Submitted:
08 March 2024
Posted:
11 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
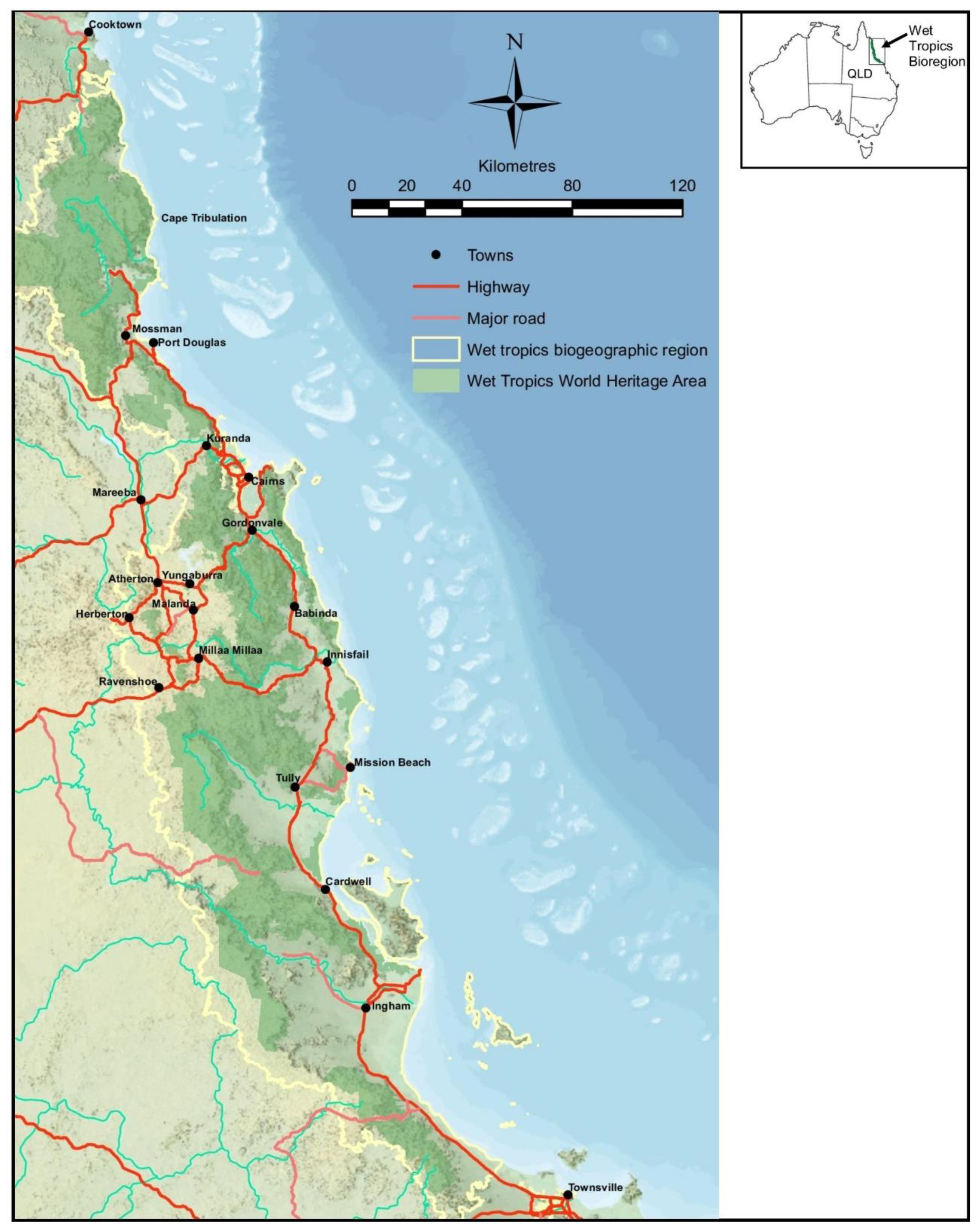
2. Climate-Affected Australian Tropical Montane Cloud Forest Plants and Their Medicinal Uses
| Botanical name, family, and synonyms | Distribution | Life Form | Medicinal uses | Metabolomics profile studied | Conservation status (QLD) |
|---|---|---|---|---|---|
| Pteridophyta | |||||
| Dryopteridaceae | |||||
|
Parapolystichum grayi (D.J.Jones) J.J.S. Gardner & Nagalingum Syn. Lastreopsis grayi D.L.Jones |
Africa, the Neotropics, north-eastern Australia, Madagascar, Pacific Island, and southern Asia | Fern | NU | No |
V |
|
Parapolystichum tinarooense (Tindale) Labiak, Sundue & R.C.Moran Syn. Lastreopsis tinarooensis Tindale |
Wet Tropics region (Australia) | Fern | NU | No | V |
| Hymenophyllaceae | |||||
| Hymenophyllum whitei Goy | Wet Tropics region (Australia) | Fern | NU | No | CR |
| Lindsaeaceae | |||||
| Lindsaea terrae-reginae K.U.Kramer | Wet Tropics region (Australia) | Fern | NU | No | E |
| Lycopodiaceae | |||||
|
Phlegmariurus creber (Alderw.) A.R.Field & Bostock Syn. Huperzia crebra (Alderw.) Holub |
Wet Tropics region (Australia), PNG, Hawaii | Epiphyte | Phlegmariurus/Huperzia species are traditionally used as vermifuge, purgative, and laxative [49]. | No | CR |
|
Phlegmariurus delbrueckii (Herter) A.R.Field & Bostock Syn. Huperzia delbrueckii (Herter) Holub |
Wet Tropics region (Australia) | Epiphyte | No | V | |
| Polypodiaceae | |||||
|
Oreogrammitis albosetosa (F.M.Bailey) Parris Syn. Polypodium albosetosum F. M.Bailey |
Wet Tropics region (Australia) | Fern | NU | No | V |
|
Oreogrammitis leonardii (Parris) Parris Syn. Grammitis leonardii Parris |
Wet Tropics region (Australia) | Fern | NU | No | V |
| Oreogrammitis reinwardtii Blume | Wet Tropics region (Australia), Sri Lanka, Philippines, Papua New Guinea, Solomon Islands, Malaysia |
Fern | NU | No | V |
|
Oreogrammitis wurunuran (Parris) Parris Syn. Grammitis wurunuran Parris |
Wet Tropics region (Australia) | Fern | NU | No | SL |
|
Magnoliophyta |
|||||
| Apiaceae | |||||
| Trachymene geraniifolia F.M.Bailey | Wet Tropics region (Australia) | Herb | NU | No | NT |
| Apocynaceae | |||||
| Parsonsia bartlensis J.B.Williams | Wet Tropics region (Australia) | Climber | NU | No | V |
| Araliaceae | |||||
| Hydrocotyle miranda A.R.Bean & Henwood | Wet Tropics region (Australia) | Herb | Hydrocotyle species are used as anti-inflammatory herbs in Taiwanese folk medicines [50]. | No | V |
| Polyscias bellendenkerensis (F.M.Bailey) Philipson | Wet Tropics region (Australia) | Shrub | Polyscias species are traditionally used to treat ailments, such as malaria, obesity, and mental disorders [51]. | No | V |
| Polyscias willmottii (F.Muell.) Philipson | Wet Tropics region (Australia) | Tree | No | LC | |
| Araucariaceae | |||||
| Agathis atropurpurea B.Hyland | Australia | Tree | Agathis species are traditionally used to treat myalgia and headaches [52]. | Yes | LC |
| Arecaceae | |||||
| Linospadix apetiolatus Dowe & A.K.Irivine | Wet Tropics region (Australia) | Tree | NU | No | LC |
| Celastraceae | |||||
| Hypsophila halleyana F.Muell. | Wet Tropics region (Australia) | Shrub | NU | No | LC |
| Clusiaceae | |||||
| Garcinia brassii C.T.White | Wet Tropics region (Australia) | Tree | Infusions prepared from fruits of Garcinia species are traditionally used to treat dysentery, ulcers, and wounds [53]. | No | LC |
| Cunoniaceae | |||||
| Ceratopetalum corymbosum C.T.White | Wet Tropics region (Australia) | Tree | NU | No | V |
| Ceratopetalum hylandii Rozefelds & R.W.Barnes | Wet Tropics region (Australia) | Tree | NU | No | LC |
| Eucryphia wilkiei B.Hyland | Wet Tropics region (Australia) | Shrub | NU | Yes | CR |
| Ebenaceae | |||||
| Diospyros granitica Jessup | Wet Tropics region (Australia) | Tree | Diospyros species are used traditionally used as sedative, astringent, carminative, febrifuge, anti-hypertensive, vermifuge, antidiuretic, and to relieve constipation [54]. | No | NT |
| Elaeocarpaceae | |||||
| Elaeocarpus linsmithii Guymer | Wet Tropics region (Australia) | Tree | Elaeocarpus species are the source of popular spiritual beads (known as Rudraksha in Asia), which are used to treat various ailments, including mental/neurological disorders (stress, depression, anxiety, hypertension, epilepsy, migraine, and neuralgia), asthma, and also used as analgesic [55]. | No | LC |
| Elaeocarpus hylobroma Y.Baba & Crayn | Wet Tropics region (Australia) | Tree | No | LC | |
| Ericaceae | |||||
| Acrotriche baileyana (Domin) J.M.Powell | Wet Tropics region (Australia) | Shrub | NU | No | NT |
| Dracophyllum sayeri F.Muell | Wet Tropics region (Australia) | Tree | NU | No | V |
|
Leucopogon malayanus subsp. novoguineensis (Sleumer) Pedley Syn. Styphelia malayana subsp. novoguineensis (Sleumer) Hislop, Crayn & Puente-Lel. |
Wet Tropics region (Australia) | Shrub | NU | No | No |
|
Rhododendron lochiae F.Muell. Syn. Rhododendron notiale, Craven |
Wet Tropics region (Australia) | Shrub | Rhododendron species are used to prevent and treat many ailments, including respiratory disorders like asthma and bronchitis, dysentery, diarrhea, constipation, fever, cardiac disorders, and inflammation [56]. | No | No |
| Rhododendron viriosum Craven | Wet Tropics region (Australia) | Tree | No | LC | |
| Trochocarpa bellendenkerensis Domin | Wet Tropics region (Australia) | Tree | NU | No | LC |
| Escalloniaceae | |||||
| Polyosma reducta F.Muell. | Wet Tropics region (Australia) | Tree | NU | No | LC |
| Gesneriaceae | |||||
| Boea kinneari (F.Muell.) B.L.Burtt | Wet Tropics region (Australia) | Herb | NU | No | E |
| Lenbrassia australiana (C.T.White) G.W.Gillett | Wet Tropics region (Australia) | Shrub | NU | No | SL |
| Lamiaceae | |||||
| Prostanthera albohirta C.T.White | Mount Emerald, Wet Tropics region (Australia) | Shrub | Some Prostanthera species are used for topical applications to treat skin sores and infections [57,58]. | No | CR |
| Prostanthera athertoniana B.J.Conn & T.C.Wilson | Wet Tropics region (Australia) | Shrub | No | CR | |
| Lauraceae | |||||
| Cinnamomum propinquum F.M.Bailey | Wet Tropics region (Australia) | Tree | Cinnamomum species are most commonly used in traditional Chinese medicines to treat multiple disorders, including indigestion, microbial infections, and cough and cold [59]. | Yes | V |
| Cryptocarya bellendenkerana B.Hyland | Wet Tropics region (Australia) | Tree | NU | Yes | LC |
| Endiandra jonesii B.Hyland | Wet Tropics region (Australia) | Tree | Endiandra species are traditionally used to treat rheumatism, headache, dysentery, pulmonary disorders, and uterine tumors [60]. | No | V |
| Litsea granitica B.Hyland | Wet Tropics region (Australia) | Tree | Litsea species are used traditionally by Aboriginal communities to treat skin infections such as sores and scabies, and also used an antiseptic [61]. | No | V |
| Myrtaceae | |||||
| Leptospermum wooroonooran F.M.Bailey | Wet Tropics region (Australia) | Tree | Leptospermum species are traditionally used in Malaysia to relieve menstrual and stomach disorders [62,63]. | Yes | LC |
| Micromyrtus delicata A.R.Bean | Wet Tropics region (Australia) | Shrub | NU | No | E |
| Pilidiostigma sessile N.Snow | Wet Tropics region (Australia) | Shrub | NU | No | LC |
| Rhodamnia longisepala N.Snow & A.J.Ford | Wet Tropics region (Australia) | Shrub | Rhodamnia species are used traditionally in Indonesia to treat scars, toothache, and cough [64]. | No | CR |
| Syzygium fratris Craven | Wet Tropic region (Australia) | Shrub | NU | No | CR |
| Uromyrtus metrosideros (F.M.Bailey) A.J.Scott | Wet Tropics region (Australia) | Shrub | NU | Yes | LC |
| Orchidaceae | |||||
| Bulbophyllum lilianiae Rendle | Wet Tropics region (Australia) | Epiphyte | Bulbophyllum species are traditionally used to treat skin diseases, cardiovascular diseases, and rheumatism [65]. | No | LC |
|
Bulbophyllum wadsworthii Dockrill Syn. Oxysepala wadsworthii (Dockrill) D.L.Jones & M.A.Clem. |
Australia | Epiphyte | No | SL | |
|
Bulbophyllum windsorense B.Gray & D.L.Jones Syn. Oxysepala windsorensis (B.Gray & D.L.Jones) D.L.Jones & M.A.Clem. |
Wet Tropics region (Australia) |
Epiphyte |
No | V | |
|
Dendrobium brevicaudum D.L.Jones & M.A.Clem. Syn. Dockrillia brevicauda (D.L.Jones & M.A.Clem.) M.A.Clem. & D.L.Jones |
Wet Tropics region (Australia) | Herb, Epiphyte | Dendrobium species are used in traditional Chinese and Indian medicine systems as a source of tonic for longevity and also as an antipyretic, analgesic, astringent, and anti-inflammatory agent [66]. | No | No |
|
Dendrobium carrii Rupp & C.T.White Syn. Australorchis carrii (Rupp & C.T.White) D.L.Jones & M.A.Clem. |
Wet Tropics region (Australia) | Herb, Epiphyte | No | SL | |
|
Dendrobium finniganense D.L.Jones Syn. Thelychiton finniganensis (D.L.Jones) M.A.Clem. & D.L.Jones |
Wet Tropics region (Australia) | Herb, Epiphyte | No | SL | |
| Liparis fleckeri Nicholls | Wet Tropics region (Australia) | Lithophyte | Liparis species are traditionally used in Chinese medicine to treat inflammatory diseases, including hemoptysis, metrorrhagia, traumatic hemorrhage, and pneumonia; it is also used to stop bleeding from wounds and to detoxify snakebite [67]. | No | No |
|
Octarrhena pusilla (F.M.Bailey) M.A.Clem. & D.L.Jones Syn. Octarrhena pusilla (F.M.Bailey) Dockrill |
Wet Tropics region (Australia) | Epiphyte | NU | No | SL |
| Piperaceae | |||||
| Peperomia hunteriana P.I.Forst. | Wet Tropics region (Australia) | Herb | Peperomia species are traditionally used for treating pain and inflammation, gastric ulcers, asthma, and bacterial infections [68,69]. | No | LC |
| Podocarpaceae | |||||
|
Prumnopitys ladei (F.M.Bailey) de Laub Syn. Stachycarpus ladei (Bailey) Gaussen, Podocarpus ladei F.M.Bailey |
Endemic to Wet Tropics Australia | Tree | Fruits and bark of Prunmnopitys species are considered medicinal [70]. | Yes | No |
| Proteaceae | |||||
| Austromuellera valida B.Hyland | Endemic to Wet Tropics region | Tree | NU | No | V |
| Helicia lewisensis Foreman | Endemic to Wet Tropics region | Tree | Helicia species are used for treating mouth and skin sores and also kidney and gastric problems [71,72,73,74]. | No | V |
| Helicia recurva Foreman | Endemic to Wet Tropics region | Tree | No | No | |
|
Hollandaea porphyrocarpa A.J.Ford & P.H.Weston Syn. Hollandaea sp. Pinnacle Rock Track (P.I.Forster PIF10714) |
Endemic to Wet Tropics region | Shrub | NU | No | CR |
|
Nothorites megacarpus (A.S.George & B.Hyland) P.H.Weston & A.R.Mast Syn. Orites megacarpa A.S.George & B.Hyland |
Endemic to Wet Tropics region | Tree | NU | No | LC |
| Rubiaceae | |||||
| Aidia gyropetala A.J.Ford and Halford | Endemic to Wet Tropics region | Tree | Aidia species are used for treating body/muscle pains and pains due to gastric disorders [75]. | No | LC |
|
Gynochthodes constipata (Halford & A.J.Ford) Razafim. & B.Bremer Syn. Morinda constipata Halford & A.J.Ford |
Endemic to Wet Tropics region | Climber | Gynochthodes/Morinda species are traditionally used for treating diabetes, inflammation, cancer, psychiatric disorders, and microbial infections [76]. | No | LC |
|
Gynochthodes podistra (Halford & A.J.Ford) Razafim. & B.Bremer Syn. Morinda podistra Halford & A.J.Ford |
Endemic to Wet Tropics region | Climber | No | LC | |
|
Ixora orophila C.T.White Syn. Psydrax montigena S.T.Reynolds & R.J.F.Hend. |
Endemic to Wet Tropics region | Shrub | Ixora species are used in Ayurvedic medicine against leucorrhoea, dys, hypertension, menstrual irregularities, sprains, bronchitis fever, sores, chronic ulcers, scabies, and skin diseases [77]. | No | No |
| Wendlandia connata C.T.White | Endemic to Wet Tropics region | Shrub | Wendlandia species are traditionally used for treating fever, dysentery, cough, hypertension, diabetes, constipation, inflammations, and hyperlipidemia [78]. | No | NT |
| Rutaceae | |||||
| Flindersia oppositifolia (F.Muell.) T.G.Hartley & Jessup | Wet Tropics region (Australia) | Tree | NU | Yes | V |
| Leionema ellipticum Paul G. Wilson | Endemic to Wet Tropics region | Shrub | NU | Yes | V |
| Zieria alata Duretto & P.I.Forst. | Endemic to Wet Tropics region | Shrub | NU | No | CR |
| Zieria madida Duretto & P.I.Forst. | Endemic to Wet Tropics region | Shrub | NU | No | CR |
| Santalaceae | |||||
| Korthalsella grayi Barlow | Endemic to Wet Tropics region | Herb | No | LC | |
| Sapindaceae | |||||
|
Mischocarpus montanus C.T.White Syn. Mischocarpus pyriformis subsp. retusus (Radlk.) R.W.Ham, Mischocarpus retusus Radlk. |
Wet Tropics region (Australia), New Guinea | Tree | NU | No | LC |
| Sapotaceae | |||||
|
Pleioluma singuliflora (C.T.White & W.D.Francis) Swenson Syn. Planchonella singuliflora (C.T.White & W.D.Francis) P.Royen, Pouteria singuliflora (C.T.White & W.D.Francis) Baehni |
Endemic to Wet Tropic region | Shrub | NU | No | LC |
|
Sersalisia sessiliflora (C.T.White) Aubrév. Syn. Pouteria sylvatica Baehni, Lucuma sessiliflora C.T.White |
Endemic to Wet Tropics region | Tree | NU | No | LC |
| Planchonella sp. Mt. Lewis (B.Hyland 14048) Qld Herbarium | Endemic to Wet Tropics region | Tree | Planchonella species have been used by Aboriginal medicine system to treat sores/sore throat and as an antiseptic for boils [61]. | No | No |
| Solanaceae | |||||
| Solanum dimorphispinum C.T.White | Endemic to Wet Tropics region | Shrub | Solanum species have been traditionally used against infectious diseases and also as anti-microbial agents and insecticidal against mosquitoes [79]. | No | LC |
| Solanum eminens A.R.Bean | Endemic to Wet Tropics region | Climber | No | LC | |
| Symplocaceae | |||||
|
Symplocos bullata Jessup Syn. Symplocos sp. North Mary (B. Gray 2543) |
Endemic to Wet Tropics region | Shrub | Symplocos species are traditionally known for treating diseases such as malaria, ulcers, leprosy, leucorrhea, menorrhagia, and gynecological disorders [80]. | No | LC |
| Symplocos graniticola Jessup | Endemic to Wet Tropics region | Shrub | No | V | |
|
Symplocos oresbia Jessup Syn. Symplocos sp. Mt Finnigan (L.J. Brass 20129) |
Endemic to Wet Tropics region | Shrub | No | NT | |
|
Symplocos wooroonooran Jessup Syn. Symplocos stawellii var. montana C.T.White, Symplocos cochinchinensis var. montana (C.T.White) Noot |
Endemic to Wet Tropics region | Shrub | No | NT | |
| Thymelaeaceae | |||||
|
Phaleria biflora (C.T.White) Herber Syn. Oreodendron biflorum C.T.White |
Endemic to Wet Tropics region | Tree | Phaleria species are used for treating stomachache, general pain, diarrhea, lowering glucose/cholesterol levels in blood, and also known for anti-cancer properties [81]. | No | V |
| Winteraceae | |||||
|
Bubbia whiteana A.C.Sm. Syn. Zygogynum semecarpoides var. whiteanum Vink, Bubbia semecarpoides var. whiteana Vink |
Endemic to Wet Tropics region | Shrub | NU | No | CR |
| Tasmannia sp. Mt Bellenden Ker (J.R.Clarkson 6571) | Wet Tropics region (Australia) | Shrub | Tasmania species are traditionally used for treating malaria, diarrhea, and cough [82]. | No | LC |
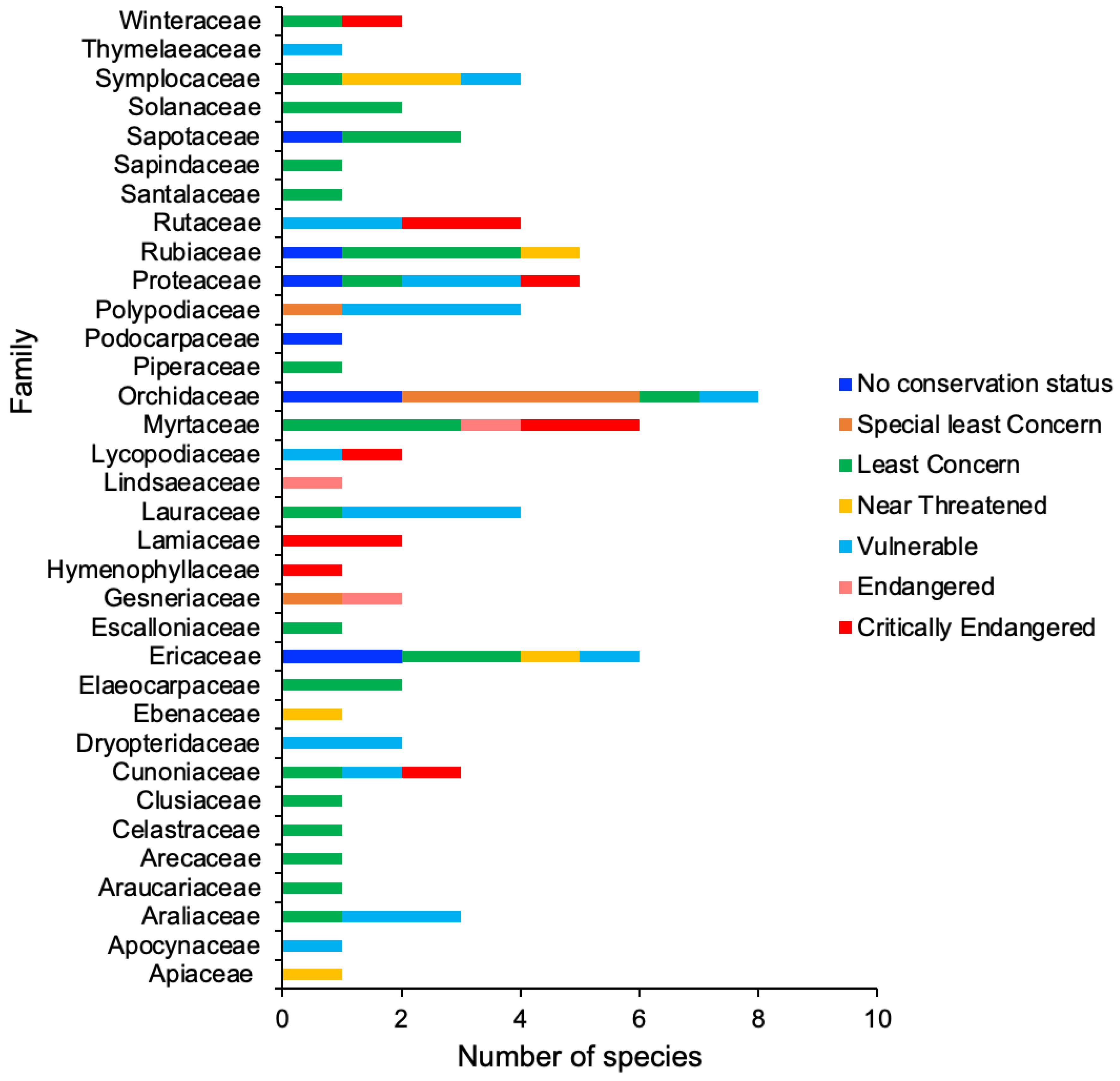
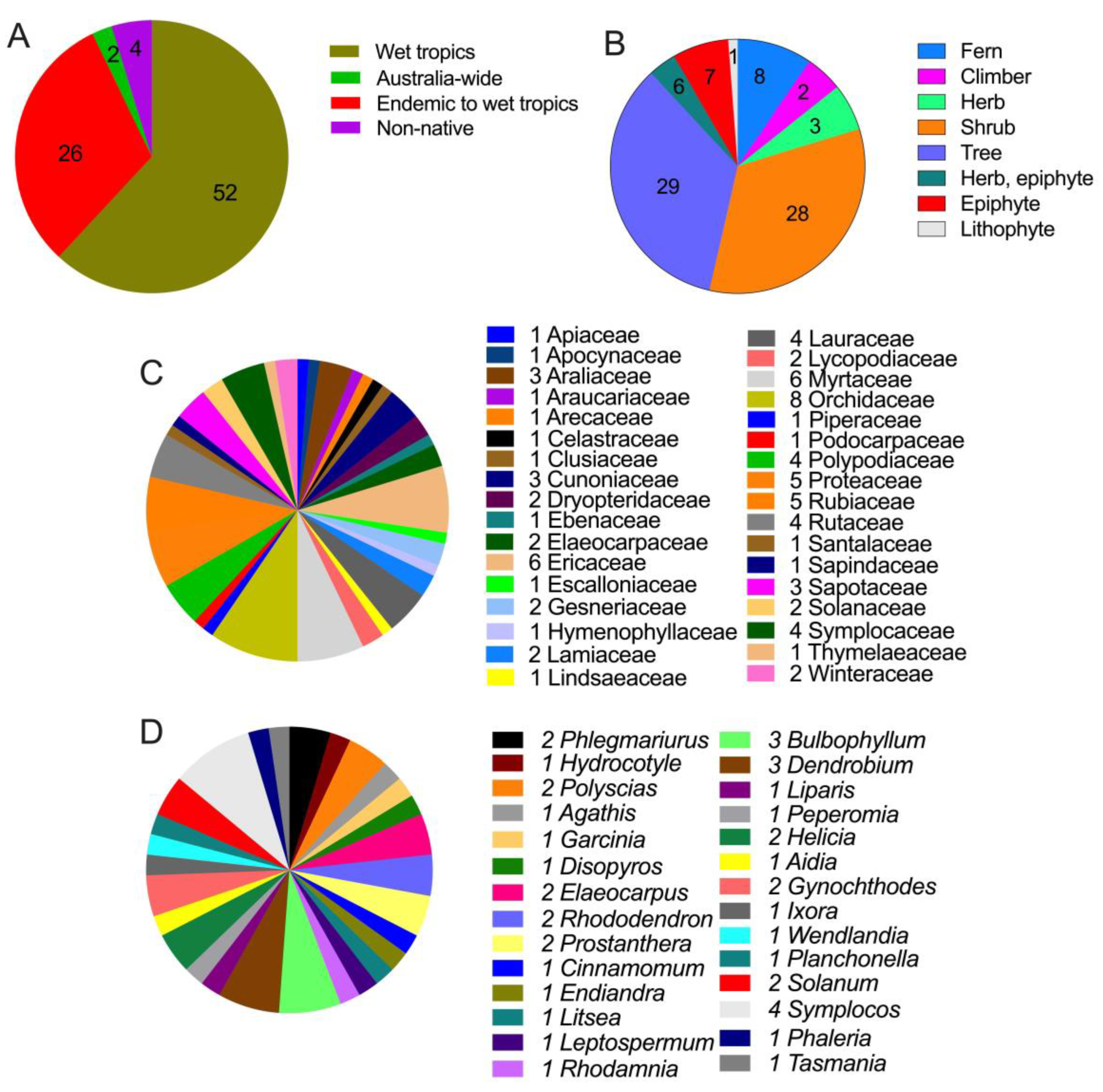
3. Metabolomic Profile of Climate-Affected Plants in WTWHA
4. Phytochemicals Isolated from Climate-Affected Plants in WTWHA
| Botanical name | Medicinal uses | Number and major metabolites identified | Isolated Compounds | Chemical class | Biological Activities of compounds |
|---|---|---|---|---|---|
| Agathis atropurpurea | Agathis species are traditionally used to treat myalgia and headaches [52]. | 27 metabolites; major metabolites are ⍺-pinene, ⍺-copaene, bicyclogermacrene, 𝛿-cadinene, phyllocladane, and 16-kaurene [115] | NA | Terpenoid | Antimicrobial, antibacterial, antiviral, anti-cancer activity (⍺-pinene) [116,117,118], antioxidant activity (⍺-copaene) [119] |
| Eucryphia wilkiei | NU | 2 unknown metabolites [120] | NA | Flavonoid | NA |
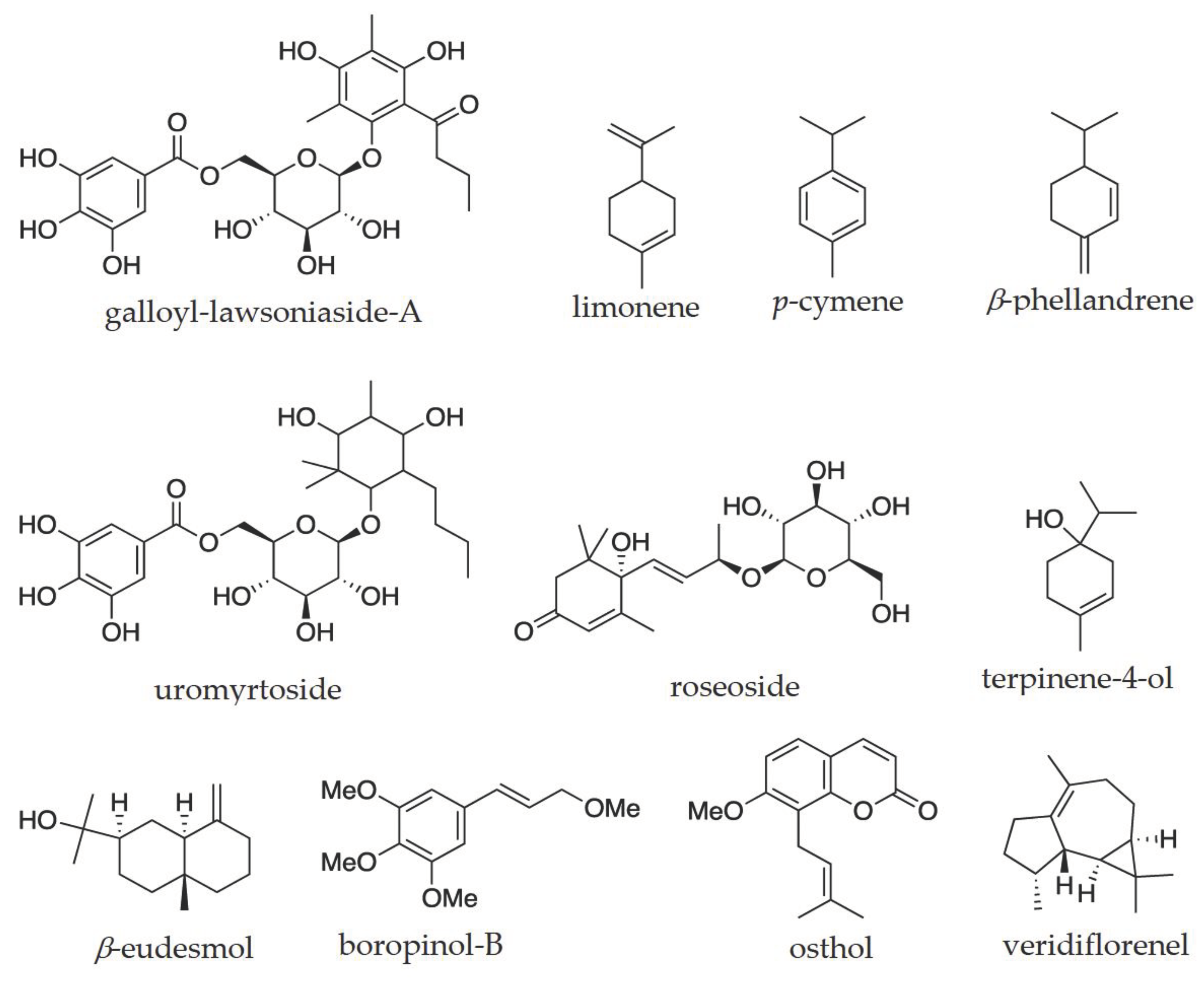
| Cinnamomum propinquum | Cinnamomum species are most commonly used in traditional Chinese medicines to treat multiple disorders, including indigestion, microbial infections, and cough and cold [59]. | 40 metabolites; Major metabolites are p-cymene, ⍺-pinene, and β-eudesmol [121] |
NA | Terpenoid | Anti-cancer activity (p-cymene) [122], anti-allergic and anti-angiogenic effect (β-eudesmol) [123,124] |
| Cryptocarya bellendenkerana | NU | 39 metabolites; major metabolites are ⍺-pinene, limonene, β-phellandrene, p-cymene, viridiflorene, E-β-farnesene, ⍺-copaene, β-and ⍺-selinene, 𝛿-cadinene, bicyclogermacrene, calamenene, and cubeban-11-ol [125]. | NA | Antioxidant, antidiabetic, anticancer, anti-inflammatory (limonene) [126,127], anti-fungal (β-phellandrene) [128], antioxidant and antitumour properties (viridiflorene) [129,130], insect repellent (E-β-farnesene) [131] antioxidant activity (copaene) [119]. | |
| Leptospermum wooroonooran | Leptospermum species are traditionally used in Malaysia to relieve menstrual and stomach disorders [62,63]. | 45 metabolites; major metabolitesare ⍺-pinene, β-pinene, sabinene, ⍺-terpinene, 𝛾-terpinene, terpinen-4-ol and ⍺-terpineol [132] | NA | Reduce skeletal muscle atrophy (sabinene) [133], antibacterial and antibiofilm activities (terpinene-4-ol) [134] | |
| Uromyrtus metrosideros | NU | 27 metabolites; major metabolites are ⍺-pinene, β-pinene, spathulenol and aromadendrene [135] | norbergenin, bergenin, (6S,9R)-roseoside, (4S)-𝜶-terpineol 8-O-β-D-(6-O-galloyl) glucopyranoside, galloyl-lawsoniaside A, and uromyrtoside [114] |
Benzopyran, Glucoside, |
Anti-inflammatory (galloyl-lawsoniaside A) [114]; reduced hypertension and allergic reaction (roseoside) [136,137] |
| Prumnopitys ladei | Fruits and bark of Prunmnopitys species are considered medicinal [70]. | 44-metabolites; major compounds are ⍺-pinene, limonene, verbenone, and p-cymene. β-caryophyllene, caryophyllene oxide, spathulenol, and ⍺-humulene [138] |
NA | Antimicrobial, anticarcinogenic, anti-inflammatory, antioxidant, and local anesthetic effects (β-caryophyllene) [139,140,141] | |
|
Flindersia oppositifolia |
NU | 37 metabolites; major compounds are β-caryophyllene and bicyclogermacrene [142]; Identified 8 alkaloids from leaf [143]. |
pimentelamine A, pimentelamine B, pimentelamine C, 2-isoprenyl-N-N-dimethyltryptamine, 4-methylborreverine, borreverine, dimethylisoborreverine, quercitrin, and carpachromene [142]; harmalan, pimentelamine B, isoborreverine, skimmianine, kokusaginine, maculosidine, flindersiamine, 8-methoxy-N-methylflindersine [143]. | Terpene, Alkaloid | Antiplasmodial (pimentelamine C) [144,145] |
| Leionema ellipticum | NU | 3,4ʹ,5-trimethoxyflavone-7-O-⍺-rhamnoside, boropinol-B, and osthol [146] |
Flavonoid | Neuroprotective (boropinol-B) [147,148]; anti-inflammatory (osthol) [149,150] | |
5. Pharmacological Activities of Isolated Phytochemicals of Climate-affected Plants in WTWHA
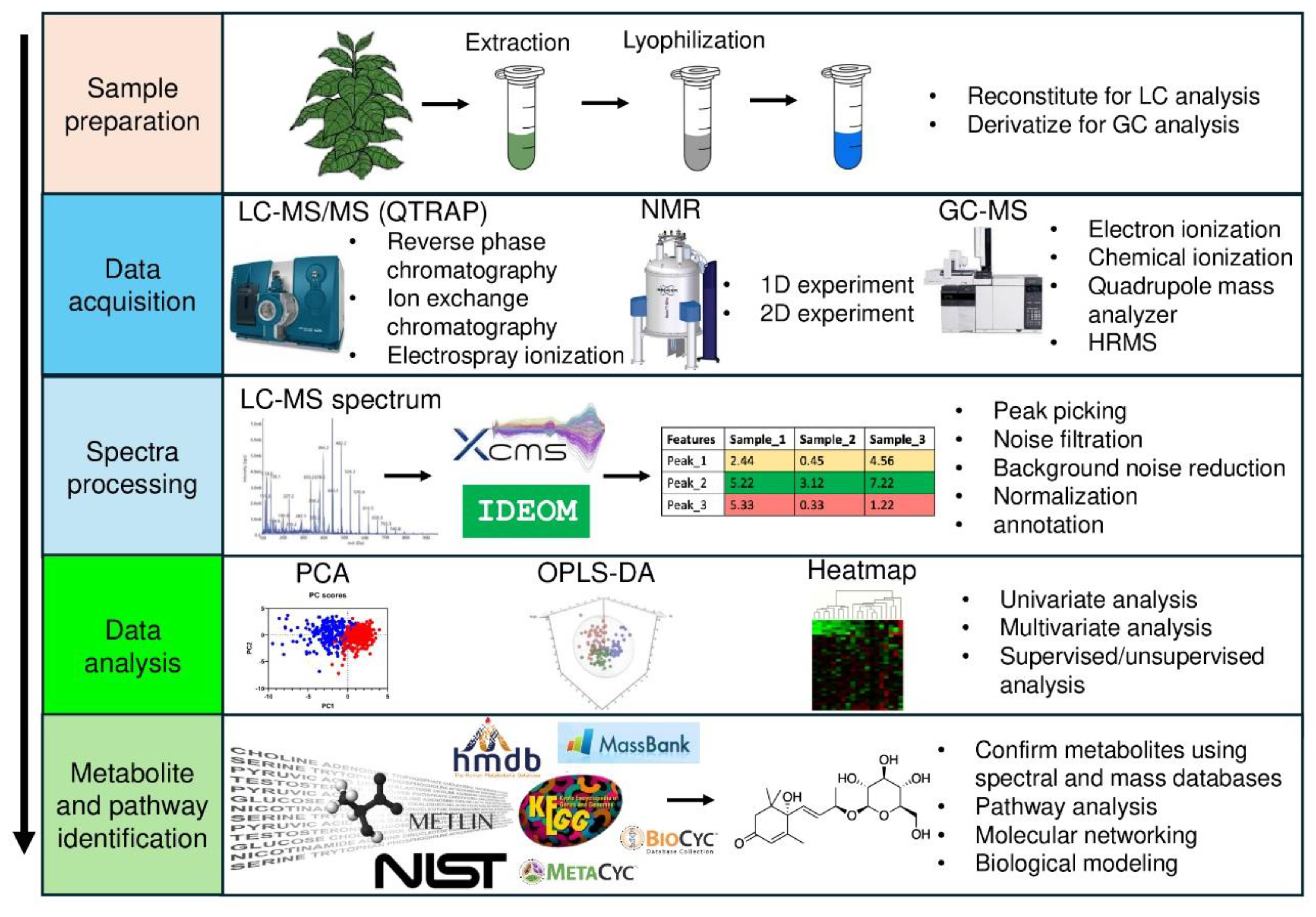
6. Metabolomics Approaches, Tools, and Techniques Used in Plant Metabolomics
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnell, N.W.; Lowe, J.A.; Challinor, A.J.; Osborn, T.J. Global and regional impacts of climate change at different levels of global temperature increase. Climatic Change 2019, 155, 377-391. [CrossRef]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiologia Plantarum 2021, 171, 66-76. [CrossRef]
- QLD. Climate change in the Far North Queensland region. 2019.
- Morris, R.J. Anthropogenic impacts on tropical forest biodiversity: a network structure and ecosystem functioning perspective. Philosophical Transactions of the Royal Society B: Biological Sciences 2010, 365, 3709-3718. [CrossRef]
- Hoyle, G.L.; Sommerville, K.D.; Liyanage, G.S.; Worboys, S.; Guja, L.K.; Stevens, A.V.; Crayn, D.M. Seed banking is more applicable to the preservation of tropical montane flora than previously assumed: A review and cloud forest case study. Global Ecology and Conservation 2023, 47. [CrossRef]
- Helmer, E.H.; Gerson, E.A.; Baggett, L.S.; Bird, B.J.; Ruzycki, T.S.; Voggesser, S.M. Neotropical cloud forests and páramo to contract and dry from declines in cloud immersion and frost. PLOS ONE 2019, 14, e0213155. [CrossRef]
- Costion, C.M.; Simpson, L.; Pert, P.L.; Carlsen, M.M.; John Kress, W.; Crayn, D. Will tropical mountaintop plant species survive climate change? Identifying key knowledge gaps using species distribution modelling in Australia. Biological Conservation 2015, 191, 322-330. [CrossRef]
- Karger, D.N.; Kessler, M.; Lehnert, M.; Jetz, W. Limited protection and ongoing loss of tropical cloud forest biodiversity and ecosystems worldwide. Nature Ecology & Evolution 2021, 5, 854-862. [CrossRef]
- Still, C.J.; Foster, P.N.; Schneider, S.H. Simulating the effects of climate change on tropical montane cloud forests. Nature 1999, 398, 608-610. [CrossRef]
- Foster, P. The potential negative impacts of global climate change on tropical montane cloud forests. Earth-Science Reviews 2001, 55, 73-106. [CrossRef]
- Hu, J.; Riveros-Iregui, D.A. Life in the clouds: are tropical montane cloud forests responding to changes in climate? Oecologia 2016, 180, 1061-1073. [CrossRef]
- Williams, S.E.; Bolitho, E.E.; Fox, S. Climate change in Australian tropical rainforests: an impending environmental catastrophe. Proc. R. Soc. B Biol. Sci. 2003, 270, 1887-1892. [CrossRef]
- Williams, S.E.; Bolitho, E.E.; Fox, S. Climate change in Australian tropical rainforests: an impending environmental catastrophe. Proc Biol Sci 2003, 270, 1887-1892. [CrossRef]
- Le Saout, S.; Hoffmann, M.; Shi, Y.; Hughes, A.; Bernard, C.; Brooks, T.M.; Bertzky, B.; Butchart, S.H.; Stuart, S.N.; Badman, T.; et al. Conservation. Protected areas and effective biodiversity conservation. Science 2013, 342, 803-805. [CrossRef]
- UNESCO World Heritage Convention. Wet Tropics of Queensland. Available online: https://whc.unesco.org/en/list/486/ (accessed on August 6 2023).
- Weber, E.T.; Catterall, C.P.; Locke, J.; Ota, L.S.; Prideaux, B.; Shirreffs, L.; Talbot, L.; Gordon, I.J. Managing a World Heritage Site in the Face of Climate Change: A Case Study of the Wet Tropics in Northern Queensland. Earth 2021, 2, 248-271. [CrossRef]
- Grossmann, G.; Krebs, M.; Maizel, A.; Stahl, Y.; Vermeer, J.E.M.; Ott, T. Green light for quantitative live-cell imaging in plants. Journal of Cell Science 2018, 131. [CrossRef]
- Awlia, M.; Alshareef, N.; Saber, N.; Korte, A.; Oakey, H.; Panzarová, K.; Trtílek, M.; Negrão, S.; Tester, M.; Julkowska, M.M. Genetic mapping of the early responses to salt stress in Arabidopsis thaliana. The Plant Journal 2021, 107, 544-563. [CrossRef]
- Berg, C.S.; Brown, J.L.; Weber, J.J. An examination of climate-driven flowering-time shifts at large spatial scales over 153 years in a common weedy annual. American journal of botany 2019, 106, 1435-1443. [CrossRef]
- Cortijo, S.; Charoensawan, V.; Brestovitsky, A.; Buning, R.; Ravarani, C.; Rhodes, D.; van Noort, J.; Jaeger, K.E.; Wigge, P.A. Transcriptional regulation of the ambient temperature response by H2A. Z nucleosomes and HSF1 transcription factors in Arabidopsis. Molecular plant 2017, 10, 1258-1273. [CrossRef]
- Sriden, N.; Charoensawan, V. Large-scale comparative transcriptomic analysis of temperature-responsive genes in Arabidopsis thaliana. Plant Molecular Biology 2022, 110, 425-443. [CrossRef]
- Zhao, Y.; Antoniou-Kourounioti, R.L.; Calder, G.; Dean, C.; Howard, M. Temperature-dependent growth contributes to long-term cold sensing. Nature 2020, 583, 825-829. [CrossRef]
- Liu, W.; Zhang, R.; Xiang, C.; Zhang, R.; Wang, Q.; Wang, T.; Li, X.; Lu, X.; Gao, S.; Liu, Z.; et al. Transcriptomic and Physiological Analysis Reveal That alpha-Linolenic Acid Biosynthesis Responds to Early Chilling Tolerance in Pumpkin Rootstock Varieties. Front Plant Sci 2021, 12, 669565. [CrossRef]
- Wang, Y.; Li, X.Y.; Li, C.X.; He, Y.; Hou, X.Y.; Ma, X.R. The Regulation of Adaptation to Cold and Drought Stresses in Poa crymophila Keng Revealed by Integrative Transcriptomics and Metabolomics Analysis. Front Plant Sci 2021, 12, 631117. [CrossRef]
- Sun, Y.; Alseekh, S.; Fernie, A.R. Plant secondary metabolic responses to global climate change: A meta-analysis in medicinal and aromatic plants. Global Change Biology 2023, 29, 477-504. [CrossRef]
- Hodges, M.; Dellero, Y.; Keech, O.; Betti, M.; Raghavendra, A.S.; Sage, R.; Zhu, X.-G.; Allen, D.K.; Weber, A.P. Perspectives for a better understanding of the metabolic integration of photorespiration within a complex plant primary metabolism network. Journal of Experimental Botany 2016, 67, 3015-3026. [CrossRef]
- Ncube, B.; Van Staden, J. Tilting plant metabolism for improved metabolite biosynthesis and enhanced human benefit. Molecules 2015, 20, 12698-12731.
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [CrossRef]
- Peng, M.; Shahzad, R.; Gul, A.; Subthain, H.; Shen, S.; Lei, L.; Zheng, Z.; Zhou, J.; Lu, D.; Wang, S. Differentially evolved glucosyltransferases determine natural variation of rice flavone accumulation and UV-tolerance. Nature communications 2017, 8, 1975. [CrossRef]
- Tohge, T.; Fernie, A.R. Leveraging natural variance towards enhanced understanding of phytochemical sunscreens. Trends in plant science 2017, 22, 308-315. [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.; Li, C.; Lee, I.H.; Lam, H.-M.; Chan, T.-F.; Hui, J.H. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020, 21, 7382. [CrossRef]
- Matsuura, H.N.; Rau, M.R.; Fett-Neto, A.G. Oxidative stress and production of bioactive monoterpene indole alkaloids: biotechnological implications. Biotechnology letters 2014, 36, 191-200. [CrossRef]
- Bakhtiari, M.; Rasmann, S. Variation in below-to aboveground systemic induction of glucosinolates mediates plant fitness consequences under herbivore attack. Journal of chemical ecology 2020, 46, 317-329. [CrossRef]
- Sardans, J.; Gargallo-Garriga, A.; Urban, O.; Klem, K.; Walker, T.W.N.; Holub, P.; Janssens, I.A.; Peñuelas, J. Ecometabolomics for a Better Understanding of Plant Responses and Acclimation to Abiotic Factors Linked to Global Change. Metabolites 2020, 10. [CrossRef]
- Ma, A.; Qi, X. Mining plant metabolomes: Methods, applications, and perspectives. Plant Commun 2021, 2, 100238. [CrossRef]
- Oh, S.-W.; Imran, M.; Kim, E.-H.; Park, S.-Y.; Lee, S.-G.; Park, H.-M.; Jung, J.-W.; Ryu, T.-H. Approach strategies and application of metabolomics to biotechnology in plants. Frontiers in Plant Science 2023, 14. [CrossRef]
- Colin, L.; Martin-Arevalillo, R.; Bovio, S.; Bauer, A.; Vernoux, T.; Caillaud, M.-C.; Landrein, B.; Jaillais, Y. Imaging the living plant cell: From probes to quantification. The Plant Cell 2021, 34, 247-272. [CrossRef]
- Hsiao, A.-S.; Huang, J.-Y. Bioimaging tools move plant physiology studies forward. Frontiers in Plant Science 2022, 13. [CrossRef]
- Uslu, V.V.; Grossmann, G. The biosensor toolbox for plant developmental biology. Current Opinion in Plant Biology 2016, 29, 138-147. [CrossRef]
- Gamalero, E.; Bona, E.; Glick, B.R. Current Techniques to Study Beneficial Plant-Microbe Interactions. Microorganisms 2022, 10. [CrossRef]
- Belbin, L.; Wallis, E.; Hobern, D.; Zerger, A. The Atlas of Living Australia: History, current state and future directions. Biodiversity Data Journal 9: e65023. Available online: (accessed on September 19).
- Zich, F.A.; Hyland, B.P.M.; Whiffin, T.; Kerrigan, R.A. Australian Tropical Rainforest Plants, Edition 8. 2020.
- Crayn, D.; Worboys, S. Personal communication. 2023.
- APC. Australian Plant Census IBIS database, Centre for Australian National Biodiversity Research, Council of Heads of Australasian Herbaria. 2024.
- WFO. World Flora Online. Version 2023.03. 2023. [CrossRef]
- CSIRO. CSIRO Annual Report 2010-11; ACT: Australia, 2011; pp. 1-176.
- E., R. Modelling the vulnerability of endemic montane flora to climate change in the Australian Wet Tropics. MSc thesis. Imperial College London, UK, 2018.
- Roeble, E. Modelling the vulnerability of endemic montane flora to climate change in the Australian Wet Tropics. Imperial College London, UK, 2018.
- Armijos, C.; Gilardoni, G.; Amay, L.; Lozano, A.; Bracco, F.; Ramirez, J.; Bec, N.; Larroque, C.; Finzi, P.V.; Vidari, G. Phytochemical and ethnomedicinal study of Huperzia species used in the traditional medicine of Saraguros in Southern Ecuador; AChE and MAO inhibitory activity. Journal of Ethnopharmacology 2016, 193, 546-554. [CrossRef]
- Hamdy, S.A.; El Hefnawy, H.M.; Azzam, S.M.; Aboutabl, E.A. Botanical and genetic characterization of Hydrocotyle umbellata L. cultivated in Egypt. Bulletin of Faculty of Pharmacy, Cairo University 2018, 56, 46-53. [CrossRef]
- Śliwińska, A.; Figat, R.; Zgadzaj, A.; Wileńska, B.; Misicka, A.; Nałęcz-Jawecki, G.; Pietrosiuk, A.; Sykłowska-Baranek, K. Polyscias filicifolia (Araliaceae) Hairy Roots with Antigenotoxic and Anti-Photogenotoxic Activity. Molecules 2021, 27. [CrossRef]
- Ho, Y.T.; Liu, I.H.; Chang, S.T.; Wang, S.Y.; Chang, H.T. In Vitro and In Vivo Antimelanogenesis Effects of Leaf Essential Oil from Agathis dammara. Pharmaceutics 2023, 15. [CrossRef]
- Espirito Santo, B.; Santana, L.F.; Kato Junior, W.H.; de Araújo, F.O.; Bogo, D.; Freitas, K.C.; Guimarães, R.C.A.; Hiane, P.A.; Pott, A.; Filiú, W.F.O.; et al. Medicinal Potential of Garcinia Species and Their Compounds. Molecules 2020, 25. [CrossRef]
- Rauf, A.; Uddin, G.; Patel, S.; Khan, A.; Halim, S.A.; Bawazeer, S.; Ahmad, K.; Muhammad, N.; Mubarak, M.S. Diospyros, an under-utilized, multi-purpose plant genus: A review. Biomedicine & Pharmacotherapy 2017, 91, 714-730. [CrossRef]
- Sudradjat, S.E.; Timotius, K.H. Pharmacological properties and phytochemical components of Elaeocarpus: A comparative study. Phytomedicine Plus 2022, 2, 100365. [CrossRef]
- Nisar, M.; Ali, S.; Qaisar, M.; Gilani, S.N.; Shah, M.R.; Khan, I.; Ali, G. Antifungal activity of bioactive constituents and bark extracts of Rhododendron arboreum. ||| Bangladesh Journal of Pharmacology 2013, 8, 218-222. [CrossRef]
- Sadgrove, N.J.; Padilla-González, G.F.; Telford, I.R.H.; Greatrex, B.W.; Jones, G.L.; Andrew, R.; Bruhl, J.J.; Langat, M.K.; Melnikovova, I.; Fernandez-Cusimamani, E. Prostanthera (Lamiaceae) as a 'Cradle of Incense': Chemophenetics of Rare Essential Oils from Both New and Forgotten Australian 'Mint Bush' Species. Plants (Basel) 2020, 9. [CrossRef]
- Lassak, E.V.; McCarthy, T. Australian medicinal plants. (No Title) 1983.
- Wang, J.; Su, B.; Jiang, H.; Cui, N.; Yu, Z.; Yang, Y.; Sun, Y. Traditional uses, phytochemistry and pharmacological activities of the genus Cinnamomum (Lauraceae): A review. Fitoterapia 2020, 146, 104675. [CrossRef]
- Salleh, W.M.N.H.W.; Farediah, A.; Khong, H.Y.; Zulkifli, R. A Review of Endiandric Acid Analogues. International Journal of Pharmacognosy and Phytochemical Research 2015, 7, 844-856.
- Cock, I.E. Medicinal and aromatic plants—Australia. In Ethnopharmacology Section, Biological, Physiological and Health Sciences, Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO; EOLSS Publishers: Oxford, UK, 2011. Available online: http://www.eolss.net (accessed on 30 April 2022).
- Caputo, L.; Smeriglio, A.; Trombetta, D.; Cornara, L.; Trevena, G.; Valussi, M.; Fratianni, F.; De Feo, V.; Nazzaro, F. Chemical Composition and Biological Activities of the Essential Oils of Leptospermum petersonii and Eucalyptus gunnii. Front Microbiol 2020, 11, 409. [CrossRef]
- Riley, M. Māori healing and herbal: New Zealand ethnobotanical sourcebook; Viking Sevenseas NZ: 1994.
- Oktavia, D.; Pratiwi, S.D.; Munawaroh, S.; Hikmat, A.; Hilwan, I. The potential of medicinal plants from heath forest: Local knowledge from Kelubi Village, Belitung Island, Indonesia. Biodiversitas Journal of Biological Diversity 2022, 23. [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Bouyahya, A.; El Menyiy, N.; El Omari, N.; Shahinozzaman, M.; Ara Haque Ovey, M.; Koirala, N.; Panthi, M.; Ertani, A.; et al. Ethnobotany, Phytochemistry, Biological Activities, and Health-Promoting Effects of the Genus Bulbophyllum. Evid Based Complement Alternat Med 2022, 2022, 6727609. [CrossRef]
- Cakova, V.; Bonte, F.; Lobstein, A. Dendrobium: Sources of Active Ingredients to Treat Age-Related Pathologies. Aging Dis 2017, 8, 827-849. [CrossRef]
- Liang, W.; Guo, X.; Nagle, D.G.; Zhang, W.-D.; Tian, X.-H. Genus Liparis: A review of its traditional uses in China, phytochemistry and pharmacology. Journal of Ethnopharmacology 2019, 234, 154-171. [CrossRef]
- Ware, I.; Franke, K.; Hussain, H.; Morgan, I.; Rennert, R.; Wessjohann, L.A. Bioactive Phenolic Compounds from Peperomia obtusifolia. Molecules 2022, 27. [CrossRef]
- Al-Madhagi, W.M.; Mohd Hashim, N.; Awad Ali, N.A.; Alhadi, A.A.; Abdul Halim, S.N.; Othman, R. -Chemical profiling and biological activity of Peperomia blanda (Jacq.) Kunth. PeerJ 2018, 6, e4839. [CrossRef]
- Inostroza-Blancheteau, C.; Sandoval, Y.; Reyes-Díaz, M.; Tighe-Neira, R.; González-Villagra, J. Phytochemical characterization and antioxidant properties of Prumnopitys andina fruits in different ripening stages in southern Chile. Chilean journal of agricultural research 2022, 82, 285-293. [CrossRef]
- Tlau, L.; Lalawmpuii, L. Commonly used medicinal plants in N. Mualcheng, Mizoram, India. Science Vision 2020, 20, 156-161. [CrossRef]
- Ray, S.; Saini, M.K. Impending threats to the plants with medicinal value in the Eastern Himalayas Region: An analysis on the alternatives to its non-availability. Phytomedicine Plus 2022, 2, 100151. [CrossRef]
- Cock, I.E. Medicinal and aromatic plants–Australia. Ethnopharmacology, Encyclopedia of Life Support Systems (EOLSS) 2011. [CrossRef]
- Palombo, E.A.; Semple, S.J. Antibacterial activity of traditional Australian medicinal plants. Journal of ethnopharmacology 2001, 77, 151-157. [CrossRef]
- Awang-Jamil, Z.; Basri, A.; Ahmad, N.; Taha, H. Phytochemical analysis, antimicrobial and antioxidant activities of Aidia borneensis leaf extracts. Journal of Applied Biology & Biotechnology 2019, 7. [CrossRef]
- Singh, B.; Sharma, R.A. Indian Morinda species: A review. Phytotherapy Research 2020, 34, 924-1007. [CrossRef]
- Baliga, M.S.; Kurian, P.J. Ixora coccinea Linn.: Traditional uses, phytochemistry and pharmacology. Chinese Journal of Integrative Medicine 2012, 18, 72-79. [CrossRef]
- Hossain, M.J.; Maliha, F.; Hawlader, M.B.; Farzana, M.; Rashid, M.A. Ethnomedicinal uses, phytochemistry, pharmacology and toxicological aspects of genus Wendlandia: an overview. Journal of Bangladesh Academy of Sciences 2023. [CrossRef]
- Chidambaram, K.; Alqahtani, T.; Alghazwani, Y.; Aldahish, A.; Annadurai, S.; Venkatesan, K.; Dhandapani, K.; Thilagam, E.; Venkatesan, K.; Paulsamy, P.; et al. Medicinal Plants of Solanum Species: The Promising Sources of Phyto-Insecticidal Compounds. J Trop Med 2022, 2022, 4952221. [CrossRef]
- Badoni, R.; Semwal, D.K.; Kothiyal, S.K.; Rawat, U. Chemical constituents and biological applications of the genus Symplocos. J Asian Nat Prod Res 2010, 12, 1069-1080. [CrossRef]
- Ahmad, R.; Khairul Nizam Mazlan, M.; Firdaus Abdul Aziz, A.; Mohd Gazzali, A.; Amir Rawa, M.S.; Wahab, H.A. Phaleria macrocarpa (Scheff.) Boerl.: An updated review of pharmacological effects, toxicity studies, and separation techniques. Saudi pharmaceutical journal : SPJ : the official publication of the Saudi Pharmaceutical Society 2023, 31, 874-888. [CrossRef]
- Mohanty, S. Bioactive properties of Australian native fruits, Tasmannia lanceolata and Terminalia ferdinandiana: The Characterization of their Active Compounds. Griffith University Australia 2016.
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet; http://www.plantsoftheworldonline.org/. 2023.
- Jones, D.L.; Hopley, T.; Duffy, S.M. Australian Tropical Rainforest Orchids. 2010.
- QLD. Nature Conservation Act 1992. 2017.
- Rivas-Ubach, A.; Pérez-Trujillo, M.; Sardans, J.; Gargallo-Garriga, A.; Parella, T.; Peñuelas, J. Ecometabolomics: optimized NMR-based method. Methods Ecol. Evol. 4, 464–473. 2013. [CrossRef]
- Rivas-Ubach, A.; Peñuelas, J.; Hódar, J.A.; Oravec, M.; Paša-Tolić, L.; Urban, O.; Sardans, J. We are what we eat: A stoichiometric and ecometabolomic study of caterpillars feeding on two pine subspecies of Pinus sylvestris. Int. J. Mol. Sci. 2018, 20, 59. [CrossRef]
- Allevato, D.M.; Kiyota, E.; Mazzafera, P.; Nixon, K.C. Ecometabolomic analysis of wild populations of Pilocarpus pennatifolius (Rutaceae) using unimodal analyses. Frontiers in plant science 2019, 10, 258. [CrossRef]
- Berini, J.L.; Brockman, S.A.; Hegeman, A.D.; Reich, P.B.; Muthukrishnan, R.; Montgomery, R.A.; Forester, J.D. Combinations of abiotic factors differentially alter production of plant secondary metabolites in five woody plant species in the boreal-temperate transition zone. Frontiers in plant science 2018, 9, 1257. [CrossRef]
- Steinbauer, M.J.; Grytnes, J.-A.; Jurasinski, G.; Kulonen, A.; Lenoir, J.; Pauli, H.; Rixen, C.; Winkler, M.; Bardy-Durchhalter, M.; Barni, E. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 2018, 556, 231-234. [CrossRef]
- Lavola, A.; Julkunen-Tiitto, R.; Aphalo, P.; de la Rosa, T.; Lehto, T. The effect of UV-B radiation on UV-absorbing secondary metabolites in birch seedlings grown under simulated forest soil conditions. The New Phytologist 1997, 137, 617-621.
- Salam, U.; Ullah, S.; Tang, Z.H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites against Different Environmental Stress Factors. Life (Basel) 2023, 13. [CrossRef]
- Sallas, L.; Luomala, E.-M.; Utriainen, J.; Kainulainen, P.; Holopainen, J.K. Contrasting effects of elevated carbon dioxide concentration and temperature on Rubisco activity, chlorophyll fluorescence, needle ultrastructure and secondary metabolites in conifer seedlings. Tree Physiology 2003, 23, 97-108. [CrossRef]
- Večeřová, K.; Klem, K.; Veselá, B.; Holub, P.; Grace, J.; Urban, O. Combined Effect of Altitude, Season and Light on the Accumulation of Extractable Terpenes in Norway Spruce Needles. Forests 2021, 12. [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejeryte, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27. [CrossRef]
- Pinto, D.M.; Blande, J.D.; Souza, S.R.; Nerg, A.M.; Holopainen, J.K. Plant volatile organic compounds (VOCs) in ozone (O3) polluted atmospheres: the ecological effects. J Chem Ecol 2010, 36, 22-34. [CrossRef]
- Schneider, G.F.; Coley, P.D.; Younkin, G.C.; Forrister, D.L.; Mills, A.G.; Kursar, T.A. Phenolics lie at the centre of functional versatility in the responses of two phytochemically diverse tropical trees to canopy thinning. J Exp Bot 2019, 70, 5853-5864. [CrossRef]
- Pinasseau, L.; Vallverdu-Queralt, A.; Verbaere, A.; Roques, M.; Meudec, E.; Le Cunff, L.; Peros, J.P.; Ageorges, A.; Sommerer, N.; Boulet, J.C.; et al. Cultivar Diversity of Grape Skin Polyphenol Composition and Changes in Response to Drought Investigated by LC-MS Based Metabolomics. Front Plant Sci 2017, 8, 1826. [CrossRef]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: a model for environmental metabolomics of plants. Sci 2016, 6, 29265. [CrossRef]
- Niinemets, Ü. Uncovering the hidden facets of drought stress: secondary metabolites make the difference. Tree Physiology 2016, 36, 129-132. [CrossRef]
- Afzal, S.F.; Yar, A.K.; Ullah, R.H.; Ali, B.G.; Ali, J.S.; Ahmad, J.S.; Fu, S. Impact of drought stress on active secondary metabolite production in Cichorium intybus roots. J Appl Environ Biol Sci 2017, 7, 39-43.
- Punia, H.; Tokas, J.; Malik, A.; Bajguz, A.; El-Sheikh, M.A.; Ahmad, P. Ascorbate-Glutathione Oxidant Scavengers, Metabolome Analysis and Adaptation Mechanisms of Ion Exclusion in Sorghum under Salt Stress. Int J Mol Sci 2021, 22. [CrossRef]
- Singiri, J.R.; Swetha, B.; Sikron-persi, N.; Grafi, G. Differential response to single and combined salt and heat stresses: Impact on accumulation of proteins and metabolites in dead pericarps of Brassica juncea. Int. J. Mol. Sci. 2021, 22. [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops–what is the cost? New phytologist 2015, 208, 668-673.
- Goche, T.; Shargie, N.G.; Cummins, I.; Brown, A.P.; Chivasa, S.; Ngara, R. Comparative physiological and root proteome analyses of two sorghum varieties responding to water limitation. Sci. Rep. 2020, 10, 11835. [CrossRef]
- Xiao, Q.; Mu, X.; Liu, J.; Li, B.; Liu, H.; Zhang, B.; Xiao, P. Plant metabolomics: a new strategy and tool for quality evaluation of Chinese medicinal materials. Chinese Medicine 2022, 17, 45. [CrossRef]
- Guy, C.; Kopka, J.; Moritz, T. Plant metabolomics coming of age. Physiol Plant 2008, 132, 113-116. [CrossRef]
- Hall, R.; Beale, M.; Fiehn, O.; Hardy, N.; Sumner, L.; Bino, R. Plant metabolomics: the missing link in functional genomics strategies. Plant Cell 2002, 14, 1437-1440. [CrossRef]
- Neilson, E.H.; Goodger, J.Q.; Woodrow, I.E.; Møller, B.L. Plant chemical defense: at what cost? Trends in plant science 2013, 18, 250-258.
- Kesselmeier, J.; Staudt, M. Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. Journal of atmospheric chemistry 1999, 33, 23-88.
- Pichersky, E.; Gang, D.R. Genetics and biochemistry of secondary metabolites in plants: an evolutionary perspective. Trends Plant Sci 2000, 5, 439-445. [CrossRef]
- Fiehn, O. Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks. Comp Funct Genomics 2001, 2, 155-168. [CrossRef]
- Kim, H.K.; Verpoorte, R. Sample preparation for plant metabolomics. Phytochem Anal 2010, 21, 4-13. [CrossRef]
- Ritmejeryte, E.; Ryan, R.Y.M.; Byatt, B.J.; Peck, Y.; Yeshi, K.; Daly, N.L.; Zhao, G.; Crayn, D.; Loukas, A.; Pyne, S.G.; et al. Anti-inflammatory properties of novel galloyl glucosides isolated from the Australian tropical plant Uromyrtus metrosideros. Chem Biol Interact 2022, 368, 110124. [CrossRef]
- Garrison, M.S.; Irvine, A.K.; Setzer, W.N. Chemical composition of the resin essential oil from Agathis atropurpurea from North Queensland, Australia. Am. J. Essent. Oils Nat. Prod 2016, 4, 4-5.
- Risner, D.; Marco, M.L.; Pace, S.A.; Spang, E.S. The Potential Production of the Bioactive Compound Pinene Using Whey Permeate. Processes 2020, 8, 263. [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; S, L.D.J.; D, A.D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9. [CrossRef]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305-6316. [CrossRef]
- Türkez, H.; Celik, K.; Toğar, B. Effects of copaene, a tricyclic sesquiterpene, on human lymphocytes cells in vitro. Cytotechnology 2014, 66, 597-603. [CrossRef]
- Wollenweber, E.; Dörr, M.; Rozefelds, A.C.; Minchin, P.; Forster, P.I. Variation in flavonoid exudates in Eucryphia species from Australia and South America. Biochemical Systematics and Ecology 2000, 28, 111-118. [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I. The Leaf Oils of the Australian Species ofCinnamomum(Lauraceae). Journal of Essential Oil Research 2001, 13, 332-335. [CrossRef]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.L.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health beneficial and pharmacological properties of p-cymene. Food and Chemical Toxicology 2021, 153, 112259. [CrossRef]
- Han, N.R.; Moon, P.D.; Ryu, K.J.; Jang, J.B.; Kim, H.M.; Jeong, H.J. β-eudesmol suppresses allergic reactions via inhibiting mast cell degranulation. Clin Exp Pharmacol Physiol 2017, 44, 257-265. [CrossRef]
- Tshering, G.; Pimtong, W.; Plengsuriyakarn, T.; Na-Bangchang, K. Anti-angiogenic effects of beta-eudesmol and atractylodin in developing zebrafish embryos. Comp Biochem Physiol C Toxicol Pharmacol 2021, 243, 108980. [CrossRef]
- Brophy, J.J.; Forster, P.I.; Goldsack, R.J. Coconut Laurels: The Leaf Essential Oils from Four Endemic Australian Cryptocarya Species: C. bellendenkerana, C. cocosoides, C. cunninghamii and C. lividula (Lauraceae). Nat Prod Commun 2016, 11, 255-258.
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A multifunctional compound with potent therapeutic effects. J Food Biochem 2021, 45, e13566. [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem Biol Interact 2018, 283, 97-106. [CrossRef]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Tiyajamorn, T.; Bharathi, M.; Chaiyasut, C. A Narrative Review on the Bioactivity and Health Benefits of Alpha-Phellandrene. Scientia Pharmaceutica 2022, 90, 57. [CrossRef]
- Ferraz, R.P.; Cardoso, G.M.; da Silva, T.B.; Fontes, J.E.; Prata, A.P.; Carvalho, A.A.; Moraes, M.O.; Pessoa, C.; Costa, E.V.; Bezerra, D.P. Antitumour properties of the leaf essential oil of Xylopia frutescens Aubl. (Annonaceae). Food Chem 2013, 141, 196-200. [CrossRef]
- Minh, P.T.H.; Tuan, N.T.; Van, N.T.H.; Bich, H.T.; Lam, D.T. Chemical Composition and Biological Activities of Essential Oils of Four Asarum Species Growing in Vietnam. Molecules 2023, 28. [CrossRef]
- Qin, Y.; Zhang, J.; Song, D.; Duan, H.; Li, W.; Yang, X. Novel (E)-β-Farnesene Analogues Containing 2-Nitroiminohexahydro-1,3,5-triazine: Synthesis and Biological Activity Evaluation. Molecules 2016, 21, 825.
- brophy2000.pdf.
- Ryu, Y.; Lee, D.; Jung, S.H.; Lee, K.J.; Jin, H.; Kim, S.J.; Lee, H.M.; Kim, B.; Won, K.J. Sabinene Prevents Skeletal Muscle Atrophy by Inhibiting the MAPK-MuRF-1 Pathway in Rats. Int J Mol Sci 2019, 20. [CrossRef]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Medeiros, D.; Nóbrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus. Int J Mol Sci 2020, 21. [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I. The Essential Oils of the Australian Species of Uromyrtus (Myrtaceae). Flavour and Fragrance Journal 1996, 11, 133-138. [CrossRef]
- Hong, E.Y.; Kim, T.Y.; Hong, G.U.; Kang, H.; Lee, J.Y.; Park, J.Y.; Kim, S.C.; Kim, Y.H.; Chung, M.H.; Kwon, Y.I.; et al. Inhibitory Effects of Roseoside and Icariside E4 Isolated from a Natural Product Mixture (No-ap) on the Expression of Angiotensin II Receptor 1 and Oxidative Stress in Angiotensin II-Stimulated H9C2 Cells. Molecules 2019, 24. [CrossRef]
- Yajima, A.; Oono, Y.; Nakagawa, R.; Nukada, T.; Yabuta, G. A simple synthesis of four stereoisomers of roseoside and their inhibitory activity on leukotriene release from mice bone marrow-derived cultured mast cells. Bioorg Med Chem 2009, 17, 189-194. [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I. Chemistry of the Australian Gymnosperms Part VIII. The Leaf Oil ofPrumnopitys ladei(Podocarpaceae). Journal of Essential Oil Research 2006, 18, 212-214. [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808-11829. [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Applied Sciences 2019, 9, 5420. [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med 2016, 5, 3007-3017. [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I. The Leaf Oils of the Australian Species of Flindersia (Rutaceae). Journal of Essential Oil Research 2005, 17, 388-395. [CrossRef]
- Robertson, L.P.; Hall, C.R.; Forster, P.I.; Carroll, A.R. Alkaloid diversity in the leaves of Australian Flindersia (Rutaceae) species driven by adaptation to aridity. Phytochemistry 2018, 152, 71-81. [CrossRef]
- Robertson, L.P.; Duffy, S.; Wang, Y.; Wang, D.; Avery, V.M.; Carroll, A.R. Pimentelamines A-C, Indole Alkaloids Isolated from the Leaves of the Australian Tree Flindersia pimenteliana. J Nat Prod 2017, 80, 3211-3217. [CrossRef]
- Robertson, L.P.; Lucantoni, L.; Avery, V.M.; Carroll, A.R. Antiplasmodial Bis-Indole Alkaloids from the Bark of Flindersia pimenteliana. Planta Med 2020, 86, 19-25. [CrossRef]
- Resch, M.; Steigel, A.; Chen, Z.-l.; Bauer, R. 5-Lipoxygenase and Cyclooxygenase-1 Inhibitory Active Compounds from Atractylodes lancea. J. Nat. Prod. 1998, 61, 347-350. [CrossRef]
- Mu, K.; Zhang, J.; Feng, X.; Zhang, D.; Li, K.; Li, R.; Yang, P.; Mao, S. Sedative-hypnotic effects of Boropinol-B on mice via activation of GABAA receptors. J Pharm Pharmacol 2023, 75, 57-65. [CrossRef]
- Hu, Q.; Luo, L.; Yang, P.; Mu, K.; Yang, H.; Mao, S. Neuroprotection of boropinol-B in cerebral ischemia-reperfusion injury by inhibiting inflammation and apoptosis. Brain Research 2023, 1798, 148132. [CrossRef]
- Liu, J.H.; Zschocke, S.; Reininger, E.; Bauer, R. Inhibitory effects of Angelica pubescens f. biserrata on 5-lipoxygenase and cyclooxygenase. Planta Med 1998, 64, 525-529. [CrossRef]
- Resch, M.; Steigel, A.; Chen, Z.L.; Bauer, R. 5-Lipoxygenase and cyclooxygenase-1 inhibitory active compounds from Atractylodes lancea. J Nat Prod 1998, 61, 347-350. [CrossRef]
- Yeshi, K.; Ruscher, R.; Miles, K.; Crayn, D.; Liddell, M.; Wangchuk, P. Antioxidant and Anti-Inflammatory Activities of Endemic Plants of the Australian Wet Tropics. Plants (Basel) 2022, 11. [CrossRef]
- Yeshi, K.; Wangchuk, P. Bush Medicinal Plants of the Australian Wet Tropics and Their Biodiscovery Potential. In Bioprospecting of Tropical Medicinal Plants, Arunachalam, K., Yang, X., Puthanpura Sasidharan, S., Eds.; Springer Nature Switzerland: Cham, 2023; pp. 357-379.
- Giménez-Bastida, J.A.; González-Sarrías, A.; Laparra-Llopis, J.M.; Schneider, C.; Espín, J.C. Targeting Mammalian 5-Lipoxygenase by Dietary Phenolics as an Anti-Inflammatory Mechanism: A Systematic Review. Int J Mol Sci 2021, 22. [CrossRef]
- Rådmark, O.; Samuelsson, B. 5-Lipoxygenase: mechanisms of regulation1. J. Lipid Res. 2009, 50, S40-S45. [CrossRef]
- Barbier de Reuille, P.; Routier-Kierzkowska, A.-L.; Kierzkowski, D.; Bassel, G.W.; Schüpbach, T.; Tauriello, G.; Bajpai, N.; Strauss, S.; Weber, A.; Kiss, A.; et al. MorphoGraphX: A platform for quantifying morphogenesis in 4D. eLife 2015, 4, e05864. [CrossRef]
- Fernandez, R.; Das, P.; Mirabet, V.; Moscardi, E.; Traas, J.; Verdeil, J.-L.; Malandain, G.; Godin, C. Imaging plant growth in 4D: robust tissue reconstruction and lineaging at cell resolution. Nature Methods 2010, 7, 547-553. [CrossRef]
- Perez de Souza, L.; Alseekh, S.; Scossa, F.; Fernie, A.R. Ultra-high-performance liquid chromatography high-resolution mass spectrometry variants for metabolomics research. Nat Methods 2021, 18, 733-746. [CrossRef]
- Jamtsho, T.; Yeshi, K.; Perry, M.J.; Loukas, A.; Wangchuk, P. Approaches, Strategies and Procedures for Identifying Anti-Inflammatory Drug Lead Molecules from Natural Products. Pharmaceuticals 2024, 17, 283. [CrossRef]
- Markley, J.L.; Brüschweiler, R.; Edison, A.S.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The future of NMR-based metabolomics. Curr Opin Biotechnol 2017, 43, 34-40. [CrossRef]
- Dunn, W.B.; Bailey, N.J.; Johnson, H.E. Measuring the metabolome: current analytical technologies. Analyst 2005, 130, 606-625. [CrossRef]
- Castrillo, J.I.; Hayes, A.; Mohammed, S.; Gaskell, S.J.; Oliver, S.G. An optimized protocol for metabolome analysis in yeast using direct infusion electrospray mass spectrometry. Phytochemistry 2003, 62, 929-937. [CrossRef]
- Maia, M.; Figueiredo, A.; Cordeiro, C.; Sousa Silva, M. FT-ICR-MS-based metabolomics: A deep dive into plant metabolism. Mass Spectrometry Reviews 2023, 42, 1535-1556. [CrossRef]
- Andrews, G.L.; Simons, B.L.; Young, J.B.; Hawkridge, A.M.; Muddiman, D.C. Performance characteristics of a new hybrid quadrupole time-of-flight tandem mass spectrometer (TripleTOF 5600). Analytical chemistry 2011, 83, 5442-5446. [CrossRef]
- Pelander, A.; Decker, P.; Baessmann, C.; Ojanperä, I. Evaluation of a high resolving power time-of-flight mass spectrometer for drug analysis in terms of resolving power and acquisition rate. Journal of the American Society for Mass Spectrometry 2011, 22, 379-385. [CrossRef]
- Ghaste, M.; Mistrik, R.; Shulaev, V. Applications of fourier transform ion cyclotron resonance (FT-ICR) and orbitrap based high resolution mass spectrometry in metabolomics and lipidomics. Int. J. Mol. Sci. 2016, 17, 816. [CrossRef]
- Glauser, G.; Veyrat, N.; Rochat, B.; Wolfender, J.-L.; Turlings, T.C. Ultra-high pressure liquid chromatography–mass spectrometry for plant metabolomics: A systematic comparison of high-resolution quadrupole-time-of-flight and single stage Orbitrap mass spectrometers. Journal of chromatography A 2013, 1292, 151-159. [CrossRef]
- Park, S.-G.; Mohr, J.P.; Anderson, G.A.; Bruce, J.E. Application of frequency multiple FT-ICR MS signal acquisition for improved proteome research. International journal of mass spectrometry 2021, 465, 116578. [CrossRef]
- Schuhmann, K.; Herzog, R.; Schwudke, D.; Metelmann-Strupat, W.; Bornstein, S.R.; Shevchenko, A. Bottom-up shotgun lipidomics by higher energy collisional dissociation on LTQ Orbitrap mass spectrometers. Analytical chemistry 2011, 83, 5480-5487. [CrossRef]
- Schuhmann, K.; Almeida, R.; Baumert, M.; Herzog, R.; Bornstein, S.R.; Shevchenko, A. Shotgun lipidomics on a LTQ Orbitrap mass spectrometer by successive switching between acquisition polarity modes. Journal of Mass Spectrometry 2012, 47, 96-104. [CrossRef]
- Allwood, J.W.; Parker, D.; Beckmann, M.; Draper, J.; Goodacre, R. Fourier Transform Ion Cyclotron Resonance mass spectrometry for plant metabolite profiling and metabolite identification. Methods Mol Biol 2012, 860, 157-176. [CrossRef]
- Barrow, M.P.; Burkitt, W.I.; Derrick, P.J. Principles of Fourier transform ion cyclotron resonance mass spectrometry and its application in structural biology. Analyst 2005, 130, 18-28. [CrossRef]
- Hiraoka, K. Fundamentals of mass spectrometry; Springer: 2013; Volume 8.
- Folli, G.S.; Souza, L.M.; Araújo, B.Q.; Romão, W.; Filgueiras, P.R. Estimating the intermediate precision in petroleum analysis by (±) electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Rapid Communications in Mass Spectrometry 2020, 34, e8861. [CrossRef]
- Hughey, C.A.; Rodgers, R.P.; Marshall, A.G. Resolution of 11 000 compositionally distinct components in a single electrospray ionization Fourier transform ion cyclotron resonance mass spectrum of crude oil. Analytical Chemistry 2002, 74, 4145-4149. [CrossRef]
- Allwood, J.W.; De Vos, R.C.; Moing, A.; Deborde, C.; Erban, A.; Kopka, J.; Goodacre, R.; Hall, R.D. Plant metabolomics and its potential for systems biology research: Background concepts, technology, and methodology. Methods in enzymology 2011, 500, 299-336.
- Shahbazy, M.; Moradi, P.; Ertaylan, G.; Zahraei, A.; Kompany-Zareh, M. FTICR mass spectrometry-based multivariate analysis to explore distinctive metabolites and metabolic pathways: A comprehensive bioanalytical strategy toward time-course metabolic profiling of Thymus vulgaris plants responding to drought stress. Plant Science 2020, 290, 110257. [CrossRef]
- Janz, D.; Behnke, K.; Schnitzler, J.-P.; Kanawati, B.; Schmitt-Kopplin, P.; Polle, A. Pathway analysis of the transcriptome and metabolome of salt sensitive and tolerant poplar species reveals evolutionary adaption of stress tolerance mechanisms. BMC Plant Biology 2010, 10, 1-17. [CrossRef]
- Kaling, M.; Kanawati, B.; Ghirardo, A.; Albert, A.; Winkler, J.B.; Heller, W.; Barta, C.; Loreto, F.; SCHMITT-KOPPLIN, P.; SCHNITZLER, J.P. UV-B mediated metabolic rearrangements in poplar revealed by non-targeted metabolomics. Plant, cell & environment 2015, 38, 892-904.
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol Biol 2002, 48, 155–171.
- Silva, L.P.; Northen, T.R. Exometabolomics and MSI: deconstructing how cells interact to transform their small molecule environment. Curr Opin Biotechnol 2015, 34, 209-216. [CrossRef]
- Mapelli, V.; Olsson, L.; Nielsen, J. Metabolic footprinting in microbiology: methods and applications in functional genomics and biotechnology. Trends in Biotechnology 2008, 26, 490-497. [CrossRef]
- Kuzina, V.; Ekstrøm, C.T.; Andersen, S.B.; Nielsen, J.K.; Olsen, C.E.; Bak, S. Identification of defense compounds in Barbarea vulgaris against the herbivore Phyllotreta nemorum by an ecometabolomic approach. Plant physiology 2009, 151, 1977-1990. [CrossRef]
- Salek, R.M.; Steinbeck, C.; Viant, M.R.; Goodacre, R.; Dunn, W.B. The role of reporting standards for metabolite annotation and identification in metabolomic studies. GigaScience 2013, 2, 13. [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211-221. [CrossRef]
- Xu, Y.; Fu, X. Reprogramming of Plant Central Metabolism in Response to Abiotic Stresses: A Metabolomics View. Int. J. Mol. Sci. 2022, 23, 5716. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





