang. Fan.; Ming. Gao.; Xiuyan. Li.; Jinghai. Yang. Eco-friendly nanostructured Zn–Al layered double hydroxide photocatalysts with enhanced photocatalytic activity. CrystEngComm 2019, 21, 4607–4619. [CrossRef]
- Ahmed, Ali.; Zainal. Abidin Talib.; Mohd. Zobir bin Hussein.; Azmi. Zakaria. Improvement of the crystallinity and photocatalytic property of zinc oxide as calcination product of Zn–Al layered double hydroxide. J. Alloys Compd 2012, 539, 154. [CrossRef]
- Tedim. J.; S. K. Poznyak.; A. Kuznetsova.; D. Raps.; T. Hack.; M. L. Zheludkevich.; M. G. S. Ferreira. Enhancement of active corrosion protection via combination of inhibitor-loaded nanocontainers. ACS Appl. Mater. Interfaces 2010, 2, 1528. [CrossRef] [PubMed]
- Prevot. V.; C. Forano.; J. P. Besse.; F. Abraham. Syntheses and thermal and chemical behaviors of tartrate and succinate intercalated Zn3Al and Zn2Cr layered double hydroxides. Inorg. Chem 1998, 37, 4293. [CrossRef] [PubMed]
- Mohapatra. Lagnamayee.; Kulamani. Parida. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem 2016, 28, 10744.
- Ye. Haoyang.; Shiyu. Liu.; Deyou. Yu.; Xuerong. Zhou.; Lei. Qin.; Cui. Lai.; Fanzhi. Qin. Regeneration mechanism, modification strategy, and environment application of layered double hydroxides: Insights based on memory effect. In Chem. Rev; 2022; Volume 450, p. 214253.
- Liu. Jiao.; Peng. Ding.; Zexuan. Zhu.; Wei. Du.; Xiaoyong. Xu.; Jingguo. Hu.; Yong. Zhou.; Haibo. Zeng. Engineering self-reconstruction via flexible components in layered double hydroxides for superior-evolving performance. Small 2021, 2021, 2021.

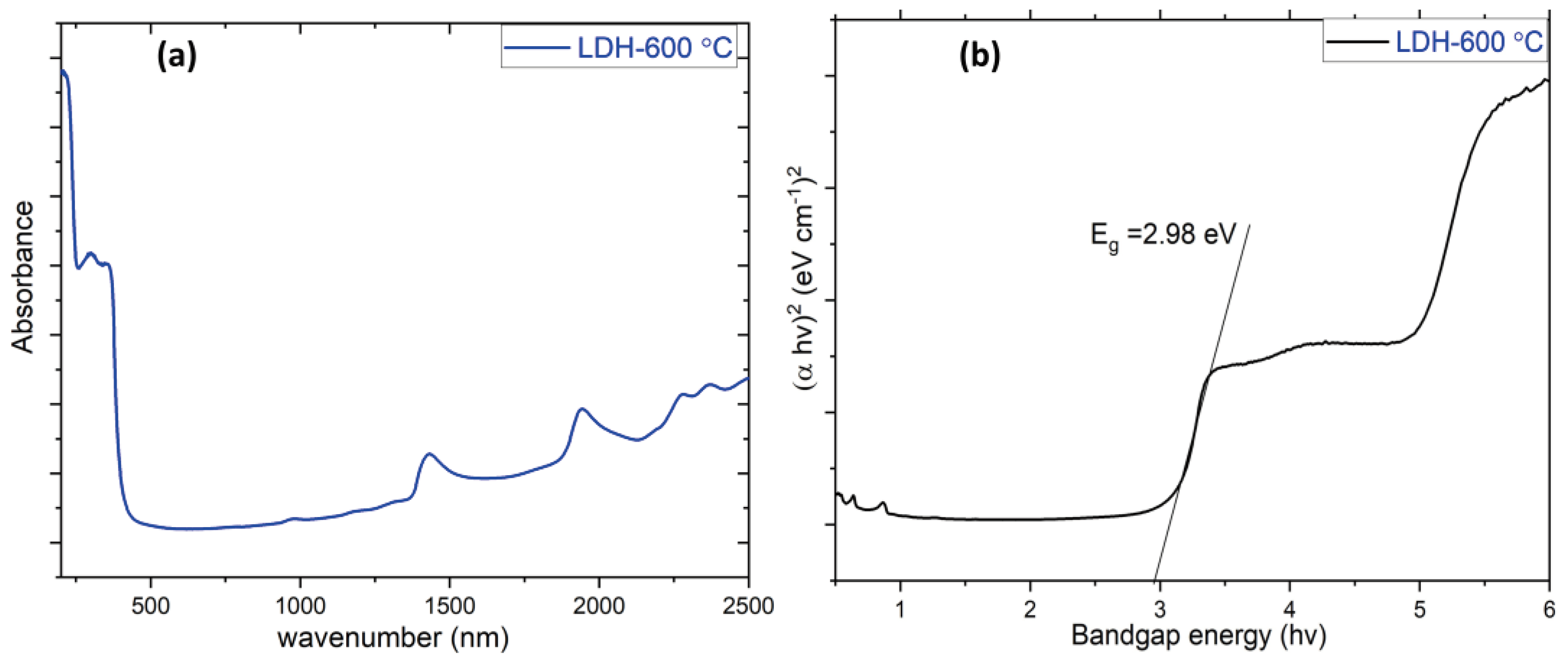
| Specimen | Lattice parameter | Interlayer distance | Crystallite size, D / nm | ||||
|---|---|---|---|---|---|---|---|
| a / nm | c / nm | d003 / nm | d006 / nm | d110 / nm | |||
| ZnAl-LDH | 0.356 | 2.71 | 0.90 | 19.7720.0120.12 | 60.322 | 3.227 | |
| LDH-400°C | 0.355 | 2.64 | 0.88 | 60.322 | 3.147 | ||
| LDH-500°C | 0.355 | 2.54 | 0.85 | 60.35 | 3.033 | ||
| Photon energy (eV) |
SBET (m2g-1) |
|
|---|---|---|
| ZnAl-LDH | 5.3 | 44.75 |
| LDH-400°C | 4.9 | 48.09 |
| LDH-500°C | 3.9 | 51.11 |
| LDH-600°C | 2.98 | 71.86 |
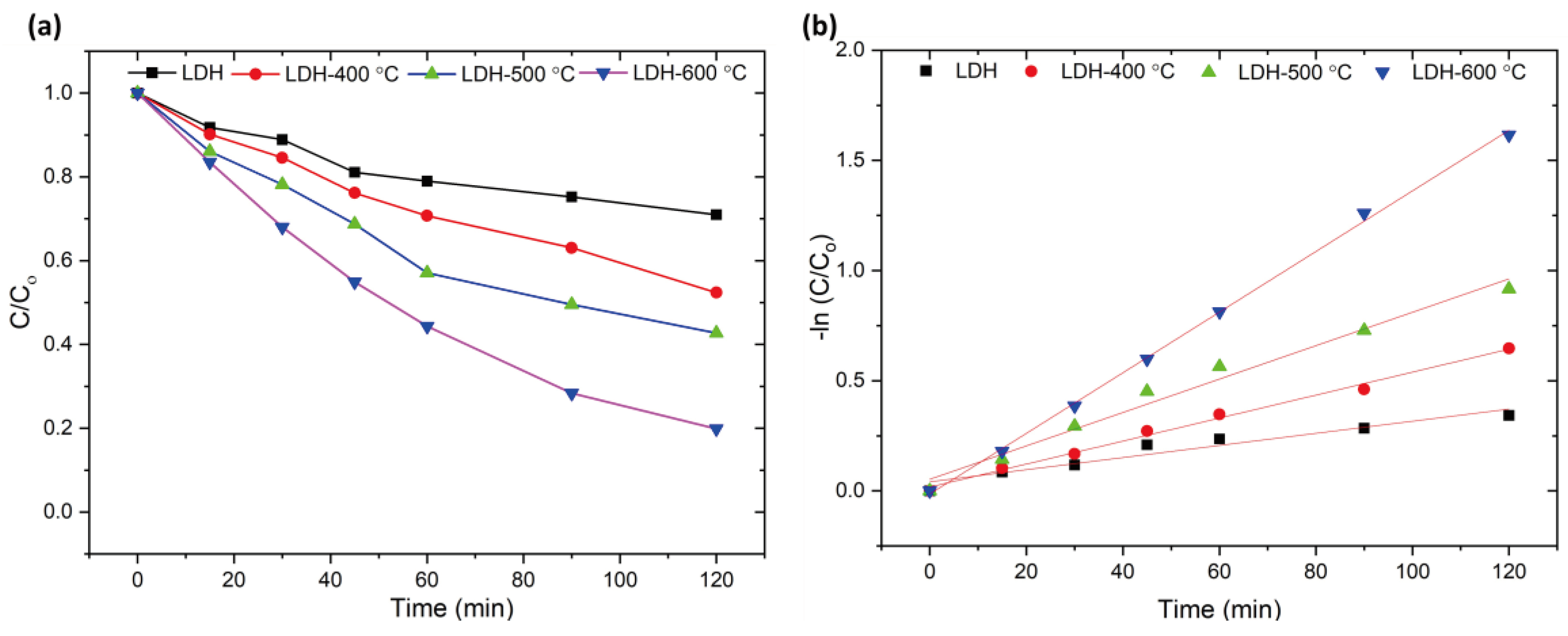
| Material | Removal Efficiency | Time | K1 (min-1) | R2 | Pseudo-first order Degradation Rate |
|---|---|---|---|---|---|
| (%) | (min) | (mM.min-1) | |||
| ZnAl-LDH | 20.19 | 120 | 0.0033 | 0.9694 | 0.000310 |
| LDH-400°C | 47.63 | 120 | 0.0051 | 0.9939 | 0.000471 |
| LDH-500°C | 59.17 | 120 | 0.0071 | 0.9951 | 0.000651 |
| LDH-600°C | 80.12 | 120 | 0.0136 | 0.9995 | 0.001268 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





