Introduction
Darier disease (DD) is an autosomal dominant disorder caused by mutation of ATP2A2 gene, located on chromosome 12q23-24.1 and encoding for sarco/endoplasmic reticulum Ca++ ATPase type 2 isoform. Mutations in the ATP2A2 gene have been shown to cause severe disruption of calcium homeostasis by altering protein expression and/or the calcium transport function.
Clinically, DD manifests with hyperkeratotic papules in seborrhoeic areas, palmo-plantar pits and distinctive nail dystrophy. At histology it is characterized by loss of adhesion and apoptosis of keratinocytes (acantolysis) [
1,
2].
Consistent extra-cutaneous manifestations of Darier disease have not been documented so far [
2] and specifically the heart has been reported as non-affected [
3].
In the present study a novel mutation of ATP2A2 is reported, severely affecting the skin and the myocardium. Clinical manifestations are supported by histological evidence of keratinocyte and cardiomyocyte disconnection. Clinical symptoms of muscle exhaustion, chest pain and cardiac arrhythmias have been attenuated by phosphodiesterase inhibitor administration.
Case Study
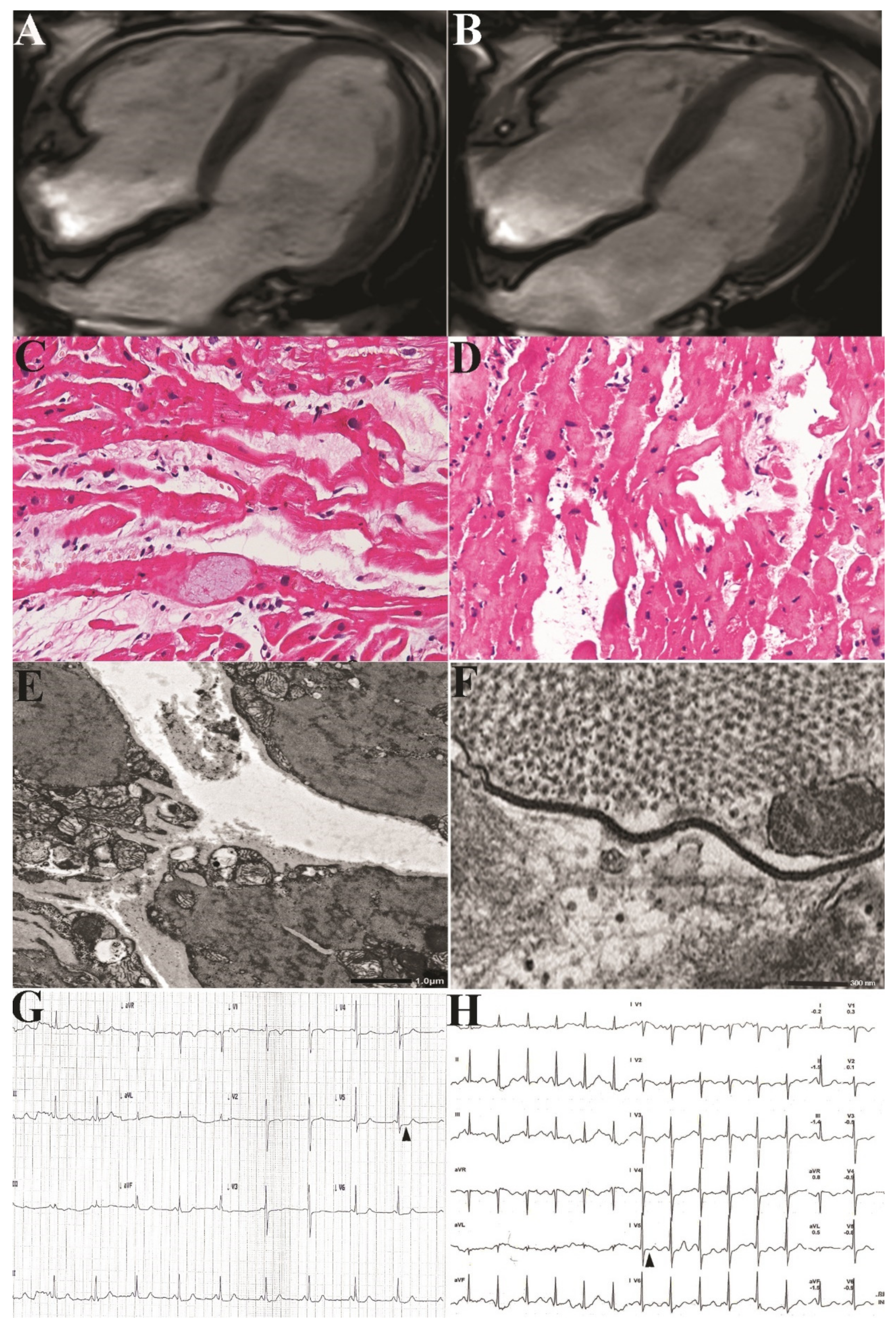
A 62-year-old lady was admitted because of palpitations and chest pain at rest, increasing with effort and limiting remarkably the quality of life and resulting in a depressive syndrome with a failed attempt of suicide. She was affected since the age of nine by Darier disease manifesting with extensive greasy, crusted, yellow-brown papules on the seborrheic areas. Beyond skin lesions, clinical examination was unremarkable, she was afebrile and her blood pressure was normal. Routine blood and chemical tests were within normal limits. Indices of inflammation like erythrocyte sedimentation rate and C reactive protein were normal. ECG showed sinus rhythm at 54 bts/min with abnormal ST segment deflection (-1,-1.5 mm) in the infero-lateral leads and negative T wave (Figure 2 Panel G).
Methods
Cardiac Studies
They included non-invasive (stress ECG, Holter monitoring, 2D-echo and Magnetic Resonance) and invasive investigations (Coronary angiography and left ventricular endomyocardial biopsy) after written informed consent. Specifically, five endomyocardial samples were drawn from left ventricular septum and processed for histology and electron microscopy as previously described [
4]. Apoptosis of cardiomyocytes was assessed by hairpin probe as described [
4].
Skin Biopsy
IT was obtained from an affected skin area after local anesthesia. It was used to confirm histological diagnosis of DD and compare skin with endomyocardial biopsy features.
Molecular Analyses
Genomic DNA was isolated from peripheral blood using the NucleoSpin Blood (Macherey-Nagel, Duren, Germany) according to the manufacturer’s protocols. An opportunely designed custom TruSight One sequencing panel kit (Illumina, San Diego, CA, USA) was used to analyze a panel of 112 selected OMIM genes, including
ATP2A2 (MIM #108740) as well as other 111 genes involved in cardiac disorders (see Supp. Figure S1) for the list of cardiac genes included in the next-generation-sequencing [NGS] panel used for this analysis). The enriched libraries were sequenced by a NextSeq 500 instrument (Illumina, San Diego, CA, USA). NGS data analysis was performed using an in-house implemented pipeline as previously described [
5,
6].
Bidirecrional Sanger sequencing was used to validate the identified variants and perform segregation studies. Sanger sequencing was performed using the ABI BigDye Terminator Sequencing Kit v.3.1 (ThermoFisher Scientific) and an ABI 3130 (ThermoFisher Scientific). Primer sequences and PCR conditions are available upon request.
RNA Studies
Total RNAs was extracted from PAXgene Blood RNA Tubes (Qiagen, Hilden, Germany) using the PAXgene Blood miRNA Kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions. cDNA was synthesized using the Suspercript III first-strand synthesis kit (ThermoFisher, Waltham, MA, USA). A SYBR green-based real-time quantitative PCR (RT-qPCR) assay was used to measure
ATP2A2 mRNA (NM_001681.3) levels in peripheral blood using two primer sets spanning the junctions between exons 1–2 and 17–18, respectively (the primer sequences are available upon request). Each primer set was run in triplicate in two independent experiments using a 7900 HT Fast Real-Time PCR Systems (Thermo Fisher, Waltham, MA, USA). The expression ratio of the target genes was calculated by using the 2
−ΔΔCt method [
7] considering actin as reference gene.
Protein Isolation and Western Blot
Heart tissue samples were treated as described [
8]. The expression of sarcoplasmic or endoplasmic reticulum calcium (SERCA-2) antibody was visualized by using a mouse monoclonal antibody (1:100, Santa Cruz Biotechnology) and Anti-α-sarcomeric actin (1:500, Sigma-Aldrich), antibody was used for normalization. Signal was visualized using a secondary horseradish peroxidase-labeled goat anti-mouse antibody (goat anti-mouse IgG-HRP 1:5000, Santa Cruz Biotechnology) and enhanced chemiluminescence (ECL Clarity Biorad). The purity as well as equal loading of the fraction was determined by measuring β-actin protein levels. Digital images of the resulting bands were quantified by the Image Lab software package (Bio-Rad Laboratories, Munchen, Germany) and expressed as arbitrary densitometric units.
Results
Cardiac Studies
Stress ECG was stopped at 2.5 min of 25 Watts (heart rate 60/bts/min, double product 6000) because of muscle exhaustion and worsening of precordial pain associated to increased (- 2 mm) deflection of ST segment in V4-V6 leads. (Figure 2, panel G).
Holter monitoring (24 hs) registered frequent (3200) supraventricular and polymorphic ventricular (2830) ectopic beats with some couples and triplets.
Cardiac Magnetic Resonance observed normal thickness of left ventricular wall (9 mm for ventricular septum and posterior wall) and normal myocardial mass (64.5 g/m2 BSA; nv 34–70), apical hypokinesis with depressed LV function (EF 40%) (Figure 2 panel A,B), while failed to show signal abnormalities in T1, T2 mapping and LGE after gadolinium infusion.
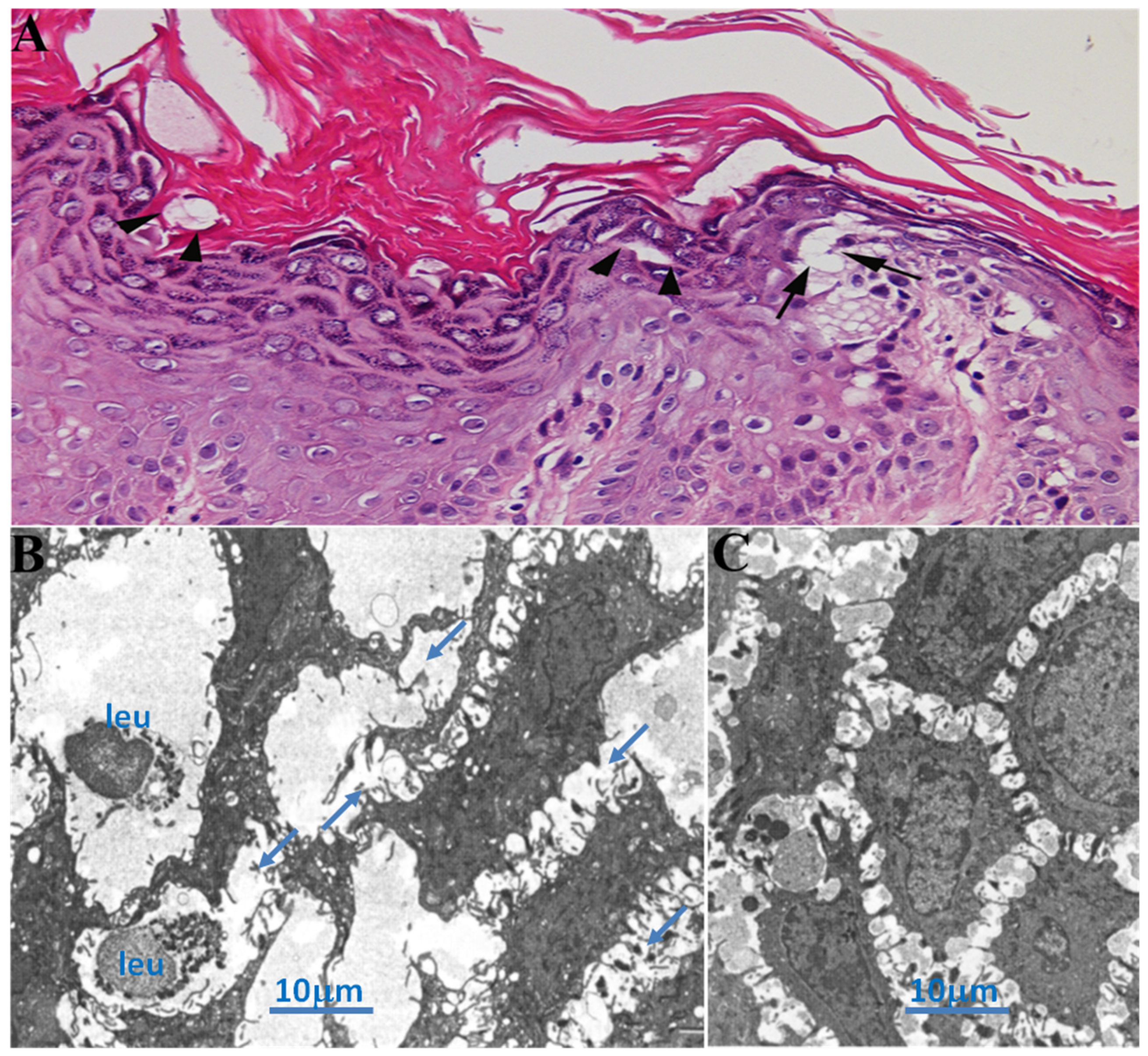
Skin biopsy showed loss of adhesion with dystrophic changes of dermal cells (
Figure 1, panel A,B) confirming the diagnosis of DD.
Likewise, endomyocardial biopsy documented at histology focal disconnection of cardiomyocytes (
Figure 2, panel C), loosing their spatial organization because of unendotheliolized spaces and appearing often in total disarray (
Figure 2, panel D). Affected myocytes showed also some swelling and cytoplasm vacuolization.
At ultrastructural examination, myocyte detachment appeared to occur at cell lateral junctions while intercalated disks appeared structurally well preserved. Cytoplasm vacuoles were due to remarkable dilatation of sarcoplasmic reticulum that was the site of mutated ATP2A2 protein and to perinuclear areas of myofibrillolysis. Myocyte apoptosis was significantly higher in our patient (1639±180 apoptotic nuclei) compared with normal controls 12±2 (p < 0.05).
Protein Studies (Western Blotting)
Protein expression of this sarcoplasmic protein failed to show significant quantitative differences compared with surgical control biopsies.
Gene Investigations
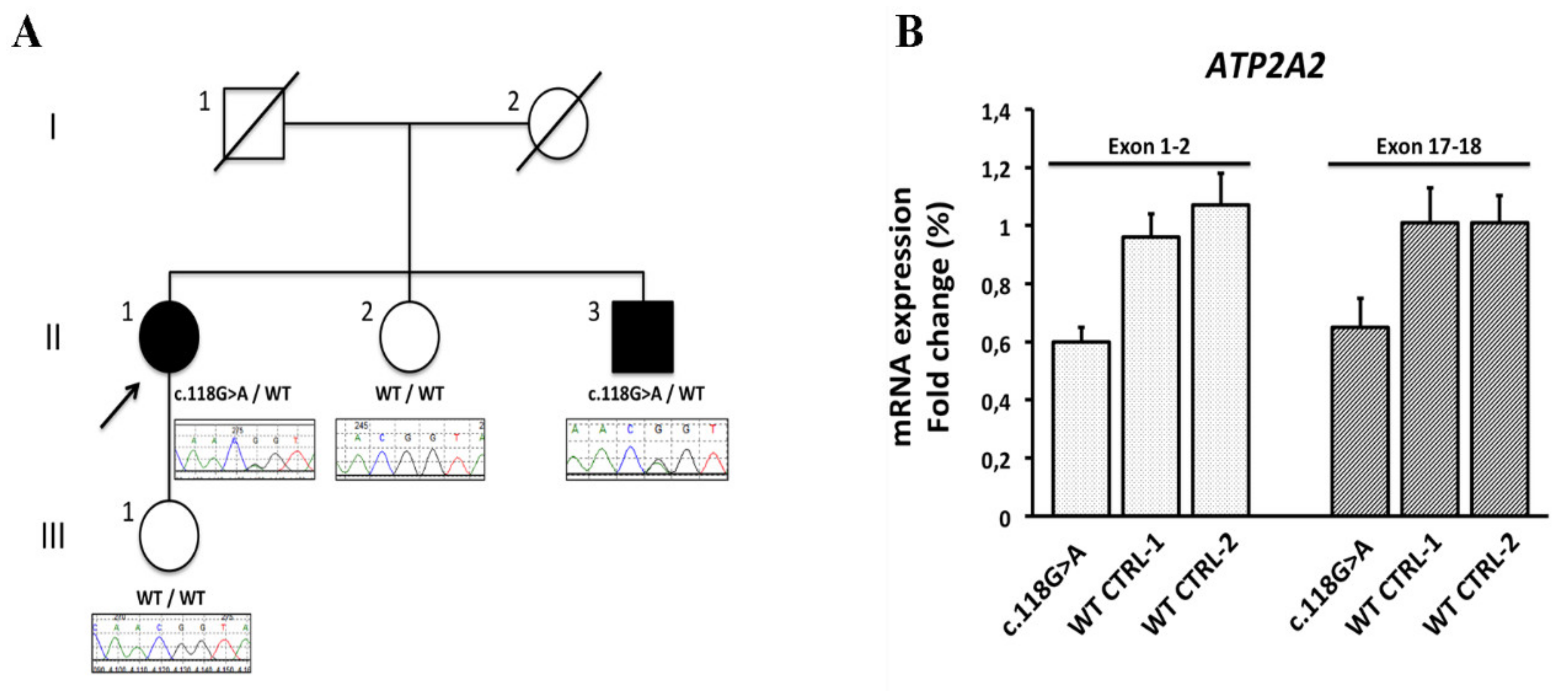
Exome sequencing on a sample from the proband identified a heterozygous G to A transition at the last nucleotide of exon 1 of
ATP2A2 gene (NM_170665.3:c.118G>A), which encodes the sarco/endoplasmic reticulum Ca(2+) ATPase type 2 isoform (SERCA2) (Supp. Figure S1). No pathogenic variant was found in the panel of cardiac genes (Supp. Table S2). Sanger sequencing confirmed segregation of the
ATP2A2 gene c.118G>A variant with DD phenotype in an affected sibling but not in two unaffected family members (
Figure 3, panel A)This variant had not previously been reported in public databases (ExAC, gnomAD, 1000 Genomes Project, and NSEuroNet) and was predicted to affect splicing by in silico tools (Alamut version 2.10.0) (Supp. Figure S3). Concordantly to the prediction, analysis of the
ATP2A2 cDNA by real-time quantitative PCR showed that c.118G>A caused 36% to 40% reduced expression of
ATP2A2 mRNA in peripheral blood leukocytes (
Figure 3, panel B), consistent with
ATP2A2 c.118G>A variant being a splicing defect associated with a decreased expression of
ATP2A2 gene. Based on these evidences, the variant was classified as pathogenic.
Treatment
Phosphodiesterase inhibitor amynophilline at the dosage of 200 mg bid was introduced to reduce degradation of cyclic AMP and improve phosphorylation and activity of ATP2A2.
After 2 weeks, the patient manifested a disappearance of chest pain, improvement in muscle function and reduction of cardiac arrhythmias (SV ectopic beats 280 and 180 V ectopic beats /24 hs without evidence of repetitive phenomena). At control stress ECG the patient was able to remarkably increase (2.3 times, heart rate.117/bts min., double product 14000) physical activity without the occurrence of precordial pain while ST segment remained at isoelectric line (
Figure 2 panel H). Cardiac contractility at 2D-echo remained unchanged.
Discussion
Although Darier disease is known as a rare disorder limited to the skin, it is likely it may affect additional systems. Indeed, incidence of psychiatric manifestations is so high [
5] that has been suggested that central nervous system might be involved as well. The present study identifies a novel
ATP2A2 (c.118G>A) gene mutation affecting both skin and myocardium. These tissues exhibit similar histological and ultrastructural changes consisting in alterations of keratinocyte and cardiomyocyte cell-to-cell interactions and suggest a pathway common to both tissues. To this regard it is known that function of proteins (such as connexins and tight junction proteins) responsible of lateral cell bonds are strictly Ca++dependent and that disruption of Ca++ homeostasis can compromise cell adhesion. Mutations in
ATP2A2 gene have been shown to cause severe disruption of CA++ homeostasis by altering protein expression and/or the transport function in vitro [
9,
10] and lead to moderate depletion of calcium stores in keratinocytes of patients [
11]. In DD, symptoms occur independently of
ATP2A2 mutation type or which aspect of SERCA2 functionality is compromised, suggesting that
ATP2A2 mutations exert their deleterious effect through haploinsufficiency, although also a dominant negative effect of the
ATP2A2 mutants has been proposed to underlie the DD phenotype [
12]. In our patient, despite the cardiac involvement, we cannot infer which specific aspect of SERCA2 functionality is compromised. We proved that the
ATP2A2 c.118G>A mutation is associated to reduced mRNA expression at real-time quantitative PCR in peripheral blood, while the SERCA2 protein was normally expressed at western blot assessment, suggesting a sort of posttranscriptional adaptation that upregulate expression of the residual SERCA2 protein. At cardiac level, mutation consequences are derangement of cardiomyocyte organization (disarray) and remarkable intracellular changes. Specifically, at ultrastructural examination, sarcoplasmic reticulum appeared dilated and perinuclear areas of myofibrillolysis were commonly seen. Noteworthy, structure of intercalated disks was preserved confirming cell detachment to occur at lateral cell junction. It may be argued that in mouse model of Darier disease [
13] there was no histological report of cardiomyocyte disconnection as well as of physiological derangements. However, it must be relieved that there was no previous endomyocardial biopsy study in human with Darier disease and that our patient developed cardiac manifestations several decades after the appearance of skin lesions. This may suggest that cardiac involvement may occur later compared with skin damage.
Clinical implications from morphological analysis are that cardiac manifestations may occur in DD. These mimic ischemic heart disease with precordial pain worsened by effort, abnormal ECG changes and polymorphic, repetitive ventricular ectopic beats.
Interestingly, phosphodiesterase inhibitors like aminophylline, reducing degradation of cyclic AMP may abolish chest pain and improve vital capacity through enhancement of SERCA2 protein phosphorylation. In our patient, in addition to disappearance of precordial pain, double product increased by 2.5 times without abnormal deflection of ST segment at on therapy stress ECG.
In conclusion, specific mutations of ATP2A2 may cause cardiac involvement in patients with DD. Cardiac pathologic changes are similar to those documented in the skin including cardiomyocyte disconnection that promotes precordial pain and cardiac arrhythmias.
Phosphodiesterase inhibitors, enhancing SERCA2 protein phosphorylation may substantially attenuate the symptoms.
Funding
The study has been supported by Ricerca corrente IRCCS L Spallanzani, and partially by Italian Health Ministry (IRCCS San Raffaele Roma–Ricerca Corrente #2020/1).
Institutional Review Board Statement
The study was conducted in accordance with the Decla-ration of Helsinki, and approved by the locally appointed ethics committee (opinion number 6/2019 and 2016-003014-28 (FARM12JCXN) and informed consent was obtained from all subjects.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
All authors.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Sehgal V, Srivastava G. Darier’s disease/keratosis follicularis. Int. J. Dermatol. 2005, 44, 84–92.
- Burge SM, Wilkinson JD. Darier-White disease: A review of the clinical features in 163 patients. J. Am. AcadDermatol 1992, 27, 40–50. [CrossRef] [PubMed]
- Tavadia S, Tait RC, McDonagh TA, Munro CS.: Platelet and cardiac function in Darier disease. ClinExpDermatol 2001, 26, 696–699.
- Chimenti C, Frustaci A. Contribution and Risks of Left Ventricular Endomyocardial Biopsy in Patients with Cardiomyopathies. A Retrospective Study Over a 28-Year Period. Circulation 2013, 128, 1531–1541. [CrossRef] [PubMed]
- Nakamura T, Kazuno A, Nakajima K, Kusumi I, Tsuboi T, Kato T. Loss of Function mutations in ATP2A2 and psychoses: A case report and literature survey. Psychiatry and Clinical Neurosciences 2016, 70, 342–350. [CrossRef] [PubMed]
- Campopiano R, Ryskalin L, Giardina E, et al. Next Generation Sequencing and ALS: Known genes, different phenotyphes. Arch. Ital. Biol. 2017, 155, 110–117. [CrossRef]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [CrossRef] [PubMed]
- Chimenti C, Verardo R, Scopelliti F, et al. Myocardial expression of Toll-like receptor 4 predicts the response to immunosuppressive therapy in patients with virus-negative chronic inflammatory cardiomyopathy. Eur. J. Heart Fail. 2017, 19, 915–925. [CrossRef] [PubMed]
- Miyauchi Y, Daiho T, Yamasaki K, et al. Comprehensive analysis of expression and function of 51 sarco(endo)plasmic reticulum Ca2+- ATPase mutants associated with Darier disease. J. Biol. Chem. 2006, 281, 22882–22895. [CrossRef] [PubMed]
- Dode L, Andersen JP, Leslie N, Dhitavat J, Vilsen B, Hovnanian A. Dissection of the functional differences between sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) 1 and 2 isoforms and characterization of Darier disease (SERCA2) mutants by steady-state and transient kinetic analyses. J. Biol. Chem. 2003, 278, 47877–47889.
- Foggia L, Hovnanian A. Calcium pump disorders of the skin. Am. J. Med. Genet. C Semin. Med. Genet. 2004, 131C, 20–31. [CrossRef] [PubMed]
- Ahn W, Lee MG, Kim KH, Muallem S. Multiple effects of SERCA2b mutations associated with Darier’s disease. J. Biol. Chem. 2003, 278, 20795–20801. [CrossRef] [PubMed]
- Prasad V, Lorenz JN, Lasko VM, et al. SERCA2 Haploinsufficiency in a Mouse Model of Darier Disease Causes a Selective. Predisposition to Heart Failure. Biomed. Res. Int. 2015, 2015, 251598. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).