1. Introduction
Microplastics pollution has become ubiquitous, so much so that these particles are now being unintentionally assimilated by both animals [
1,
2] and humans [
3]. With the rise in microplastics contamination, concerns have also risen regarding the potential health risks of such exposure. While most recent scientific reports focused on microplastics (MPs) toxicity in terms of teratogenic effects [
4], as well as their accumulation in organs such as the placenta [
5], lungs [
6] or the intestinal tissues [
7], including microplastic-related toxicity represented by endocrine disrupting effects [
8,
9], fewer scientific releases reported MPs potential as microbial carriers.
The most frequent MPs pollution occurs in the aquatic environment, due to anthropogenic factors [
10,
11]. Once the aquatic environment has been contaminated, the MPs are scaling up the food chain as they are mistakenly ingested as food by the aquatic fauna, or via trophic transfer [
12,
13]. Fish are the main consumers of MPs, with emerging evidence suggesting that MPs accumulate in the gastrointestinal tract of various fish species [
14,
15]. Predatory fish species in particular present higher MPs accumulation compared to omnivorous species, as Sequeira et al. [
16] reported. In the study that involved 198 species examined across 24 countries, MPs were detected in 60% of wild-caught fish and 14% of aquaculture fish [
16].
Once ingested, MPs can block the gastrointestinal tract resulting in physical damage [
17] or can induce fake satiation [
18], resulting in the starvation or even potential death of the animal [
19,
20]. Furthermore, MPs ingestion can induce intestinal dysbiosis in both humans [
19] and animals [
21].
When the MPs are excreted, they may carry along a bacterial load represented by the hosts’ intestinal microbiota. Although several reports have stated the interaction of MPs with the intestinal microbiota as in toxicological effects [
22] and even in regard to a potential biodegradation through digestion and intestinal bacteria [
23], colonization of MPs by bacteria, especially pathogens, should not be overlooked. Zettler et al., 2010 [
24] coined the term “Plastisphere” which represent the diverse microbial community inhabiting plastic particles. Some inhabitants of the plastisphere are opportunistic pathogens, such as members of the genus
Vibrio, of which strains are capable of reproducible biofilm formation on multiple types of polymers [
25].
Although evidence bulked-up during the past decade, MPs have only recently started to be strongly suspected of spreading disease [
26], mainly due to the fact that the pollution with such agents is growing, and pathogens may use these durable fragments to hitchhike long distances in water [
27,
28]. Therefore, the purpose of this study was to evaluate under controlled condition to which extent, if any, nylon MPs are colonized by bacteria, especially by fish and waterborne pathogens.
2. Materials and Methods
2.1. Specimens and Exposure
Wild specimens of
Oreochromis niloticus (Nile tilapia) weighting
≈400 grams each, were grouped by five in three water tanks of 300 L each, under similar conditions (
Supplementary Table 1), and food deprived for one week prior to the exposure. Feed was grinded and reformed into pellets, with each pellet containing one strip of thermo-resistant nylon (nylon 6) measuring 2 mm width and 4 mm length. The nylon-containing feed pellets were left to dry at room temperature for 24 h and then autoclaved (121 °C, 15 psi, 20 minutes). Control was represented by the same nylon-containing feed pellets, which were inserted in the water tanks in sieved recipients that prevented their consumption, yet allowed contact with the aquatic environment. Each group received and fully ingested 50 nylon-containing feed pellets, weighting
≈10 grams. After their ingestion, at every 24 h each group received 10 grams of sterilized non-nylon feed pellets, to facilitate bowel movement and the excretion of the nylon strips over the course of one week. The nylon strips were recovered immediately after excretion and stored at 4 °C prior analysis.
No specimens were harmed during the experiment and all actions were performed in conformity with the Guide for the Use and Care of Laboratory Animals recommendations [
29] regarding reduction of animal suffering.
2.2. Microbiota Recovery
The excreted nylon strips of the groups, as well as their respective controls, were tapped dried using sterile paper and then bathed through three successive sterile water baths to remove non-adherent microbiota. For microbiota recovery, the dried nylon strips were inserted in liquid Amies media, shaken and incubated for 2 hours at room temperature prior to plate inoculations. Wet faeces samples were also prelevated per group, diluted in liquid Amies media (100 mg/mL) and inoculated on the bellow stated media plates, with the use of a 10 µL sterile loop. Amies media nylon samples were spread at 100 µL on: Chapman agar, 5% blood agar, bile aesculin agar, Salmonella-Shigella agar, Mac Conkey sorbitol agar, DCL agar, Yersinina agar and xylose lysine deoxycholate agar plates, with the use of an Drigalski spatula and a Petri dish turntable (Schuett-Biotec, Göttingen, Germany). The media plates were incubated at 23 °C for 36 h and the developed colonies were isolated and inoculated on fresh media plates, followed by a macroscopic observation of the colonies and their morphology at 48 h post-incubation 23 °C.
2.3. Taxonomic Identification
Final taxonomic identification was performed with an Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight mass spectrometer (Bruker MALDI Biotyper, Bruker Daltonics, Bremen, Germany), the data acquisition was performed using the MBT Compass IVD software (Server Version: 4.1.80 PYTH) coupled with Species/Entry List MBT IVD Library (Revision J, Bruker Daltonics, Bremen, Germany). To perform the assay, MSP 96 target polished steel plate spots (Bruker Daltonics, Bremen, Germany) were loaded with isolated colonies, and on each spot α- Cyano-4-hydroxycinnamic acid (Bruker Daltonics, Bremen, Germany) matrix was added among with the stock solution (acetonitrile, trifluoracetic acid, and distilled water).
2.4. Polymer Analysis
2.4.1. Differential Scanning Calorimetry Analysis
To investigate the thermal behavior of the excreted nylon strips versus controls, a Differential Scanning Calorimetry (DSC) analysis was conducted using a Netzsch 204 F1 Phoenix instrument (Netzsch Gerätebau GmbH, Selb, Germany). The samples, weighing between 3.5–4 mg, were placed in individual aluminum pans and positioned on the sample platform of the DSC instrument. The experiments were carried out in a controlled nitrogen environment with a purity of 99.99%. The samples were heated from 0 to 250 °C at a heating rate of 5 °C/min, with a continuous nitrogen flow rate of 20 mL/min. Two complete heating-cooling cycles were performed for each sample, and the resulting thermograms were analyzed using Proteus analysis software (version 4.8.5).
2.4.2. Fourier-Transform Infrared Spectroscopy
Fourier Transform Infrared Spectroscopy (FTIR) was performed on the negative control (NC) represented by a pristine nylon sample, the positive controls (PCs) represented by nylon kept under water and the samples (S) represented by nylon pieces that transited the digestive system of fish wand was recovered from the water tanks. Measurements were performed on three nylon strips corresponding to each condition (NC, PC and S) using a Bruker Tensor 27 spectrometer (Bruker Optik GmbH, Ettlingen, Germany) with attenuated total reflection (ATR) module. The analyzed nylon strips were tightly pressed on the ATR crystal in order to perform the measurement. The absorbance of samples was recorded in the 4000 – 400 cm-1 range with a resolution of 4 cm-1, during 1 minute. For an easy comparison, spectra were normalized by 0 to 1. Spectra acquision and analysis were performed with OPUS software version 7.2 (Bruker Optik GmbH, Ettlingen, Germany).
Carbonyl indices (I
CO) were calculated based on the measured spectra as the ratio between the maximum absorbance of the carbonyl group found at ~1634 cm
-1 and the maximum absorbance of the C-H band at ~684 cm
-1 [
30]. The triplicate measurements performed in each condition (CN, CP and S) were used to calculate mean indices and their standard deviations.
3. Results
3.1. Statistical Analysis of MPs Excretion
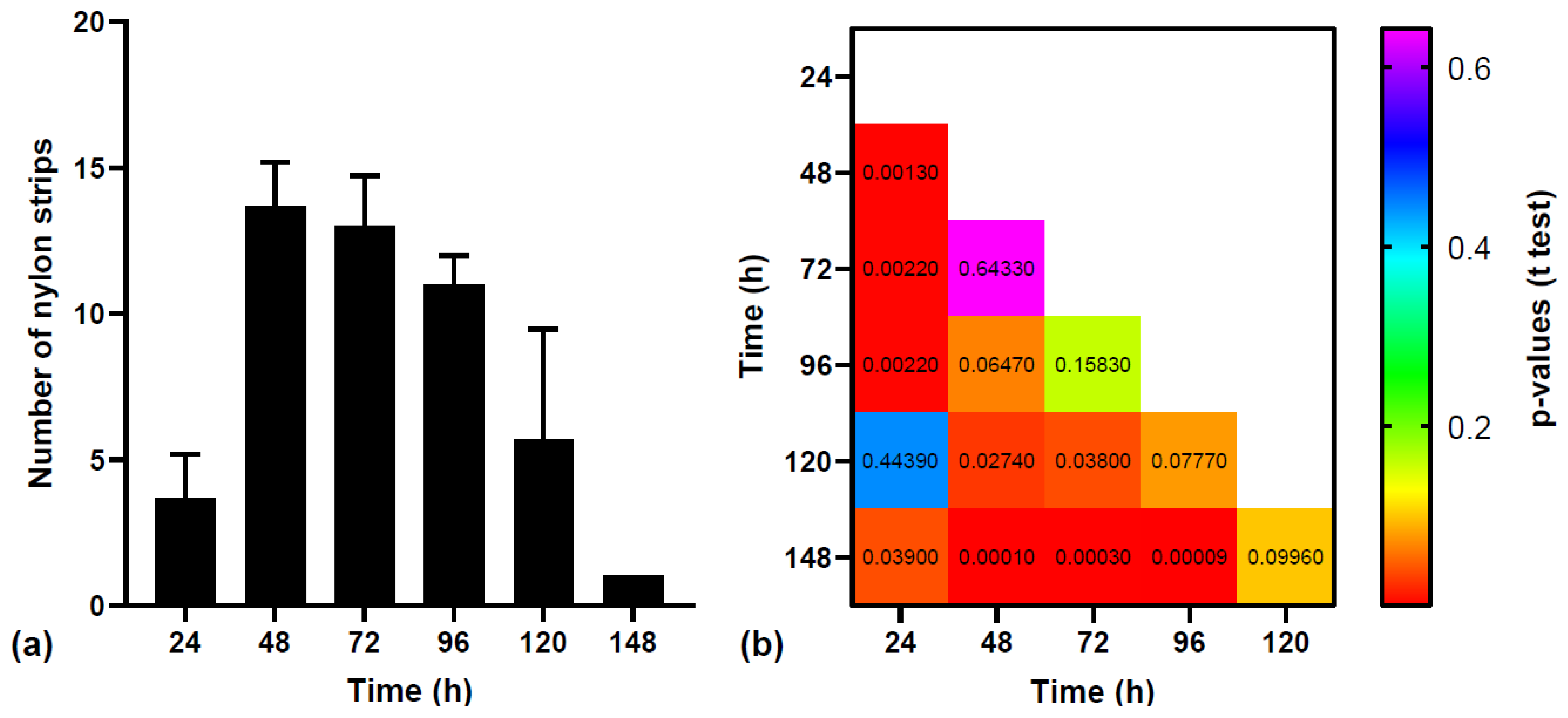
The mean number of nylon strips that were recovered from the three groups of fish are shown in
Figure 1a. From the 150 nylon strips that were fed to the three groups of fish (50/group), only 6 strips were not recovered: 3 from the first group, 1 from the second group and 2 from the third group. The differences between the three groups of fish were not statistically significant (ANOVA test F value was 0.0053 and p-value was 0.9946), showing a similar excretion rate of the nylon strips. When addressing the differences between the numbers of nylon strips recovered in time, ANOVA test showed that there are significant differences between these datasets (ANOVA test F value was 21.81 and p-value was <0.0001). By performing pairwise t-tests (
Figure 1b), we identified significant differences between the following numbers of strips: (i) recovered after 24 hours and those recovered after 48, 72, 96 and 148 hours, (ii) recovered after 48 hours and those recovered after 120 and 148 hours, (iii) recovered after 72 hours and those recovered after 120 and 148 hours and (iv) recovered after 96 hours and those recovered after 148 hours. These results show that the largest numbers of nylon strips were excreted by fish after 48 and 72 hours after ingestion (not statistically significant, p-value = 0.643 >> 0.05). The number slightly decreases after 96 hours, but still not significantly different from the numbers at 48 and 72 hours (p-value of ~0. 158 >> 0.05). Lower numbers of strips were recovered at 24, 120 and at 148 hours. The number of strips excreted at 24 hours and 120 hours are similar (not statistically significant, p-value = ~ 0.444 >> 0.05). The number of strips recovered after 148 hours is the smallest and is statistically different from the numbers of strips recovered after all time intervals except at 120 hours.
3.2. Microbial Diversity
Aeromonas jandaei and
Aeromonas hydrophila were the predominant species found on all samples and controls.
Pantoea agglomerans and
Pseudomonas umsongensis were detected only on group 1 samples, and grew strictly on Mac Conkey sorbitol agar plates.
Plesiomonas shigelloides was detected on both group 1 samples and group 1 control and grew on xylose lysine deoxicolat. Compared to group 1 samples, control 1 differentiated by presented
Acinetobacter johnsonii colonies. No species grew on Chapman agar for all groups and their controls, yet such growth was present from faeces samples. The wet faeces samples revealed
Staphylococcus xylosus on Chapman agar,
Bacillus cereus on blood agar,
Aeromonas jandaei on bile aesculine agar,
Aeromonas hydrophila on both Salmonella-shigella and Mac Conkey sorbitol agar plates,
Aeromonas caviae only on DCS agar,
Aeromonas veronii on Yersinia agar, and interestingly,
Edwardsiella tarda on xylose lysine deoxicholat agar. Detected species as well as the CFU/mL of the initial innoculation are present in
Supplementary Table 2.
3.3. Polymer Structural Integrity
DSC first cooling and second heating cycles revealed no discernible differences among the nylon samples excreted by the fish, their respective controls, and the standard nylon. The phase transition was recorded at 214 °C during the first cooling, corresponding to the crystallization of the nylon polymer as it cooled down from its melted state. This process involves the reorganization of polymer chains into a more ordered, crystalline structure. The second heating pointed a melting temperature around 220 °C for all samples and their controls. Only some slight changes were observed, associated with the sample’s foil shape, which, by its nature of thin and irregular form, possess the potential to introduce fluctuations in both heat transfer and surface effects during the course of DSC analysis [
31].
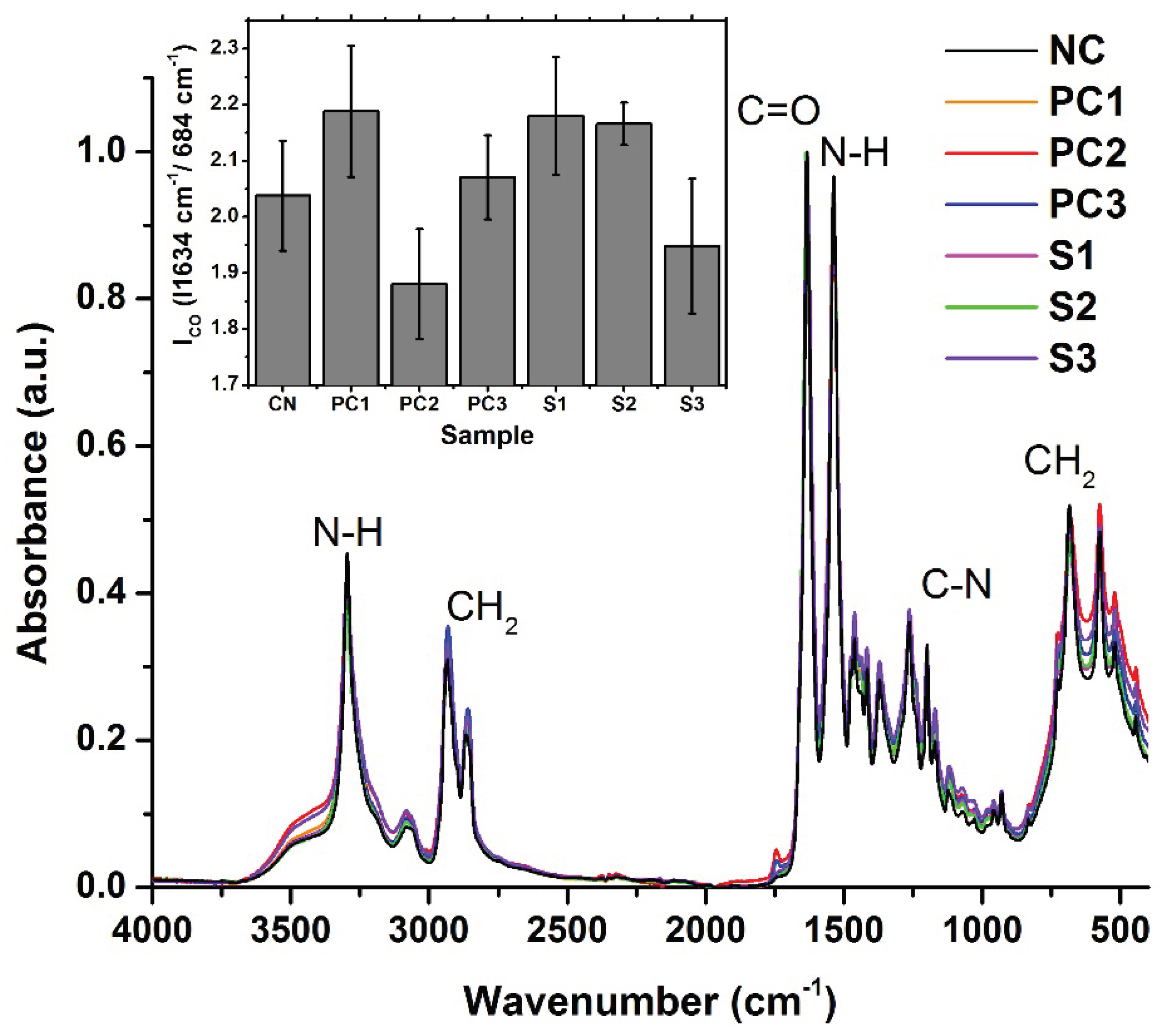
The FTIR spectra recorded on the samples are presented in
Figure 2. The spectrum of the negative control is in good agreement with the FTIR spectra of nylon 6 that were previously published [
32,
33,
34]. Its main absorption peaks are located at 3296 cm
-1 (N-H stretching), 2933 cm
-1 and 2866 cm
-1 (C-H stretching), 1634 cm
-1 (C=O stretching), 1537 cm
-1 (deformation of N-H), 1199 cm
1 (stretching of C-N) and 684 cm
-1 (CH
2 group deformation). The peaks assignment was performed according to [
33]. In what concerns the positive controls and samples, the spectra present the same peaks as the negative control, without the occurrence of new peaks. This shows that no major transformations occurred in the structure of nylon in these samples.
To assess the degradation of nylon strips, we used the measured spectra to calculate the carbonyl index (I
CO). These are based on the ratio between the height of the carbonyl absorption band (~1634 cm
-1) that is expected to increase if the samples are oxidized and the height of one CH
2 absorption band (like the one at ~684 cm
-1) that is expected to remain constant regardless of oxidation [
30]. Results presented in the insert in
Figure 2 show that mean I
CO vary over the considered conditions. Pairwise t-tests conducted on the negative control versus positive controls and samples returned p-values that exceeded the confidence interval of 0.05. This shows that no statistically significant differences are seen in the oxidation states of the nylon strips that underwent a type of treatment relative to the pristine nylon strips.
Altogether, the results suggest that the polymer structure remained unchanged despite the digestion, bacterial and aquatic environment exposure.
4. Discussion
The present study investigated the colonization of nylon MPs by bacteria, particularly focusing on pathogens association with MPs. The findings indicate that nylon MPs can serve as carriers for various bacterial species, suggesting potential implications for environmental and public health.
The excretion dynamics of nylon MPs by O. niloticus were examined over a one-week period with the excretion rates varying significantly over time. The majority of nylon strips were excreted within the first 72 hours post-ingestion, with a gradual decrease observed thereafter. This suggests a potential retention or accumulation of MPs within the gastrointestinal tract of fish, which merits further investigation.
Analysis of the microbiota recovered from excreted nylon strips as well as controls revealed the presence of various pathogens, mainly Aeromonas jandaei and Aeromonas hydrophila as predominant species across all samples. Additionally, Pantoea agglomerans, Pseudomonas umsongensis, and Plesiomonas shigelloides were detected in specific groups, highlighting the diverse microbial community associated with nylon MPs and the potential role of MPs as carriers for pathogens in aquatic environments.
DSC and FTIR analyses were conducted to assess the structural integrity of nylon MPs following exposure to fish digestion and aquatic environments. The DSC results revealed no discernible differences in the thermal behavior of excreted nylon MPs compared to controls and the reference sample, indicating the preservation of polymer structure. Similarly, FTIR spectra demonstrated minimal alterations in the chemical composition of nylon MPs, further supporting the resilience of polymer integrity.
Nylon MPs colonization by diverse pathogens raises concerns regarding their potential transmission through MPs contaminated aquatic environments. Given the widespread distribution of microplastics in aquatic ecosystems, there is a heightened risk of microbial dissemination and associated health hazards for both aquatic organisms and humans.
Although with its limitations, this study opens future research to explore the colonization dynamics of various microplastic polymers, and assess the potential transfer of antibiotic resistance genes and virulence factors by MP-associated bacteria.
5. Conclusions
The results of this study support the concept that MPs can be colonized by diverse bacterial species and raises concerns regarding the transmission of pathogens through MPs contaminated aquatic environments. Given the widespread distribution of microplastics in aquatic habitats, there is a pressing need for comprehensive risk assessment and mitigation strategies to safeguard environmental and public health.
In conclusion, this study contributes to our understanding of the complex interactions between microplastics and microbial communities in aquatic environments. The findings underscore the urgent need for interdisciplinary research and collaborative efforts to address the challenges posed by microplastics pollution and mitigate its impacts on ecosystems and human health.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: title; Table S1: title; Video S1: title.
Author Contributions
Conceptualization, A.J. and C.-E.P.; methodology, A.J., C.-E.P., M.M. and D.C.; software, D.F.M.; validation, G.D., N.C. and A.C.S.; formal analysis, S.F.; investigation, A.J. and I.S.; resources, C.-E.P.; data curation, G.D., D.F.M. and S.F.; writing—original draft preparation, A.J. and I.S; writing—review and editing, C.-E.P and M.M.; visualization, D.F.M.; supervision, G.D and D.F.M.; project administration, C.-E.P; funding acquisition, C.-E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Non-Governmental Research Organization Biologic, Bucharest, Romania.
Institutional Review Board Statement
This research was approved by University of Bucharest Ethics Committee of Research, decision no. 42/07.07.2021.
Data Availability Statement
Data is available via the coresponding author upon resonable request.
Acknowledgments
The authors would like to thank Hanganu Dorin for providing the Oreochromis niloticus specimens.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- de Vries, A.N.; Govoni, D.; Árnason, S.H.; Carlsson, P. Microplastic ingestion by fish: Body size, condition factor and gut fullness are not related to the amount of plastics consumed. Marine Pollution Bulletin 2020, 151, 110827. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liang, W.; Liu, Q.-X.; Fu, S.; Ma, C.; Chen, Q.; Su, L.; Craig, N.J.; Shi, H. Fish Ingest Microplastics Unintentionally. Environmental Science & Technology 2021, 55, 10471–10479. [Google Scholar] [CrossRef]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the mass of microplastics ingested – A pivotal first step towards human health risk assessment. Journal of Hazardous Materials 2021, 404, 124004. [Google Scholar] [CrossRef] [PubMed]
- Hofstede, L.T.; Vasse, G.F.; Melgert, B.N. Microplastics: A threat for developing and repairing organs? Cambridge Prisms: Plastics 2023, 1, e19. [Google Scholar] [CrossRef]
- Weingrill, R.B.; Lee, M.-J.; Benny, P.; Riel, J.; Saiki, K.; Garcia, J.; Oliveira, L.F.A.d.M.; Fonseca, E.J.d.S.; Souza, S.T.d.; D’Amato, F.d.O.S.; et al. Temporal trends in microplastic accumulation in placentas from pregnancies in Hawaiʻi. Environment International 2023, 180, 108220. [Google Scholar] [CrossRef] [PubMed]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; Dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J Hazard Mater 2021, 416, 126124. [Google Scholar] [CrossRef]
- Ibrahim, Y.S.; Tuan Anuar, S.; Azmi, A.A.; Wan Mohd Khalik, W.M.A.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of microplastics in human colectomy specimens. JGH Open 2021, 5, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Ahmad, S.; Guo, X.; Ullah, S.; Ullah, S.; Nabi, G.; Wanghe, K. A review of the endocrine disrupting effects of micro and nano plastic and their associated chemicals in mammals. Front Endocrinol (Lausanne) 2022, 13, 1084236. [Google Scholar] [CrossRef]
- EndocrineSociety. Plastics pose threat to human health. Available online: https://www.endocrine.org/news-and-advocacy/news-room/2020/plastics-pose-threat-to-human-health (accessed on 8th of March, 2024).
- D’Avignon, G.; Gregory-Eaves, I.; Ricciardi, A. Microplastics in lakes and rivers: an issue of emerging significance to limnology. Environmental Reviews 2022, 30, 228–244. [Google Scholar] [CrossRef]
- Issac, M.N.; Kandasubramanian, B. Effect of microplastics in water and aquatic systems. Environmental Science and Pollution Research 2021, 28, 19544–19562. [Google Scholar] [CrossRef]
- Nair, H.T.; Perumal, S. Trophic Transfer and Accumulation of Microplastics in Freshwater Ecosystem: Risk to Food Security and Human Health. International Journal of Ecology 2022, 2022, 1234078. [Google Scholar] [CrossRef]
- Garrido Gamarro, E.; Ryder, J.; Elvevoll, E.O.; Olsen, R.L. Microplastics in fish and shellfish–a threat to seafood safety? Journal of Aquatic Food Product Technology 2020, 29, 417–425. [Google Scholar] [CrossRef]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.-C.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Scientific reports 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Walkinshaw, C.; Lindeque, P.K.; Thompson, R.; Tolhurst, T.; Cole, M. Microplastics and seafood: lower trophic organisms at highest risk of contamination. Ecotoxicology and Environmental Safety 2020, 190, 110066. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, I.F.; Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Worldwide contamination of fish with microplastics: A brief global overview. Marine Pollution Bulletin 2020, 160, 111681. [Google Scholar] [CrossRef]
- Gregory, M.R. Environmental implications of plastic debris in marine settings—entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philosophical Transactions of the Royal Society B: Biological Sciences 2009, 364, 2013–2025. [Google Scholar] [CrossRef]
- Uy, C.A.; Johnson, D.W. Effects of microplastics on the feeding rates of larvae of a coastal fish: direct consumption, trophic transfer, and effects on growth and survival. Marine Biology 2022, 169, 27. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Igwaran, A. Microplastics in seafood: Implications for food security, safety, and human health. Journal of Sea Research 2023, 194, 102410. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Anderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene spherules in coastal waters. Science 1972, 178, 749–750. [Google Scholar] [CrossRef]
- Souza-Silva, T.G.d.; Oliveira, I.A.; Silva, G.G.d.; Giusti, F.C.V.; Novaes, R.D.; Paula, H.A.d.A. Impact of microplastics on the intestinal microbiota: A systematic review of preclinical evidence. Life Sciences 2022, 294, 120366. [Google Scholar] [CrossRef] [PubMed]
- Parsaeimehr, A.; Miller, C.M.; Ozbay, G. Microplastics and their interactions with microbiota. Heliyon 2023, 9, e15104. [Google Scholar] [CrossRef]
- Tamargo, A.; Molinero, N.; Reinosa, J.J.; Alcolea-Rodriguez, V.; Portela, R.; Bañares, M.A.; Fernández, J.F.; Moreno-Arribas, M.V. PET microplastics affect human gut microbiota communities during simulated gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion. Scientific Reports 2022, 12, 528. [Google Scholar] [CrossRef] [PubMed]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: microbial communities on plastic marine debris. Environmental science & technology 2013, 47, 7137–7146. [Google Scholar]
- Mincer, T.J.; Bos, R.P.; Zettler, E.R.; Zhao, S.; Asbun, A.A.; Orsi, W.D.; Guzzetta, V.S.; Amaral-Zettler, L.A. Sargasso Sea Vibrio bacteria: Underexplored potential pathovars in a perturbed habitat. Water Research 2023, 242, 120033. [Google Scholar] [CrossRef] [PubMed]
- Beans, C. Are microplastics spreading infectious disease? Proceedings of the National Academy of Sciences 2023, 120, e2311253120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hong, X.; Chai, J.; Wan, B.; Zhao, K.; Han, C.; Zhang, W.; Huan, H. Interaction between Microplastics and Pathogens in Subsurface System: What We Know So Far. Water 2024, 16, 499. [Google Scholar] [CrossRef]
- Zhong, H.; Wu, M.; Sonne, C.; Lam, S.S.; Kwong, R.W.M.; Jiang, Y.; Zhao, X.; Sun, X.; Zhang, X.; Li, C.; et al. The hidden risk of microplastic-associated pathogens in aquatic environments. Eco-Environment & Health 2023, 2, 142–151. [Google Scholar] [CrossRef]
- Close, B.; Banister, K.; Baumans, V.; Bernoth, E.-M.; Bromage, N.; Bunyan, J.; Erhardt, W.; Flecknell, P.; Gregory, N.; Hackbarth, H. Recommendations for euthanasia of experimental animals: Part 2. Laboratory animals 1997, 31, 1–32. [Google Scholar] [CrossRef]
- Benítez, A.; Sánchez, J.J.; Arnal, M.L.; Müller, A.J.; Rodríguez, O.; Morales, G. Abiotic degradation of LDPE and LLDPE formulated with a pro-oxidant additive. Polymer Degradation and Stability 2013, 98, 490–501. [Google Scholar] [CrossRef]
- UserCom. Information for users of Mettler Toledo thermal analysis systems 2000, 11.
- Bhullar, S.K.; Rana, D.; Ozsel, B.K.; Orhan, M.; Jun, M.B.G.; Buttar, H.S.; Ostrovidov, S.; Ramalingam, M. Development of Silver-Based Bactericidal Composite Nanofibers by Airbrushing. J Nanosci Nanotechnol 2018, 18, 2951–2955. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; Vázquez-Vélez, E.; Martinez, H.; Torres, A. Superficial Surface Treatment using Atmospheric Plasma on Recycled Nylon 6,6. Journal of Nuclear Physics, Material Sciences, Radiation and Applications 2021, 8, 191–196. [Google Scholar] [CrossRef]
- Polyamide (Nylon 6). Available online: https://spectra.chem.ut.ee/textile-fibres/polyamide/ (accessed on 9 March 2024).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).