1. Introduction
Infectious pathogens, comprising viruses, bacteria, and protozoa, have had a profound impact on global public health and the economy. Among these pathogens, the influenza A virus (IAV) poses a persistent threat to various hosts, highlighting the significance of its pandemic potential and prompting international surveillance networks to monitor its emergence and circulation [
1]. The virus's ability to rapidly adapt to multiple hosts and evade the host immune system has resulted in significant challenges in preventing and controlling outbreaks [
2]. Furthermore, its remarkable genetic diversity, such as antigenic drift and shift, can potentially lead to a pandemic, which can transmit across a broad spectrum of hosts, including humans, mammals, and birds, as evidenced by historical records [
3].
On the other hand, flaviviruses represent the next significant pathogen for future pandemics and cause medically relevant illnesses in various hosts. For instance, Zika virus (ZIKV) and dengue virus (DENV) pose considerable health threats in Latin America [
4]. Following the ZIKV outbreak in the Americas, which resulted in a dramatic increase in microcephaly and brain malformations in newborns, ZIKV disease became an international public health emergency. Similarly, a significant increase in DENV cases has been reported in the Americas in recent decades, with an estimated 390 million infections per year [
5]. While many DENV infections resolve without complications, severe dengue remains a significant cause of illness and death in some Asian and Latin American countries [
4]. Therefore, the pandemic potential of problematic RNA viruses has recently attracted considerable attention in many governments and diverse industries, necessitating the development of antiviral strategies to tackle these challenges.
Numerous attempts have been made to discover antiviral compounds in natural resources with evidence of safety and strong effectiveness, which could be applied to clinical studies [
6,
7,
8]. These trials have led to exploring various sources, such as herbs and microorganisms from fresh or seawater. Notably, many studies in marine microbe revealed that carotenoids abundantly produced by the family Erythrobacteraceae exhibit antiviral functions by inhibiting viral genome transcription, virus-induced pro-inflammatory response, or cell death regardless of RNA or DNA viruses [
9,
10,
11,
12]. Carotenoids from
Qipengyuania pacifica, another member of the Erythrobacteraceae family, have also been demonstrated to have anti-bacterial capabilities, especially against bacterial infections that are resistant to a broad array of antibiotics [
13]. Collectively, compounds sourced from marine bacteria have become an active area of investigation in antiviral research.
Interestingly, infectious RNA viruses, including IAV, ZIKV, and DENV, induce necroptosis, while apoptosis is a limiting factor for producing infectious virus particles [
14,
15]. Host immune reaction develops a formation of apoptotic bodies containing intracellular organelles, other cytosolic components, and premature and mature viral particles [
16]. Subsequently, newly expressed ligands of apoptotic bodies stimulate phagocytosis, preventing the release of infection progeny and protecting the entire organism [
17]. Indeed, an extract from a marine microbe exhibited tremendous in vitro antiviral activity with guaranteed safety against IAV and influenza B virus (IBV) [
18]. Therefore, apoptosis-mediated host protection has been a new antiviral target, providing important insight into developing novel therapeutic strategies against RNA viruses.
The present study carried out antiviral screening using extracts of various marine bacteria. Among them is an extract from Parerythrobacter sp. M20A3S10, belonging to the family Erythrobacteraceae, which showed remarkable antiviral efficacies in vitro against influenza virus A/Puerto Rico/8/34 (H1N1) (A/PR8) [half maximal inhibitory concentration (IC50) = 51.1 µg/mL, selectivity index (SI) = 24.0], influenza virus A/Wisconsin/15/2009 (H3N2) (A/Wisconsin) (IC50 = 40.6 µg/mL, SI = 30.1), influenza virus B/Florida/78/2015 Victoria lineage (B/Florida) (IC50 = 32.1 µg/mL, SI = 38.2), ZIKV (IC50 = 69.6 µg/mL, SI = 22.5) and Dengue virus type 2 (DENV2) (IC50 = 65.1 µg/mL, SI = 24.1), namely a broad spectrum of antiviral potential.
Notably, the
Parerythrobacter extract is expected to contain large amounts of carotenoids [
9,
10,
19], which have been shown to exhibit antiviral functions by attenuating cholesterol synthesis, affecting the general replication cycle of enveloped viruses. However, the specific compound that affects apoptosis and viral replication is yet to be identified and quantified.
Nevertheless, the M20A3S10 extract suppresses viral replication by stimulating apoptosis-mediated host immunity in IAV, ZIKV, and DENV2 infections. These findings provide important insights for developing novel therapeutic strategies against enveloped RNA viruses. With further studies to identify and quantify the specific chemical within the Parerythrobacter extract, the antiviral evaluation in this study sheds light on a promising strategy for targeting broad infection by future pandemics.
2. Materials and Methods
2.1. Bacterial Isolation and Culture Conditions
Several bacterial strains, including Parasphingopyxis, Roseibium, Parerythrobacter, Neo-rhizobium, and Qipengyuania species, were collected from seawater in Seosan (Chungnam Province, KR) on March 16, 2020. The water sample was spread on ZoBell medium (0.5 g peptone, 0.1 g yeast extract, and 0.001 g ferric phosphate [FePO4] per liter of 20% distilled water and 80% filtered seawater) using the standard dilution-plating method. The inoculated plates were incubated at 25°C for 5 days, and individual colonies were streaked on marine agar 2216 for purification. The purified strains were routinely cultured on MA at 25°C and preserved with 20% (v/v) glycerol at -80°C. The bacterial isolates were deposited under the numbers MI00005948, MI00006287, MI00006290, MI00006301, MI00007026, and MI00007049, respectively, at the Microbial Marine BioBank (MMBB) of the National Marine Biodiversity Institute of Korea (MABIK).
2.2. Phylogeny of 16S rRNA Gene Sequences
The genomic DNA of the bacterial strains was extracted using an Exgene DNA extraction kit, and the 16S rRNA gene was PCR amplified using bacteria-specific universal primers 27F and 1492R [
20]. The amplified partial 16S rRNA gene sequences were sequenced and assembled by Geneious program v9.0.5 to obtain a nearly full-length 16S rRNA gene sequence. The EzBioCloud server was used to identify the phylogenetic position of strain M20A3S10, and the 16S rRNA gene sequence was compared with validly published species in the server [
21]. The 16S rRNA gene sequence was deposited into GenBank under the accession OR481698. Phylogenetic trees based on 1,419 unambiguously aligned sequences were reconstructed using the neighbor-joining (NJ), maximum-likelihood (ML), and maximum-parsimony (MP) algorithms in the MEGA X [
22,
23,
24,
25]. The genetic correlation among bacterial strains used in this study and reference strains was calculated using the Kimura-2 parameter model at the nucleotide level, and phylogenetic trees were constructed using the neighbor-joining method with 1000 bootstrap replicates [
26].
2.3. Preparation of the Bacterial Extracts
Bacterial extracts were prepared using a modified method from Xu et al. [
9]. The bacterial including
Parasphingopyxis, Roseibium, Parerythrobacter, Neorhizobium, and
Qipengyuania species, were cultured in 2.5 L Erlenmeyer flasks containing 1 L of marine broth under LED light at 25°C. Subsequently, 20 L of culture using a panel or column-type photobioreactor was inoculated with the initial inoculum at a concentration of 104 CFU mL-1 in the same culture condition for 10-20 days with shaking at 150 rpm. At the end of the culture period, culture broth was extracted twice with the same volume of ethyl acetate (EtOAc). The EtOAc soluble component of bacterial culture broth was combined and dried using a vacuum evaporator. A crude total of 150 mg of bacterial extract was obtained from each species. The extracts were dissolved in dimethyl sulfoxide (DMSO) for the antiviral evaluation.
2.4. Cells and Viruses
Madin-Darby canine kidney epithelium (MDCK; ATCC CCL-34) and African green monkey kidney epithelium (Vero; ATCC CRL-1586) E6 cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM) form WELGENE (Seoul, KR) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin(P/S) at 37°C in a 5% CO2 atmosphere. Two strains of influenza A virus (IAV) and one strain of influenza B virus (IBV) from ATCC were used: influenza virus A/Puerto Rico/8/34 (H1N1) (A/PR8), A/Wisconsin/15/2009 (H3N2) (A/Wisconsin) strain, and B/Florida/78/2015 Victoria lineage (B/Florida). For influenza virus propagation, MDCK cells were infected with influenza viruses at a multiplicity of infection (MOI) of 1 (1 MOI), supplemented with 1 µg/mL N-tosyl-L-phenylalanine chloromethyl ketone (TPCK)-treated trypsin and incubated at 37°C in a 5% CO2 atmosphere for 48 h. Both types of flavivirus were provided from the National Culture Collection for Pathogens (Cheongju, KR): Dengue virus type 2 (DENV2) isolated from serum samples from Korean patients traveling to Singapore, India, and Thailand in 2015 and Zika virus (ZIKV) strains of Asian/American lineage, PRVABC59. For the propagation of two flaviviruses, Vero E6 cells were infected with ZIKV and DENV2 (1 MOI) and incubated at 37°C in a 5% CO2 atmosphere for 48 h.
2.5. Cell Viability Assay
MDCK and Vero E6 cell viability was measured with extracts using the WST assay per the manufacturer's instructions (Abfrontier, Seoul, KR) [
27]. A confluent monolayer of MDCK and Vero E6 cells was prepared in a 96-well plate at a healthy density of 5.0 x 10
4 cells. The extracts were prepared using a continuous dilution method with an adjusted dilution factor, resulting in the following concentrations: 1000, 500, 250, 100, 50, 25, 10, 5, 2, 1, 0.5, 0.1, 0.01 µg/mL. MDCK cells and Vero E6 cells were treated with each extract concentration using the experimental scheme outlined and incubated at 37°C in a 5% CO
2 atmosphere for 48 h. The cells were then gently washed with DPBS, and 50 μL of CellVia working solution (90 μL of DMEM with 10 μL of CellVia stock solution) was added to each well. The cells were then incubated for 30 min, and absorbance was measured at 450nm with an ELISA microplate reader (Thermo Fisher Scientific, Waltham, MA, USA) Cell viability was calculated as a percentage in comparison with DMSO-treated cells. GraphPad Prism software v9. 5. 1 (GraphPad Software, CA, USA) was used to generate nonlinear regression curves and calculate median cell cytotoxicity concentration (CC
50) as described previously [
28].
2.6. Antiviral Assay
To screen for antiviral activity, the above-diluted extracts (100, 50, 25, 10, 5, 2, 1, 0.5, 0.1, 0.01 µg/mL) were mixed with A/PR8 strain (0.1 MOI) supplemented with 1% P/S and 1 µg/mL of TPCK-treated trypsin at RT for 1 h. The mixtures were then absorbed in confluent MDCK cells in 96-well plates for 1 h and incubated at 37°C in a 5% CO
2 atmosphere for 48 h. Cell viability was measured by the inhibition level of CPE induced by the A/PR8 strain using the MTT assay, per the manufacturer's instructions [
29]. The cells were washed with DPBS and received 200 μL of MTT solution (0.4 mg/mL) in each well. After incubation at 37°C in a 5% CO
2 atmosphere for 2 h, DMSO was added to dissolve formazan for 10 min at RT. Relative cell viability was then measured at 570nm.
Different treatment methods were applied to identify the mechanism of action of an extract, with inoculation with A/PR8 strain (0.1 MOI) as previously described [
18]: treatment 1 h before virus infection (pre-treatment), simultaneously with virus inoculum (co-treatment), or after virus absorption (post-treatment). Infected cells were incubated for 48 h, and tissue culture supernatants were collected to measure the level of mRNA and progeny virus titers. Antiviral activity was calculated based on the cell viability of infection using an MTT assay. Nonlinear regression curves and the median inhibitory concentration (IC
50) were determined as described above.
ZIKV and DENV2 were used to determine whether the extract had antiviral effects on other viruses. The serial-diluted extracts were mixed with ZIKV and DENV2 (0.1 MOI) supplemented with 1% P/S at RT for 1 h. The mixtures were then absorbed in confluent Vero E6 cells in 96-well plates for 1 h and incubated at 37°C in a 5% CO2 atmosphere for 48 h.
2.7. RT-qPCR
The quantitative RT-qPCR method was used to measure the virus in infected cells, and cell supernatants were analyzed at 48 h after treatment. Virus RNA was extracted using the QIAamp Viral RNA Mini kit (QIAGEN, Hilden, DE) per the manufacturer's guidelines [
30]. PCR was performed with SensiFAST™ SYBR® Lo-ROX One-Step Kit (Bioline Meridian BioScience, Cincinnati, OH, USA). For this reaction, a mixture was assembled in a total volume of 20 μL as follows: 0.2 μL reverse transcriptase (RT), 0.4 μL Ribosafe RNase inhibitor, 0.8 μL of a primer pair (10 μM), 1X SYBR Green, 100 ng of viral RNA, and nuclease-free water. The detecting primers are as follows: forward (5’-GGCCCTTCAGTTGTTCATC-3'), and reverse primers (5’-GCAGACTTCAGGAATGTG-3') against IAV PB1, forward (5’-GGTCATGATACTGCTGATTGC-3'), and reverse primers (5’-CCACTAACGTTCTTTTGCAGAC-3') against ZIKV NS5, (5’-AGTTGTTAGTCTRYGTGGACCGAC-3'), and reverse primers (5’-TTGCACCAACAGTCAATGTCTTCAGGTTC-3') against DENV prM-. After incubation at 42°C for 30 min, PCR amplification was carried out with the following conditions: For IAV, the denaturation step (95°C, 10 s), annealing step (60°C, 10 s), and extension step (72°C, 15 s) were repeated 40 times; For ZIKV and DENV2, the denaturation step (95°C, 10s), annealing step (55°C, 15 s), and extension step (72°C, 15 s) were repeated 35 times.
2.8. Virus Titration
To confirm the progeny virus production of the supernatants, a 50% tissue culture infective dose (TCID
50) was used, as described previously [
16]. The supernatants were prepared in a 10-fold dilution series with DMEM and absorbed in a confluent MDCK cell for 1h at 37°C in a 5% CO
2 atmosphere. At 5 days post-infection, cells were fixed with 4% neutral paraformaldehyde (4% PFA, Bio-solution, Seoul, KR), stained with 0.5% crystal violet solution, and TCID
50 was determined by the Reed–Muench method.
2.9. Attachment and Penetration Assays
The attachment and penetration assays were performed as described previously [
18,
31]. For the attachment assay, confluent MDCK cells in a 24-well plate were treated with extracts (100 µg/mL) at 4°C before infection. After 30 minutes, the inoculum was washed thoroughly with cold DPBS. Then, the A/PR8 strain (1 MOI) was absorbed for 1h on ice. After thorough washing with cold DPBS, the cells were incubated for 20 h at 37°C in a 5% CO
2 atmosphere. MDCK cells in a 24-well plate received A/PR8 strain (1 MOI) for 1 h on ice for penetration assay. After thorough washing, the cells were treated with extracts for 10 minutes and incubated for 20 hours at 37°C in a 5% CO
2 atmosphere.
2.10. Cell Culture Immunofluorescence Assay
MDCK cells were prepared in an 8-well chamber to check viral protein synthesis and infected with the A/PR8 strain (0.1 MOI). Vero E6 cells were also prepared and infected with ZIKV, and DENV2 (0.1 MOI) as described elsewhere [
14,
32]. After fixation with 4% PFA for 10 min at RT, the chamber was washed and blocked with 5% Bovine Serum Albumin (BSA) at RT for 1 h to reduce non-specific reaction. Subsequently, a primary antibody against IAV M2, flavivirus envelope protein 4G2, was applied overnight at 4°C. The next day, a secondary antibody, goat-anti mouse Alexa Flour® 488 conjugated to a fluorescent dye, was added, and the cells were incubated for 1 h at RT. The 96-well plates were washed and analyzed using a fluorescence microscope.
2.11. Flow Cytometry
The present study utilized flow cytometry to assess the induction of viral protein and apoptosis. The methodology adopted was in accordance with previously established protocols [
33]. Briefly, Madin-Darby Canine Kidney (MDCK) cells were inoculated with influenza A Virus (IAV) at 0.1 MOI and incubated at 37°C for an hour in a 5% CO2 atmosphere. Following this, a vehicle or an extract at a concentration of 50 µg/mL was added to each well, and the cells were then incubated at 37°C for 48 hours. Subsequently, the cells were washed with Dulbecco's Phosphate Buffered Saline (DPBS) and centrifuged at 3000 rpm at 4°C. This procedure resulted in the formation of a cell pellet, which was subjected to the TUNEL assay to detect apoptosis using the In Situ Cell Death Detection Kit (Roche, Basel, CH) after blocking with 5% BSA at room temperature for an hour, per the manufacturer's instructions [
16]. Additionally, primary antibodies against IAV M2 and secondary antibodies conjugated with AF647 were applied to evaluate viral protein. Similarly, Vero E6 cells were inoculated with ZIKV and DENV2 at 0.1 MOI and incubated at 37°C for an hour in a 5% CO2 atmosphere. Flavivirus envelope protein 4G2 was utilized as the primary antibody against ZIKV and DENV2, and a secondary antibody conjugated with AF947 was applied. Flow cytometry was performed using the AttuneTM NxT flow cytometer (Thermo Scientific), and the AttuneTM NxT software v3.1.2 was used to digitize the data from each sample.
2.12. Statistical Analysis
Statistical analysis was carried out using GraphPad Prism software, with one-way ANOVA being employed. The data was expressed as the mean ± standard deviation (SD) of at least three independent experiments. Statistical significance was considered at the following levels: *, P < 0.05; **, P < 0.01; ***, P < 0.001, ****, P < 0.0001. The selective index (SI) was calculated using sigmoidal dose-response curves, with the following equation: SI = mean CC50 / mean IC50. The ratio of CC50 to IC50, i.e., CC50/IC50, was employed to calculate SI.
4. Discussion
The COVID-19 pandemic has brought to light the importance of pandemic preparedness. The next pandemic is expected to attract even more historical attention, particularly with respect to the influenza virus and flavivirus, which have continuously posed a threat to human society due to their high contagiousness and potential to undermine public health and economies worldwide [
35]. Those viruses have high mutation rates and lack proofreading mechanisms, leading to genetic diversity [
36]. This diversity can give rise to mutant or variant viruses that can infect humans via zoonotic transmission, thereby raising serious public health concerns regarding climate change and newly emerging reservoirs such as bats, mosquitos, and birds [
35]. Consequently, the pandemic potential of these pathogens highlights the need to develop new antiviral strategies and drugs. In this regard, marine microbes have gained significant attention, particularly as a source of natural compounds with antiviral, anti-inflammatory, anti-microbial, and anti-malarial properties [
37,
38,
39], some of which have entered clinical studies or advanced stages for commercial uses.
An extract of
Mameliella sp., a Gram-negative, non-motile, rod-shaped marine bacterium, recently exhibited significant antiviral activity against IAV and IBV [
18]. In parallel, we conducted preliminary antiviral screening with 200 marine bacteria isolated from the coastal seawater. We identified
Parerythrobacter sp. M20A3S10 was the bacterium whose extract exhibited the most significant safety and effectiveness as an antiviral against A/PR8 infection. This novel strain of the rare genus
Parerythrobacter was isolated from the seawater closest to
Parerythrobacter lutipelagi (98.11%). Notably, the Erythrobacteraceae family, including
Parerythrobacter, is known to produce carotenoids [
9]. Carotenoids are organic pigments studied for their antioxidant properties, which could interfere with viral replication mechanisms [
12,
40]. Carotenoids from
Qipengyuania pacifica, another member of the Erythrobacteraceae family, have also been demonstrated to have anti-bacterial capabilities, especially against bacterial infections that are resistant to a broad array of antibiotics [
13].
In other words, influenza viruses have lipid envelopes that are susceptible to disruption [
40], so it is plausible that carotenoid-rich extracts could destabilize the viral membrane. Additionally, some members of the Erythrobacteraceae family have been found in marine environments rich in bioactive compounds that could have broader antiviral effects [
13]. While empirical studies are needed to substantiate these claims, the unique biochemical profile of carotenoid-producing bacteria within the Erythrobacteraceae family offers a promising target for developing novel antiviral agents against influenza viruses [
13,
40].
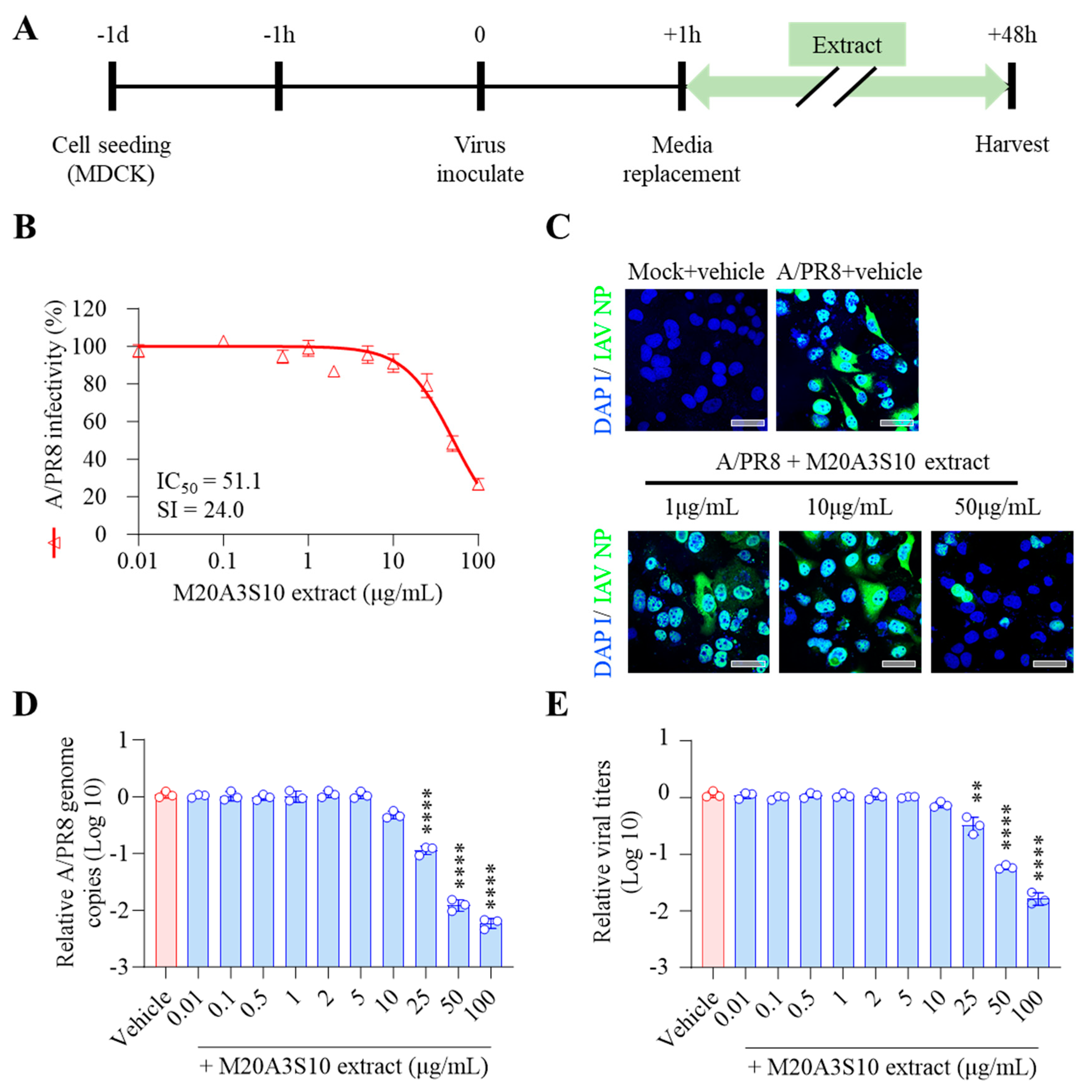
Post-treatment of M20A3S10 extract exhibited remarkable antiviral activity against A/PR8 infection (IC50 = 51.1 µg/mL, SI = 24.0), which was more significant than chloroquine, a commercialized drug. In addition, dose-dependent antiviral activity was found in viral protein synthesis, genome synthesis, and progeny production. However, pre-and co-treatment of the extract did not suppress the influenza virus. Even if the penetration assay revealed a significant reduction of the viral genome, it is likely due to some residual components from the extract within the cell during pre-treatment. Collectively, it indicates that the antiviral action of the extract is supposedly involved in post-entry stages, suggesting post-treatment therapy is available after viral infection, which is more adaptable in clinical uses and more feasible for controlling epidemic diseases than prophylactic therapy.
Regarding compounds of marine extracts, carotenoids could exert antiviral activities in the late stage of viral replication, primarily during genome replication and caspase-mediated apoptotic clearance [
41,
42]. Accordingly, we found that M20A3S10 extract remarkably suppressed virus replication by enhancing apoptotic reactions. In many viral and bacterial infections, apoptosis prevents the growth of intracellular microbes as an innate defense mechanism [
34]. In other words, apoptosis exhibits a significant antiviral reaction by inhibiting IAV replication and respiratory immunopathology [
43].
Interestingly, flavivirus shares common characteristics with the influenza virus during its replication. Flavivirus has cholesterol as a component of the envelope, which is critical for viral binding, entry, and fusion processes. Therefore, a drug modulating cholesterol synthesis, such as statins, could efficiently control the influenza virus and flavivirus [
4]. Indeed, M20A3S10 extract significantly inhibits viral protein synthesis, replication, and progeny production against either ZIKV or DENV infection in vitro. The therapeutic indexes are IC
50 = 69.6 µg/mL and SI = 22.5 against ZIKV, IC
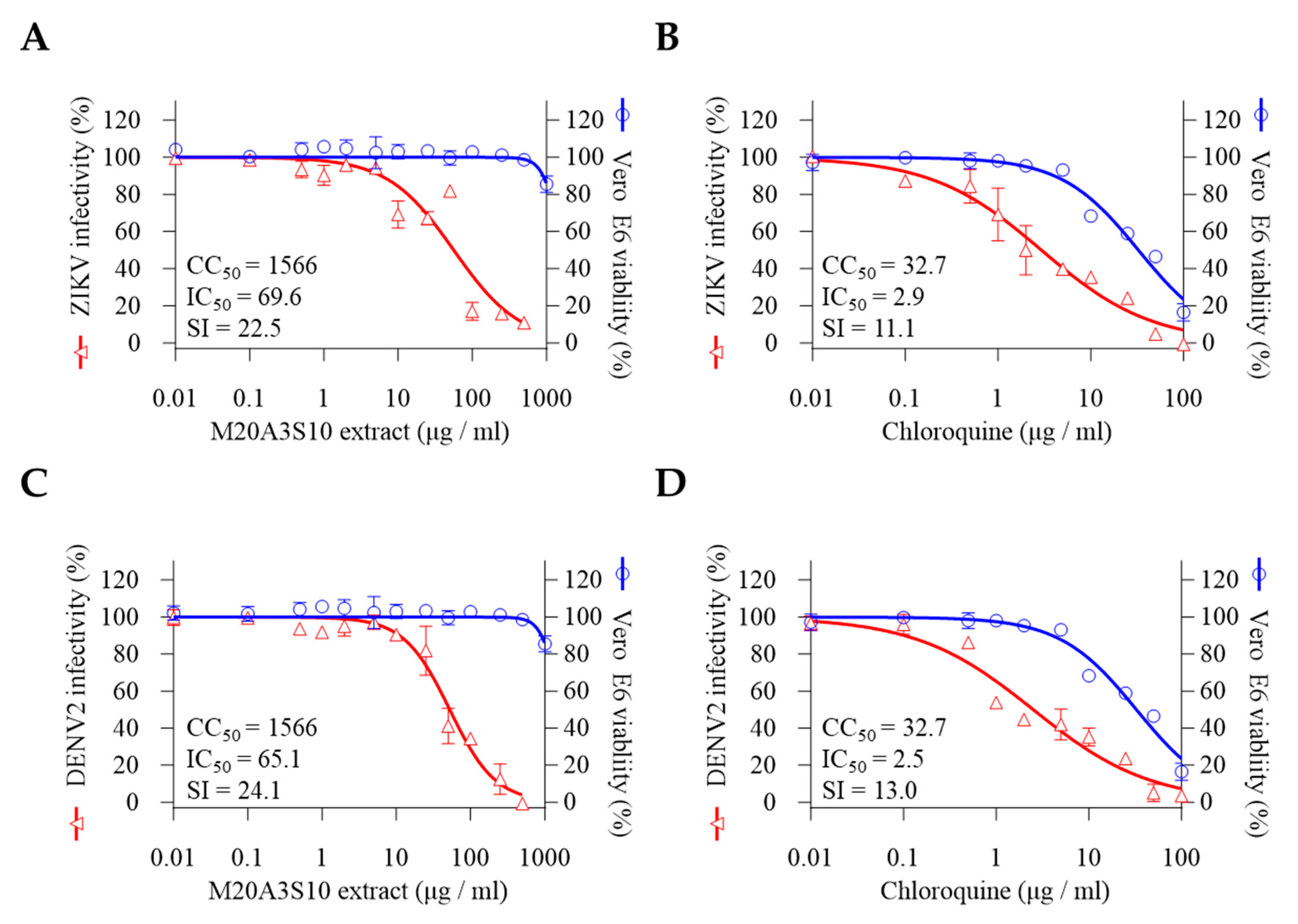
50 = 65.1 µg/mL, and SI = 24.1 against DENV, suggesting a cholesterol-destabilizing component within the extract should be further investigated to control influenza virus and flavivirus.
Regarding the replication mechanism, IAV, ZIKV, and DENV induce necroptosis through the NLRP3 inflammasome while evading apoptosis, thereby allowing more reproduction of progeny. However, hosts exhibit an essential innate immune reaction, where viral RNA is detected by RIG-I-like receptors, triggering IRF3-mediated apoptosis, which limits viral propagation [
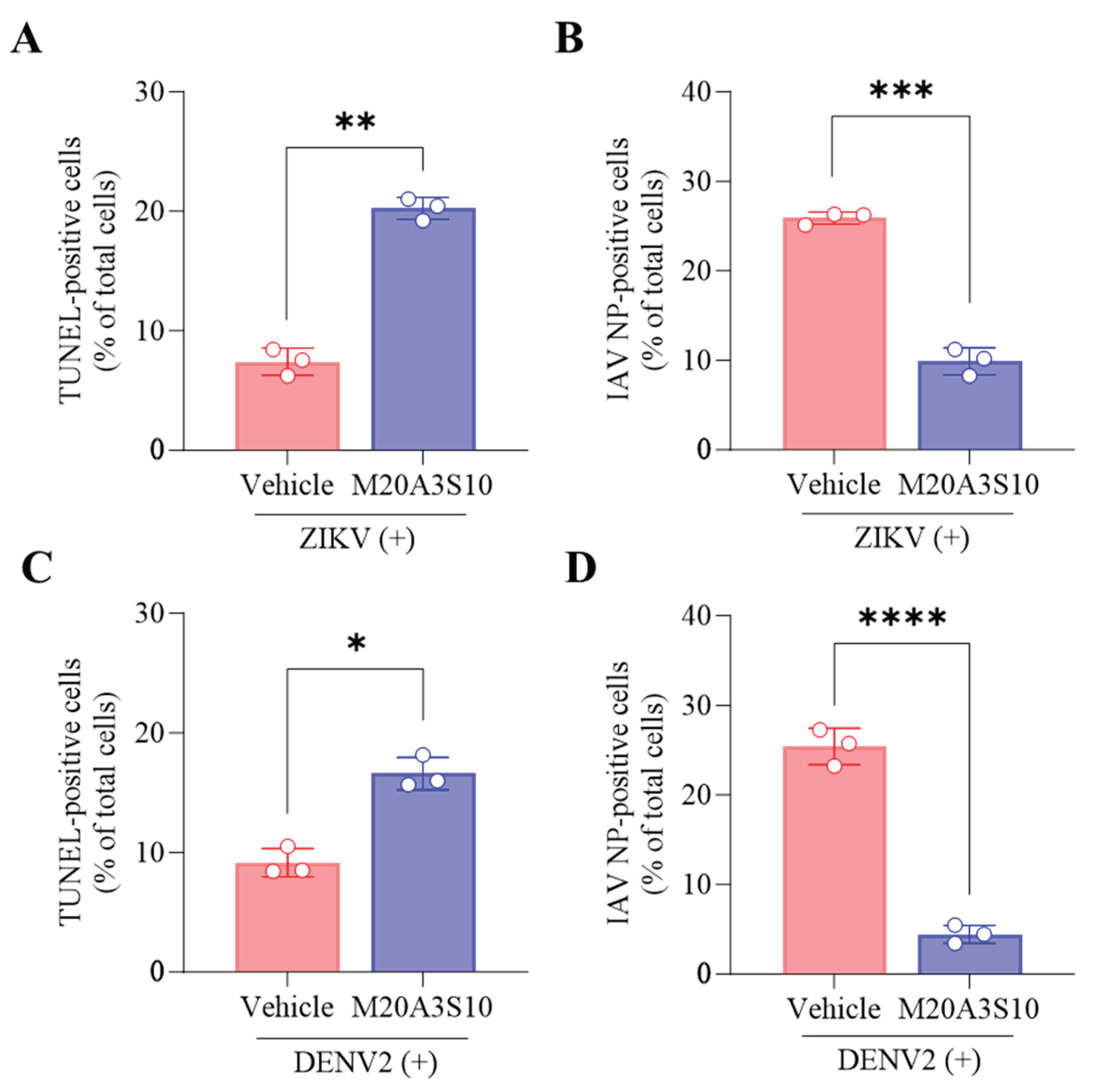
44]. As expected, post-treatment of the M20A3S10 extract enhanced apoptotic reaction, reducing viral replication in ZIKV and DENV infections.
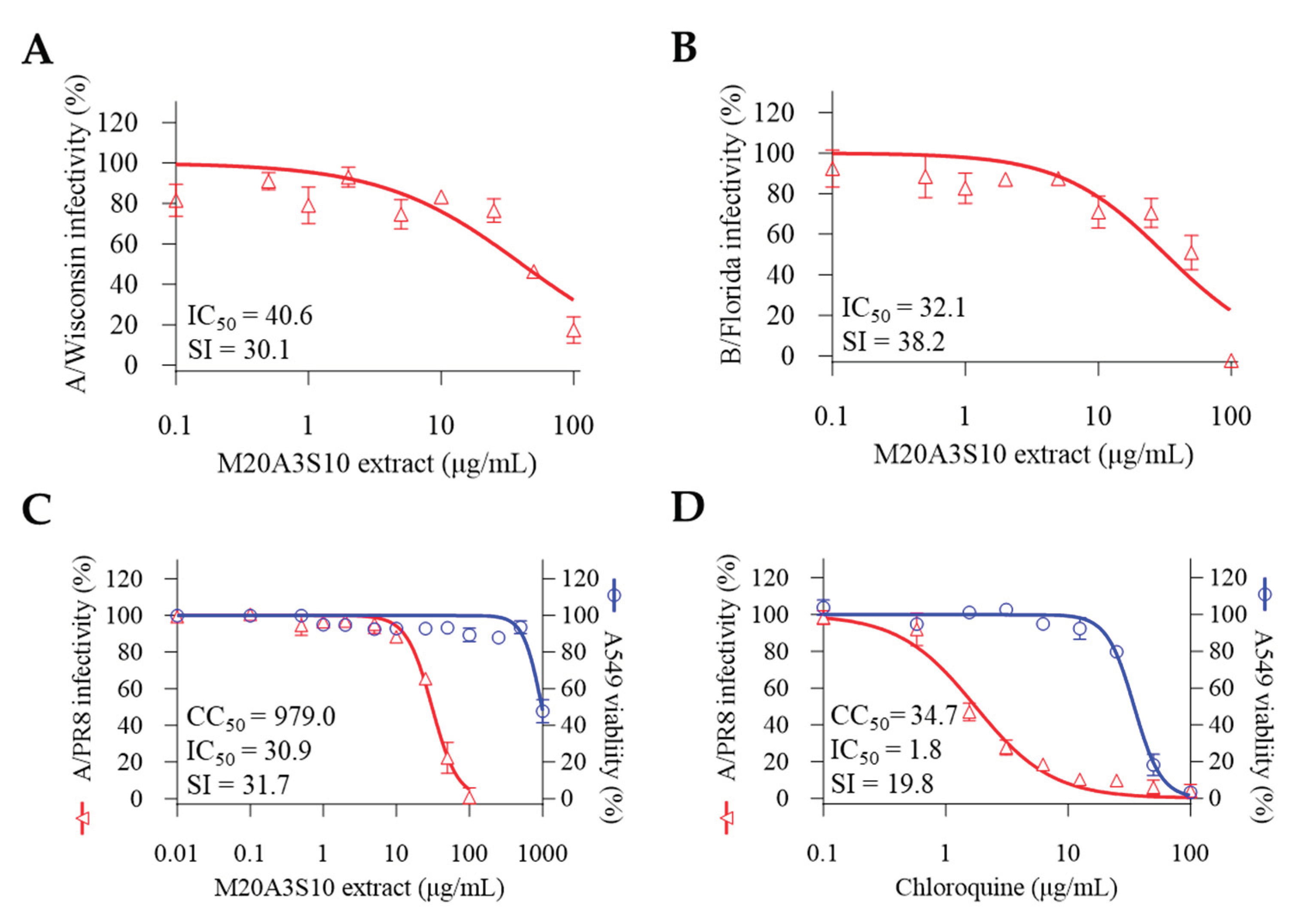
A broad antiviral mechanism was investigated using multiple strains of influenza viruses, resulting in promising therapeutic indexes against A/Wisconsin (IC50 = 40.6 µg/mL, SI = 30.1) and B/Florida (IC50 = 32.1 µg/mL, SI = 38.2). Moreover, the extract's effectiveness was guaranteed in a lung epithelial cell line against A/PR8 infection (IC50 = 30.9 µg/mL, SI = 31.7), greater than chloroquine. Given the similarity of the viral architecture and replication mechanism, it is plausible that apoptosis-mediated innate immunity is the limiting factor in controlling broad-spectrum infections by IAV, IBV, ZIKV, and DENV, which the M20A3S10 extract could efficiently induce.
Since The Great Flu of 1918, the influenza virus has been the focus of active investigation in public health, requiring significant effort for antiviral treatment and drug development. Multidisciplinary research, including microbiology, natural product chemistry, and molecular biology, has been undertaken [
45,
46]. Currently, antiviral studies have focused on creating a novel lead agent from natural compounds due to their notable efficacy and safety in controlling various viral diseases [
18,50]. The extract of
Parerythrobacter sp. M20A3S10 has been identified as a promising source of broad-spectrum antiviral agents that are both effective and safe.
Further research is required to identify antiviral components within the extract, including carotenoids, and to explore their properties and profiles using LC-MS/MS-based metabonomics. Animal experiments will also be required to evaluate the in vivo antiviral potential. Nevertheless, given its promising efficacy, possible antiviral mechanism of action, and broad-spectrum activity, antiviral research with the M20A3S10 extract could be extended to pre-clinical studies targeting current viral diseases and emerging pandemics in the future. Additionally, the antiviral mechanism of carotenoid-producing bacteria from the Erythrobacteraceae family against influenza viruses will be an active area of further investigation.
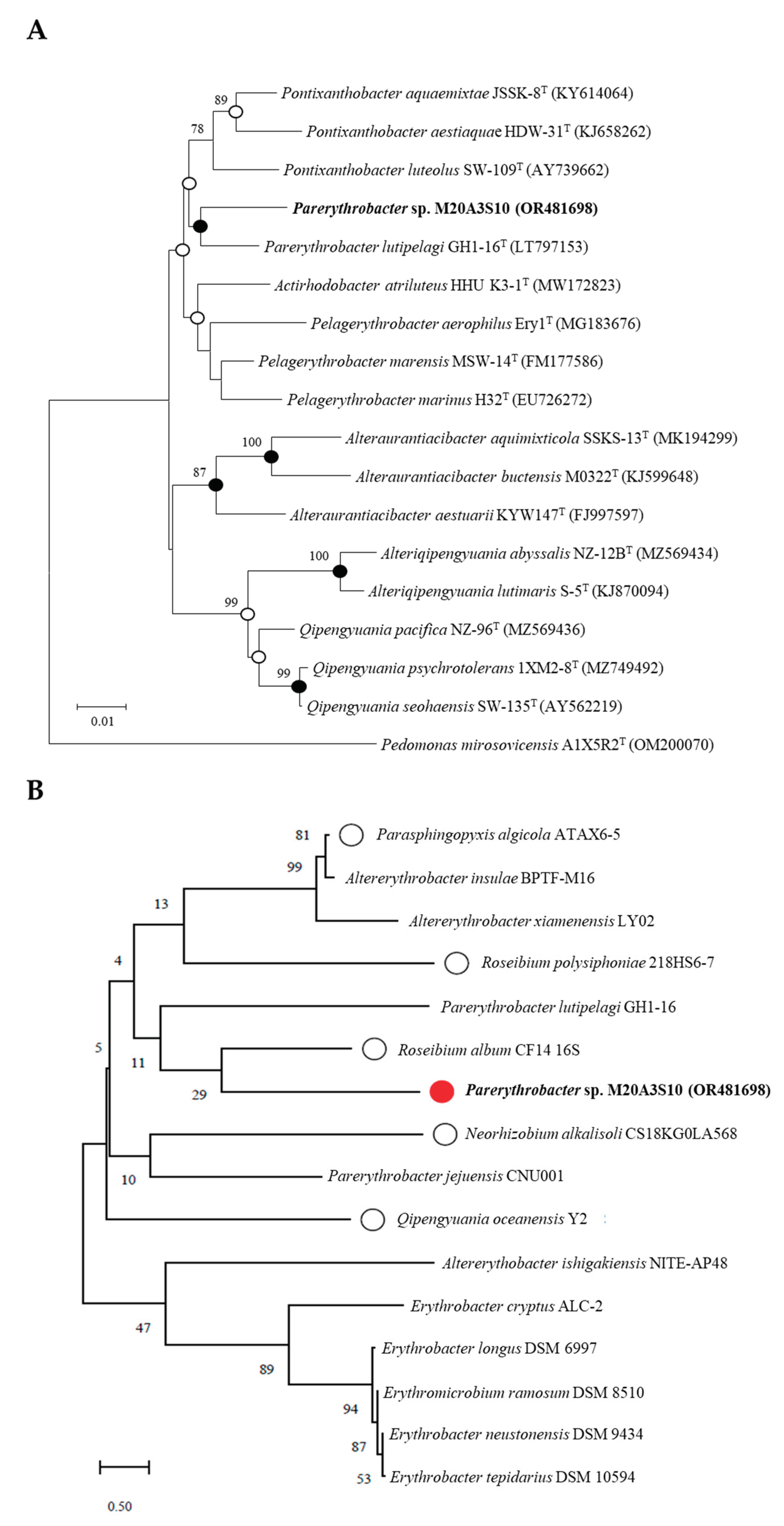
Figure 1.
Phylogenetic analysis of marine bacteria either in use or for comparison with isolated strains. (A) A neighbor-joining tree based on 16S rRNA gene, showing the phylogenetic relationships of strain M20A3S10 (in bold type) and closely related taxa with validly published names. GenBank accession numbers are given in parentheses. Bootstrap values above 70 % are shown on nodes in percentages of 1,000 replicates. Bar, 0.01 changes per nucleotide position. (B) Marine bacteria whose extracts were subjected to preliminary antiviral screening. Bar, 0.04 changes per nucleotide position.
Figure 1.
Phylogenetic analysis of marine bacteria either in use or for comparison with isolated strains. (A) A neighbor-joining tree based on 16S rRNA gene, showing the phylogenetic relationships of strain M20A3S10 (in bold type) and closely related taxa with validly published names. GenBank accession numbers are given in parentheses. Bootstrap values above 70 % are shown on nodes in percentages of 1,000 replicates. Bar, 0.01 changes per nucleotide position. (B) Marine bacteria whose extracts were subjected to preliminary antiviral screening. Bar, 0.04 changes per nucleotide position.
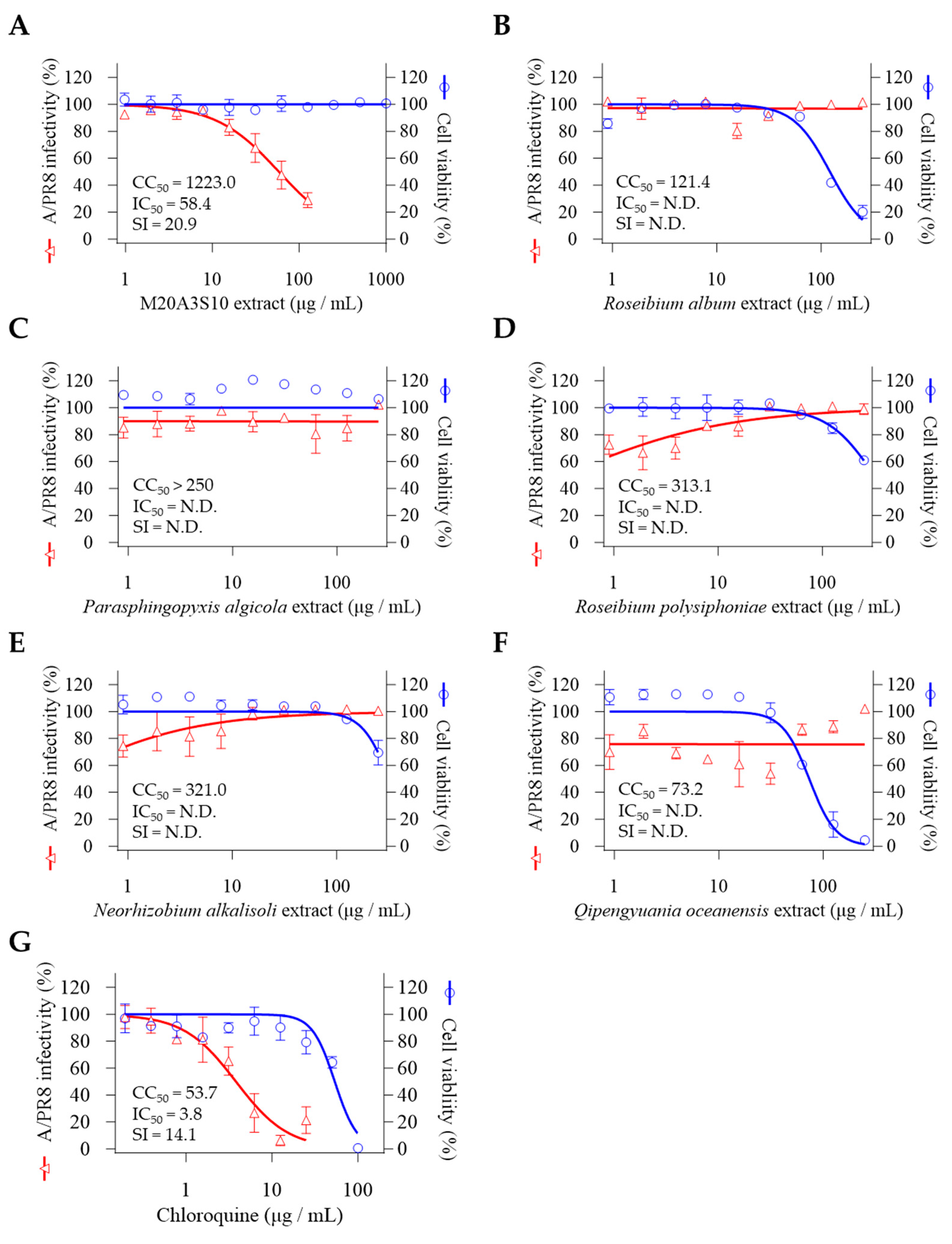
Figure 2.
Marine bacteria whose extracts were subjected to preliminary antiviral screening. (A) Parerythrobacter sp. M20A3S10, (B) Roseibium album, (C) Parasphingopyxis algicola, (D) Roseibium polysiphoniae, (E) Neorhizobium alkalisoli, (F) Qipengyuania oceanensis.
Figure 2.
Marine bacteria whose extracts were subjected to preliminary antiviral screening. (A) Parerythrobacter sp. M20A3S10, (B) Roseibium album, (C) Parasphingopyxis algicola, (D) Roseibium polysiphoniae, (E) Neorhizobium alkalisoli, (F) Qipengyuania oceanensis.
Figure 3.
Antiviral effect of M20A3S10 extract by post-treatment. (A) Schematic diagram of virus inoculation and extract treatment. (B) IC50 and SI of M20A3S10 extract measured by CPE-inhibition assay. (C) Viral protein synthesis detected by IFA. (D) Viral genome copies detected by RT-qPCR. (E) Progeny virus production measured by TCID50. All data in the graphs are presented as arithmetic means ± S.D. from 3 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001, ****, P < 0.0001.
Figure 3.
Antiviral effect of M20A3S10 extract by post-treatment. (A) Schematic diagram of virus inoculation and extract treatment. (B) IC50 and SI of M20A3S10 extract measured by CPE-inhibition assay. (C) Viral protein synthesis detected by IFA. (D) Viral genome copies detected by RT-qPCR. (E) Progeny virus production measured by TCID50. All data in the graphs are presented as arithmetic means ± S.D. from 3 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001, ****, P < 0.0001.
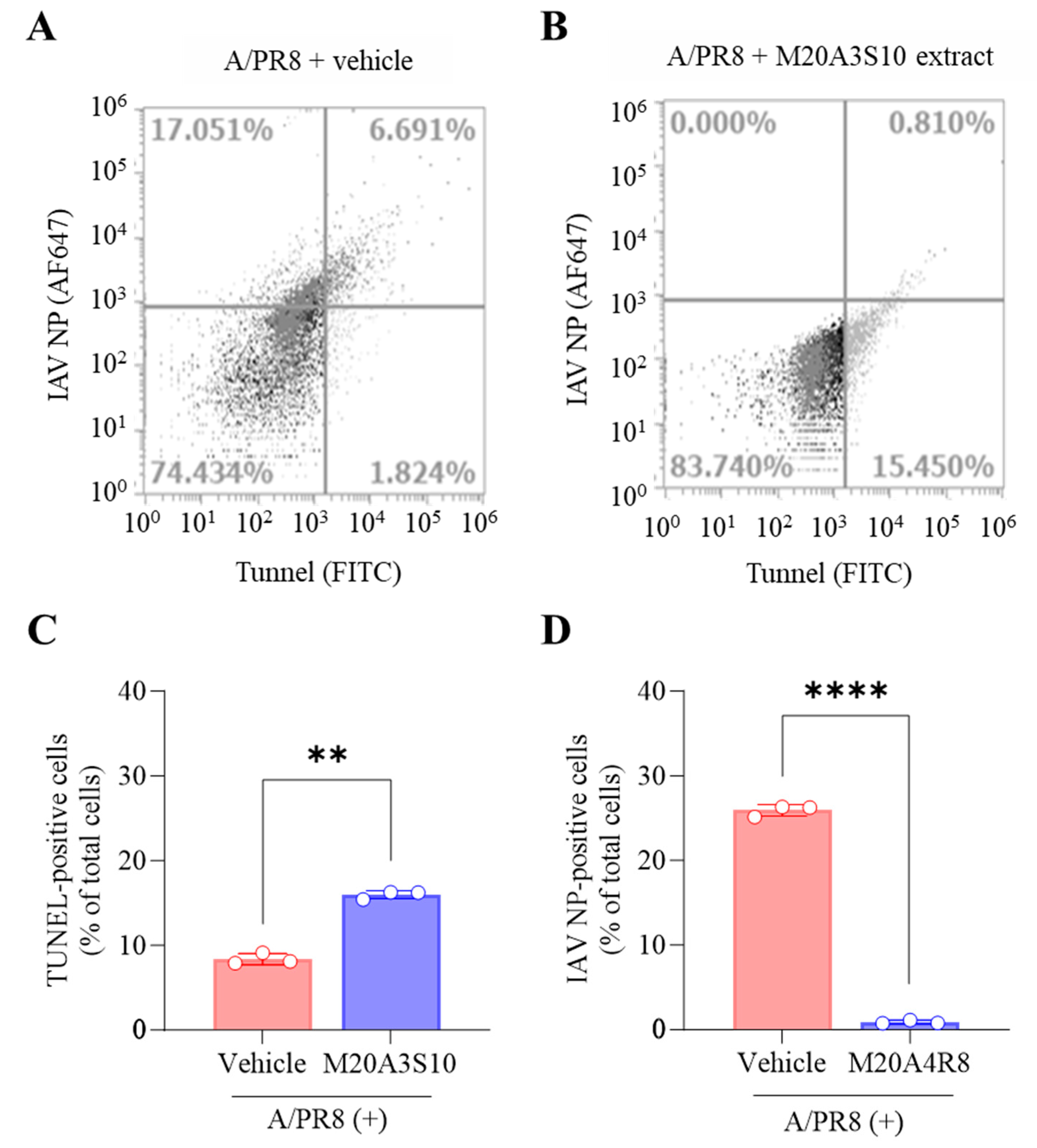
Figure 4.
Evaluation of apoptotic response by M20A3S10 extract using flow cytometry. Apoptosis was measured by TUNEL assay and virus protein by an antibody against IAV NP. (A) A/PR8-infected (0.1 MOI) vehicle-treated group. (B) A/PR8-infected (0.1 MOI), M20A3S10 extract-treated group. Quantification of TUNEL- (C) and virus-positive cells (D) treated with vehicle or M20A3S10 extract. **, P < 0.01; ****, P < 0.0001.
Figure 4.
Evaluation of apoptotic response by M20A3S10 extract using flow cytometry. Apoptosis was measured by TUNEL assay and virus protein by an antibody against IAV NP. (A) A/PR8-infected (0.1 MOI) vehicle-treated group. (B) A/PR8-infected (0.1 MOI), M20A3S10 extract-treated group. Quantification of TUNEL- (C) and virus-positive cells (D) treated with vehicle or M20A3S10 extract. **, P < 0.01; ****, P < 0.0001.
Figure 5.
Antiviral activity against flaviviruses by full-treatment of M20A3S10 extract. Antiviral actions were measured against ZIKV (A,B) and DENV (C,D) infections, treated with either the M20A3S10 extract or chloroquine.
Figure 5.
Antiviral activity against flaviviruses by full-treatment of M20A3S10 extract. Antiviral actions were measured against ZIKV (A,B) and DENV (C,D) infections, treated with either the M20A3S10 extract or chloroquine.
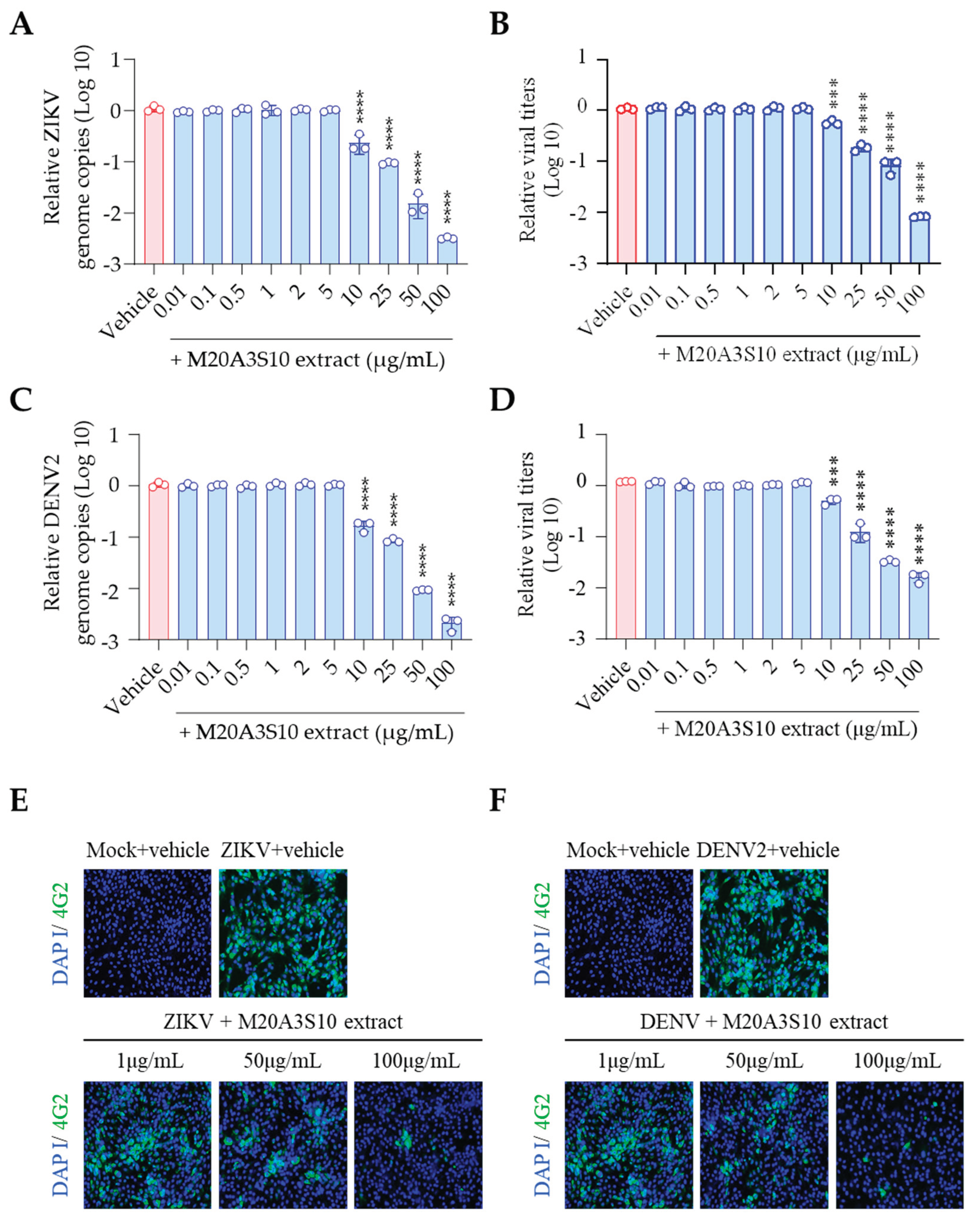
Figure 6.
ZIKV and DENV of progeny virus inhibition effect according to M20A3S10 extract treatment (A,B) ZIKV and DENV genome copies detected by RT-qPCR. (C,D) ZIKV and DENV protein synthesis detected by IFA. ***, P < 0.001; ****, P < 0.0001.
Figure 6.
ZIKV and DENV of progeny virus inhibition effect according to M20A3S10 extract treatment (A,B) ZIKV and DENV genome copies detected by RT-qPCR. (C,D) ZIKV and DENV protein synthesis detected by IFA. ***, P < 0.001; ****, P < 0.0001.
Figure 7.
Evaluation of apoptotic response by M20A3S10 extract using flow cytometry. Apoptosis was measured by TUNEL assay and virus protein by an antibody against flavivirus group envelope protein, respectively. (A-D) Summary of flow cytometry data. Quantification of TUNEL- (A,C) and virus-positive cells (B,D) treated with the vehicle or M20A3S10 extract. All data in the graphs are presented as the arithmetic mean ± S.D. from three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001, ****, P < 0.0001.
Figure 7.
Evaluation of apoptotic response by M20A3S10 extract using flow cytometry. Apoptosis was measured by TUNEL assay and virus protein by an antibody against flavivirus group envelope protein, respectively. (A-D) Summary of flow cytometry data. Quantification of TUNEL- (A,C) and virus-positive cells (B,D) treated with the vehicle or M20A3S10 extract. All data in the graphs are presented as the arithmetic mean ± S.D. from three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001, ****, P < 0.0001.
Figure 8.
The post-treatment of the M20A3S10 extract shows a broad spectrum of antiviral potential against multiple influenza viruses. Antiviral actions were measured against the A/H3N2 strain (A) and the B/Yamagata strain (B). Antiviral responses in A549 cells by the post-treatment of M20A3S10 extract (C) and chloroquine (D).
Figure 8.
The post-treatment of the M20A3S10 extract shows a broad spectrum of antiviral potential against multiple influenza viruses. Antiviral actions were measured against the A/H3N2 strain (A) and the B/Yamagata strain (B). Antiviral responses in A549 cells by the post-treatment of M20A3S10 extract (C) and chloroquine (D).